Abstract
Chemists’ ability to synthesize structurally complex, high-value organic molecules from simple starting materials is limited by a paucity of methods to selectively activate and functionalize strong covalent bonds. Recent activity in this field has focused on the activation of abundant C–O, C–N, and C–C bonds via a mechanistic paradigm of oxidative addition of a low-valent, electron-rich transition metal. This approach typically employs nickel(0), rhodium(I), ruthenium(0), and iron(I) catalysts under conditions that are finely tuned for specific, electronically activated substrates, sometimes assisted by chelating functional groups or ring strain. For instance, in the context of C–O oxidative addition, common substrates include esters or aryl, benzyl, or allyl ethers. By adopting a distinct strategy involving palladium(II)-catalyzed C–H activation followed by β-heteroatom/carbon elimination, we describe a novel catalytic method to activate alkyl C(sp3)–oxygen, nitrogen, carbon, fluorine, and sulfur bonds. Directed hydrofunctionalization of the resultant palladium(II)-bound alkene intermediate leads to a formal functional group metathesis. The method is applied toward amino acid upconversion with complete regioselectivity and moderate to high retention of enantiomeric excess. Low-strain five- and six-membered heterocycles undergo strong bond activation and substitution to give ring-opened products. High selectivity is obtained with substrates containing multiple potentially reactive C–heteroatom bonds by virtue of the high site-selectivity of the directed C–H activation step.
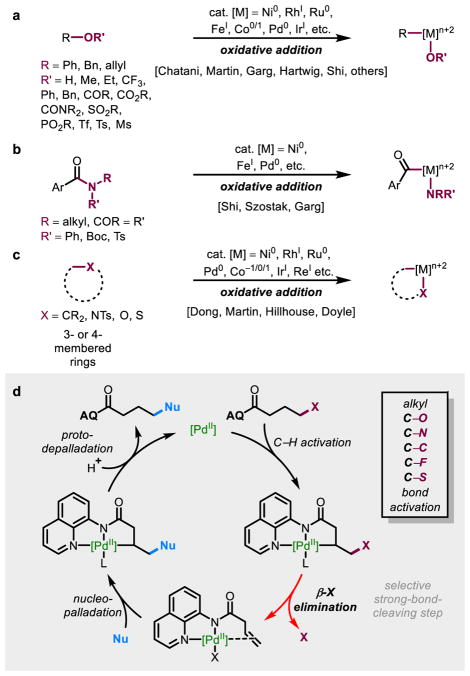
The selective activation of strong covalent bonds via transition metal catalysis allows functionalization of traditionally inert starting materials and enables novel retrosynthetic disconnections. In recent years, immense effort has focused on the use of transition metals for activating C–O, C–N, and C–C bonds via an oxidative addition pathway using mainly nickel(0), rhodium(I), ruthenium(0), and iron(I) catalysts, allowing the respective starting materials to serve as electrophiles in catalytic transformations, such as cross-coupling (Figure 1a–c).1–20 Oxidative addition to a C–heteroatom or C–C bond requires an appropriately electron-rich metal center, a directing group proximal to the bond of interest, an activated bond (e.g., a strained ring or a system that contains an adjacent heteroatom), or a combination of these features. Consequently, the substrate scope and synthetic utility are limited. In regards to catalytic C–O bond activation via oxidative addition, for instance, substrates are limited to aryl, vinyl, benzyl, or allyl C–O bonds1, 2, 5–7 or strained-ring systems, such as epoxides4. Successful cases of oxidative addition to an unactivated alkyl C–O bond generally involve stoichiometric transition metal species and reactions that are driven by the high stability of the resulting well-defined organometallic complexes.21–27 These limitations are largely a consequence of an oxidative addition approach to strong covalent bond activation, pointing to the need for alternative catalytic approaches to C–heteroatom and C–C cleavage that would complement the selectivity and reactivity patterns of existing methods. To this end, we envisioned a strategy that would take advantage of β-heteroatom/carbon (β-X) elimination from an alkylpalladium(II) intermediate28–35 generated from an initial C(sp3)–H activation step (Figure 1d). This approach takes inspiration from non-classical C–F and C–O bond activation by stoichiometric iridium pincer complexes36–38 and iron catalysts39. To ensure selectivity, we chose to access this intermediate from a well-established C–H activation step enabled by Daugulis’s bidentate 8-aminoquinoline (AQ) directing group.40 Though the fundamental β-X elimination step has been previously documented28–35, examples of its application toward small molecule synthesis are rare, with the Catellani reaction being a notable exception30.
Figure 1. Common approaches to activate strong bonds via oxidative addition and this new strategy for activating alkyl C–O, N, C, F, and S bonds.
A brief overview of well-known methods to activate strong bonds a, oxidative addition into aryl, benzyl, or allyl C–O bonds, b, oxidative addition into protected amide C–N bonds, c, oxidative addition facilitated by ring strain release. d, This work, a different approach through C–H activation followed by β-elimination to break strong bonds selectively.
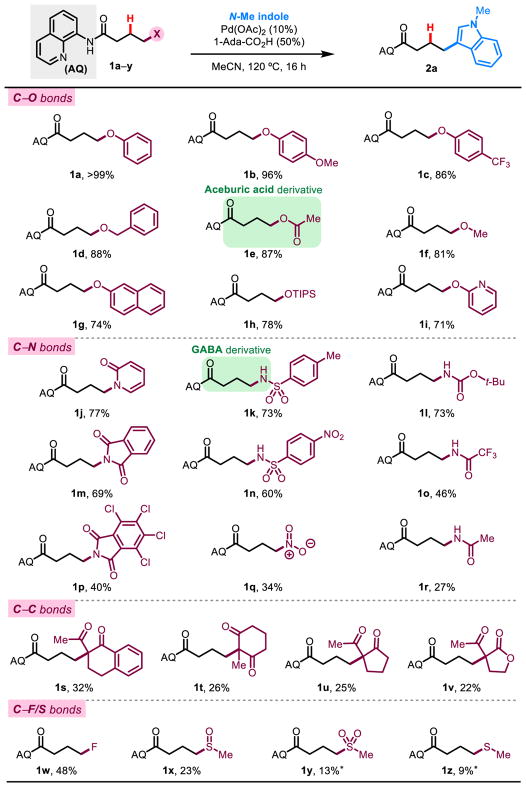
To initiate the study, we examined a series of butanoic acid substrates bearing the AQ group and various leaving groups at the γ-position. With the AQ group, we sought to access an alkyl palladium species through well-precedented C–H activation at the β-position,40 leading to β-X elimination and thus selective activation of the γ C–X bond. In the presence of a suitable nucleophile, the putative alkene intermediate would undergo hydrofunctionalization41–43 to effect net functional group metathesis and render the process catalytic. We optimized the reaction using phthalimide as the leaving group (see SI), as it had intermediate reactivity compared to other leaving groups, and identified optimal conditions with Pd(OAc)2 (10 mol%) as catalyst, 1-adamantane carboxylic acid (1-Ada-CO2H) (50 mol%) as promoter, and MeCN as solvent at 120 °C for 16 h. Notably, other inexpensive carboxylic acid promoters, such as acetic acid, functioned nearly as well. We then proceeded to survey an array of oxygen-, nitrogen-, carbon-, fluorine-, and sulfur-based leaving groups with N-methylindole as the nucleophile (Figure 2). Surprisingly, reaction efficiency under optimized conditions does not closely follow classical leaving group ability trends. Comparison of substrates 1e (acetate) and 1f (methoxide) demonstrates this point, as the respective leaving group conjugate acids have pKa values that are greater than 10 log units apart, yet perform similarly in the reaction. At the time, these trends cannot be easily explained. We found oxygen-based leaving groups to be most efficient with nearly quantitative yields for phenol leaving groups (1a–c). Groups on which SN2 reactions are untenable such as benzyloxide (1d), methoxide (1f), and siloxide (1h) functioned well under our conditions. Additionally, C–O bond activation in these cases were selective for the γ-position regardless of classic oxidative addition trends. For example, an oxidative addition approach would be unselective if possible in the case of 1f (a dialkyl ether) and selective for the undesired benzylic position in the case of 1d. For C–N bond activation, yields were slightly lower. Interestingly, the nitro leaving group in 1q was operative in our reaction. Carbon-based leaving groups42 were significantly lower yielding, with 1s proving the highest yielding at 32%. However, substrates 1s–v demonstrate that activation of sterically encumbered C–C bonds without assistance of ring strain is possible in our system. Furthermore, alkyl C–F and C–S bond activation were also demonstrated in moderate yields via β-fluoride44–45 and β-sulfur elimination with sulfoxide, sulfone, and sulfide containing substrates (1x–z).
Figure 2. Leaving group scope.
An array of alkyl C–O, C–N, C–C, C–F, and C–S bonds that can be activated using β-elimination. *Yields determined by 1H NMR analysis of the crude reaction mixture using CH2Br2 as internal standard.
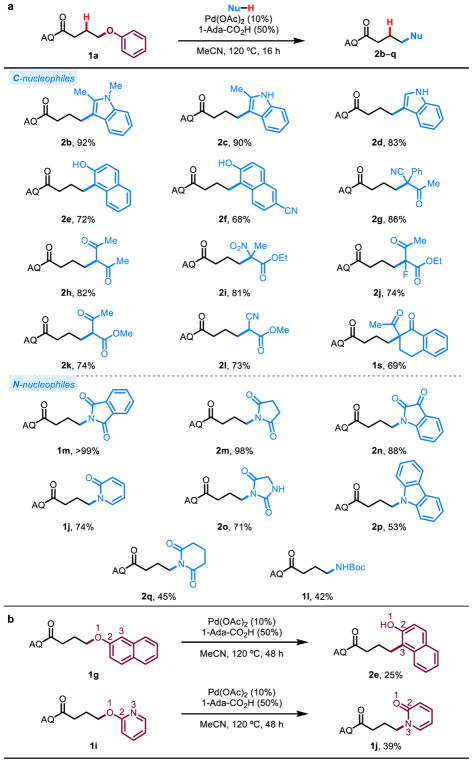
To assess different nucleophiles, we chose 1a as our standard substrate due to its excellent performance with N-methylindole as nucleophile. Various nucleophiles our laboratory previously employed in alkene hydrofunctionalization gave good to excellent yields with electron-rich arenes and 1,3-dicarbonyl-type (pro)nucleophiles (Figure 3a).42 Moderate to excellent yields were also obtained with nitrogen nucleophiles.41, 43 This method of interchanging C–O or other C–X bonds with C–C or C–N bonds is synthetically enabling where the saturated starting material with a leaving group is more readily available than the corresponding alkene (Figure 4a). The stability and ubiquity of the compatible leaving groups enable orthogonal reactivity and fewer protecting group interconversions.
Figure 3. Nucleophile scope.
a, Various operative carbon and nitrogen nucleophiles. b, Examples of formal [1,3]-rearrangments following C–O bond activation.
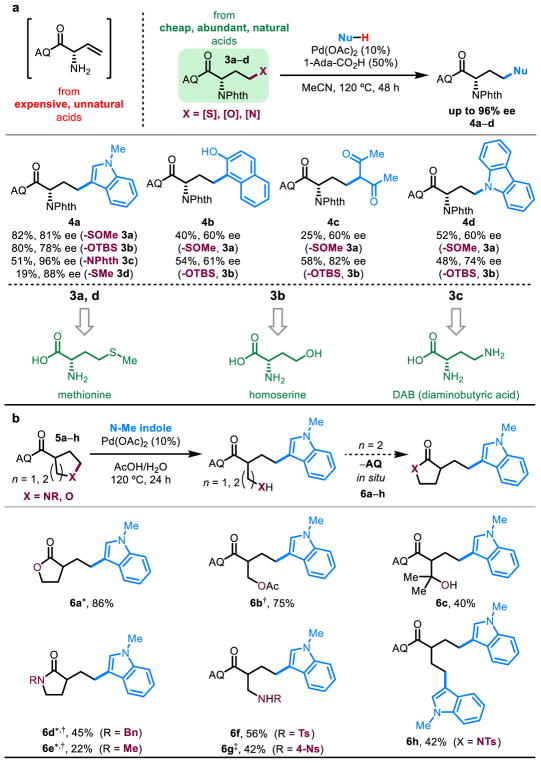
Figure 4. Applications toward small molecule synthesis.
a, Amino acid upconversion through selective C–S, C–O, and C–N activation. b, Ring opening and substitution of unstrained rings through selective C–O and C–N activation. *1 equiv CuSO4•5H2O. †AcOH as solvent. ‡50% AcOH, HFIP as solvent.
Inspired by the power of the Fries and Claisen rearrangements of phenol derivatives, we next sought to leverage the fact that some of our leaving groups can also double as nucleophiles (1g, 1i). By reengaging with the putative alkene through a different nucleophilic site, we hypothesized that a formal [1,3]-rearrangement could be achieved (Figure 3b). In the event, when 1g and 1i were subjected to the reaction conditions without exogenous nucleophile for 48 h, we isolated 25% and 39% of the formal [1,3]-rearrangement products 2e and 1j, respectively.
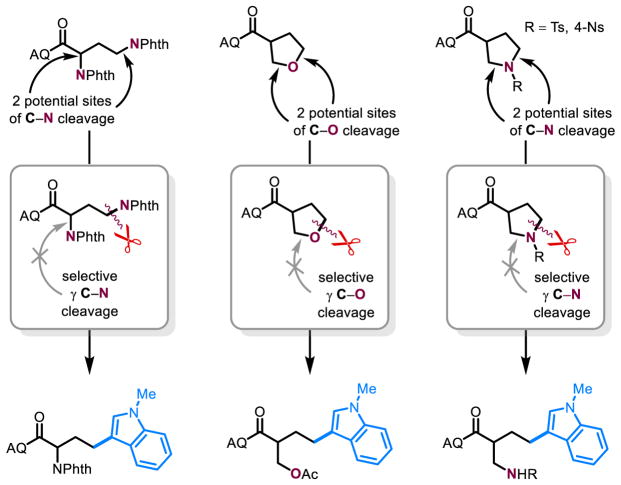
Most substrates with nitrogen leaving groups can be easily synthesized from GABA (γ-aminobutyric acid). We reasoned that this strong bond activation reaction could thus be expanded to chiral pool α-amino acids with γ-heteroatom substitution to generate an array of enantioenriched unnatural amino acids that would be difficult to access otherwise (Figure 4a).46 By comparison, enantiopure vinyl glycine is expensive from commercial suppliers or must be prepared in several synthetic steps.47 To explore this “amino acid upconversion,” we started with a DAB (2,4-diaminobutyric acid) derivative, bearing phthalimide (-NPhth) groups at the α- and γ-positions. At the outset, we were concerned that either of the two C–N bonds could be activated; however, upon subjection of 3c to the reaction conditions, we observed not only regioselective substitution of exclusively the γ-phthalimide but almost complete preservation of the α-stereocenter, demonstrating high selectivity (Figure 5). In hopes of using more abundant natural amino acid starting materials, we synthesized the γ-methylsulfoxide variant of 3c (3a) from methionine and found it to work efficiently in our reaction. The corresponding γ-methylsulfide (3d) and γ-methylsulfone (S7) were also synthesized but did not perform nearly as well. Lastly, our method is also compatible with a homoserine derivative (3b). Suitable nucleophiles include N-methylindole, 2-naphthol, acetylacetone, and carbazole, albeit with moderate epimerization of the α-stereocenter.
Figure 5. Exceptional selectivity.
Regioselective strong bond activation of alkyl C–O and C–N bonds exclusively at the γ-position.
Furthermore, this β-elimination pathway was applied towards nucleophilic opening of unstrained rings. In previous reports of catalytic ring opening from alkyl-palladium intermediates, success has been limited to strained systems, such as oxonorbornadienes.48 With focus on catalytic opening of unstrained heterocycles, we subjected a pyran motif 5a to our conditions and observed the unexpected product of in situ lactonization with the intermediate pendant alcohol following ring opening. Sequestration of the resulting free AQ group by a stoichiometric copper(II) additive and solvent optimization provided conditions for nucleophilic ring opening of unstrained six-membered and five-membered rings with oxygen- (5a–c) and nitrogen- (5d–h) based leaving groups (Figure 4b). Again, the method is selective only for the C–O or C–N bond at the γ-position without requirement for a symmetric five-membered ring, as is the case for an oxidative addition approach (Figure 5). With six-membered rings and nucleophilic leaving groups, lactonization/lactamization is observed (6a, 6d, 6e). In the case of 5h, the resulting tosylamide is presumably too weak of a nucleophile to displace the AQ group. However, the tosylamide intermediate is not observed due to a rapid second β-elimination event, leading to a bis-indole substituted product 6h. Lastly, 6b is isolated after Fischer esterification with the solvent.
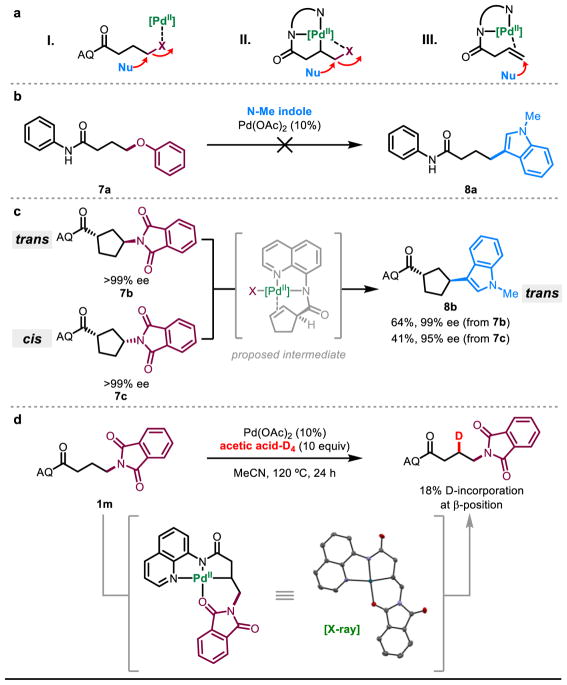
Though a detailed mechanistic investigation is outside of the scope of the present work, we performed several preliminary experiments (Figure 6). Three limiting scenarios could be envisioned: (I) Pd(II) serves as a hard Lewis acid to activate the leaving group for SN2-type displacement, (II) following C–H activation to form an alkylpalladium(II) intermediate, the nucleophile displaces the leaving group in an SN2 fashion, possibly facilitated by Pd(II) coordination, (III) after C–H activation, β-X elimination leads to a Pd(II)-π-alkene complex, and hydrofunctionalization occurs as previously described. The β-elimination step could take place with (III.A) syn (inner sphere) or (III.B) anti (outer sphere) geometry with respect to the metal center (See SI).
Figure 6. Mechanistic experiments.
a, Three proposed mechanistic scenarios, b, Directing group control. c, Testing possibilities of anti and syn β-elimination. d, Demonstrating feasibility of C–H activation step.
To examine these pathways, we prepared substrate 7a, which we expected would not undergo C–H activation. When we exposed 7a to the reaction conditions, no product formation was observed by 1H-NMR. This is inconsistent with pathway (I). Next, we prepared enantiopure trans (7b) and cis (7c) versions of a cyclopentane substrate 7 and subjected them to the reaction conditions with N-methylindole as nucleophile. With 7b (trans), we observed a moderately high yield of 8b and determined its relative stereochemistry to be trans. With 7c (cis), we again observed the same product 8b with trans stereochemistry, but with significantly lower yield. In both cases, the enantiopurity was largely preserved, ruling out epimerization that would convolute the analysis (see SI). The fact that trans 7b is converted to trans 8b is inconsistent with pathways (I) and (II). Collectively, the data support a mechanistic model in which β-X elimination proceeds in a predominantly anti fashion but the syn-pathway is also energetically accessible in cases where anti elimination is geometrically forbidden (7c). The trans stereochemistry of the final product 8b regardless of the starting material stereochemistry (cis or trans) is consistent with anti-nucleopalladation from a common Pd(II)-bound alkene intermediate, as characterized previously.41–43 In attempts to isolate the proposed alkene intermediate, we subjected 1a to the reaction conditions without addition of nucleophile, but only observed quantitative amounts of starting material (See SI). This could potentially be due to nucleophile assistance in the C–H activation or β-X elimination steps or due to rapid reinsertion into the intermediate alkene. We observed deuterium exchange at the β-position of substrate 1m (see SI) and obtained a crystal structure of the proposed C–H activated intermediate without added nucleophile. Additionally, the results from Fig. 3b indicate that the overall reaction can take place in the absence of exogenous nucleophile. Thus, we believe that nucleophile is not necessary for the C–H activation or β-X elimination steps and that rapid reinsertion into the proposed alkene is the main reason behind our inability to observe this intermediate. Independently prepared alkene was found to be a competent starting material in accessing 2a under these reaction conditions (see SI). These preliminary mechanistic experiments are consistent with the catalytic cycle shown in Figure 1.
In conclusion, we have designed a palladium(II)-catalyzed reaction system that allows for activation of strong alkyl C(sp3)–O, N, C, F, and S bonds by strategic application of a rarely used elementary step in catalysis, β-X elimination. Following activation of the strong covalent bond, non-classical nucleophilic substitution can be achieved with a range of carbon and nitrogen nucleophiles. Specifically, this C–H activation/β-X elimination/hydrofunctionalization cascade enabled novel [1,3]-rearrangements, diversification of abundant amino acids, and nucleophilic ring opening of unstrained heterocycles. Each of these reactions serves as a specific example of how a new approach toward strong covalent bond activation through β-X elimination can be strategically employed in catalytic reaction development.
Supplementary Material
Acknowledgments
This work was financially supported by TSRI, Pfizer, Inc., and the National Institutes of Health (1R35GM125052). We also gratefully acknowledge the following graduate fellowship programs: Frank J. Dixon Fellowship (V.T.T.), Donald E. and Delia B. Baxter Foundation (J.A.G.), and the National Science Foundation (NSF/DGE-1346837) (J.A.G.). We thank Dr. Jason S. Chen for determination of enantiomeric excess, along with Dr. Dee-Hua Huang and Dr. Laura Pasternack for assistance with NMR spectroscopy. We also thank Prof. Arnold L. Rheingold, Dr. Milan Gembicky, and Dr. Curtis E. Moore (UCSD) for X-ray crystallographic analysis. Prof. Jin-Quan Yu is thanked for helpful discussions.
Footnotes
Author Contributions: V.T.T., J.A.G., K.S.Y., and K.M.E. conceived this work. V.T.T. and J.A.G. performed the experiments and analyzed the data. V.T.T. and K.M.E. wrote the manuscript with input from J.A.G. and K.S.Y.
References
- 1.Rosen BM, Quasdorf KW, Wilson DA, Zhang N, Resmerita AM, Garg NK, Percec V. Nickel-catalyzed cross-coupling involving carbon–oxygen bonds. Chem Rev. 2011;111:1346–1416. doi: 10.1021/cr100259t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cornella J, Zarate C, Martin R. Metal-catalyzed activation of ethers via C–O bond cleavage: a new strategy for molecular diversity. Chem Soc Rev. 2014;43:8081–8097. doi: 10.1039/c4cs00206g. [DOI] [PubMed] [Google Scholar]
- 3.Dermenci A, Coe JW, Dong G. Direct activation of relatively unstrained carbon–carbon bonds in homogeneous systems. Org Chem Front. 2014;1:567–581. doi: 10.1039/c4qo00053f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang CY, Doyle AG. The chemistry of transition metals with three-membered ring heterocycles. Chem Rev. 2014;114:8153–8198. doi: 10.1021/cr500036t. [DOI] [PubMed] [Google Scholar]
- 5.Tobisu M, Chatani N. Cross-couplings using aryl ethers via C–O bond activation enabled by nickel catalysis. Acc Chem Res. 2015;48:1717–1726. doi: 10.1021/acs.accounts.5b00051. [DOI] [PubMed] [Google Scholar]
- 6.Tobisu M, Chatani N. Nickel-catalyzed cross-coupling reactions of unreactive phenolic electrophiles via C–O bond activation. Top Curr Chem. 2016;374:41. doi: 10.1007/s41061-016-0043-1. [DOI] [PubMed] [Google Scholar]
- 7.Zarate C, van Gemmeren M, Somerville RJ, Martin R. Phenol derivatives: modern electrophiles in cross-coupling reactions. Adv Organomet Chem. 2016;66:143–222. [Google Scholar]
- 8.Chen PH, Billett BA, Tsukamoto T, Dong G. “Cut and sew” transformations via transition-metal-catalyzed carbon–carbon bond activation. ACS Catal. 2017;7:1340–1360. doi: 10.1021/acscatal.6b03210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dander JE, Garg NK. Breaking amides using nickel catalysis. ACS Catal. 2017;7:1413–1423. doi: 10.1021/acscatal.6b03277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsunaga PT, Hillhouse GL. Thianickelacycles by ring-opening reactions of cyclic thioethers and their subsequent carbonylation to thioesters. Angew Chem Int Ed. 1994;33:1748–1749. [Google Scholar]
- 11.Lin BL, Clough CR, Hillhouse GL. Interactions of aziridines with nickel complexes: oxidative-addition and reductive-elimination reactions that break and make C–N bonds. J Am Chem Soc. 2002;124:2890–2891. doi: 10.1021/ja017652n. [DOI] [PubMed] [Google Scholar]
- 12.Dankwardt JW. Nickel-catalyzed cross-coupling of aryl Grignard reagents with aromatic alkyl ethers: an efficient synthesis of unsymmetrical biaryls. Angew Chem Int Ed. 2004;43:2428–2432. doi: 10.1002/anie.200453765. [DOI] [PubMed] [Google Scholar]
- 13.Ueno S, Mizushima E, Chatani N, Kakiuchi F. Direct observation of the oxidative addition of the aryl carbon–oxygen bond to a ruthenium complex and consideration of the relative reactivity between aryl carbon–oxygen and aryl carbon–hydrogen bonds. J Am Chem Soc. 2006;128:16516–16517. doi: 10.1021/ja067612p. [DOI] [PubMed] [Google Scholar]
- 14.Quasdorf KW, Riener M, Petrova KV, Garg NK. Suzuki-Miyaura coupling of aryl carbamates, carbonates, and sulfamates. J Am Chem Soc. 2009;131:17748–17749. doi: 10.1021/ja906477r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbero N, Martin R. Ligand-free Ni-catalyzed reductive cleavage of inert carbon–sulfur bonds. Org Lett. 2012;14:796–799. doi: 10.1021/ol2033306. [DOI] [PubMed] [Google Scholar]
- 16.Sergeev AG, Webb JD, Hartwig JF. A heterogeneous nickel catalyst for the hydrogenolysis of aryl ethers without arene hydrogenation. J Am Chem Soc. 2012;134:20226–20229. doi: 10.1021/ja3085912. [DOI] [PubMed] [Google Scholar]
- 17.Juliá-Hernández F, Ziadi A, Nishimura A, Martin R. Nickel-catalyzed chemo-, regio- and diastereoselective bond formation through proximal C–C cleavage of benzocyclobutenones. Angew Chem Int Ed. 2015;54:9537–9541. doi: 10.1002/anie.201503461. [DOI] [PubMed] [Google Scholar]
- 18.Liu XW, Echavarren J, Zarate C, Martin R. Ni-catalyzed borylation of aryl fluorides via C–F cleavage. J Am Chem Soc. 2015;137:12470–12473. doi: 10.1021/jacs.5b08103. [DOI] [PubMed] [Google Scholar]
- 19.Hie L, Nathel NFF, Hong X, Yang YF, Houk KN, Garg NK. Nickel-catalyzed activation of acyl C–O bonds of methyl esters. Angew Chem Int Ed. 2016;55:2810–2814. doi: 10.1002/anie.201511486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moragas T, Gaydou M, Martin R. Nickel-catalyzed carboxylation of benzylic C–N bonds with CO2. Angew Chem Int Ed. 2016;55:5053–5057. doi: 10.1002/anie.201600697. [DOI] [PubMed] [Google Scholar]
- 21.van der Boom ME, Liou SY, Ben-David Y, Shimon LJW, Milstein D. Alkyl– and aryl–oxygen bond activation in solution by rhodium(I), palladium(II), and nickel(II). Transition-metal-based selectivity. J Am Chem Soc. 1998;120:6531–6541. [Google Scholar]
- 22.Lara P, Paneque M, Poveda ML, Salazar V, Santos LL, Carmona E. Formation and cleavage of C–H, C–C, and C–O bonds of ortho-methyl-substituted anisoles by late transition metals. J Am Chem Soc. 2006;128:3512–3513. doi: 10.1021/ja0586790. [DOI] [PubMed] [Google Scholar]
- 23.Fulmer GR, Muller RP, Kemp RA, Goldberg KI. Hydrogenolysis of palladium(II) hydroxide and methoxide pincer complexes. J Am Chem Soc. 2009;131:1346–1347. doi: 10.1021/ja807936q. [DOI] [PubMed] [Google Scholar]
- 24.Santos LL, Mereiter K, Paneque M. Reaction of 2-methylanisole with TpMe2Ir(C6H5)2(N2): a comprehensive set of activations. Organometallics. 2013;32:565–569. [Google Scholar]
- 25.Edouard GA, Kelley P, Herbert DE, Agapie T. Aryl ether cleavage by group 9 and 10 transition metals: stoichiometric studies of selectivity and mechanism. Organometallics. 2015;34:5254–5277. [Google Scholar]
- 26.Chu T, Boyko Y, Korobkov I, Nikonov GI. Transition metal-like oxidative addition of C–F and C–O bonds to an aluminum(I) center. Organometallics. 2015;34:5363–5365. [Google Scholar]
- 27.Crimmin MR, Butler MJ, White AJP. Oxidative addition of carbon–fluorine and carbon–oxygen bonds to Al(I) Chem Commun. 2015;51:15994–15996. doi: 10.1039/c5cc07140b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henry PM. Palladium(II)-catalyzed exchange and isomerization reactions. IV exchange of vinylic chloride with radioactive lithium chloride catalyzed by palladium(II) chloride in acetic acid. J Org Chem. 1972;37:2443–2447. [Google Scholar]
- 29.Henry PM. Palladium(II)-catalyzed exchange and isomerization reactions. Acc Chem Res. 1973;6:16–24. [Google Scholar]
- 30.Catellani M, Fagnola MC. Palladacycles as intermediates for selective dialkylation of arenes and subsequent fragmentation. Angew Chem Int Ed. 1994;33:2421–2422. [Google Scholar]
- 31.Zhao H, Ariafard A, Lin Z. β-Heteroatom versus β-hydrogen elimination: a theoretical study. Organometallics. 2006;25:812–819. [Google Scholar]
- 32.Yang J, Mercer GJ, Nguyen HM. Palladium-catalyzed glycal imidate rearrangement: formation of α- and β-N-glycosyl trichloroacetamides. Org Lett. 2007;9:4231–4234. doi: 10.1021/ol701778z. [DOI] [PubMed] [Google Scholar]
- 33.White PB, Stahl SS. Reversible alkene insertion into the Pd-N bond of Pd(II)-sulfonamidates and implications for catalytic amidation reactions. J Am Chem Soc. 2011;133:18594–18597. doi: 10.1021/ja208560h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jana R, Pathak TP, Jensen KH, Sigman MS. Palladium(II)-catalyzed enantio- and diastereoselective synthesis of pyrrolidine derivatives. Org Lett. 2012;14:4074–4077. doi: 10.1021/ol3016989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.For a comprehensive list of examples of β-X elimination from an alkylpalladium species see Supporting Information.
- 36.Choi J, Choliy Y, Zhang X, Emge TJ, Krogh-Jespersen K, Goldman AS. Cleavage of sp3 C–O bonds via oxidative addition of C–H bonds. J Am Chem Soc. 2009;131:15627–15629. doi: 10.1021/ja906930u. [DOI] [PubMed] [Google Scholar]
- 37.Choi J, Wang DY, Kundu S, Choliy Y, Emge TJ, Krogh-Jespersen K, Goldman AS. Net oxidative addition of C(sp3)–F bonds to iridium via initial C–H bond activation. Science. 2011;332:1545–1548. doi: 10.1126/science.1200514. [DOI] [PubMed] [Google Scholar]
- 38.Haibach MC, Lease N, Goldman AS. Catalytic cleavage of ether C–O bonds by pincer iridium complexes. Angew Chem Int Ed. 2014;53:10160–10163. doi: 10.1002/anie.201402576. [DOI] [PubMed] [Google Scholar]
- 39.Luo S, Yu DG, Zhu RY, Wang X, Wang L, Shi ZJ. Fe-promoted cross coupling of homobenzylic methyl ethers with Grignard reagents of via sp3 C–O bond cleavage. Chem Commun. 2013;49:7794–7796. doi: 10.1039/c3cc43616k. [DOI] [PubMed] [Google Scholar]
- 40.Zaitsev VG, Shabashov D, Daugulis O. Highly regioselective arylation of sp3 C–H bonds catalyzed by palladium acetate. J Am Chem Soc. 2005;127:13154–13155. doi: 10.1021/ja054549f. [DOI] [PubMed] [Google Scholar]
- 41.Gurak JA, Jr, Yang KS, Liu Z, Engle KM. Directed, regiocontrolled hydroamination of unactivated alkenes via protodepalladation. J Am Chem Soc. 2016;138:5805–5808. doi: 10.1021/jacs.6b02718. [DOI] [PubMed] [Google Scholar]
- 42.Yang K, Gurak JA, Jr, Liu Z, Engle KM. Catalytic, regioselective hydrocarbofunctionalization of unactivated alkenes with diverse C–H nucleophiles. J Am Chem Soc. 2016;138:14705–14712. doi: 10.1021/jacs.6b08850. [DOI] [PubMed] [Google Scholar]
- 43.Gurak JA, Jr, Tran VT, Sroda MM, Engle KM. N-alkylation of 2-pyridone derivatives via palladium(II)-catalyzed directed alkene hydroamination. Tetrahedron. 2017;73:3636–3642. [Google Scholar]
- 44.Wada S, Jordan RF. Olefin insertion into a Pd–F bond: catalyst reactivation following β-F elimination in ethylene/vinyl fluoride copolymerization. Angew Chem Int Ed. 2017;56:1820–1824. doi: 10.1002/anie.201611198. [DOI] [PubMed] [Google Scholar]
- 45.Ichikawa J, Nadano R, Ito J. 5-endo Heck-type cyclization of 2-(trifluoromethyl)allyl ketone oximes: synthesis of 4-difluoromethylene-substituted 1-pyrrolines. Chem Commun. 2006:4425–4427. doi: 10.1039/b610690k. [DOI] [PubMed] [Google Scholar]
- 46.For select examples of relevant unnatural amino acid synthesis, see Supplementary Information.
- 47.For information on commercial prices and synthetic routes towards vinyl glycine, see Supplementary Information.
- 48.Li Y, Yang W, Cheng G, Yang D. Palladium-catalyzed syn-stereocontrolled ring-opening of oxabicyclic alkenes with sodium arylsulfinates. J Org Chem. 2016;81:4744–4750. doi: 10.1021/acs.joc.6b00667. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.