Abstract
Objective
To characterize the independent association between antibiotic exposure in the first week of life and the risk of bronchopulmonary dysplasia (BPD) or death among very preterm infants without culture-confirmed sepsis.
Methods
Retrospective cohort study using the Optum Neonatal Database. Infants without culture-confirmed sepsis born less than 1500g and less than 32 weeks gestation between 1/2010 and 11/2016 were included. The independent association between antibiotic therapy during the first week of life and BPD or death prior to 36 weeks postmenstrual age (PMA) was assessed by multivariable logistic regression.
Results
Of 4950 infants, 3946 (79.7%) received antibiotics during the first week of life. Rates of BPD or death (41.5% vs. 31.1%, p<0.001) and the two individual outcomes were significantly higher among antibiotic treated infants. After adjusting for potential confounding variables, antibiotic use in the first week of life was not associated with increased risk of BPD or death (OR 0.96, 95% CI [0.76,1.21]) or BPD among survivors (OR 0.86, 95% CI [0.67,1.09]). Antibiotic use was associated with increased risk of death prior to 36 weeks PMA (OR 3.01, 95% CI [1.59,5.71]), however, secondary analyses suggested this association may be confounded by unmeasured illness severity.
Conclusions
Antibiotic exposure in the first week of life among preterm infants without culture-confirmed sepsis was not independently associated with increased risk of BPD or death.
Keywords: prematurity, antibiotics, bronchopulmonary dysplasia, death
Introduction
The incidence of culture-confirmed sepsis in the first week of life among very low birthweight infants (VLBW; <1500 grams) is low (<2%) and may be decreasing over time.(1–3) However, VLBW infants frequently receive antibiotics soon after birth for suspected sepsis, with treatment courses that are often continued for more than 48-72 hours.(4–7) Early, prolonged antibiotic exposure among preterm infants without culture-confirmed sepsis is associated with increased risk for several adverse outcomes including subsequent colonization with resistant bacterial organisms, late onset sepsis, necrotizing enterocolitis (NEC), invasive fungal infection, and mortality.(7–13) Recent observational studies also suggest that such antibiotic use may increase the risk of bronchopulmonary dysplasia (BPD).(4, 5, 14) One hypothesized etiology for this association is that early antibiotic exposure may alter the neonatal microbiome and disrupt innate mechanisms meant to prevent airway and systemic inflammation, leading to increased risk of BPD.(4, 5, 15) Alternatively, it is also possible that the reported association is confounded by unmeasured differences in illness severity and early respiratory disease. We conducted the present study to characterize the independent association between antibiotic exposure in the first week of life and the risk of BPD or death among VLBW infants without culture-confirmed sepsis. We hypothesized that any potential association between early antibiotic use and increased risk for BPD or death would no longer be present after adjustment for severity of illness, particularly markers of early respiratory disease.
Methods
Study Data Source and Population
This retrospective cohort study used data from the Optum Neonatal Database (Eden Prairie, MN). The Optum Corporation provides neonatal care management services for multiple private, government, and self-insured employer health plans throughout the United States. Optum data has been used previously in both pediatric and adult research studies.(16, 17) The database utilized in the present analysis comprises predefined daily clinical, socio-demographic, and cost-related information abstracted multiple times per week by trained neonatal nurses. All data were collected prospectively using written protocols and were subject to routine validation and quality audits. Infants whose insurer contracts with the Optum Corporation to provide care management services and are hospitalized in a Level II or higher academic or community hospital-based neonatal intensive care unit (NICU) are included in the database. In general, this results in a small number of infants sampled from a large number of hospitals located throughout the United States. Infants who died in the delivery room and were not admitted to a NICU are not included in the database.
For the present study, we evaluated VLBW infants with gestational ages less than 32 weeks who were born between January 1, 2010 and November 22, 2016. Infants diagnosed with culture-confirmed bacterial or fungal sepsis during the first week of life and those with major congenital anomalies were excluded. The Institutional Review Board at Thomas Jefferson University Hospital certified the use of this de-identified dataset as non-human subjects research.
Study Outcomes and Exposure Definitions
The primary study outcome was BPD or death prior to 36 weeks postmenstrual age (PMA). We evaluated this morbidity-mortality composite outcome because early deaths among preterm infants are most commonly attributed to respiratory causes, and therefore have competing risks for BPD.(18) The individual outcomes of death prior to 36 weeks PMA and BPD among survivors to 36 weeks PMA were evaluated as secondary outcomes.
Our primary analysis compared the risks for the study outcomes between infants who received antibiotic therapy for any duration during the first week of life and those who did not. In pre-specified secondary analyses, we evaluated total days of antibiotic exposure in the first week of life as a continuous variable and as a 3-level categorical variable (no antibiotic exposure, ≤ 48 hours of antibiotic exposure, and 3-7 days of antibiotic exposure). Of note, the 3-level categorical variable summarized total days of exposure during the first week of life and does not necessarily represent contiguous treatment days.
BPD was defined as the use of supplemental oxygen or respiratory support at 36 weeks PMA. NEC was defined as Bell’s stage 2 or higher.(19) Sepsis was defined as a culture-confirmed bacterial or fungal bloodstream infection. Small for gestational age was defined as a birthweight less than the 10th percentile for gestational age and sex using the Olsen infant growth curves.(20) Chorioamnionitis included clinical and histological diagnoses; the method of diagnosis was not recorded. Infants considered to have been exclusively fed human milk did not receive formula during the admission, but may have received parental nutrition and formula based milk fortifiers. Use of donor human milk versus mother’s own milk was not recorded in the database.
Statistical Analysis
Standard descriptive analyses of the demographic and clinical data were performed using Student t-tests, Mann-Whitney U-tests, or chi-square tests as appropriate. Hierarchical, logistic regression was used to explore the independent association between antibiotic exposure in the first week of life and the risk of the study outcomes. Three regression models were developed to sequentially adjust for a priori specified perinatal factors known to affect the risk of the study outcomes, or hypothesized to influence the neonatal microbiome. Model 1 was an unadjusted bivariable analysis. Model 2 adjusted for the following infant and maternal factors: gestational age, birth weight, sex, race/ethnicity, prenatal steroids, chorioamnionitis, gestational hypertension or pre/eclampsia, gestational diabetes, mode of delivery, small for gestational age, multiple gestation pregnancy, and exclusive human milk feeding. Model 3 adjusted for the same variables as model 2, with the addition of indicators for treatment with caffeine, surfactant, mechanical ventilation on the first day of life, and the total duration (days) of mechanical ventilation during the first week of life. Given the large number of centers, each contributing few infants, a robust sandwich variance estimator for cluster-correlated data was used in each of the above logistic regression models to relax the assumption of independence among infants cared for in the same center.(21)
We evaluated 3 infant subgroups for potential differences in the association between early antibiotic exposure and the study outcomes: birth weight (<1000 grams vs. ≥1000 grams), NEC, and culture-confirmed sepsis developing after day of life 7. Subgroup testing was conducted by adding an interaction term between the primary exposure and the subgroup variable to model 3. No adjustment for multiple comparisons was performed.
The independent association between the two pre-specified alternative categorizations of antibiotic exposure described above and the risks for the study outcomes were evaluated using logistic regression, adjusting for all covariates included in model 3. Kaplan-Meier failure functions were used to graphically assess the chronological age at death for the infants who died prior to 36 weeks PMA based on the 3-level categorical definition of early antibiotic exposure. Equality of the 3 curves was assessed using the log-rank test. A post-hoc analysis evaluated the independent association between antibiotic exposure in the first week and the risks for the study outcomes among the infants who survived the first 7 days of life. P <0.05 was considered statistically significant, and all reported P values are 2-sided. Statistical analyses were performed using Stata statistical software version 13.1 (StataCorp, College Station, TX).
Results
Characteristics of the Study Subjects
We excluded 232 infants with major congenital anomalies or culture-confirmed sepsis diagnosed in the first week of life, leaving 4950 infants sampled from 603 NICUs (Figure 1; median number of infants contributed per hospital: 3, range 1-147). Of these, 3946 (79.7%) received antibiotics during the first week of life and 1004 (20.3%) did not. A total of 4617 infants (93.3%) survived to 36 weeks PMA.
Figure 1.
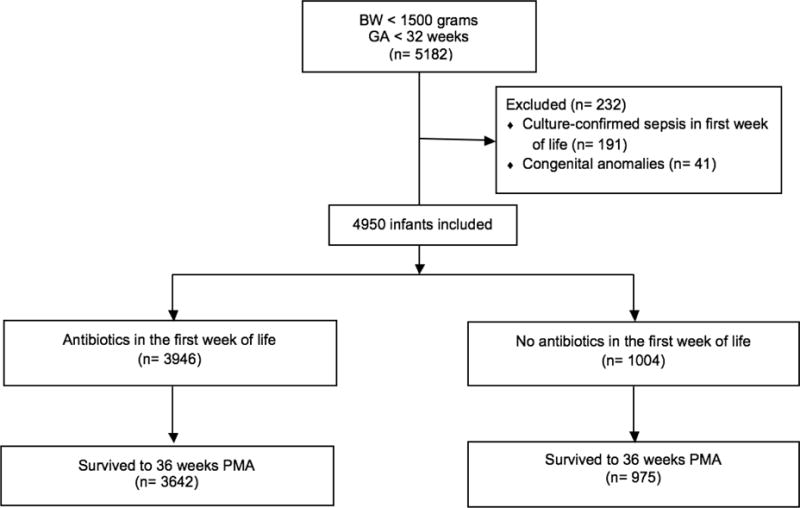
Flow diagram of the infants included in the analysis
BW (birthweight); GA (gestational age); n (number); PMA (postmenstrual age)
Table 1 compares the characteristics (values shown without risk-adjustment) of infants who did and did not receive antibiotic therapy during the first week of life. Infants treated with antibiotics had modestly lower gestational ages, were less often small for gestational age, and were more commonly treated with caffeine, surfactant, and mechanical ventilation. Mothers of infants in the early antibiotic group had lower rates of gestational hypertension and higher rates of chorioamnionitis.
Table 1.
Subject characteristics and study outcomes
| Early antibiotics (n=3946) | No early antibiotics (n=1004) | p | |
|---|---|---|---|
| Infant demographics | |||
| Gestational age, wk - mean±SD | 27.9 ± 2.3 | 28.3 ± 2.4 | <0.001 |
| Birthweight, g - mean±SD | 1032 ± 286 | 1016 ± 277 | 0.11 |
| Male sex, n (%) | 2015 (51.1) | 488 (48.6) | 0.16 |
| Small for gestational age, n (%) | 534 (13.5) | 192 (19.1) | <0.001 |
| Singleton, n (%) | 2849 (72.2) | 750 (74.7) | 0.11 |
| Race/Ethnicity, n (%) | 0.01 | ||
| African-American | 793 (20.1) | 196 (19.5) | |
| Caucasian | 1281 (32.5) | 308 (30.7) | |
| Hispanic | 726 (18.4) | 157 (15.6) | |
| Other/unknown | 1146 (29.0) | 343 (34.2) | |
| Cesarean section, n (%) | 2705 (68.6) | 719 (71.6) | 0.06 |
| Exclusive human milk feeding, n (%) | 874 (22.2) | 219 (21.8) | 0.82 |
| Maternal factors | |||
| Prenatal steroids, n (%) | 2026 (51.3) | 468 (46.6) | 0.007 |
| Chorioamnionitis, n (%) | 203 (5.1) | 23 (2.3) | <0.001 |
| Gestational hypertension or pre/eclampsia, n (%) | 761 (19.3) | 336 (33.5) | <0.001 |
| Gestational diabetes, n (%) | 143 (3.6) | 41 (4.1) | 0.49 |
| Respiratory characteristics | |||
| Caffeine therapy, n (%) | 3447 (87.4) | 721 (71.8) | <0.001 |
| Surfactant therapy, n (%) | 2283 (57.9) | 199 (19.8) | <0.001 |
| Invasive ventilation on day 0 of life, n (%) | 2460 (62.3) | 183 (18.2) | <0.001 |
| Ventilation days week 1 of life, mean±SD | 2.8 ± 2.9 | 0.7 ± 1.7 | <0.001 |
| Outcome rates | |||
| BPD or death prior to 36 wk PMA or, n (%) | 1639 (41.5) | 312 (31.1) | <0.001 |
| BPD among survivors to 36 wk PMA1, n (%) | 1336 (36.7) | 283 (29.0) | <0.001 |
| Death prior to 36 wk PMA, n (%) | 302 (7.7) | 29 (2.9) | <0.001 |
Outcome calculated among survivors to 36 wk PMA (3642 received early antibiotics, 975 did not)
wk (weeks); SD (standard deviation); g (grams); BPD (bronchopulmonary dysplasia); PMA (postmenstrual age)
Primary Analyses
The unadjusted rates of the primary outcome, BPD or death prior to 36 weeks PMA, and the individual secondary outcomes were significantly higher among the infants treated with antibiotics in the first week of life (Table 1). In sequential, hierarchical modeling (Table 2), antibiotic exposure was associated with an increased risk of all three study outcomes in the unadjusted model (model 1) and after adjustment for infant demographic characteristics and maternal factors (model 2). After accounting for differences in early infant respiratory characteristics (model 3), antibiotic exposure in the first week of life was not associated with increased risk of BPD or death (adjusted odds ratio [aOR] 0.96, 95% CI [0.76,1.21]), or BPD among survivors (aOR 0.86, 95% CI [0.67,1.09]). However, early antibiotic exposure remained associated with increased risk for death prior to 36 weeks PMA (aOR 3.01, 95% CI [1.59,5.71]).
Table 2.
Adjusted hierarchical logistic regression analysis* (adjusted odds ratios, 95% confidence intervals)
| Model 11 | Model 22 | Model 33 | |
|---|---|---|---|
| BPD or death prior to 36wk PMA | 1.58 [1.29,1.93] | 1.70 [1.35,2.14] | 0.96 [0.76,1.21] |
| BPD among survivors to 36 wk PMA | 1.42 [1.14,1.76] | 1.59 [1.25,2.00] | 0.86 [0.67,1.09] |
| Death prior to 36wk PMA | 2.79 [1.98,3.92] | 3.42 [2.18,5.38] | 3.01 [1.59,5.71] |
Robust sandwich variance estimator for cluster-correlated data used in all models to relax assumption of independence among babies cared for in same center
Unadjusted bivariable analysis
Adjusted for gestational age, birthweight, sex, race/ethnicity, prenatal steroids, chorioamnionitis, gestational hypertension or pre/eclampsia, gestational diabetes, mode of delivery, small for gestational age, and exclusive human milk feeding
Adjusted for same variables as model 2 plus treatment with caffeine, surfactant, mechanical ventilation on the first day of life, and the total duration (days) of mechanical ventilation during the first week of life
PMA (postmenstrual age); BPD (bronchopulmonary dysplasia); wk (weeks)
There was evidence of one statistically significant subgroup effect among the 9 subgroup analyses conducted. Antibiotic use in the first week of life was independently associated with increased risk of death among infants born <1000g (14.8% vs. 4.3%, p<0.001; aOR 5.94, 95% CI [2.61,13.5]) but not ≥1000g (2.0% vs. 1.7%, p=0.67; aOR 0.67, 95% CI [0.24,1.89]) (interaction p=0.02). Of note, this finding would not reach statistical significance if Bonferroni correction for multiple comparisons was performed (required P <0.006). There was no evidence of significant subgroup effects based on birthweight for the other 2 study outcomes, or for culture-confirmed sepsis developing after day of life 7 or NEC for any of the study outcomes (interaction p-values: 0.24-0.90).
Secondary Analyses using Alternative Categorization of Early Antibiotic Exposure
The secondary analyses did not show a consistent, independent relationship between the duration of early antibiotic exposure and risk of the study outcomes. Increasing duration of antibiotic therapy not was not associated with higher or lower risk-adjusted odds of BPD or death when treatment duration was considered as a 3-level categorical variable (Table 3) or as continuous variable (aOR 0.97, 95% CI [0.93,1.01]).
Table 3.
Outcomes analysis based on duration of early antibiotic exposure
| No antibiotics (n=1004) | ≤48h of antibiotics (n=1366) | 3-7d of antibiotics (n=2580) | p | |
|---|---|---|---|---|
| BPD or death prior to 36 wk PMA | 312 (31.1%) | 529 (38.7%) | 1110 (43.0%) | <0.001 |
| Adjusted OR [95%CI]1 | Reference | 1.19 [0.88,1.62] | 0.82 [0.65,1.04] | |
| BPD among survivors to 36 wk PMA2 | 283 (29%) | 368 (30.5%) | 968 (39.7%) | <0.001 |
| Adjusted OR [95% CI]1 | Reference | 1.00 [0.71,1.42] | 0.78 [0.62,0.97] | |
| Death prior to 36 wk PMA | 29 (2.9%) | 160 (11.7%) | 142 (5.5%) | <0.001 |
| Adjusted OR [95%CI]1 | Reference | 6.01 [3.11,11.55] | 1.65 [0.82,3.33] |
Adjusted for gestational age, birthweight, sex, race/ethnicity, prenatal steroids, chorioamnionitis, gestational hypertension or pre/eclampsia, gestational diabetes, mode of delivery, small for gestational age, and exclusive human milk feeding, treatment with caffeine, surfactant, mechanical ventilation on the first day of life, and the total duration (days) of mechanical ventilation during the first week of life
Denominators for survivors to 36 wk PMA: 975 (no antibiotics); 1205 (≤48h antibiotics); 2437 (3-7d antibiotics)
BPD (bronchopulmonary dysplasia); wk (weeks); h (hours); d (days); OR (odds ratio); CI (confidence interval); PMA (postmenstrual age)
Relative to infants who did not receive early antibiotic therapy, those treated for 3-7 days but not ≤ 48 hours had lower adjusted odds of BPD among survivors to 36 weeks PMA (Table 3). However, antibiotic therapy during the first week of life was not associated with differences in the risk of BPD when the duration of therapy was explored as a continuous variable (aOR 0.99, 95% CI [0.95,1.03]).
Compared to no antibiotic therapy, treatment with antibiotics for ≤ 48 hours, but not 3-7 days, was associated with increased risk-adjusted odds of death (Table 3). The majority of deaths among infants who received ≤ 48 hours of antibiotic therapy occurred earlier than those in the other two groups (Figure 2). Of the 160 deaths among infants who received antibiotics for ≤ 48 hours, 98 (61.3%) died in the first week of life and 130 (81.3%) were receiving the same antibiotic course started during the first week of life on the date of death. Conversely, only 8 (5.6%) of the deaths among babies treated with 3-7 days of antibiotics occurred in the first week of life and only 9 (6.3%) were receiving the course of antibiotics started in the first week of life on the date of death. When considered as a continuous variable, each additional day of antibiotic therapy during the first week of life was associated with decreased risk-adjusted odds of death prior to 36 weeks PMA (aOR 0.82, 95% CI [0.76,0.88]).
Figure 2.
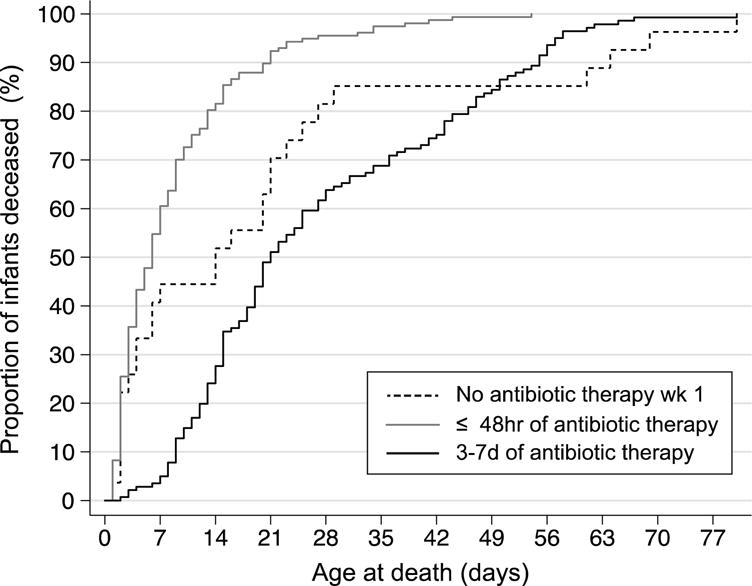
Kaplan-Meier hazard function depicting the timing of death among the infants who died prior to 36 weeks postmenstrual age
Log-rank test for the comparison of the 3 hazard function curves P<0.001
Wk (week); hr (hours); d (days)
Post-Hoc Analysis Among Infants Surviving the First Week of Life
Of the 4633 infants who survived the first 7 days of life, 3654 (78.9%) received antibiotics during the first week. Rates of BPD or death in this sub cohort were higher among the infants who received antibiotics during the first week of life compared to those who did not (Supplemental Table 1). Rates of death prior to 36 weeks PMA were similar between the groups. After risk adjustment (using the covariates included in model 3), early antibiotic use among infants who survived the first 7 days was not associated with higher or lower odds of any of the study outcomes (Supplemental Table 1).
Discussion
Preterm infants without culture-confirmed sepsis in the first week of life are commonly treated with early and prolonged antibiotic therapy.(7, 22) Previous studies suggested that this potentially unnecessary antibiotic use may increase the risk of several adverse outcomes, including BPD.(4, 5, 14) In this large cohort of VLBW infants without culture-confirmed sepsis, antibiotic treatment in the first week of life was not associated with higher or lower risk-adjusted odds of the composite outcome of BPD or death, or BPD among survivors to 36 weeks PMA.
The discrepancy between our findings and those reports showing a potential association between early antibiotic use and BPD may result from differences in how these studies adjusted for early disease severity. Two single center studies found an association between early antibiotic exposure and the risk of BPD among VLBW infants.(5, 14) Both studies controlled for early illness acuity using standardized disease severity scores, and one (14) adjusted for the total duration of mechanical ventilation during the entire hospitalization.(5, 14) However, neither study adjusted for early respiratory support characteristics.(5, 14) In a large, multi-center study, Ting et al. reported an association between greater antibiotic exposure during the entire hospitalization (defined as the number of antibiotic treatment days dived by the length of stay) among VLBW infants without sepsis or NEC and higher odds of BPD.(4) A secondary analysis revealed a similar association between higher antibiotic use rates in the first 7 days of life and BPD.(4) These investigators also adjusted for several demographic factors and measures of early illness severity, but not early respiratory support characteristics.(4) The results of our stepwise hierarchical analyses suggest that measures of respiratory disease severity are key factors confounding the association between early antibiotic exposure and BPD.
Our secondary analysis suggests a potential protective effect of a 3-7 day antibiotic course during the first week of life for prevention of BPD among survivors to 36 weeks PMA. This result should be interpreted with caution. Compared to infants who did not receive antibiotics during the first week and those treated for ≤ 48 hours, infants treated for 3-7 days experienced the highest, not the lowest, unadjusted rates of BPD. In addition, when the duration of antibiotic use was evaluated as a continuous variable, longer treatment was not independently associated with higher or lower risk of BPD. This suggests a beneficial dose-response effect is unlikely and that the observed statistically significant finding may arise from chance alone.
Similar to our primary analysis, several prior studies and a recent meta-analysis reported an association between antibiotic exposure in preterm infants without culture-confirmed infection and increased risk of mortality.(4, 7, 23, 24) However, the results of our secondary and post-hoc analyses strongly suggest this association is confounded by unmeasured differences in early illness severity. Firstly, when the duration of antibiotic therapy was considered as a continuous variable, longer treatment was associated with lower, not higher, risk-adjusted odds of death. This result is likely explained by the more than 2-fold higher death rates observed among the infants treated with antibiotics for ≤ 48 hours compared to those treated for 3-7 days. Further evaluation of the deaths among the infants treated with antibiotics for ≤ 48 hours showed that most occurred in the first week of life. Moreover, antibiotic use in the first week of life was not independently associated with higher or lower risk of death prior to 36 weeks PMA once infants who died in the first 7 days of life were excluded. Together, these results indicate that antibiotic treatment in the first week of life is likely a marker of illness severity and may not be a true contributor to the pathophysiology of mortality.
Previous investigators hypothesized that antibiotic use in preterm infants may disrupt the developing neonatal microbiome and lead to an increased risk of BPD.(4, 5, 15) Although our data do not support an independent relationship between early antibiotic therapy and BPD, there are numerous disease processes in which alteration of the neonatal microbiome secondary to antibiotic exposure may play a role.(7, 9–13, 24–27) Limiting potentially harmful, unnecessary antibiotic use in the NICU remains an important goal. Moreover, a better understanding of how antibiotic use affects disease risk during the newborn period may help define the interaction between the developing microbiome and neonatal illness.
We acknowledge several study limitations. Our data are observational and cannot prove causality. However, randomized controlled trials investigating liberal versus conservative antibiotic treatment strategies may be difficult to conduct in the neonatal population. As a result, well-designed cohort studies are an essential tool to further understand the risks and benefits of antibiotic therapy. We were not able to include common composite scores of early illness severity in our risk adjustment models, as the necessary physiological data are not captured in the Optum database. Importantly, inclusion of these or other unaccounted for covariates in the final regression models is unlikely to change the conclusion that early antibiotic exposure among infants without culture proven sepsis is not associated with increased BPD risk. All maternal data were abstracted directly from the neonatal record, which may lead to imprecision of some perinatal variables. We also acknowledge that the infants who received antibiotics in the first week of life may have been sicker that those who were not treated. However, our observation that nearly 20% of the non-treated infants received invasive mechanical ventilation during the first 24 hours of life and that over 30% died before 36 weeks PMA suggest these were not uniformly “well” infants. The Optum database does not include specific microbiology data (only the presence or absence of culture-confirmed sepsis) or information on the type or dosage of the prescribed antibiotics. Lastly, we limited our analysis to antibiotic treatment in the first week of life as severity of illness adjustment over longer exposure windows may be less reliable when utilizing administrative datasets.
The main strengths of this study are the large sample size of infants cared for in both academic and community hospitals over a broad geographic region. Although our analyses were retrospective, the study data were collected prospectively at regular intervals during each infant’s hospitalization. In addition, the use of hierarchical modeling and inclusion of several secondary and post-hoc analyses enabled a robust, sequential analysis to better understand the etiology of the observed increase in death and BPD among antibiotic treated infants.
Conclusions
After adjusting for early respiratory support, we did not find an independent association between early antibiotic exposure among preterm infants without culture-confirmed sepsis and the risk of BPD or the composite of BPD or death. These results suggest the previously reported relationship between early antibiotic exposure and the increased risk of BPD may be confounded by greater illness severity, particularly early respiratory disease. We did find an independent association between early antibiotic exposure and the risk of death. However, the higher rates of early death among infants treated with antibiotics strongly suggest this finding may also be confounded by unmeasured differences in illness severity. Although our results do not support a clear link between early antibiotic use and BPD, judicious use of antibiotics in preterm infants remains important to avoid other adverse outcomes and the emergence of antibiotic resistant organisms.
Supplementary Material
Acknowledgments
DDF was supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (T32HD060550). EAJ was supported by a grant from the National Health, Lung, and Blood Institute (K23HL136843).
Abbreviations
- BPD
bronchopulmonary dysplasia
- CI
confidence interval
- NEC
necrotizing enterocolitis
- NICU
neonatal intensive care unit
- OR
odds ratio
- PMA
postmenstrual age
- VLBW
very low birthweight
Footnotes
Disclosure Statements:
The authors report no conflicts of interest and no sources of support. No honorarium, grant, or other form of payment was given to anyone to produce the manuscript.
Supplementary information is available at JPER’s website.
Conflicts of Interest
The authors report no other acknowledgements or conflicts of interest including no competing financial interests in relation to the described work.
References
- 1.Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants. N Engl J Med. 2002;347(4):240–7. doi: 10.1056/NEJMoa012657. [DOI] [PubMed] [Google Scholar]
- 2.Stoll BJ, Hansen NI, Higgins RD, Fanaroff AA, Duara S, Goldberg R, et al. Very low birth weight preterm infants with early onset neonatal sepsis: the predominance of gram-negative infections continues in the National Institute of Child Health and Human Development Neonatal Research Network, 2002-2003. Pediatr Infect Dis J. 2005;24(7):635–9. doi: 10.1097/01.inf.0000168749.82105.64. [DOI] [PubMed] [Google Scholar]
- 3.Stoll BJ, Hansen NI, Sanchez PJ, Faix RG, Poindexter BB, Van Meurs KP, et al. Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics. 2011;127(5):817–26. doi: 10.1542/peds.2010-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ting JY, Synnes A, Roberts A, Deshpandey A, Dow K, Yoon EW, et al. Association Between Antibiotic Use and Neonatal Mortality and Morbidities in Very Low-Birth-Weight Infants Without Culture-Proven Sepsis or Necrotizing Enterocolitis. JAMA Pediatr. 2016;170(12):1181–7. doi: 10.1001/jamapediatrics.2016.2132. [DOI] [PubMed] [Google Scholar]
- 5.Cantey JB, Huffman LW, Subramanian A, Marshall AS, Ballard AR, Lefevre C, et al. Antibiotic Exposure and Risk for Death or Bronchopulmonary Dysplasia in Very Low Birth Weight Infants. J Pediatr. 2017;181:289–93.e1. doi: 10.1016/j.jpeds.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Tolia VN, Desai S, Qin H, Rayburn PD, Poon G, Murthy K, et al. Implementation of an Automatic Stop Order and Initial Antibiotic Exposure in Very Low Birth Weight Infants. Am J Perinatol. 2017;34(2):105–10. doi: 10.1055/s-0036-1584522. [DOI] [PubMed] [Google Scholar]
- 7.Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sanchez PJ, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123(1):58–66. doi: 10.1542/peds.2007-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuppala VS, Meinzen-Derr J, Morrow AL, Schibler KR. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr. 2011;159(5):720–5. doi: 10.1016/j.jpeds.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cotten CM, McDonald S, Stoll B, Goldberg RN, Poole K, Benjamin DK., Jr The association of third-generation cephalosporin use and invasive candidiasis in extremely low birth-weight infants. Pediatrics. 2006;118(2):717–22. doi: 10.1542/peds.2005-2677. [DOI] [PubMed] [Google Scholar]
- 10.Benjamin DK, Jr, Stoll BJ, Gantz MG, Walsh MC, Sanchez PJ, Das A, et al. Neonatal candidiasis: epidemiology, risk factors, and clinical judgment. Pediatrics. 2010;126(4):e865–73. doi: 10.1542/peds.2009-3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Man P, Verhoeven BA, Verbrugh HA, Vos MC, van den Anker JN. An antibiotic policy to prevent emergence of resistant bacilli. Lancet. 2000;355(9208):973–8. doi: 10.1016/s0140-6736(00)90015-1. [DOI] [PubMed] [Google Scholar]
- 12.Alexander VN, Northrup V, Bizzarro MJ. Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. J Pediatr. 2011;159(3):392–7. doi: 10.1016/j.jpeds.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel SJ, Saiman L. Antibiotic resistance in neonatal intensive care unit pathogens: mechanisms, clinical impact, and prevention including antibiotic stewardship. Clin Perinatol. 2010;37(3):547–63. doi: 10.1016/j.clp.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novitsky A, Tuttle D, Locke RG, Saiman L, Mackley A, Paul DA. Prolonged early antibiotic use and bronchopulmonary dysplasia in very low birth weight infants. Am J Perinatol. 2015;32(1):43–8. doi: 10.1055/s-0034-1373844. [DOI] [PubMed] [Google Scholar]
- 15.Neu J, Douglas-Escobar M, Lopez M. Microbes and the developing gastrointestinal tract. Nutr Clin Pract. 2007;22(2):174–82. doi: 10.1177/0115426507022002174. [DOI] [PubMed] [Google Scholar]
- 16.Hadland SE, Wharam JF, Schuster MA, Zhang F, Samet JH, Larochelle MR. Trends in Receipt of Buprenorphine and Naltrexone for Opioid Use Disorder Among Adolescents and Young Adults, 2001-2014. JAMA Pediatr. 2017;171(8):747–55. doi: 10.1001/jamapediatrics.2017.0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeffery MM, Hooten WM, Hess EP, Meara ER, Ross JS, Henk HJ, et al. Opioid Prescribing for Opioid-Naive Patients in Emergency Departments and Other Settings: Characteristics of Prescriptions and Association With Long-Term Use. Ann Emerg Med. 2017 doi: 10.1016/j.annemergmed.2017.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel RM, Kandefer S, Walsh MC, Bell EF, Carlo WA, Laptook AR, et al. Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med. 2015;372(4):331–40. doi: 10.1056/NEJMoa1403489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kliegman RM, Walsh MC. Neonatal necrotizing enterocolitis: pathogenesis, classification, and spectrum of illness. Curr Probl Pediatr. 1987;17(4):213–88. doi: 10.1016/0045-9380(87)90031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125(2):e214–24. doi: 10.1542/peds.2009-0913. [DOI] [PubMed] [Google Scholar]
- 21.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56(2):645–6. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 22.Cordero L, Ayers LW. Duration of empiric antibiotics for suspected early-onset sepsis in extremely low birth weight infants. Infect Control Hosp Epidemiol. 2003;24(9):662–6. doi: 10.1086/502270. [DOI] [PubMed] [Google Scholar]
- 23.Esaiassen E, Fjalstad JW, Juvet LK, van den Anker JN, Klingenberg C. Antibiotic exposure in neonates and early adverse outcomes: a systematic review and meta-analysis. J Antimicrob Chemother. 2017 doi: 10.1093/jac/dkx088. [DOI] [PubMed] [Google Scholar]
- 24.Abdel Ghany EA, Ali AA. Empirical antibiotic treatment and the risk of necrotizing enterocolitis and death in very low birth weight neonates. Ann Saudi Med. 2012;32(5):521–6. doi: 10.5144/0256-4947.2012.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alm B, Erdes L, Mollborg P, Pettersson R, Norvenius SG, Aberg N, et al. Neonatal antibiotic treatment is a risk factor for early wheezing. Pediatrics. 2008;121(4):697–702. doi: 10.1542/peds.2007-1232. [DOI] [PubMed] [Google Scholar]
- 26.Mbakwa CA, Scheres L, Penders J, Mommers M, Thijs C, Arts IC. Early Life Antibiotic Exposure and Weight Development in Children. J Pediatr. 2016;176:105–13.e2. doi: 10.1016/j.jpeds.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 27.Greenwood C, Morrow AL, Lagomarcino AJ, Altaye M, Taft DH, Yu Z, et al. Early empiric antibiotic use in preterm infants is associated with lower bacterial diversity and higher relative abundance of Enterobacter. J Pediatr. 2014;165(1):23–9. doi: 10.1016/j.jpeds.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


