Abstract
Fibrinogen (Fg)-containing plaques are associated with memory loss during various inflammatory neurodegenerative diseases such as Alzheimer’s disease, multiple sclerosis, stroke, and traumatic brain injury. However, mechanisms of its action in neurovascular unit are not clear. As Fg is a high molecular weight blood protein and cannot translocate far from the vessel after extravasation, we hypothesized that it may interact with astrocytes first causing their activation. Cultured mouse cortical astrocytes were treated with Fg in the presence or absence of function-blocking anti-mouse ICAM-1 antibody, or with medium alone (control). Expressions of intercellular adhesion molecules 1 (ICAM-1) and tyrosine receptor kinase B (TrkB) as markers of astrocyte activation, and phosphorylation of TrkB (pTrkB) were assessed. Fg dose-dependently increased activation of astrocytes defined by their shape change, retraction of processes, and enhanced expressions ICAM-1 and TrkB, and increased pTrkB. Blocking of ICAM-1 function ameliorated these Fg effects. Data suggest that Fg interacts with astrocytes causing overexpression of ICAM-1 and TrkB, and TrkB phosphorylation, and thus, astrocyte activation. Since TrkB is known to be involved in neurodegeneration, interaction of Fg with astrocytes and the resultant activation of TrkB can be a possible mechanism involved in memory reduction, which were observed in previous studies and were associated with formation of complexes of Fg deposited in extravascular space with proteins such as Amyloid beta or prion, the proteins involved in development of dementia.
1. Introduction
Traumatic brain injury (TBI) that is caused by head trauma is a devastating disease that may cause death and disability (Davis, 2000). Brain trauma results in primary and secondary injuries. Primary injuries include mechanical damage to neurons, glial cells, and vasculature. Secondary injuries, which may act individually or cooperatively, include release of neurotransmitters, neuronal degradation, neuro inflammation and disruption of the blood brain barrier (BBB) (Davis, 2000; Giza and Hovda, 2001; Werner and Engelhard, 2007). The most common TBI, mild TBI can lead to long-term gray/white matter atrophy, neurodegeneration, and increased risk of developing neurological disorders (McKee and Robinson, 2014). Short-term memory (STM) impairment is one of the major problems of people with head injury (Cernich et al., 2010). Transient long-term and longer lasting STM deficits occur during mild TBI (Gorman et al., 1993). STM impairments persist longer in patients with mild to moderate TBI (Chuah et al., 2004). However, precise mechanisms of these effects are not clear.
The BBB is a complex structure consisting of endothelial cells (ECs), glial cells, mural cells, and neurons (Alves, 2014; Chodobski et al., 2011). The BBB serves to maintain neuronal function as well as prevent blood toxins, inflammatory cell and pathogens from reaching the central nervous system (CNS) (Abbott, 2000; Zlokovic, 2008). The neurovascular unit, which is comprised of ECs, astrocytes, and neurons, functions as the link between neuronal circuity and blood vessels (Attwell et al., 2010; Gordon et al., 2011; Hawkins and Davis, 2005). Disruption of this delicate barrier can lead to CNS pathologies such as Alzheimer’s disease (AD) (Rosenberg, 2014; Sweeney et al., 2018), dementia (van de Haar et al., 2015), or multiple sclerosis (MS) (Sweeney et al., 2018; Vos et al., 2005).
TBI causes complex biological responses resulting in systemic inflammation (Chhabra et al., 2010; Muradashvili et al., 2015; Pahatouridis et al., 2010; Sun et al., 2011) associated with increased release of cytokines and chemokines from damaged vascular cells, glial cells, and neurons and the resultant production of the high molecular weight (340 kD) blood plasma protein fibrinogen (Fg) by hepatic cells (Humphries, 1995; Vasse et al., 1996). High blood level of Fg, called hyperfibrinogenemia (HFg), is considered not only a marker of inflammation (Ross, 1999) but also a cause of inflammatory responses (Kerlin et al., 2004; Muradashvili et al., 2011; Muradashvili et al., 2012a; Patibandla et al., 2009; Tyagi et al., 2008). HFg accompanies inflammatory diseases such as stroke (D’Erasmo et al., 1993; del Zoppo et al., 2009), hypertension (Letcher et al., 1981; Lominadze et al., 1998), diabetes (Lee et al., 2007) and TBI (Chhabra et al., 2010; Muradashvili et al., 2015; Pahatouridis et al., 2010; Sun et al., 2011). Some studies suggest that HFg can be a causative factor in certain cerebrovascular events (Ernst et al., 1988; Shenhar-Tsarfaty et al., 2008) besides the TBI.
Fg/fibrin-containing plaque formations are the hallmark of neurodegenerative diseases associated with memory impairment such as AD (Ahn et al., 2010), MS (Vos et al., 2005) and TBI (Johnson et al., 2010). Fibrin deposits were found many years after head injury in humans (Hay et al., 2015). However, the initial steps in mechanisms of Fg appearance in extravascular space and subsequent formation of Fg/fibrin-containing plaques, in the absence of vascular rupture, are not clear. Recently, great attention is given to studies related to vascular contributions to cognitive impairment (Corriveau et al., 2016; Gorelick Philip et al., 2011; Muradashvili et al., 2012a; Muradashvili and Lominadze, 2013; Muradashvili et al., 2016; Murphy et al., 2016). However, mechanisms involved in vascular-mediated astrocyte activation resulting in neurodegeneration and memory impairment are not known. Increased cerebrovascular permeability, the only mechanism (Muradashvili et al., 2012a; Muradashvili and Lominadze, 2013; Muradashvili et al., 2016) for Fg deposition in subendothelial matrix (Sahni et al., 2009), was found in a mouse model of AD (Paul et al., 2007) and in patients with MS (Vos et al., 2005) and TBI (Hay et al., 2015). At an elevated level, Fg itself is the main culprit in increased cerebrovascular permeability (Muradashvili et al., 2012a; Muradashvili and Lominadze, 2013; Muradashvili et al., 2016), as it remains elevated for more than 20 days after inflammatory insult (Gabay and Kushner, 1999).
Blood levels of Fg is significantly associated with development of AD (Ahn et al., 2010; Cortes-Canteli and Strickland, 2009; Cortes-Canteli et al., 2010), and correlatedwith loss of memory during AD (Ahn et al., 2010) and with STM reduction during TBI (Muradashvili et al., 2015; Muradashvili et al., 2017). The presence of amyloid beta (Aβ)-Fg complexes have been shown to have a detrimental effect on memory retention (Ahn et al., 2010; Cortes-Canteli and Strickland, 2009; Cortes-Canteli et al., 2010; van Oijen et al., 2005 ). However, some studies indicate that cellular prion protein (PrP) has a greater effect on memory than content of Aβ (Chung et al., 2010; Gimbel et al., 2010). Interaction of Fg with non-digested scrapie prion protein (PrPSc) was shown (Fischer et al., 2000). We found a strong association of Fg-PrPC complex formation and reduction in STM after cortical contusion injury (CCI) (Muradashvili et al., 2015) and during HFg (Muradashvili et al., 2016). Upregulation of Fg results in increased blood viscosity (Lominadze et al., 1998) and shear stress (Chien et al., 1966; Davies et al., 2003; Lowe et al., 1997). This activiates ECs and induces expression of plasma adhesion molecules and integrins including intercellular adhesion molecule-1 (ICAM-1) (Altieri et al., 1995; Languino et al., 1993; Plow et al., 2000; Springer, 1990; Suehiro et al., 1997). In a previous study, it was demonstrated that Fg binds to ECs through its receptor ICAM-1 (Lominadze et al., 2005). This Fg-ICAM-1 binding further activates ECs and via extracellular signal-regulated kinases 1 and 2 signaling (Pluskota and D’Souza, 2000; Sen et al., 2009) results in enhanced exocytosis (Lominadze et al., 2005; Sen et al., 2009). It has been shown that astrocytes that express ICAM-1 are found in AD and some other neurodegenerative disorders (Akiyama et al., 1993). However, its functional implication of is not clear.
Although Fg is a large molecule, it can translocate to the extravascular space during TBI (Muradashvili and Lominadze, 2013; Muradashvili et al., 2017). In a previous study, it was demonstrated that 14 days after CCI, Fg could be found deposited between the vascular wall and endfeet of an adjacent astrocyte (Muradashvili et al., 2017). These Fg deposits led to astrocyte activation defined by their shape changes and retraction of endfeet (Muradashvili et al., 2017) as it usually happens with activated astrocytes (Schachtrup et al., 2010). It is known that activated astrocytes express tropomyosin receptor kinase B (or tyrosine receptor kinase B, TrkB), which causes neuronal degeneration (Colombo et al., 2012).
In the present study, we define specific mechanisms of Fg-mediated astrocyte activation that can be a premise for neuronal degeneration and STM reduction during mild TBI seen previously (Muradashvili et al., 2017). HFg-induced astrocyte activation and possible role of ICAM-1 in this process were studied in cultured mouse cortical astrocytes (MCAs).
2. Results
Our data show that Fg dose-dependently activated astrocytes (Fig. 1). Astrocyte activation was defined by changes in their shape and retraction of their processes and was measured by levels of GFAP intensity and expression of ICAM-1 (Fig. 1). Fg-induced astrocyte activation was reduced in the presence of function-blocking antibody against ICAM-1 (Fig. 1). The antibody alone did not have an effect on astrocytes (Fig. 1). Similarly, co-localization of Fg and GFAP, ICAM-1 and Fg, and GFAP and ICAM-1 were increased in astrocytes treated with high dose of Fg (Fig. 1). These effects were reduced by function-blocking antibody against ICAM-1 (Fig. 1).
Figure 1. Fibrinogen (Fg) induced activation of astrocytes.
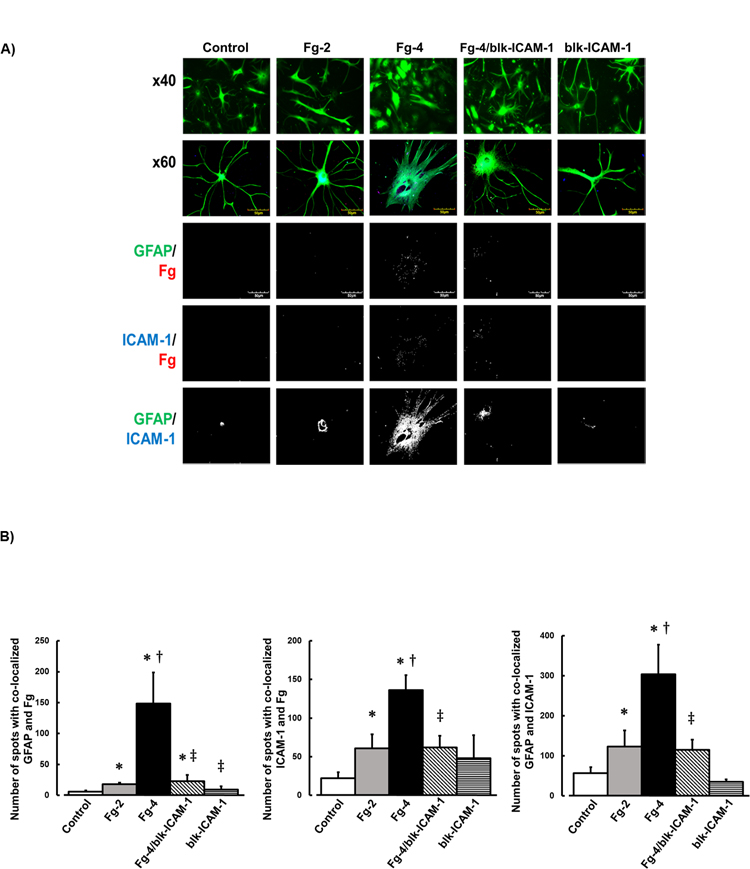
A) Examples of images of cultured mouse brain astrocytes treated with serum free media alone (control), 2 mg/ml of Fg (Fg-2), 4 mg/ml of Fg (Fg-4), 4 mg/ml of Fg in the presence of function blocking anti-intercellular adhesion molecule-1 (ICAM-1) antibody (Fg-4/blk-ICAM-1), or with function blocking anti-ICAM-1 antibody alone (blk-ICAM-1).
Astrocytes were identified by expression of glial fibrillary acidic protein (GFAP, green); ICAM-1 is shown in blue, and Fg -red.
First row: images taken with objective x40. Rows 2–5: images taken with objective x60. Rows 3–5: represent images after deconvolution.
Fg dose-dependently activated astrocytes (see shape change and retraction of processes), increased association of Fg with astrocytes and with ICAM-1, and increased expression of ICAM-1 in astrocytes.
B) Summary of number of spots with co-localized GFAP and Fg (GFAP/Fg); ICAM-1 and Fg (ICAM-1/Fg), and GFAP and ICAM-1 (GFAP/ICAM).
P < 0.05 for all. * - vs. Control, †- vs. Fg-2, ‡- vs. Fg-4; n=4
In another series of experiments, besides confirming that Fg dose-dependently activates astrocytes (defined by enlarged shape and retracted processes that were identified by GFAP), we found that Fg dose-dependently increased expressions of ICAM-1, TkB, and pTrkB (also markers of astrocyte activation) (Fig. 2, A, B, and C). It is noteworthy to mention that Fg-induced enhanced expressions of ICAM-1 and TrkB were found mainly in the periphery of cells (Fig. 2, A and B). Similarly, co-localization of GFAP and ICAM-1, GFAP and TrkB, and GFAP, TrkB, and pTrkB were increased in cells treated with higher dose of Fg (Fig. 2, A and B). Function-blocking antibody against ICAM-1 reduced, while the detecting antibody did not affect these effects (Fig. 2). Function-blocking and detecting antibodies against ICAM-1 alone did not have any significant effect on astrocytes (Fig. 2).
Figure 2. Fibrinogen (Fg) induced overexpression of intercellular adhesion molecule-1 (ICAM-1), tyrosine receptor kinase B (TrkB) and its phosphorylation (pTrkB).
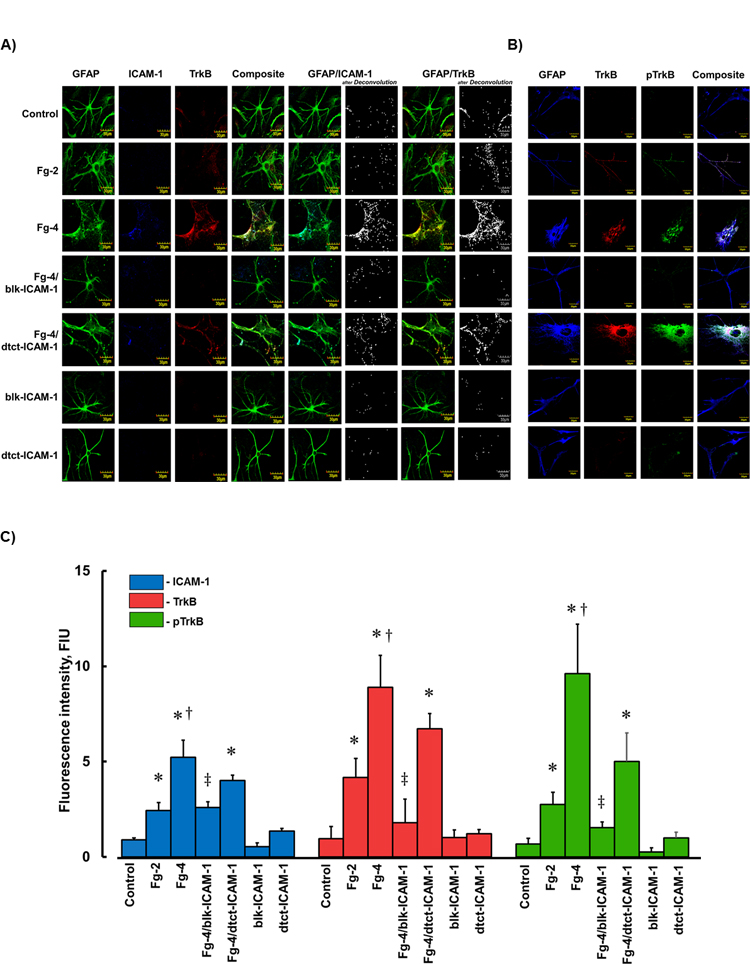
A and B) Examples of images of cultured mouse brain astrocytes (MCAs) treated with serum free media alone (control), 2 mg/ml of Fg (Fg-2), 4 mg/ml of Fg (Fg-4), 4 mg/ml of Fg in the presence of function blocking anti-ICAM-1 antibody (Fg-4/blk-ICAM-1), 4 mg/ml of Fg in the presence of detecting anti-ICAM-1 antibody (Fg-4/dtct-ICAM-1), or with function blocking or detecting anti-ICAM-1 antibodies alone (blk-ICAM- 1 and dtct-ICAM-1, respectively).
A) Astrocytes were identified by expression of glial fibrillary acidic protein (GFAP, green); ICAM-1 -blue, and TrkB -red.
B) Astrocytes were identified by GFAP -blue, TrkB -red, and pTrkB - green.
C) Summary of fluorescence intensity changes for ICAM-1, TrkB, and pTrkB in cultured MCAs treated with Fg-2, Fg-4, Fg-4/blk-ICAM-1, Fg-4/dtct-ICAM-1, blk-ICAM-1, or dtct-ICAM-1.
P < 0.05 for all. * - vs. Control, †- vs. Fg-2, ‡- vs. Fg-4; n=4
Western blot analyses confirmed results of Fg effects on astrocytes obtained by immunohistochemistry (Fig. 3, A, B, C, D, E, and F).
Figure 3. Fibrinogen (Fg) induced overexpression of intercellular adhesion molecule-1 (ICAM-1), tyrosine receptor kinase B (TrkB) and its phosphorylation (pTrkB) detected by Western blot analysis.
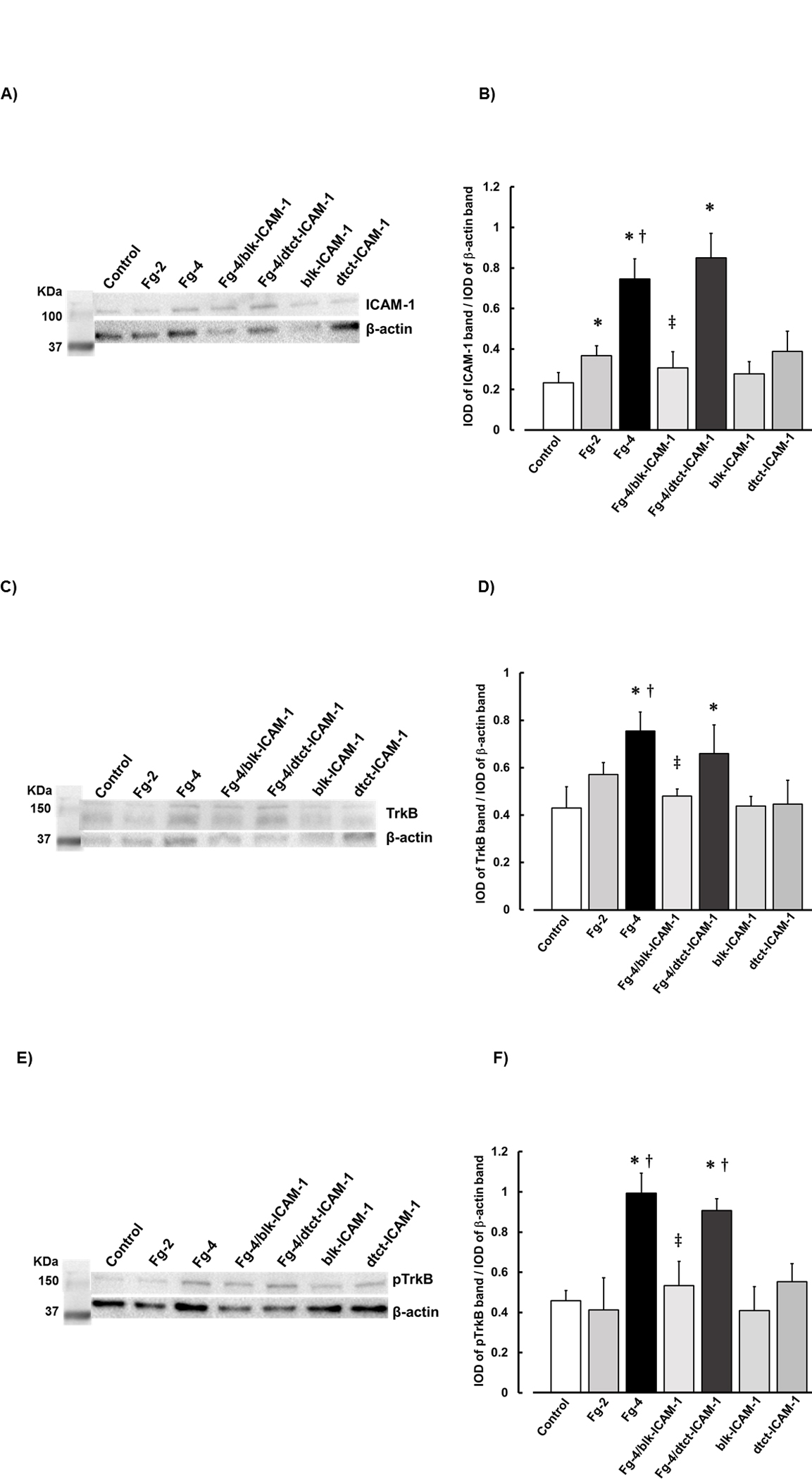
Examples of images of Western blot analyses of levels of ICAM-1 (A), TrkB (C) and pTrkB (E) of cultured MCAs treated with serum free media alone (control), 2 mg/ml of Fg (Fg-2), 4 mg/ml of Fg (Fg-4), 4 mg/ml of Fg in the presence of function blocking anti-ICAM-1 antibody (Fg-4/blk-ICAM-1), 4 mg/ml of Fg in the presence of detecting anti-ICAM-1 antibody (Fg-4/dtct-CAM-1), or with function blocking or detecting anti-ICAM-1 antibodies alone (blk-ICAM-1 and dtct-ICAM-1, respectively). A total of 10 μg protein was placed in each well of the SDS gel.
Molecular weight standard (Std.) is presented in the left lane.
Summary of Western blot results of ICAM-1 (B), TrkB (D) and pTrkB (F).
P < 0.05 for all. * - vs. Control, † - vs. Fg-2, ‡ -vs Fg-4; n=4
3. Discussion
Although precise mechanisms are still not clear, Fg deposited in extravascular space has been implicated in memory reduction during various neurodegenerative diseases (Hay et al., 2015; Paul et al., 2007). Recently, we showed that Fg crosses wall of non-ruptured microvessels and deposits between the vessel and astrocyte endfeet during TBI (Muradashvili et al., 2017). This was associated with activation of astrocytes defined by their enlargement and retraction of processes (Muradashvili et al., 2017). We also found that enhanced deposition of Fg in extravascular space was correlated with neuronal degeneration (Muradashvili et al., 2017) and reduction in STM (Muradashvili et al., 2015; Muradashvili et al., 2017). All these findings suggest that extravasated and deposited in vascular and astrocyte endfeet interface Fg results neuronal degeneration via activation of astrocytes. However, mechanisms of this Fg-induced astrocyte activation are not clear. The present study was designed to fill some gaps in Fg-astrocyte interaction.
To specifically define possible mechanisms of Fg-astrocyte association in vivo presented previously (Muradashvili et al., 2017) we performed in vitro study on cultured astrocytes. Of course, in culture, astrocytes may lose some of their properties; however, data presented here coincide with results we have seen in our previous study: that is astrocyte activation in the presence of Fg. Therefore; most likely, Fg-astrocyte interaction in vitro does not change significantly from that seen in vivo. This association was also confirmed in another study (Hsiao et al., 2013)
Our results show that at high level Fg activates astrocytes resulting in their swelling, endfeeet retraction, and overexpression of ICAM-1 and TrkB. These data provide a strong premise for findings that Fg, leaked from ruptured vasculature causes astrocyte scar formation (Schachtrup et al., 2010) and axonal damage (Davalos et al., 2012). However, absence of degradation of exogenous Fg used in the latter studies was not documented (Davalos et al., 2012) masking the effects of Fg. In our studies, we observed effects of Fg extravasated from non-ruptured cerebral vessels and deposited in in extravascular space (Muradashvili et al., 2015; Muradashvili et al., 2016; Muradashvili et al., 2017).
In the present study we found that Fg dose-dependently activated astrocytes, which was defined by their swelling and retraction of processes. Moreover, activated astrocytes overexpressed ICAM-1 and TrkB and generated pTrkB, suggesting changes in their functional properties. Co-localization of GFAP with ICAM-1, TrkB, and pTrkB clearly indicated that Fg activated astrocytes and triggered their functional effects.
It is known that ICAM-1 expression is enhanced in activated astrocytes (Akiyama et al., 1993; Brosnan et al., 1995; Lee et al., 2000). However, its functional role was not clear. Here we confirmed that astrocyte ICAM-1 expression was increased with their activation induced by Fg. Activation of astrocytes was demonstrated not only by their shape change and endfeet retraction but it was also shown by enhanced expressions of ICAM-1 and TrkB and formation of pTrkB. Further, we found that this Fg-induced astrocyte activation was reduced by blocking the ICAM-1. Moreover, we found that Fg was dose-dependently bound to astrocytes, and this binding was reduced by blocking of ICAM-1 function. Thus, our results indicate that Fg affects astrocytes via possibly interacting with overexpressed ICAM-1.
It has been shown, that astrocytes remove surface-coating Fg (Hsiao et al., 2013). Although it was not discussed, data of this study show that removal of Fg by astrocytes results in their activation and disappearance (which can be a result of their death) (Hsiao et al., 2013). Thus, Fg and astrocyte interaction may result in astrocyte activation and Fg digestion by the activated astrocytes. Activation of astrocytes leads to expression of chondroitin sulfate proteoglycans (Hsiao et al., 2013) and TrkB seen in the present study. Overexpression of TrkB and its phosphorylation (formation of pTrkB) can be detrimental to the adjacent neurons as they are involved in neuronal degeneration and loss of memory via production of nitric oxide (Colombo et al., 2012).
It is possible that removal of accumulated Fg from extravascular space by astrocytes (Hsiao et al., 2013) occurs constantly. Although it results in decrease of astrocyte number, this process overall is beneficial to the CNS as it may prevent formation of Fg-containing protein complexes, such as Fg-Aβ and Fg-PrP (Muradashvili et al., 2016), which could lead to plaque formations seen in various diseases (Ahn et al., 2010; Johnson et al., 2010; Vos et al., 2005). However, in the case of systemic inflammation that occurs during several neurodegenerative pathologies such as AD, TBI, and others, increase in blood level of Fg results in its enhanced extravasation (Muradashvili et al., 2011; Muradashvili et al., 2012a; Muradashvili et al., 2012b; Muradashvili and Lominadze, 2013; Muradashvili et al., 2016; Muradashvili et al., 2017). During inflammatory diseases the level of plasminogen activator inhibitor (mainly produced by ECs), is also increased (Landin et al., 1990; Lip and Beevers, 2007; Pankow et al., 1998) resulting in decreased fibrinolysis. In general, digestion of Fg by plasmin in vivo is quite rare (Boutcher et al., 1996; Gaffney, 2001). In the excess of extravascular Fg, astrocytes may not be as effective. Therefore, this may result in neuronal degeneration and STM reduction during TBI seen previously (Muradashvili et al., 2017) and formation Fg-containing plaques during other neurodegenerative diseases leading to loss of memory (Johnson et al., 2010).
In conclusion, the present study demonstrated a possible mechanism of Fg-astrocyte interaction and its effect, which may occur via Fg activation of its receptor ICAM-1 that is found on astrocyte surface (Akiyama et al., 1993; Brosnan et al., 1995; Lee et al., 2000). In addition, based on data of this and our previous studies we suggest that Fg-astrocyte interaction can have a functional implication such as overexpression of TrkB, which is known to be involved in memory impairment (Colombo et al., 2012). In fact, we have previously found that STM was reduced in mice with head trauma 14 days after CCI (Muradashvili et al., 2015; Muradashvili et al., 2017), which was associated with increased blood level of Fg (Muradashvili et al., 2015) and its increased deposition between vessels and endfeet of activated astrocytes resulting in physical detachment of the vessel and astrocyte endfeet (Muradashvili et al., 2017).
4. Methods and Materials
4.1. Antibodies and reagents.
Human Fg depleted of plasminogen, von-Willebrand factor, and fibronectin was from Enzyme Research Laboratories (South Bend, IN). Polyclonal rabbit antibody against human Fg (crossreacts with mouse) was purchased from DakoCytomation (Carpentaria, CA). Rat purified detecting and function blocking (clone: YN1/1.7.4) antibodies against mouse ICAM-1 (CD-54) were obtained from BioLegend (San Diego, CA). In vitro Animal Astrocyte Medium (AM-a) was purchased from ScienCell Research Laborites (Carlsbad, CA). Chicken antibody against glial fibrillary acidic protein (GFAP) was from Antibody Online. Rabbit polyclonal antibody against TrkB and Rabbit antibody against phosphorylated TrkB (pTrkB) was from Millipore (Burlington, MA). Secondary antibodies conjugated with Alexa-Fluor 488, Alexa-Fluor 594, or Alexa-Fluor 647 were purchased from Invitrogen (Carlsbad, CA). Bovine serum albumin (BSA), Hirudin, and Poly-L-ornithine were from Sigma Aldrich Chemicals Co. (St. Louis, MO). 8-well chambered glass-bottomed plates were purchased from Nalge Nunc International (Rochester, NY). 4,6-diamidino-2-phenyl-indole HCl (DAPI) were from Santa Cruz Biotechnology (Santa Cruz, CA).
4.2. Astrocyte culture.
MCAs were purchased from ScienCell. The MCAs were grown in AM-a complete media either in glass-bottomed, 8-well chambers or 6-well plastic plates coated with L-ornithine for better astrocyte attachment. Cells were kept at 37 °C with 5% CO2/air in a humidified environment as recommend by the manufacturer and were used at the 3rd or 4th passage for the experiments.
4.3. Experimental setups and groups.
Experiments were conducted once MCAs in 8-well chambers reached 80% confluence. The complete media was removed the day of the experiment and replaced with serum free media (SFM) for 2 hours. Serum-starved cells were then treated for 20 hours with SFM alone, Fg 2 mg/ml, Fg 4 mg/mL, Fg 4 mg/mL with function blocking anti-ICAM-1 antibody (100 μg/ml), Fg 4 mg/mL with detecting anti-ICAM-1 antibody (100 μg/ml), and with SFM in the presence of function-blocking or detecting anti-ICAM-1 antibodies. The content of Fg was chosen based on the normal level (2 mg/ml) of Fg in human plasma and the level of Fg (4 mg/ml) during inflammation (Letcher et al., 1981). Each experimental group contained Hirudin (2 μl/ml) to block possible conversion of Fg to fibrin. The experimental cells were kept in the incubator at 37 °C overnight. After the experiment, MCAs washed with phosphate buffered saline (PBS), fixed with 3.7% formaldehyde, and processed for immunochemical analysis.
In separate series of experiments, cells grown in 6-well pales were treated as described above. After experiments, cells were washed with PBS and processed for Western blot analysis. A total of 4 experiments were performed. Each experimental group was tested in duplicate. All treatments and analyses were performed by researchers blinded (as it was possible) to the treatment groups.
4.4. Immunohistochemistry.
The fixed MCAs were treated with a permeability/blocking solution containing Tween 20 (0.5 μL/mL) and BSA (20 mg/mL) in PBS for 1 hour. After washing three times with PBS, MCAs were incubated with appropriate amounts (1:100 dilution) of primary antibodies against GFAP, ICAM-1, TrkB, and pTrkB. The chambers were kept in the dark at 4°C overnight. MCAs were then washed three times with PBS and treated with appropriate secondary antibodies conjugated with Alexa Fluor 488/594/or 647 fluorescent dyes (1:200 dilution) for 2 hours. DAPI (dilution: 1:10,000) was added for a period of 10 min to stain the cell nuclei. Then the cells were washed and stored in 1% PBS in the dark at 4°C.
4.5. Confocal Microscopy.
Glass-bottomed 8-well chambers were viewed. Digital images were taken with a laser scanning confocal microscope (Olympus, Fluoview IV1000, objective x60). Cell nuclei and GFAP (DAPI and Alexa-488, respectively) were visualized using a multiline argon-ion laser (458/488/515 nm) to excite the dye, while emissions were observed above 519 nm. DAPI was used for initial detection of cells by observing their nuclei (they are not presented in final images). TrkB (Alexa-594) was visualized using a HeNe-Green laser (543 nm) to excite the dye, while emission was observed above 620 nm. ICAM-1 and pTrkB (Alexa-647nm) were visualized using a HeNe-Red laser (633 nm), while emission was observed above 667 nm.
4.6. Image Analysis
Fg-induced expressions of GFAP, ICAM-1, TrkB, and pTrkB were assessed for each experimental group using offline image analysis software (Image-Pro Plus, Media Cybernetics, Inc., Bethesda, MD) as previously described (Muradashvili et al., 2012a; Muradashvili et al., 2014b; Muradashvili et al., 2015; Muradashvili et al., 2017). Briefly, cells samples were imaged randomly (at least 4–5 times in each well). To determine expressions of ICAM-1, TrkB, and pTrkB, using the Image Pro-Plus software, rectangular areas of interests (AOIs) of the same size were placed over the treated astrocyte images of all respective experimental groups. Fluorescence intensity in 4 randomly placed AOIs were measured. The results were averaged for each experimental group and presented as fluorescence intensity units (FIU). To identify co-localization of GFAP with ICAM-1, TrkB, or pTrkB, the images were de-convoluted with Image Pro-Plus and “masks” were formed, which demonstrated overlap of different colors as previously described (Muradashvili et al., 2014a; Muradashvili et al., 2017). The numbers of co-localized objects were automatically counted by the software.
4.7. Western Blot Analysis.
At the end of the experiments, cells were lysed and levels of target proteins in astrocyte samples were assessed as described previously (Muradashvili et al., 2011; Patibandla et al., 2009; Sen et al., 2009). Briefly, equal volume (30 µl) of astrocyte protein from cell samples were loaded onto 10% SDS-PAGE gels and electrophoresed under reducing conditions and then transferred onto polyvinylidene difluoride (PVDF) membranes. Non-specific sites on membranes were blocked with 5% non-fat dry milk in TBS-T and membranes were incubated with antibodies against target proteins overnight at 4°C. Then, probing with appropriate secondary antibodies for 2 hours at room temperature, the blots were developed using a BioRad Molecular Imager (ChemiDoc XRS+, Hercules, CA). Obtained blot images were analyzed with Image Pro Plus. Levels of β-actin were used as loading controls. Levels of target proteins were assessed by measuring an integrated optical density (IOD) of their bands in each sample lane profile and presented relative to the IOD of the respective β-actin band.
4.8. Data Analysis.
All data are expressed as mean ± SEM. The experimental groups were compared by one-way ANOVA. If ANOVA indicated a significant difference (P < 0.05), Tukey’s multiple comparison test was used to compare group means. Differences were considered significant if P < 0.05.
Highlights.
Fibrinogen (Fg) dose-dependently activates astrocytes
Fg-activated astrocytes overexpress ICAM-1 and TrkB, result in TrkB phosphorylation.
Fg may bind the activated astrocytes via ICAM-1
5. Acknowledgements
Supported in part by grants from NIH (NS-084823), AHA (17SDG33670372), University of Louisville (UofL) SOM (Basic 57139) and UoL Summer Cardiovascular Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NJ, 2000. Inflammatory mediators and modulation of blood-brain barrier permeability. Cellular and Molecualr Neurobiology 20, 131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn HJ, Zamolodchikov D, Cortes-Canteli M, Norris EH, Glickman JF, Strickland S, 2010. Alzheimer’s disease peptide β-amyloid interacts with fibrinogen and induces its oligomerization. Proc Natl Acad Sci USA 107, 21812–21817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H, Kawamata T, Yamada T, Tooyama I, Ishii T, McGeer P, 1993. Expression of intercellular adhesion molecule (ICAM)-1 by a subset of astrocytes in Alzheimer disease and some other degenerative neurological disorders. Acta Neuropathologica 85, 628–634. [DOI] [PubMed] [Google Scholar]
- Altieri DC, Duperray A, Plescia J, Thornton GB, Languino LR, 1995. Structural recognition of a novel fibrinogen gamma chain sequence (117–133) by intercellular adhesion molecule-1 mediates leukocyte-endothelium interaction. J Biol Chem 270, 696–699. [DOI] [PubMed] [Google Scholar]
- Alves JL, 2014. Blood–brain barrier and traumatic brain injury. Journal of Neuroscience Research 92, 141–147. [DOI] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, MacVicar BA, Newman EA, 2010. Glial and neuronal control of brain blood flow. Nature 468, 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutcher PA, Gaffney PJ, Raut S, O’Regan RG, McLoughlin P, 1996. Effects of early plasmin digests of fibrinogen on isometric tension development in isolated rings of rat pulmonary artery. Thrombosis Research 81, 231–239. [DOI] [PubMed] [Google Scholar]
- Brosnan C, Cannella B, Battistini L, Raine C, 1995. Cytokine localization in multiple sclerosis lesions: correlation with adhesion molecule expression and reactive nitrogen species. Neurology 45, S16–S21. [DOI] [PubMed] [Google Scholar]
- Cernich A, Kurtz S, Mordecai K, Ryan P, 2010. Cognitive rehabilitation in traumatic brain injury. Current Treatment Options in Neurology 12, 412–423. [DOI] [PubMed] [Google Scholar]
- Chhabra G, Rangarajan K, Subramanian A, Agrawal D, Sharma S, Mukhopadhayay A, 2010. Hypofibrinogenemia in isolated traumatic brain injury in Indian patients. Neurology India 58, 756–757. [DOI] [PubMed] [Google Scholar]
- Chien S, Usami S, Taylor HM, Lundberg JL, Gregersen MI, 1966. Effects of hematocrit and plasma proteins on human blood rheology at low shear rates. Journal of Applied Physiology 21, 81–87. [DOI] [PubMed] [Google Scholar]
- Chodobski A, Zink BJ, Szmydynger-Chodobska J, 2011. Blood-brain barrier pathophysiology in traumatic brain injury. Translational stroke research 2, 492–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuah YML, Maybery MT, Fox AM, 2004. The long-term effects of mild head injury on short-term memory for visual form, spatial location, and their conjunction in well-functioning university students. Brain and Cognition 56, 304–312. [DOI] [PubMed] [Google Scholar]
- Chung E, Ji Y, Sun Y, Kascsak R, Kascsak R, Mehta P, Strittmatter S, Wisniewski T, 2010. Anti-PrPC monoclonal antibody infusion as a novel treatment for cognitive deficits in an Alzheimer’s disease model mouse. BMC Neurosci 11, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo E, Cordiglieri C, Melli G, Newcombe J, Krumbholz M, Parada L, Medico E, Hohlfeld R, Meinl E, Farina C, 2012. Stimulation of the neurotrophin receptor TrkB on astrocytes drives nitric oxide production and neurodegeneration. Journal of Experimental Medicine 209, 521–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corriveau RA, Bosetti F, Emr M, Gladman JT, Koenig JI, Moy CS, Pahigiannis K, Waddy SP, Koroshetz W, 2016. The Science of Vascular Contributions to Cognitive Impairment and Dementia (VCID): A Framework for Advancing Research Priorities in the Cerebrovascular Biology of Cognitive Decline. Cellular and Molecular Neurobiology 36, 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes-Canteli M, Strickland S, 2009. Fibrinogen, a possible key player in Alzheimer’s disease. Journal of Thrombosis and Haemostasis 7, 146–150. [DOI] [PubMed] [Google Scholar]
- Cortes-Canteli M, Paul J, Norris EH, Bronstein R, Ahn HJ, Zamolodchikov D, Bhuvanendran S, Fenz KM, Strickland S, 2010. Fibrinogen and β-Amyloid association alters thrombosis and fibrinolysis: A possible contributing factor to Alzheimer’s Disease. Neuron 66, 695–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Erasmo E, Acca M, Celi FS, Medici F, Palmerini T, Pisani D, 1993. Plasma fibrinogen and platelet count in stroke. Journal of Medicine 24, 185–191. [PubMed] [Google Scholar]
- Davalos D, Kyu Ryu J, Merlini M, Baeten KM, Le Moan N, Petersen MA, Deerinck TJ, Smirnoff DS, Bedard C, Hakozaki H, Gonias Murray S, Ling JB, Lassmann H, Degen JL, Ellisman MH, Akassoglou K, 2012. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat Commun 3, 1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PF, Zilberberg J, Helmke BP, 2003. Spatial microstimuli in endothelial mechanosignaling. Circulation Research 92, 359–370. [DOI] [PubMed] [Google Scholar]
- Davis AE, 2000. Mechanisms of traumatic brain injury: biomechanical, structural and cellular considerations. Critical Care Nursing Quarterly 23, 1–13. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ, Levy DE, Wasiewski WW, Pancioli AM, Demchuk AM, Trammel J, Demaerschalk BM, Kaste M, Albers GW, Ringelstein EB, 2009. Hyperfibrinogenemia and functional outcome from acute ischemic stroke. Stroke 40, 1687–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst E, Matrai A, Marshall M, 1988. Blood rheology in patients with transient ischemic attacks. Stroke 19, 634–636. [DOI] [PubMed] [Google Scholar]
- Fischer MB, Roeckl C, Parizek P, Schwarz HP, Aguzzi A, 2000. Binding of disease-associated prion protein to plasminogen. Nature 408, 479–483. [DOI] [PubMed] [Google Scholar]
- Gabay C, Kushner I, 1999. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 340, 448–454. [DOI] [PubMed] [Google Scholar]
- Gaffney PJ, 2001. Fibrin degradation products: A review of structures found in vitro and in vivo. Annals of the New York Academy of Sciences 936, 594–610. [PubMed] [Google Scholar]
- Gimbel DA, Nygaard HB, Coffey EE, Gunther EC, Laurén J, Gimbel ZA, Strittmatter SM, 2010. Memory impairment in transgenic Alzheimer mice requires cellular prion protein. The Journal of Neuroscience 30, 6367–6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giza CC, Hovda DA, 2001. The Neurometabolic Cascade of Concussion. Journal of Athletic Training 36, 228–235. [PMC free article] [PubMed] [Google Scholar]
- Gordon GR, Howarth C, MacVicar BA, 2011. Bidirectional control of arteriole diameter by astrocytes. Experimental Physiology 96, 393–399. [DOI] [PubMed] [Google Scholar]
- Gorelick Philip B, Scuteri A, Black S, Decarli C, Greenberg S, Iadecola C, Launer L, Laurent S, Lopez O, Nyenhuis D, Petersen R, Schneider J, Tzourio C, Arnett D, Bennett D, Chui H, Higashida R, Lindquist R, Nilsson P, Roman G, Sellke F, Seshadri S, 2011. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke 42, 2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman L, Shook B, Becker D, 1993. Traumatic brain injury produces impairments in long-term and recent memory. Brain research 614, 29–36. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP, 2005. The blood-brain barrier/neurovascular unit in health and disease. Pharmacological Reviews 57, 173–185. [DOI] [PubMed] [Google Scholar]
- Hay J,R, Johnson VE, Young AM, Smith DH, Stewart W, 2015. Blood-brain barrier disruption is an early event that may persist for many years after traumatic brain injury in humans. Journal of Neuropathology and Experimental Neurology 74, 1147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao TW, Swarup VP, Kuberan B, Tresco PA, Hlady V, 2013. Astrocytes specifically remove surface-adsorbed fibrinogen and locally express chondroitin sulfate proteoglycans. Acta Biomaterialia 9, 7200–7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries SE, 1995. Genetic regulation of fibrinogen. European Heart Journal 16, 16–20. [DOI] [PubMed] [Google Scholar]
- Johnson VE, Stewart W, Smith DH, 2010. Traumatic brain injury and amyloid-[beta] pathology: a link to Alzheimer’s disease? Nat Rev Neurosci 11, 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerlin B, Cooley BC, Isermann BH, Hernandez I, Sood R, Zogg M, Hendrickson SB, Mosesson MW, Lord S, Weiler H, 2004. Cause-effect relation between hyperfibrinogenemia and vascular disease. Blood 103, 1728–1734. [DOI] [PubMed] [Google Scholar]
- Landin K, Tengborn L, Smith U, 1990. Elevated fibrinogen and plasminogen activator inhibitor (PAI-1) in hypertension are related to metabolic risk factors for cardiovascular disease. J Intern Med 227, 273–278. [DOI] [PubMed] [Google Scholar]
- Languino LR, Plescia J, Duperrray A, Brian AA, Plow EF, Geltosky JE, Alteri DC, 1993. Fibrinogen mediates leukocyte adhesion to vascular endothelium through an ICAM-1-dependent pathway. Cell 73, 1423–1434. [DOI] [PubMed] [Google Scholar]
- Lee AJ, Lowe GDO, Woodward M, Tunstall-Pedoe H, 2007. Fibrinogen in relation to personal history of prevalent hypertension, diabetes, stroke, intermittent claudication, coronary heart disease, and family history: the Scottish Heart Health Study. British Heart Journal 69, 338–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Drabik K, Van Wagoner NJ, Lee S, Choi C, Dong Y, Benveniste EN, 2000. ICAM-1-induced expression of proinflammatory cytokines in astrocytes: involvement of extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways. The Journal of Immunology 165, 4658–4666. [DOI] [PubMed] [Google Scholar]
- Letcher RL, Chien S, Pickering TG, Sealey JE, Laragh JH, 1981. Direct relationship between blood pressure and blood viscosity in normal and hypertensive subjects. Role of fibrinogen and concentration. The American Journal of Medicine 70, 1195–1202. [DOI] [PubMed] [Google Scholar]
- Lip GYH, Beevers DG, 2007. Abnormalities of rheology and coagulation in hypertension. Journal of Human Hypertension 8, 693–702. [PubMed] [Google Scholar]
- Lominadze D, Joshua IG, Schuschke DA, 1998. Increased erythrocyte aggregation in spontaneously hypertensive rats. Am J Hypertens 11, 784–789. [DOI] [PubMed] [Google Scholar]
- Lominadze D, Tsakadze N, Sen U, Falcone JC, D’Souza SE, 2005. Fibrinogen- and fragment D-induced vascular constriction. American Journal of Physiology 288, H1257–H1264. [DOI] [PubMed] [Google Scholar]
- Lowe GDO, Lee AJ, Rumley A, Price JF, Fowkes FGR, 1997. Blood viscosity and risk of cardiovascular events: the Edinburgh Artery Study. British Journal of Haematology 96, 168–173. [DOI] [PubMed] [Google Scholar]
- McKee AC, Robinson ME, 2014. Military-related traumatic brain injury and neurodegeneration. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 10, S242–S253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muradashvili N, Tyagi N, Tyagi R, Munjal C, Lominadze D, 2011. Fibrinogen alters mouse brain endothelial cell layer integrity affecting vascular endothelial cadherin. Biochemical and Biophysical Research Communications 413, 509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muradashvili N, Qipshidze N, Munjal C, Givvimani S, Benton RL, Roberts AM, Tyagi SC, Lominadze D, 2012a. Fibrinogen-induced increased pial venular permeability in mice. J Cereb Blood Flow Metab 32, 150–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muradashvili N, Tyagi R, Lominadze D, 2012b. A dual-tracer method for differentiating transendothelial transport from paracellular leakage in vivo and in vitro. Front Physiol 3, 166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muradashvili N, Lominadze D, 2013. Role of fibrinogen in cerebrovascular dysfunction after traumatic brain injury. Brain Injury 27, 1508–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muradashvili N, Benton R, Tyagi R, Tyagi S, Lominadz D, 2014a. Elevated level of fibrinogen increases caveolae formation; Role of matrix metalloproteinase-9. Cell Biochem Biophys 69, 283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muradashvili N, Tyagi R, Metreveli N, Tyagi SC, Lominadze D, 2014b. Ablation of MMP9 gene ameliorates paracellular permeability and fibrinogen-amyloid beta complex formation during hyperhomocysteinemia. J Cereb Blood Flow Metab 34, 1472–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muradashvili N, Benton RL, Saatman KE, Tyagi SC, Lominadze D, 2015. Ablation of matrix metalloproteinase-9 gene decreases cerebrovascular permeability and fibrinogen deposition post traumatic brain injury in mice. Metabolic Brain Disease 30, 411–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muradashvili N, Tyagi R, Tyagi N, Tyagi SC, Lominadze D, 2016. Cerebrovascular disorders caused by hyperfibrinogenemia. The Journal of Physiology 594, 5941–5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muradashvili N, Tyagi SC, Lominadze D, 2017. Localization of fibrinogen in the vasculo-astrocyte interface after cortical contusion injury in mice. Brain Sciences 7, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M, Corriveau R, Wilcock D, 2016. Vascular contributions to cognitive impairment and dementia (VCID). Biochim. Biophys. Acta 1862, 857–859. [DOI] [PubMed] [Google Scholar]
- Pahatouridis D, Alexiou G, Zigouris A, Mihos E, Drosos D, Voulgaris S, 2010. Coagulopathy in moderate head injury. The role of early administration of low molecular weight heparin. Brain Injury 24, 1189–1192. [DOI] [PubMed] [Google Scholar]
- Pankow JS, Folsom AR, Province MA, Rao DC, Williams RR, Eckfeldt J, Sellers TA, 1998. Segregation analysis of plasminogen activator inhibitor-1 and fibrinogen levels in the NHLBI family heart study. Arterioscler.Thromb.Vasc.Biol 18, 1559–1567. [DOI] [PubMed] [Google Scholar]
- Patibandla PK, Tyagi N, Dean WL, Tyagi SC, Roberts AM, Lominadze D, 2009. Fibrinogen induces alterations of endothelial cell tight junction proteins. Journal of Cellular Physiology 221, 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J, Strickland S, Melchor JP, 2007. Fibrin deposition accelerates neurovascular damage and neuroinflammation in mouse models of Alzheimer’s disease. The Journal of Experimental Medicine 204, 1999–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleasant J, Carlson S, Mao H, Scheff S, Yang K, Saatman K, 2011. Rate of neurodegeneration in the mouse controlled cortical impact model is influenced by impactor tip shape: implications for mechanistic and therapeutic studies. Journal of Neurotrauma [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow EF, Haas TA, Zhang L, Loftus J, Smith JW, 2000. Ligand binding to integrins. The Journal of Biological Chemistry 275, 21785–21788. [DOI] [PubMed] [Google Scholar]
- Pluskota E, D’Souza SE, 2000. Fibrinogen interactions with ICAM-1 (CD54) regulate endothelial cell survival. European Journal of Biochemistry 267, 4693–4704. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, 2014. Blood-Brain Barrier Permeability in Aging and Alzheimer’s Disease. The journal of prevention of Alzheimer’s disease 1, 138–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R, 1999. Mechanisms of disease - Atherosclerosis - An inflammatory disease. N Engl J Med 340, 115–126. [DOI] [PubMed] [Google Scholar]
- Sahni A, Arévalo MT, Sahni SK, Simpson-Haidaris PJ, 2009. The VE-cadherin binding domain of fibrinogen induces endothelial barrier permeability and enhances transendothelial migration of malignant breast epithelial cells. International Journal of Cancer 125, 577–584. [DOI] [PubMed] [Google Scholar]
- Schachtrup C, Ryu JK, Helmrick MJ, Vagena E, Galanakis DK, Degen JL, Margolis RU, Akassoglou K, 2010. Fibrinogen triggers astrocyte scar formation by promoting the availability of active TGF-β after vascular damage. The Journal of Neuroscience 30, 5843–5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen U, Tyagi N, Patibandla PK, Dean WL, Tyagi SC, Roberts AM, Lominadze D, 2009. Fibrinogen-induced endothelin-1 production from endothelial cells. Am J Physiol Cell Physiol 296, C840–C847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhar-Tsarfaty S, Ben Assayag E, Bova I, Shopin L, Cohen M, Berliner S, Shapira I, Bornstein N, 2008. Persistent hyperfibrinogenemia in acute ischemic stroke / transient ischemic attack (TIA). Thrombosis and Haemostasis 99, 169–173. [DOI] [PubMed] [Google Scholar]
- Springer TA, 1990. Adhesion receptors of the immune system. Nature 346, 425–434. [DOI] [PubMed] [Google Scholar]
- Suehiro K, Gailit J, Plow EF, 1997. Fibrinogen is a ligand for integrin alpha beta 5 beta1 on endothelial cells. The Journal of Biological Chemistry 272, 5360–5366. [DOI] [PubMed] [Google Scholar]
- Sun Y, Wang J, Wu X, Xi C, Gai Y, Liu H, Yuan Q, Wang E, Gao L, Hu J, Zhou L, 2011. Validating the incidence of coagulopathy and disseminated intravascular coagulation in patients with traumatic brain injury – analysis of 242 cases. British Journal of Neurosurgery 25, 363–368. [DOI] [PubMed] [Google Scholar]
- Sweeney MD, Sagare AP, Zlokovic BV, 2018. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nature Reviews Neurology 14, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi N, Roberts AM, Dean WL, Tyagi SC, Lominadze D, 2008. Fibrinogen induces endothelial cell permeability. Molecular & Cellular Biochemistry 307, 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Haar HJ, Burgmans S, Hofman PAM, Verhey FRJ, Jansen JFA, Backes WH, 2015. Blood–brain barrier impairment in dementia: Current and future in vivo assessments. Neuroscience & Biobehavioral Reviews 49, 71–81. [DOI] [PubMed] [Google Scholar]
- van Oijen M, Witteman JC, Hofman A, Koudstaal PJ, Breteler MMB, 2005. Fibrinogen is associated with an increased risk of Alzheimer disease and vascular dementia. Stroke 36, 2637–2641. [DOI] [PubMed] [Google Scholar]
- Vasse M, Paysant J, Soria J, Collet JP, Vannier JP, Soria C, 1996. Regulation of fibrinogen biosynthesis by cytokines, consequences on the vascular risk. Haemostasis 26, 331–339. [DOI] [PubMed] [Google Scholar]
- Vos CMP, Geurts JJG, Montagne L, van Haastert ES, Bö L, van der Valk P, Barkhof F, de Vries HE, 2005. Blood–brain barrier alterations in both focal and diffuse abnormalities on postmortem MRI in multiple sclerosis. Neurobiology of Disease 20, 953–960. [DOI] [PubMed] [Google Scholar]
- Werner C, Engelhard K, 2007. Pathophysiology of traumatic brain injury. BJA: British Journal of Anaesthesia 99, 4–9. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV, 2008. The Blood-Brain Barrier in Health and Chronic Neurodegenerative Disorders. Neuron 57, 178–201. [DOI] [PubMed] [Google Scholar]


