Abstract
The stem cell transcription factor Sox2 is highly expressed in many cancers where it is thought to mark cancer stem cells (CSC). In osteosarcomas, the most common bone malignancy, high Sox2 expression marks and maintains a fraction of tumor initiating cells that show all the properties of CSC. Knock down of Sox2 expression abolishes tumorigenicity and suppresses the CSC phenotype. Here we show that, in a mouse model of osteosarcoma, osteoblast-specific Sox2 conditional knockout (CKO) causes a drastic reduction in the frequency and onset of tumors. The rare tumors detected in the Sox2 CKO animals were all Sox2 positive, indicating that they arose from cells that had escaped Sox2 deletion. Furthermore Sox2 inactivation in cultured osteosarcoma cells by CRISPR/CAS technology leads to a loss of viability and proliferation of the entire cell population. Inactivation of the YAP gene, a major Hippo Pathway effector which is a direct Sox2 target, causes similar results and YAP overexpression rescues cells from the lethality caused by Sox2 inactivation. These effects were osteosarcoma-specific, suggesting a mechanism of cell “addiction” to Sox2 initiated pathways. The requirement for Sox2 for osteosarcoma formation as well as for the survival of the tumor cells suggests that disruption of Sox2-initiated pathways could be an effective strategy for the treatment of osteosarcoma.
INTRODUCTION
The initiation and development of tumors is determined by multiple factors, including genetic and epigenetic events, and the expression of genes that control the nature and fate of the original target cell. The transcription factor Sox2, which plays a major role in development and in controlling the embryonic stem cell state, is also highly expressed in many cancers where it is thought to mark cancer stem cells (CSC) (1–6). In osteosarcomas, the most common bone tumor (7), high Sox2 expression marks and maintains a variable fraction of tumor initiating cells that show all the properties of CSC, including high expression of stem cell antigens, ability to form colonies in suspension, high expression of proliferation genes and a blockage in osteoblastic differentiation with a concomitant retention of the ability to enter the adipogenic fate. Such properties are absent in the low Sox2, non CSC population that are not tumorigenic and can be easily induced into osteoblastic differentiation (3,6,8,) Knock down (KD) of Sox2 expression by shRNA abolishes tumorigenicity in mouse xenografts and Sox2 KD cells behave very similarly to the non CSC fraction of the tumor cell population (6). It was not known however whether Sox2 was necessary for tumor initiation in vivo, or whether osteosarcomas can arise from cells which do not express Sox2. In this report we show that, in a mouse model of spontaneous osteosarcoma, osteoblast-specific Sox2 knockout causes a drastic reduction in the frequency and onset of tumors. The tumors that did occur in the Sox2 CKO animals were all Sox2 positive, and no Sox2 negative tumor was ever identified. Furthermore Sox2 inactivation in cultured osteosarcoma cells using CRISPR/CAS technology leads to loss of viability and proliferation of the entire cancer cell population, including CSC and non CSC cells. Inactivation of the YAP gene, a major Hippo Pathway effector (9–11) which is a direct Sox2 target (8,12), causes similar results and YAP overexpression rescues cells from the lethality caused by Sox2 inactivation. Thus Sox2 is required for osteosarcoma initiation or development in a mouse tumor model and Sox2 as well as its YAP target are essential for the survival and proliferation of osteosarcoma cells.
RESULTS AND DISCUSSION
RB and p53 knock out in the mouse osteoblast lineage induce a very high incidence of osteosarcomas, which occur early and frequently metastasize to other tissues and organs (13,14). We therefore tested whether the conditional KO (CKO) of Sox2 in the osteogenic lineage affected the insurgence of osteosarcomas in this mouse tumor model. We bred mice with ‘floxed” Rb, P53 and Sox2 genes combined with an Osterix (OSX) driven transgene expressing the Cre recombinase in the osteogenic lineage to obtain the desired genotypes. We compared Cre bearing mice with identical Rb and p53 genotypes in a background of wild type or floxed (deleted) Sox2 conditional knockout alleles (Sox2 CKO). In all the genotypes examined tumor formation was greatly reduced in the Sox2 CKO mice, and their appearance was delayed (FIG. 1). Animals bearing a wild type allele of Sox2 had reduced survival due to spontaneous osteosarcoma development compared with animals where Sox2 was deleted and this was true for all genotypes examined (Fig.1A), Since Cre mediated excision of floxed Sox2 (as well of Rb and p53) is not 100% efficient and these animals are largely mosaic, a reduction but not a complete suppression of tumor incidence was not surprising. Indeed we did find tumors arising in the Sox2 CKO animals. When examined (red arrows Fig1A) these tumors were uniformly Sox2 positive (FIG 1B). No Sox2 negative tumor was ever isolated.
Figure 1. Sox2 deletion is incompatible with OS development.
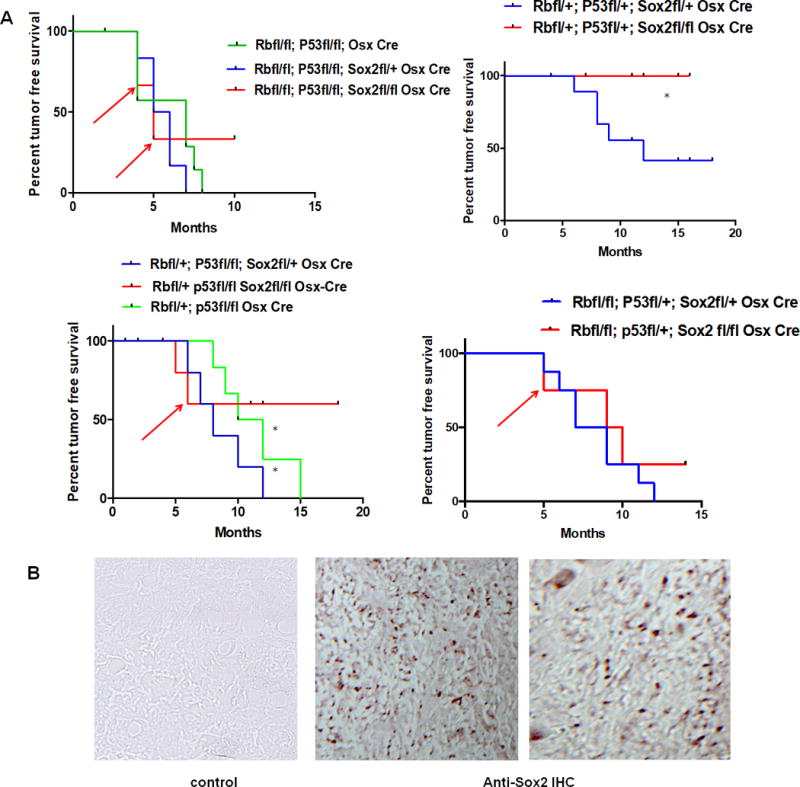
Animal Model: the animal model employed to assess the effect of SOX2 deletion on spontaneous osteosarcoma development was obtained from Dr S. H. Orkin, Harvard Medical School, Boston, MA. In this model the mice have a floxed (fl) RB and P53 that is specifically deleted in the osteoblast lineage by introduction of an OSX-Cre allele (Osterix driven transgene expressing the Cre recombinase) (14). The SOX2 floxed mice were obtained from Dr Silvia Nicolis, Bicocca University, Milan and have been previously described (15). The mice were bred in a C57Bl/6 background for several rounds to obtain SOX2-deleted (flox/flox) mice in a P53 null, RB null, OSX-Cre expressing background. Animals genotyped by tail DNA PCR analysis were observed twice a week for spontaneous tumor development. Animals with spontaneous tumors were sacrificed and identified by number only. Genotypes were blinded to the observing researcher. SOX2–deleted animals (n=20) were compared with SOX2 heterozygous or wild type animals (n=49) in the RB and P53 null backgrounds and each animal was plotted as a time-point. Cre bearing animals with increasing homozygosity for the three floxed alleles were assessed for the appearance of spontaneous osteosarcoma (OS).). A) Kaplan Meier survival plots. Kaplan Meier plots were generated and statistical analysis of P-values determined by one way ANOVA using GraphPad Prism. Red arrows indicate animals with OS that were harvested and found to be positive for SOX2 expression. Power analysis for the proposed animal numbers was computed to achieve significance of p<0.05 (error probability) and 80% power (G power 3.1 power analysis program).. B) Spontaneous tumors in a Sox2 CKO background are positive for Sox2 expression. Representative immunohistochemistry staining on two tumors arising in a Sox2 flox/flox genetic background using anti-Sox2 antibody. Mag 20X
The absence of tumors in the Sox2 CKO mice could be due to the inability of the cell which is targeted by the initial transforming event to maintain or establish a CSC phenotype, or to some proliferation defects in the otherwise transformed cell clones. In our previous experiments aimed at determining the importance of Sox2 expression for the adult stem cell state (15) we had observed that Cre-mediated deletion of the Sox2 gene in preosteoblasts or MSC led to exhaustion of proliferative capacity with the appearance of a senescent-like phenotype. In contrast, Sox2 knockdown (KD) in osteosarcoma cells produced cells which grew more slowly, exhibited a more differentiated phenotype with activation of the tumor suppressive Hippo pathway and of the osteogenic Wnt pathway, but were still capable of indefinite propagation in culture (6). This different response could have been due to the fact that while Cre-mediated Sox2 deletion in osteoblasts led to total suppression of Sox2 expression in each affected cell, shRNA-mediated knockdown produced a significant but not complete loss of Sox2 activity in osteosarcoma cells. Alternatively it could have reflected the different nature of adult stem cells such as preosteoblasts and MSC and that of CSC. Normal adult multipotent or unipotent stem cells are relatively quiescent and tend to divide asymmetrically, but are not immortal. In contrast CSC tend to divide symmetrically and have unlimited self renewal capacity. In this view, loss of Sox2 expression could induce distinct fates in adult and cancer stem cells.
To answer this question we introduced inactivating deletions in the Sox2 gene in murine osteosarcoma (mOS) cells (6, 14) using CRISPR/CAS technology (16) and determined their effect on cell proliferation and ability to form colonies in culture. Initially we introduced the Cas9 nuclease and Sox2 guide RNA (gRNA) into mOS cells by plasmid transfection and observed a considerable reduction in the number of colonies formed by the targeted population with respect to controls. Since OS cells are not easily transfectable, we created mOS cell lines constitutively expressing CAS9 and then introduced gRNAs using lentivirus vectors into these cells. The gRNAs were designed to create deletions in the single exon of the Sox2 gene. Following the introduction of these gRNAS in 202 CAS9 mOS cells, sequencing of the Sox2 region in the initial polyclonal cell population selected for guide RNA expression showed clear evidence of a mixed population, containing wild type cells and cells with variable length indels in the Sox2 exon (FIG.2). These mixed cell populations were propagated in culture for 4-5 passages and the Sox2 DNA region sequenced at every passage. By the 4th passage the Sox2 DNA sequence showed no evidence of deletions or mutations and was undistinguishable from the wild type, suggesting that Sox2 deficient OS cells are strongly selected against during proliferation in culture. (FIG.2).
Figure 2. Sox2 negative cells can not survive during culture propagation.
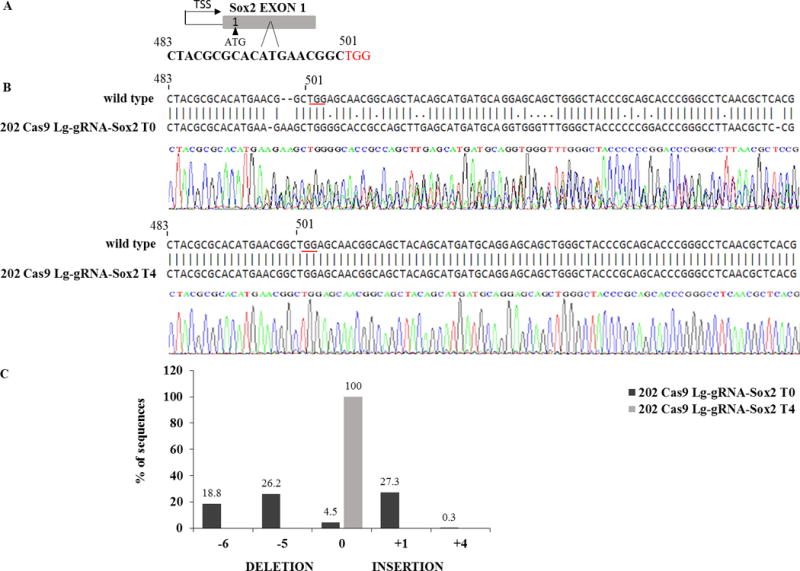
mOS-202 cells expressing CAS9 were infected with lentiguide viral vectors carrying resistance to puromycin and a gRNA targeting Sox2. Following infection the cells were grown in the presence of puromycin (2 μg/ml) at low density and the Sox2 locus sequenced at every culture passage. (A) shows the gRNA sequence used to target Sox2 (PAM sequence is in red). (B) Following infection, DNA was harvested and amplified by PCR with specific primers for the Sox2 region (FW: 5’-GAAGCGGCCGTTCATCGACG-3’, RV: 5’-GTGCTGGGCCATGTGCAGTC-3’). The PCR products were purified and sequenced and alignment data were analysed by ClustalW software. The sequence of one part of the amplified Sox2 exon is shown compared to the wild type murine sequence. Sequence tracing of first passage cells (T0) shows a clearly mixed cell population with numerous alterations in the Sox2 sequence starting around the region targeted by the gRNA. After four culture passages (T4) the sequence is undistinguishable from wild type. (C) mixed DNA sequence deconvolution using TIDE software (31) shows the percentage of cells with deletions and insertions at T0 and T4; at least 77% (p<0.001) of cells showed deletions and insertions within the Sox2 region at T0 and only 4.5% of cells expressed a wild type sequence. At T4 100% (p<0.001) of cells contained Sox2 wild type DNA sequences without deletions or insertions. Methods: the mOS cell lines were previously described (6, 14). Lentiguide-Puro/LentiCas9-Blast (two vectors system) was purchased from Addgene and used following manufacturer instructions. Lentiguide-Puro plasmids express gRNA and puromycin resistance, while a separate lentiviral construct LentiCas9-Blast that delivers hSpCas9 and blasticidin resistance was used to first integrate Cas9 into mOS-202 and 3T3 cell lines. Lentiviruses were generated as previously described (12). Briefly, the Lentiguide-Puro vector was digested using BsmBI enzyme, and a pair of annealed oligos were cloned into the single guide RNA (gRNA) scaffold. Lentiviral transfer plasmids were transformed into Stbl3 bacteria, purified using MaxiPrep Kit (Qiagen) and DNA concentration was evaluated by Nanodrop (Thermo Fisher Scientific). To make lentivirus, the transfer plasmids Lentiguide-Puro and LentiCas9-Blast were co-transfected into HEK293FT cells with the packaging plasmids PLP1, PLP2, and VSVG. To evaluate lentivirus concentration, the virus ability to induce puromycin resistant colonies was determined in mOS-202 and HEK293FT cell lines. To obtain cells knockout for Sox2, mOS-202 cells expressing CAS9 were infected with lentiguide viral vectors carrying resistance to puromycin and a gRNA targeting Sox2 (5’-CTACGCGCACATGAACGGC-3’) in the presence of 8 mg/ml polybrene for 24 hours.
To better understand and quantitate this result, we coinfected 202 CAS9 cells with lentivirus vectors encoding the gRNA for Thymidine kinase (TK) together with an excess of lentiguide vectors targeting Sox2. TK deficiency makes cells resistant to bromodeoxyuridine (BrDU) (17), and thus infected cells were selected for colony formation in the presence of BrDU . We assumed that the ability of these cells to form colonies in the presence of BrDU would have been substantially impaired if the concomitant introduction of Sox2 deletions would have had a deleterious effect. Indeed the number of colonies formed by these cell populations was drastically reduced with respect to control samples (infection with TK vectors and an excess of “empty” lentiguide vectors). The few BrDU resistant colonies detected in the Sox2 targeted populations were all Sox2 positive, indicating that they had escaped Sox2 inactivation (FIG.3). A similar experiment using a gRNA directed against the HGPRT gene, whose inactivation leads to thioguanine resistance (18), gave identical results (Not shown).
Figure 3. Sox2 or Yap1 inactivation abolish OS cells colony formation ability.
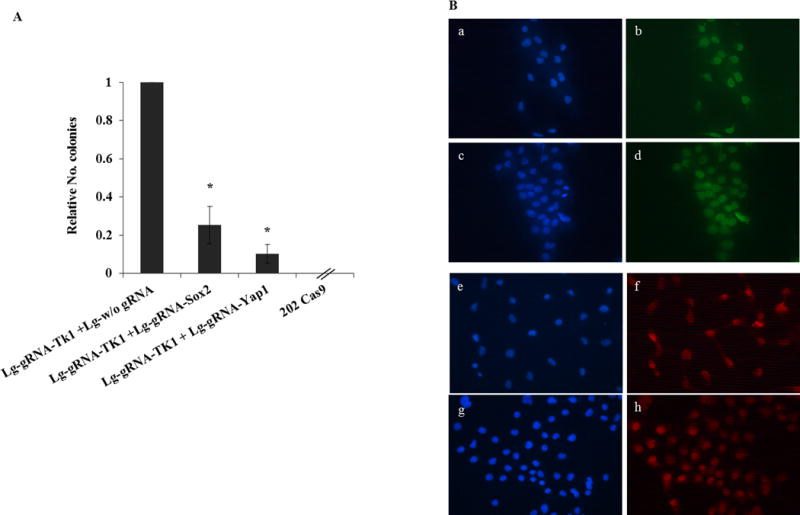
Lentiguide-Puro (Lg) vectors described above were used to infect mOS-202 Cas9 cells to obtain cells knockout for SOX2, YAP1 and Thymidine kinase (TK). Lentiguide-Puro virus without gRNA was used as a control. (A) 202 Cas9 cells were infected with an excess (10X) Lg w/o gRNA together with Lg-gRNA-TK1, Lg-gRNA-SOX2 (10X) with Lg-gRNA-TK1, Lg-gRNA-YAP1 (10X) with Lg-gRNA-TK1. gRNAs sequences were as follow: SOX2-gRNA 5’-CTACGCGCACATGAACGGC-3’; YAP1-gRNA 5’-TGATGTACCACTGCCAGC-3’; TK1-gRNA 5’-TTCCCAGTTTCCTGACATTG-3’; HGPRT-gRNA 5’-TATACCTAATCATTATGCCG-3’. Cells were preselected with puromycin, blasticidin and then with BrDU (50 micrograms/ml), and colonies were counted. (The experiment was repeated 3 times seeding cells at different cell density). Results were analysed by T-test. (*) p<0.05. Error bars= s.d. (B) All the colonies obtained were harvested and checked for Sox2 and Yap1 expression by immunofluorescence staining as previously described (8), using anti Sox2 (MilliporeAb5603) or anti YAP (Cell signaling 4912) antibodies. All the 202 Cas9 colonies harvested had escaped Sox2 and Yap1 knockout, staining in green and red, respectively; nuclei were marked in blue with Dapi. In (a, b, e, f) 202 Cas9 cells had been infected with Lg w/o gRNA and Lg-gRNA-TK1; in (c, d) 202 Cas9 cells had been infected with Lg-gRNA-Sox2 and Lg-gRNA-TK1; in (g, h) 202 Cas9 cells were infected with Lg-gRNA-Yap1 and Lg-gRNA-TK1.
Thus these results indicate that Sox2 is essential for the survival and proliferation of mOS cells, and that, surprisingly, this requirement is not unique to the CSC population but also applies to the non CSC, more differentiated cells. In addition to determine the effect of Sox2 inactivation, we thought to test also the requirement for YAP, a Sox2 target gene which is a major effector of the Hippo pathway and plays an important role in the Sox2-mediated antagonism of this pathway (8–12). Furthermore YAP is an important player in the maintenance of Stem cells pluripotency (19). As also shown in Fig.3, YAP inactivation produces results identical to that of Sox2. Importantly, inactivation of either YAP or Sox2 in murine fibroblasts has no discernible effect (data not shown), indicating that the requirement for these activities is OS cells-specific. To verify that the effects of Sox2 or YAP deletion were not a peculiarity of the 202 cell line we introduced Sox2 or YAP gRNAs together with Cas 9 into mOS 482 cells using multicistronic lentiviral vectors and compared the results to those obtained with gRNAs targeting the ROSA locus, which is not necessary for cell proliferation or survival. Colony forming ability was substantially impaired in both 202 and 482 cells following the transduction of YAP or Sox2 gRNAs (Suppl. Fig 1).
To rule out off-target effects and assess the relative importance of these genes in OS cells maintenance, we assessed whether overexpression of either Sox2 or YAP could recue cells from the lethality induced by their inactivation (FIG. 4). While YAP can rescue cells from the result of Sox2 deletion, the opposite was not true, placing YAP downstream of Sox2 in the OS cells survival hierarchy, and supporting the notion that YAP is an essential effector of at least the self renewal function of Sox2 in OS.
Figure 4. Yap1 overexpression can rescue OS cells from the effect of Sox2 deletion.
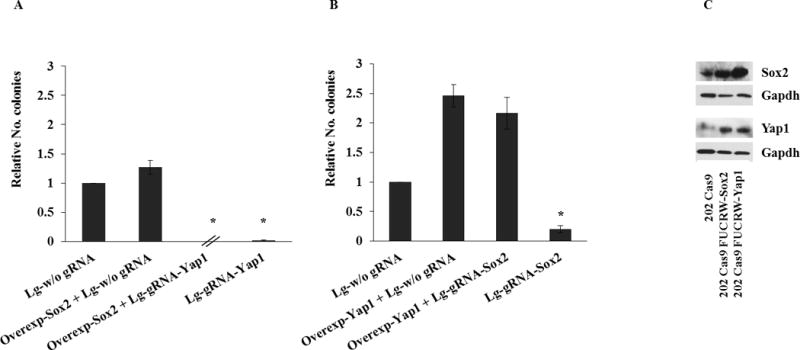
mOS-202 Cas9 cells were infected either with Lg w/o gRNA or Lg-gRNA-SOX2 or Lg-gRNA-YAP1. Cells were selected with puromycin and colonies were counted. (The experiment was repeated 3 times seeding cells at different cell density). Results were analysed by T-test. (*) p<0.05. Error bars= s.d. To over-express SOX2 and YAP1, FUCRW lentivirus vectors (12) expressing RFP and either -SOX2 or YAP1 were used to infect the cells. (A) 202 Cas9 cells were infected with FUCRW-SOX2 and Lg-gRNA-YAP1 lentivirus to overexpress SOX2 and knockout YAP1, respectively (B) 202 Cas9 cells were infected with FUCRW YAP1 and Lg-gRNA-SOX2 lentiviruses to overexpress YAP1 and knockout SOX2, respectively. A low number of colonies was obtained when cells were infected with FUCRW-SOX2 and Lg-gRNA-YAP1, showing that the overexpression of SOX2 can not rescue cells from the lethality induced by YAP1 inactivation. On the contrary, a high number of colonies was obtained when cells were infected with FUCRW-YAP1 and Lg-gRNA-SOX2, showing that YAP1 can rescue cells from the result of SOX2 deletion. (The experiment was repeated 3 times seeding cells at different cell density). Results were analysed by T-test. (*) p<0.05. Error bars= s.d. (C) Western Blot analysis of 202 Cas9 infected with FUCRW-SOX2 and FUCRW-YAP1 lentivirus, respectively was performed as previously described (8).
The results presented here establish two important notions. They show that Sox2 is essential for tumor initiation in a mouse model of spontaneous osteosarcomas, in line with the notion that this transcription factor is a key player in the maintenance of the CSC phenotype and the consequent tumor forming potential. Indeed many of the genes that are differentially expressed between the CSC and non-CSC fractions of murine osteosarcomas are direct Sox2 targets (3,6,8,12). Sox2 is probably a universal marker and maintenance factor for CSC. It has been shown to be highly expressed in other solid tumors, including glioblastomas, melanomas and squamous cell carcinomas (20–27), and in experiments similar to those described here it was shown to be required for the insurgence of the latter type of tumors in a mouse squamous carcinoma model (28,29). In the case of osteosarcomas, the drastically reduced tumor incidence and the absence of Sox2-negative tumors in mice with an osteoblast specific KO of the Sox2 gene bears out our initial hypothesis that Sox2 is indispensible for sarcomagenesis. However the finding that Sox2 deletion by CRISPR/CAS technology abolishes the ability of OS cells to proliferate and form colonies was intriguing and somewhat unexpected. Sox2 or YAP are not essential genes for cell proliferation and indeed their inactivation in murine fibroblasts has no effect (data not shown). It is likely that OS cells have become “addicted” to a gene expression cascade which is initiated by Sox2 and includes induction of YAP expression. Interestingly Sox2 deletion seems to affect equally the survival of the CSC and non-CSC populations, and the same is true of the YAP deletion. Thus the expression of these two genes is required for the survival and proliferation of all cells in the OS cell line. This result is somewhat at odd with the notion (6,8) that Sox2 (and YAP) expression only maintains the CSC population. Clearly this was not the case, as apparently the low levels of Sox2 expression that are detected in the non CSC population are still required for their viability and proliferation.
The observation that deletion of the Sox2 target YAP gene has the same effect as deletion of Sox2 indicates that the Sox2-mediated antagonism of the Hippo pathway is very important for the maintenance for the proliferation and tumorigenicity of OS cells. Importantly, the fact that the expression of these genes is crucial for both the CSC and the non CSC populations suggest that in spite of the different tumorigenicity of the two cell types, these cells share important properties and requirements, that probably reflect the origin of the non CSC from the CSC fraction by asymmetric division (30). In this view the original transformed clone is an adult stem cell that is the target of an oncogenic event, that transforms it into a cancer cell which maintains stem cell properties. Its progenitor-like descendants lose tumorigenicity together with other stem cell properties, but are still capable of indefinite self -renewal, a property that may require Sox2 activation of specific genes but not antagonism of the Hippo pathway, that requires very high levels of Sox2 expression. As discussed above, the loss of viability of OS cells following Sox2 inactivation resembles the behavior of immature osteoblasts/osteoprogenitors that also lose viability upon Sox2 deletion. (15). Sox2 is required therefore for the viability of adult stem cells as well of the cancer stem cells which originate from them.
The finding that Sox2 and YAP are required for the proliferation of the CSC as well of the non CSC portion of the OS cell population highlight the common origin of these two cell types in osteosarcomas and the notion that the differences between CSC and non CSC in solid tumors has perhaps been overly emphasized. These results and the lack of tumors in a mouse model of osteosarcomas in the absence of SOX2 strongly indicate that drugs that interfere with the activity of these two transcription factors would be very effective in targeting and killing the entire tumor cell population in osteosarcomas and perhaps other cancers.
Supplementary Material
Acknowledgments
This investigation was supported by NYSTEM contract CO29560 and NIH/NCI-R21CA186031. We thank Dr. Stuart Orkin for providing us with the Tp53, Rb mutant mice, Matthew Murtha for his contribution to some of the initial experiments and Upal BasuRoy for helpful discussions. We also wish to acknowledge the Histopathology core services at NYU Langone Medical Center
Footnotes
Conflict of interest
The authors declare no conflict of interest
References
- 1.Arnold K, Sarkar A, Yram MA, Polo JM, Bronson R, Senqupta S, et al. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell. 2011;9:317–329. doi: 10.1016/j.stem.2011.09.001. doi:S1934-5909(11)00432-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basu-Roy U, Basilico C, Mansukhani A. Perspectives on cancer stem cells in osteosarcoma. Cancer Lett. 2013;338:158–167. doi: 10.1016/j.canlet.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Driessens G, Blanpain C. Long live sox2: sox2 lasts a lifetime. Cell Stem Cell. 2011;9:283–284. doi: 10.1016/j.stem.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Niwa H. How is pluripotency determined and maintained? Development. 2007;134:635–646. doi: 10.1242/dev.02787. doi:dev.02787 [pii] 10.1242/dev.02787. [DOI] [PubMed] [Google Scholar]
- 6.Basu-Roy U, Seo E, Ramanathapuram L, Rapp TB, Perry JA, Orkin SH, et al. Sox2 maintains self renewal of tumor-initiating cells in osteosarcomas. Oncogene. 2012;31:2270–2282. doi: 10.1038/onc.2011.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rainusso N, Wang LL, Yustein JT. The adolescent and young adult with cancer: state of the art – bone tumors. Curr Oncol Rep. 2013;15:296–307. doi: 10.1007/s11912-013-0321-9. [DOI] [PubMed] [Google Scholar]
- 8.Basu-Roy U, Bayin NS, Rattanakorn K, Han E, Placantonakis DG, Mansukhani A, et al. Sox2 antagonizes the Hippo pathway to maintain stemness in cancer cells. Nat Commun. 2015 Apr 2;6:6411. doi: 10.1038/ncomms7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev. 2013;27:355–371. doi: 10.1101/gad.210773.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes Dev. 2016;30:1–17. doi: 10.1101/gad.274027.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP1 and TAZ in cancer. Nat Rev Cancer. 2015;15:73–79. doi: 10.1038/nrc3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seo E, Basu-Roy U, Gunaratne PH, Coarfa C, Lim DS, Basilico C, et al. SOX2 regulates YAP1 to maintain stemness and determine cell fate in the osteo-adipo lineage. Cell Rep. 2013 Jun 27;3(6):2075–87. doi: 10.1016/j.celrep.2013.05.029. Epub 2013 Jun 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berman S, Calo E, Landman AS, Danelian PS, Miller ES, West JC, et al. Metastatic osteosarcoma induced by inactivation of Rb and p53 in the osteoblast lineage. Proc Natl Acad Sci U S A. 2008;105:11851–11856. doi: 10.1073/pnas.0805462105. doi:0805462105 [pii]10.1073/pnas.0805462105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walkley CR, Qudsi R, Sankaran VG, Perry JA, Gostissa M, Roth SI, et al. Conditional mouse osteosarcoma, dependent on p53 loss and potentiated by loss of Rb, mimics the human disease. Genes Dev. 2008;22:1662–1676. doi: 10.1101/gad.1656808. doi:22/12/1662 [pii]10.1101/gad.1656808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basu-Roy U, Ambrosetti D, Favaro R, Nicolis SK, Mansukhani A, Basilico C. The transcription factor Sox2 is required for osteoblast self-renewal. Cell Death Differ. 2010 Aug;17(8):1345–53. doi: 10.1038/cdd.2010.57. Epub 2010 May 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guernet A, Grumolato L. CRISPR/Cas9 editing of the genome for cancer modeling. Methods. 2017 Mar 10; doi: 10.1016/j.ymeth.2017.03.007. pii: S1046-2023(16)30293-6. [DOI] [PubMed] [Google Scholar]
- 17.Basilico C, Matsuya Y, Green H. Origin of the thymidine kinase induced by polyoma virus in productively infected cells. J Virol. 1969 Feb;3(2):140–5. doi: 10.1128/jvi.3.2.140-145.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao S, Tammaro M, Yan H. Enriching CRISPR-Cas9 targeted cells by co-targeting the HPRT gene. Nucleic Acids Res. 2015 Nov 16;43(20):e134. doi: 10.1093/nar/gkv675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lian I, Kim J, Okazawa H, Zhao B, Yu J, Chinnayan A, et al. The role of YAP1 transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010;24:1106–1118. doi: 10.1101/gad.1903310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tafani M, Perrone GA, Pucci B, Russo A, Bizzarri M, Mechanick JI, et al. Reprogramming cancer cells in endocrine-related tumors: open issues. Curr Med Chem. 2014;21(9):1146–51. doi: 10.2174/0929867321666131129125624. [DOI] [PubMed] [Google Scholar]
- 21.Palumbo P, Miconi G, Cinque B, Lombardi F, La Torre C, Dehcordi SR, et al. NOS2 expression in glioma cell lines and glioma primary cell cultures: correlation with neurosphere generation and SOX-2 expression. Oncotarget. 2017 Apr 11;8(15):25582–25598. doi: 10.18632/oncotarget.16106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song WS, Yang YP, Huang CS, Lu KH, Liu WH, et al. Sox2, a stemness gene, regulates tumor-initiating and drug-resistant properties in CD133-positive glioblastoma stem cells. J Chin Med Assoc. 2016 Oct;79(10):538–45. doi: 10.1016/j.jcma.2016.03.010. Epub 2016 Aug 13. [DOI] [PubMed] [Google Scholar]
- 23.Mansouri S, Nejad R, Karabork M, Ekinci C, Solaroglu I, Aldape KD, et al. Sox2: regulation of expression and contribution to brain tumors. CNS Oncol. 2016 Jul;5(3):159–73. doi: 10.2217/cns-2016-0001. Epub 2016 May 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santini R, Pietrobono S, Pandolfi S, Montagnani V, D’Amico M, Penachioni JY, et al. SOX2 regulates self-renewal and tumorigenicity of human melanoma-initiating cells. Oncogene. 2014 Sep 18;33(38):4697–708. doi: 10.1038/onc.2014.71. Epub 2014 Mar 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan Y, Tajik A, Chen J, Jia Q, Chowdhury F, Wang L, Chen J, et al. Matrix softness regulates plasticity of tumour-repopulating cells via H3K9 demethylation and Sox2 expression. Nat Commun. 2014 Aug 6;5:4619. doi: 10.1038/ncomms5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weina K, Wu H, Knappe N, Orouji E, Novak D, Bernhardt M, et al. TGF-ß induces SOX2 expression in a time-dependent manner in human melanoma cells. Pigment Cell Melanoma Res. 2016 Jul;29(4):453–8. doi: 10.1111/pcmr.12483. [DOI] [PubMed] [Google Scholar]
- 27.Ferone G, Song JY, Sutherland KD, Bhaskaran R, Monkhorst K, Lambooij JP, et al. SOX2 Is the Determining Oncogenic Switch in Promoting Lung Squamous Cell Carcinoma from Different Cells of Origin. Cancer Cell. 2016 Oct 10;30(4):519–532. doi: 10.1016/j.ccell.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siegle JM, Basin A, Sastre-Perona A, Yonekubo Y, Brown J, Sennett R, et al. SOX2 is a cancer-specific regulator of tumour initiating potential in cutaneous squamous cell carcinoma. Nat Commun. 2014 Jul 31;5:4511. doi: 10.1038/ncomms5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boumahdi S, Driessens G, Lapouge G, Rorive S, Nassar D, Lemercier M, et al. Sox2 controls tumor initiation and cancer stem-cell functions in squamous cell carcinoma. Nature. 2014 Jul 10;511(7508):246–50. doi: 10.1038/nature13305. Epub 2014 Jun 8. [DOI] [PubMed] [Google Scholar]
- 30.Shahriyari L, Komarova NL. Symmetric vs. asymmetric stem cell divisions: an adaptation against cancer? PLoS One. 2013 Oct 29;8(10):e76195. doi: 10.1371/journal.pone.0076195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brinkman EK, Chen T, Amendola M, van Steensel B. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res. 2014 Dec 16;42(22):e168. doi: 10.1093/nar/gku936. Epub 2014 Oct 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


