Abstract
Thrombocytopenia, a serious complication of myelosuppressive chemotherapy in cancer patients, is managed with platelet transfusions until recovery of platelet counts. However, children receiving chemotherapy can rarely develop immune thrombocytopenia (ITP) that is refractory to transfused platelets. This limits the ability to achieve adequate platelet counts and administer further myelosuppressive chemotherapy safely, especially if first-line ITP therapy is ineffective. We report two cases of IVIG-refractory ITP in children receiving chemotherapy for high-risk neuroblastoma. ITP was successfully treated with the thrombopoietin-receptor-agonist romiplostim, allowing safe and timely continuation of anti-neuroblastoma therapies in these high-risk patients.
Keywords: neuroblastoma, platelet, cancer, thrombopoietin
Introduction
Myelosuppressive chemotherapy in children with cancer often results in thrombocytopenia which can be managed with platelet transfusions. Rarely, autoimmune thrombocytopenia (ITP) may develop. In ITP, auto-antibodies and/or T-cells directed against platelet antigens cause platelet destruction and inhibit platelet production.1 In particular, platelet transfusions are futile due to rapid immune-mediated clearance of infused platelets. Front-line ITP therapies, i.e. intravenous immunoglobulin (IVIG) and steroids, in our experience, are often not effective in these patients with cancer and/or chemotherapy-mediated secondary ITP.
Romiplostim, a thrombopoietin-receptor-agonist licensed for chronic ITP in adults in 2008, is generally safe and often effective as second-line therapy in children with ITP.2,3 Other anecdotal applications of romiplostim or eltrombopag include treatment of thrombocytopenia associated with aplastic anemia,4 liver disease,5 hematopoietic stem cell transplantation,6 and graft-versus-host disease.7 Romiplostim is being investigated in adults with chemotherapy-induced thrombocytopenia in an attempt to reduce the need for platelet transfusions and allow for timely reinstitution of chemotherapy.8 A report described romiplostim treatment post-chemotherapy in five pediatric oncology patients.9 While the findings were encouraging, the role of thrombopoietin-receptor-agonist for chemotherapy-induced thrombocytopenia remains poorly defined.
We encountered ITP in two patients with high-risk neuroblastoma (NB) in whom romiplostim therapy led to improvement in platelet counts and allowed continuation of anti-NB therapy.
Case Reports
Patient One
A three year-old male was diagnosed with stage 4 MYCN-amplified NB. Treatment included N7 induction chemotherapy for high risk NB,10 surgery, high-dose cyclophosphamide plus camptothecins, murine anti-GD2 monoclonal antibody 3F8 (m3F8) plus granulocyte-macrophage colony stimulating factor (GMCSF),11 and radiotherapy. He developed human anti-mouse antibody (HAMA), which subsided after treatment with rituximab and cyclophosphamide. Treatment for NB relapses included irinotecan and temozolomide, haplo-identical NK cells and m3F8 on a phase I study (NCT00877110), additional m3F8 plus GMCSF12 (developing HAMA after two cycles), bivalent anti-NB vaccine13 and humanized 3F8 (hu3F8) plus GMCSF (NCT01757626) without development of HAMA or human anti-human antibodies. At the age of nine years, 12 months after the last chemotherapy and 11 months following his third complete remission, he developed ITP with an acute drop in platelet count to 33×109/L. He had no bleeding other than petechiae at the site of tourniquet application. Bone marrow (BM) was normocellular with mildly increased megakaryocytes, no evidence of NB or myelodysplasia, and normal karyotype. There was no evidence of fibrosis on hematoxylin and eosin staining. Further clinical work up of thrombocytopenia included a positive indirect platelet-antibody screen with detection of antibodies to glycoprotein Ib/IX and glycoprotein IIb/IIIa. He was initially treated with four doses of IVIG with poor response. Steroids were not administered due to the risk of impairing the anti-NB effect of hu3F8. Instead he was started on 1μg/kg romiplostim subcutaneously with platelet response to 158×109/L; subsequent 1μg/kg doses were less effective and he required intermittent dosing at 3μg/kg approximately every two weeks to maintain platelet counts (Figure 1A). A lower romiplostim dose was initially tested because at the time of development of ITP he was in remission and treatment for NB with myelosuppressive chemotherapy was not imminent.
Figure 1.
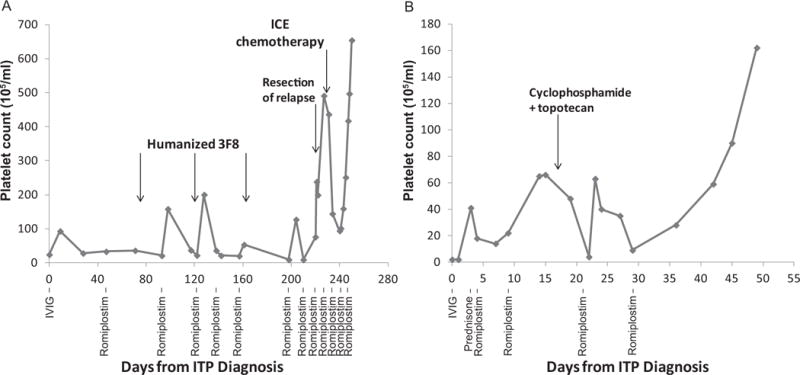
Platelet counts for patients #1 (A) and #2 (B) over time, measured in days after ITP onset, which was 49 and 11 months after NB diagnosis in patients #1 and 2, respectively. Therapies for neuroblastoma are noted above the line graph; therapies for ITP are noted below the line graph.
ICE = Ifosfamide, carboplatin, etoposide, IVIG = Intravenous immune globulin.
Six months later the patient had an abdominal NB recurrence. With this high risk recurrence, immediate surgery and chemotherapy was required and thus two doses of romiplostim (3μg/kg and 4μg/kg) were administered three days apart to facilitate a rapid platelet rise prior to surgery, which was followed by ifosfamide, carboplatin and etoposide (ICE) with romiplostim support. Two months later the platelet count was 654×109/L. Romiplostim was weaned and discontinued (Figure 1A). He received anti-NB treatment including chemotherapy, immunotherapy and palbociclib, and at age 12 years, he is currently in remission both from NB and ITP.
Patient Two
A three year-old female diagnosed with stage 4 MYCN-amplified NB underwent induction therapy with cisplatin, vincristine, carboplatin, etoposide, and cyclophosphamide (rapid COJEC)14 and surgery at an outside institution. This was followed by one cycle each of cyclophosphamide, doxorubicin, and vincristine;10 and cyclophosphamide, topotecan, and vincristine after which she was in complete remission. She received m3F8 plus GMCSF12 but developed an early, soft tissue NB relapse. Subsequent treatment included ICE, surgical resection, irinotecan and temozolomide, and radiotherapy to the primary site. Eleven months after diagnosis and three weeks after last chemotherapy, she precipitously developed severe thrombocytopenia with acute onset ecchymoses, petechiae, and gum bleeding. Platelet count was <2×109/L. BM examination revealed maturing myeloid and erythroid elements without dysplasia, mild decrease in megakaryocytes, without metastatic NB or secondary leukemia. Platelet antibody screen was positive; anti-HLA antibodies and HAMA were undetectable. Post-transfusion platelet count remained <2×109/L. For treatment of ITP, she received two doses of IVIG and a five-day course of prednisone without response. In view of the urgent need for neuroblastoma treatment, she was treated with a higher dose of 5μg/kg romiplostim with improvement in platelet count to 22×109/L. Given the need to start chemotherapy urgently, 7μg/kg romiplostim was administered, resulting in a platelet count increase to 65×109/L. Cyclophosphamide and topotecan were given six days after the romiplostim (Figure 1B). She received further therapy for NB including surgery, high-dose ICE and MIBG therapy, requiring only two further doses of romiplostim. ITP did not recur six months after stopping romiplostim, despite multiple NB relapses.
Discussion
Two patients receiving intensive therapy for high-risk NB developed prolonged ITP-like thrombocytopenia unresponsive to front-line ITP treatment. In primary ITP, responses to IVIG are seen in 70-90% of children within 2-4 days and to steroids within days to several weeks.15 However, in the above cases IVIG yielded minimal response; steroids were contraindicated in patient #1 and ineffective in patient #2. Romiplostim, however, was effective in increasing and maintaining platelet counts in both patients. For patient #1, thrombocytopenia was not profound and treatment of NB not urgent, so romiplostim was initiated at a low dose and titrated up to 4 μg/kg/week. Patient #2, due to profound thrombocytopenia and urgency to continue anti-NB therapy, had romiplostim started at 5μg/kg, then escalated to 7μg/kg. In both cases, NB treatment could be resumed within two weeks after initiating romiplostim.
Romiplostim has been shown in multiple randomized placebo controlled studies in ITP in both adults and children to increase platelet counts at doses of 1-10 μg/kg, with higher doses often being required in children, as in patient #2.2,3 However ITP that is secondary to an underlying disease such as malignancy in a patient who is receiving chemotherapy has not been well described and the number of children previously reported receiving a thrombopoietin receptor agonist (romiplostim or eltrombopag) is very small. Romiplostim-related adverse events were not seen in our patients (with the exception of transient self-limited thrombocytosis). Adverse events are infrequent in children receiving romiplostim for ITP, apparently much less than those seen in adults, and include headache, myalgia, and development of a romiplostim neutralizing antibody.16 Thrombosis has been very rarely reported in children, even in the presence of romiplostim-induced thrombocytosis.
We chose romiplostim rather than eltrombopag for treatment of these seriously ill children since we anticipated problems with compliance with oral medication. Moreover, they were being seen weekly for management of NB, so the need for weekly subcutaneous administration of romiplostim was not a drawback. Recent studies in ITP have suggested that patients not responding to romiplostim may respond better to eltrombopag and vice versa.19 Therefore if there had not been a sufficient response to romiplostim, it would have been reasonable to attempt treatment with eltrombopag. The clinical evidence for the lack of overlap of response of these two agents is substantiated by work suggesting that their mechanisms of effect are different in certain ways.20
Despite the immunosuppressive effect of chemotherapy, ITP has been reported in pediatric patients receiving chemotherapy.1 A possible mechanism for development of ITP in patients receiving chemotherapy could be suppression of regulatory T-cell function and number, leading to immune dysregulation. In one of the patients, ITP was associated with the development of HAMA. We speculate that autoantibodies against 3F8 could have cross-reacted with platelet epitopes.17 However, ITP associated with 3F8-induced HAMA has previously been described in only two prior patients.18 The development of ITP in children receiving chemotherapy poses significant diagnostic and management challenges. Thrombocytopenia resulting in delay in anti-cancer therapy can prove detrimental, especially in patients with aggressive MYCN-amplified NB. In primary ITP, extensive diagnostic evaluations are not always indicated; however when ITP is suspected in patients with cancer, careful evaluation to exclude other causes of thrombocytopenia is required, including in particular BM evaluation to exclude metastatic disease, secondary leukemia, and marrow aplasia secondary to chemotherapy. Furthermore diagnosing ITP can be difficult as chemotherapy can reduce megakaryocytes; only one of our patients had increased BM megakaryocytes which is usually seen with classical ITP, while the other had normal but not decreased BM megakaryocyte numbers. Platelet antibodies were tested and positive in our patients but this test is not considered useful in the diagnosis of primary ITP. More specifically, neither patient responded to IVIG. However, platelet transfusions did not lead to increase in platelet counts and antibody studies excluded post-transfusion purpura providing diagnostic evidence for ITP.
ITP treatment in patients with cancer can be complicated as, in our opinion, standard therapies (e.g. anti-D immune globin in anemic patients or steroids in patients receiving immunotherapy) may often not be effective or safe. By tailoring the use of romiplostim to platelet count recovery, we could resume myelosuppressive chemotherapy, despite inadequate responses to front-line ITP therapy. Our report indicates that romiplostim may be effective for ITP in patients receiving chemotherapy. However, our experience does not provide general recommendations regarding dosing of romiplostim. Factors that guided romiplostim dosing in our patient were platelet count nadirs, urgency to resume anti-NB therapy, and BM megakaryocyte status. It is anticipated that romiplostim will shortly be licensed for use in children with primary ITP. The role of romiplostim in the management of chemotherapy-related thrombocytopenia is formally undergoing investigation in adults and will require study in children as well.
Acknowledgments
We thank Joe Olechnowicz for editorial support. We acknowledge support from the NCI Cancer Center Support Grant P30 CA008748.
Disclosures: Dr. Bussel has received clinical research support from Amgen and Novartis and served on advisory boards for them. We acknowledge support of the MSK Cancer Center Support Grant P30 CA008748.
Footnotes
The authors have no other conflicts to disclose.
References
- 1.Bayhan T, Ünal Ş, Gümrük F, Çetin M. Immune Thrombocytopenic Purpura During Maintenance Phase of Acute Lymphoblastic Leukemia: A Rare Coexistence Requiring a High Degree of Suspicion, a Case Report and Review of the Literature. Turkish J Hematol. 2015;32(4):363–366. doi: 10.4274/tjh.2014.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tarantino MD, Bussel JB, Blanchette VS, et al. Romiplostim in children with immune thrombocytopenia: a phase 3, randomised, double-blind, placebo-controlled study. Lancet (London, England) 2016;388(10039):45–54. doi: 10.1016/S0140-6736(16)00279-8. [DOI] [PubMed] [Google Scholar]
- 3.Bussel JB, Buchanan GR, Nugent DJ, et al. A randomized, double-blind study of romiplostim to determine its safety and efficacy in children with immune thrombocytopenia. Blood. 2011;118(1):28–37. doi: 10.1182/blood-2010-10-313908. [DOI] [PubMed] [Google Scholar]
- 4.Olnes M, Scheinberg P, Calvo K, et al. Eltrombopag and Improved Hematopoiesis in Refractory Aplastic Anemia. N Engl J Med. 2012;367:11–19. doi: 10.1056/NEJMoa1200931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodeghiero F, Carli G. Beyond immune thrombocytopenia : the evolving role of thrombopoietin receptor agonists. Ann Hematol. 2017 doi: 10.1007/s00277-017-2953-6. [DOI] [PubMed] [Google Scholar]
- 6.Maximova N, Zanon D, Rovere F, Maestro A, Schillani G, Paparazzo R. Romiplostim for secondary thrombocytopenia following allogeneic stem cell transplantation in children. Int J Hematol. 2015;102(5):626–632. doi: 10.1007/s12185-015-1821-1. [DOI] [PubMed] [Google Scholar]
- 7.Ruiz-Delgado GJ, Lutz-Presno J, Ruiz-Arguelles GJ. Romiplostin may revert the thrombocytopenia in graft-versus-host disease. Hematology. 2011;16(2):108–109. doi: 10.1179/102453311X12940641877885. [DOI] [PubMed] [Google Scholar]
- 8.Parameswaran R, Lunning M, Mantha S, et al. Romiplostim for management of chemotherapy-induced thrombocytopenia. Support Care Cancer. 2014;22(5):1217–1222. doi: 10.1007/s00520-013-2074-2. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson AE, Shah N, Setty BA. Romiplostim for therapy-related thrombocytopenia in pediatric malignancies. Pediatr Blood Cancer. 2017 Nov;:e26473. doi: 10.1002/pbc.26473. 2016. [DOI] [PubMed] [Google Scholar]
- 10.Cheung NK, Kushner BH, LaQuaglia M, et al. N7: A Novel Multi-Modality Therapy of High Risk Neuroblastoma (NB) in Children Diagnosed Over 1 Year of Age. Med Pediatr Oncol. 2001;36(1):227–230. doi: 10.1002/1096-911X(20010101)36:1<227::AID-MPO1055>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 11.Cheung NKV, Cheung IY, Kramer K, et al. Key role for myeloid cells: Phase II results of anti-GD2 antibody 3F8 plus granulocyte-macrophage colony-stimulating factor for chemoresistant osteomedullary neuroblastoma. Int J Cancer. 2014;135(9):2199–2205. doi: 10.1002/ijc.28851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheung NKV, Cheung IY, Kushner BH, et al. Murine Anti-GD2 Monoclonal Antibody 3F8 Combined With Granulocyte-Macrophage Colony-Stimulating Factor and 13-Cis-Retinoic Acid in High-Risk Patients With Stage 4 Neuroblastoma in First Remission. J Clin Oncol. 2012;30(26):3264–3270. doi: 10.1200/JCO.2011.41.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kushner BH, Cheung IY, Modak S, Kramer K, Ragupathi G, Cheung NKV. Phase I Trial of a Bivalent Gangliosides Vaccine in Combination with β-Glucan for High-Risk Neuroblastoma in Second or Later Remission. Clin Cancer Res. 2014;20(5):1375–1382. doi: 10.1158/1078-0432.CCR-13-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peinemann F, Tushabe DA, van Dalen EC, Berthold F. Rapid COJEC versus standard induction therapies for high-risk neuroblastoma. Cochrane database Syst Rev. 2015;5(5):CD010774. doi: 10.1002/14651858.CD010774.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Provan D, Stasi R, Newland AC, et al. Review article International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115(2):168–187. doi: 10.1182/blood-2009-06-225565. [DOI] [PubMed] [Google Scholar]
- 16.Neunert C, Despotovic J, Haley K, et al. Thrombopoietin Receptor Agonist Use in Children: Data From the Pediatric ITP Consortium of North America ICON2 Study. Pediatr Blood Cancer. 2016;63(1):1407–1413. doi: 10.1002/pbc.26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheung NKV, Guo HF, Cheung IY. Correlation of anti-idiotype network with survival following anti-G(D2) monoclonal antibody 3F8 therapy of stage 4 neuroblastoma. Med Pediatr Oncol. 2000;35(6):635–637. doi: 10.1002/1096-911x(20001201)35:6<635::aid-mpo32>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 18.Modak S, Kushner BH, Kramer K, Vickers A, Cheung IY, Cheung N-KV. Anti-GD2 antibody 3F8 and barley-derived (1 → 3),(1 → 4)-β-D-glucan: A Phase I study in patients with chemoresistant neuroblastoma. Oncoimmunology. 2013;2(3):e23402. doi: 10.4161/onci.23402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Depre F, Aboud N, Mayer B, Salama A. Bidirectional inefficacy or intolerability of thrombopoietin receptor agonists: new data and a concise review. Blood Transfus. 2017 doi: 10.2450/2012.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Buduo CA, Currao M, Pecci A, David L, Balduini CL, Balduini A. Revealing eltrombopag’s promotion of human megakaryopoiesis through AKT/ERK-dependent pathway activation. Haematologica. 2016;101(12):1479–1488. doi: 10.3324/haematol.2016.146746. [DOI] [PMC free article] [PubMed] [Google Scholar]


