Abstract
Aluminum oxide nanoparticles (Al2O3 NPs) are among the most widely used nanomaterials; however, relatively little information about their risk identification and assessment is available. In the present study, we aimed to investigate the potential toxicity of Al2O3 NPs following repeated inhalation exposure in male Sprague-Dawley rats. Rats were exposed to Al2O3 NPs for 28 days (5 days/week) at doses of 0, 0.2, 1, and 5 mg/m3 using a nose-only inhalation system. During the experimental period, we evaluated the clinical signs, body weight change, hematological and serum biochemical parameters, necropsy findings, organ weight, and histopathology findings. Additionally, we analyzed the bronchoalveolar lavage fluid (BALF), including differential leukocyte counts, and aluminum contents in the major organs and blood. Aluminum contents were the highest in lung tissues and showed a dose-dependent relationship in the exposure group. Histopathology showed alveolar macrophage accumulation in the lungs of rats in the 5 mg/m3 group during exposure and recovery. These changes tended to increase at the end of the recovery period. In the BALF analysis, total cell and neutrophil counts and lactate dehydrogenase, tumor necrosis factor-α, and interleukin-6 levels significantly increased in the 1 and 5 mg/m3 groups during exposure. Under the present experimental conditions, we suggested that the no-observed-adverse-effect level of Al2O3 NPs in male rats was 1 mg/m3, and the target organ was the lung.
Keywords: Inhalation toxicity, Aluminum oxide, Target organ, No-observed-adverse-effect level
INTRODUCTION
Aluminum is a silver-white, soft, non-magnetic, ductile, and relatively abundant metal, accounting for approximately 8% of the earth’s crust. This metal is used in aerospace, transportation, and building industries because of its low density and ability to resist corrosion (1). Aluminum has a relatively low toxicity and is not classified according to its carcinogenicity. Additionally, the time-weighted average (TWA) of aluminum is 5 mg/m3 by inhalation. However, aluminum production has been classified as carcinogenic to humans by the International Agency for Research on Cancer (IARC) (2). The sources of human exposure to aluminum are very diverse, including food additives, pharmaceuticals, personal care products, paints, and fuel additives, amongst others (1). In particular, occupational exposure to aluminum occurs during the refining process of primary metals and in secondary industries utilizing aluminum products. Moreover, it is a common metal component in ultrafine airborne particles in the ambient environment, and is relatively stable in the form of aluminum oxide (Al2O3) (3). An example of occupational exposure to Al2O3 involves the use of sandpaper for polishing, where inhalable Al2O3 dust is generated when Al2O3 is used as an abrasive material on sandpaper.
Nanotechnology has advanced exponentially over the past decade, with nanoscale materials being exploited in several applications and disciplines. However, nano-sized materials exhibit different properties from those of conventional materials because the size and corresponding large specific surface area can influence the physiochemical properties (4).
Al2O3 nanoparticles (NPs) are among the most widely used nanomaterials owing to the advantages provided by their thermal, chemical, and physical properties (5,6). As such, Al2O3 NPs have been used for the modification of polymers, functionalization of textiles, heat transfer fluids, treatment of waste water, biosensors, biofiltration, drug delivery, and antigen delivery for immunization purposes.
Owing to the increasing use of Al2O3 NPs, concerns about their human health risks and associated need for safety research are increasing. Li et al. demonstrated that Al2O3 NPs induced mitochondrial-dependent apoptosis and oxidative stress in vitro and in vivo. Additionally, Kwon et al. evaluated the cytotoxicity of Al2O3 NPs in rat lung epithelial cells (3,7) and demonstrated that intratracheal instillation of Al2O3 NPs could trigger an inflammatory response in the lungs. Generally, NPs can be inhaled more deeply than large particles, leaving sediment on the surface of the trachea, bronchi, and alveoli. The lung is thus considered the primary target organ for inhaled NPs (3). Al2O3 NPs have previously been investigated; however, studies focused on inhalation exposure remain lacking. In a previous study, inhaled Al2O3 has been associated with pulmonary fibrosis (8). There is thus an urgent need for further occupational exposure toxicity data since workers can be directly exposed to Al2O3 NPs through inhalation. For this reason, we conducted a 28-day repeated inhalation toxicity study to assess the health and safety of Al2O3 NPs exposure, particularly as it relates to occupational exposure in the workplace. To achieve this, we examined the toxicity and determined the no-observed-adverse-effect level (NOAEL) after Al2O3 NPs inhalation in male Sprague-Dawley rats.
MATERIALS AND METHODS
Test materials
Al2O3 powder was purchased from Sigma-Aldrich (St. Louis, MO, USA). Particle size was estimated by assuming the particles to have the same spherical shape and size, as per the formula used in a previous study (9). The Brunauer-Emmett-Teller (BET) method (Micromeritics ASAP 2420, Micromeritics Inc., Norcross, GA, USA) was used to determine the BET surface area under the following conditions: test materials were degassed for 4 hr at 300°C; adsorptive analysis used dinitrogen, analysis bath temperature was 77.300 K, and the equilibration interval was 10 s. The surface area of Al2O3 used in the present study was 127.25 m2/g, and the particle size was 11.94 nm.
Test animals
Seventy specific pathogen-free (SPF) Sprague-Dawley (SD) male rats (6 weeks old) were purchased from Japan SLC Inc. (Tokyo, Japan), and acclimatized for two weeks before initial exposure, including restraining tube acclimatization. Rats were housed in a room maintained at 22 ± 3°C, with a relative humidity of 50 ± 20%, ventilation of 13–18 air changes/hr, and 12-hr light/12-hr dark cycle with 150~300 Lux. Four or less rats were housed in a solid bottom polysulfone cage (235 × 380 × 175 mm) containing sterilized bedding. Animals were provided with irradiation-sterilized pellet food (18% protein rodent diet 2918C, ENVIGO RMS Inc., IN, USA), and UV-sterilized and filtered water ad libitum. The study protocol was approved by the Institutional Animal Care and Use Committee of the Chemicals Toxicity Research Bureau (IACUC-1703).
Study design
After a 2 week acclimatization period, 64 healthy rats (8 weeks old) were used. Four groups of SD rats were exposed to Al2O3 NPs for 28 days (6 hr/day, 5 days/week) at doses of 0, 0.2, 1, and 5 mg/m3. These doses were selected based on the results of a previous repeated inhalation toxicity study of metal NPs, including neodymium oxide, cerium oxide, and lanthanum oxide (10,11).
Each dose group consisted of 16 rats, with 8 rats/group sacrificed after the 28-day exposure period. The remaining rats were maintained as the recovery groups and sacrificed after a 28-day recovery period to identify reversibility, persistence, and delayed toxic effects.
Generation, analysis, and inhalation chamber monitoring
Al2O3 nanopowder was suspended in distilled water at concentrations of 0.08 to 0.49% w/v and sonicated for 30 min (5-s sonication/3-s rest cycle, 19 mm probe, 40% amplitude) using a probe-type ultrasonicator (VC750, Sonics & Materials Inc., Newtown, CT, USA) on ice to avoid heat generation. Hydrodynamic diameters were measured using dynamic light scattering (Zetasizer Nano ZS 90, Malvern, UK) to check particle size and dispersity. The resulting dispersion was aerosolized through a 0.6~0.8 mm diameter orifice at an airflow of 4~8 L/min in a nose-only inhalation chamber (NITC system, HCT Co., Icheon, Korea) under constant agitation. The total airflow for each chamber was set at 20 L/min to achieve a 1 L/min flow/rat. Chamber conditions (temperature, relative humidity, and oxygen concentration) were automatically measured.
The concentration of Al2O3 NPs were measured using a personal air sampling pump (Air Chek XR, SKC, Somerset, NJ, USA) and glass fiber filter (GF-C, HG00025C, HYUNDAI Micro, Korea). The mass median aerodynamic diameter (MMAD) and geometric standard deviation (GSD) were measured using a cascade impactor (Model-135 Mini MOUDI™ Impactor, MSP Co., MN, USA). Samples were collected from the middle part of the port at a flow rate of 1 L/min. During the exposure period, the mass concentrations of the aerosols in the chamber were measured at least three times daily. Aerosol particles were also sampled for transmission electron microscopy (TEM; H-7100FA, Hitachi, Tokyo, Japan). TEM was performed at a magnification of 10,000 ×, and the particles analyzed using an energy dispersive X-ray spectrometer (EDS, EX200, Horiba, Kyoto, Japan) at an accelerating voltage of 75 kV.
Clinical symptoms and body weight
All animals were observed daily for mortality and the development of clinical symptoms, including respiratory, dermal, behavioral, nasal, and genitourinary changes before and after exposure. The type, date of occurrence, and severity of these symptoms were individually recorded. Body weight was measured using an electronic balance (QUINTIX3102, Sartorius Co., Göttingen, Lower Saxony, Germany) once/week throughout the experimental period.
Hematology and serum biochemistry
Blood samples (approximately 2 mL each) were placed into a complete blood count (CBC) bottle (vacutainer 3 mL, BD, NJ, USA), containing dipotassium ethylenediaminetetraacetic acid (EDTA-2K) as an anticoagulant, and analyzed using an automatic hematology analyzer (ADVIA 2120i, Siemens Diagnostics, Tarrytown, NY, USA). Hematology analysis included the following parameters: red blood cell count, hemoglobin concentration, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, red cell distribution width, mean platelet volume, platelet count, white blood cell count, and white blood cell differential count (neutrophils, lymphocytes, monocytes, eosinophils, and basophils). An additional 3 mL of blood was collected and placed into a 5-mL vacutainer tube (BD Pharmingen, San Diego, CA, USA), containing a clot activator. Blood samples were maintained at 20 ± 5°C for 15~20 min to allow coagulation, and then centrifuged at 3,000 rpm (LABMASTER ABC-CB200R, HANLAB, Cheongju, Korea) for 10 min. Using a serum biochemistry analyzer (TBA-120FR, Toshiba Co., Tokyo, Japan), the following parameters were measured: aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, creatine phosphokinase, total bilirubin, glucose, total cholesterol, triglycerides, total protein, albumin, blood urea nitrogen, creatinine, inorganic phosphorus, lactate dehydrogenase (LDH), and calcium ions.
Necropsy and organ weight
Before the scheduled necropsy, all surviving rats were fasted overnight and euthanized using a small animal inhaler with isoflurane (Ilsung Pharm. Co., Seoul, Korea) on the day of necropsy. Blood was collected from the posterior vena cava for hematology and serum biochemistry analyses after confirming euthanasia. The abdominal aorta and posterior vena cava were cut for exsanguination. Gross examination of the body surface, subcutis, and all internal organs in the head, abdominal and thoracic cavities was performed. Thereafter the kidneys, spleen, lungs, brain, and liver were removed and weighed using an electronic balance (QUINTIX313, Sartorius Co.). The relative organ weight was determined based on the ratio of absolute organ weight to fasted body weight.
Histopathology
Microscopic examination of the weighed organs was performed. All gross lesions, as defined by the study pathologist, were included in the examination. Organs and tissues (the left lung was used for lung histopathology) were fixed in 10% neutral buffered formalin solution and stained with hematoxylin and eosin. The samples were examined under a light microscope at 100X or 200X magnification.
Bronchoalveolar lavage fluid (BALF) analysis
The right lung was lavaged five times with 3 mL cold sterile saline solution by tracheal cannulation with a PE-90 tube (Clay Adams, NJ, USA). Lavaged fluids were centrifuged at 1,500 rpm for 10min (Micro 17R, Hanil Scientific, Gimpo, Korea). Supernatants from the first lavage fluid were stored at −80°C for subsequent albumin, interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and LDH assays. Precipitated cells were counted using an automatic hematology analyzer (ADVIA 2120i, Siemens Diagnostics). The remaining pellet was used for differential leukocyte count determination after centrifugation at 1,500 rpm for 10 min (Hanil Cellspin, Incheon, Korea) and Diff-Quick staining (Sysmex, Kobe, Japan). Approximately 300 leukocytes (macrophages, neutrophils, lymphocytes, and eosinophils) were counted under a light microscope at 400X magnification. LDH and albumin concentrations in the lavage fluid were measured using a biochemistry analyzer (TBA-120FR, Toshiba Co.), while TNF-α and IL-6 concentrations were measured using commercial assay kits (RTA00 and R6000B, respectively, R&D systems, MN, USA).
Oxidative stress analysis
The right lung of animals was homogenized in five volumes of 50 mM phosphate buffer solution (pH 7.0), and the resulting supernatant was used for analysis after centrifugation (Micro 17R, Hanil Science). Catalase (Cayman catalase kit 707002, Cayman Chemical, Ann Arbor, MI, USA), reduced glutathione (GSH) (Cayman glutathione assay kit 703002, Cayman Chemical), and thiobarbituric acid reactive substance (TBARS) (Cayman TBARS assay kit 10009055, Cayman Chemical) were measured in the supernatant following adjustment to a final protein concentration of 2 mg/mL.
Aluminum content measurement
Aluminum content was measured using inductively coupled plasma mass spectrometry (ICP-MS; 7500CE, Agilent Technologies, Santa Clara, CA, USA). The right lung, liver, spleen, kidneys, whole blood, and brain tissues were digested in 5 or 10 volumes of 69% nitric acid (Merck, Whitehouse Station, NJ, USA) before analysis. ICP-MS analytical conditions are shown in Table 1.
Table 1.
Aluminum analysis condition of inductively coupled plasma mass spectrometry
| Parameter | Unit | Value |
|---|---|---|
| RF power | W | 1,600 |
| Sampling depth | mm | 8 |
| Torch-H | mm | −0.4 |
| Carrier gas | L/min | 0.7 |
| Makeup gas | L/min | 0.5 |
| S/C temp | °C | 2 |
| He gas | mL/min | 5 |
RF, radio frequency; He, helium.
Statistical analysis
Data are presented as the means ± standard deviation (SD). Body weight, hematology, serum biochemistry, organ weights, BALF analysis, and aluminum content were assumed to be normally distributed and analyzed by one-way analysis of variance (ANOVA) followed by Dunnett’s T3 post hoc test. SPSS 22.0K software was used for all statistical analyses (IBM SPSS Statistics, Armonk, NY, USA). A p-value < 0.05 indicated statistical significance.
RESULTS
Monitoring of inhalation chamber and analysis of Al2O3
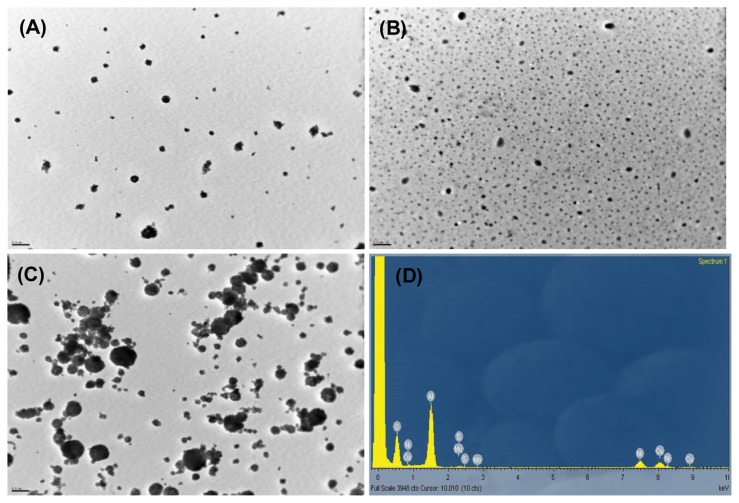
The concentration of Al2O3 was maintained as constant by daily adjustment of dispersion concentration and aeration flow in the inhalation exposure system throughout the exposure period. Al2O3 dispersion was prepared daily during the exposure period, with its associated properties shown in Table 2. The concentration of Al2O3 dispersion was 0.08 ± 0.02, 0.26 ± 0.06, and 0.49 ± 0.04% for the 0.2, 1, and 5 mg/m3 groups, respectively. After sonication, the dispersion particle sizes were 206 ± 31, 267 ± 14.8, and 273 ± 13.2 nm, while the polydispersity indices were 0.29 ± 0.03, 0.33 ± 0.06, and 0.36 ± 0.06 for the 0.2, 1, and 5 mg/m3 groups, respectively. The applied energy to dispersions was 279.95 ± 10.21, 271.19 ± 9.27, and 269.47 ± 8.14 J/mL for the 0.2, 1, and 5 mg/m3 groups, respectively. The actual concentrations for the 0.2, 1, and 5 mg/m3 groups were 0.24 ± 0.13, 1.27 ± 0.43, and 4.99 ± 1.2 mg/m3, respectively. The MMAD was 0.378, 0.48, and 0.515 μm, and the GSD 1.7, 4.61, and 3.28 for the 0.2, 1, and 5 mg/m3 groups, respectively. TEM of particles collected from the inhalation chamber showed aciniform aggregates and agglomerates (Fig. 1).
Table 2.
Characterization of Al2O3 nanoparticles dispersion during the exposure period
| Parameter | Dose (mg/m3) | ||
|---|---|---|---|
|
| |||
| 0.2 | 1 | 5 | |
| Concentration (%) | 0.08 ± 0.02 | 0.26 ± 0.06 | 0.49 ± 0.04 |
| Energy (Jule/mL) | 279.95 ± 10.21 | 271.19 ± 9.27 | 269.47 ± 8.14 |
| Size (nm) | 186.90 ± 8.38 | 191.50 ± 9.95 | 193.69 ± 7.00 |
| PDI | 0.29 ± 0.03 | 0.33 ± 0.06 | 0.36 ± 0.06 |
PDI, polydispersity index.
Values are expressed as means ± SD (n=20).
Fig. 1.
Transmission electron microscopy (TEM) images (A-C, 10,000 ×) and energy dispersive spectroscopy (EDS) analysis (D) of Al2O3 nanoparticles collected from the exposure chambers. TEM images were taken from the 0.2mg/m3 (A), 1mg/m3 (B) and 5mg/m3 (C) exposure group. Bar = 0.5 μm.
Clinical symptoms and body weight
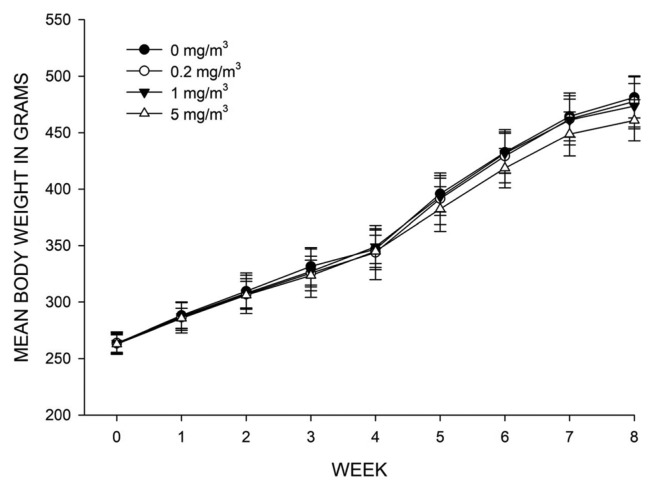
No treatmentrelated clinical symptoms were observed during the exposure and recovery periods (data not shown). Similarly, no significant differences were observed in body weight among groups (Fig. 2).
Fig. 2.
Mean body weight of rats exposed to 0mg/m3, 0.2 mg/m3, 1mg/m3 and 5mg/m3 Al2O3 nanoparticles during the experimental period. Values are expressed as means ± SD (n = 16, 0~4 week; n = 8, 5~8 week; n=8).
Hematology and serum biochemistry
Hematology analysis showed no significant differences among the test and control groups during exposure (Table 3). By contrast, the mean corpuscular hemoglobin concentration significantly decreased in the 1 mg/m3 recovery group compared with the control group (p < 0.01). Additionally, neutrophil count significantly increased in the 1 mg/m3 recovery group, compared with the control group (p < 0.05) (Table 4). Serum biochemistry analysis indicated no significant differences among the test and control groups during both exposure and recovery (Table 5, 6).
Table 3.
Hematological values of rats after 28-day exposure of Al2O3 nanoparticles
| Parameter | Dose (mg/m3) | |||
|---|---|---|---|---|
|
| ||||
| 0 | 0.2 | 1 | 5 | |
| No. of animals | 8 | 8 | 8 | 8 |
| RBC (106/μL) | 8.10 ± 0.29 | 8.49 ± 0.49 | 8.42 ± 0.31 | 8.28 ± 0.23 |
| Hemoglobin (g/dL) | 14.49 ± 0.38 | 14.96 ± 0.77 | 14.94 ± 0.44 | 14.73 ± 0.32 |
| Hematocrit (%) | 41.54 ± 1.06 | 43.35 ± 2.21 | 43.05 ± 1.39 | 42.76 ± 1.12 |
| MCV (fL) | 51.29 ± 1.28 | 51.10 ± 1.11 | 51.18 ± 1.00 | 51.65 ± 0.63 |
| MCH (pg) | 17.89 ± 0.61 | 17.64 ± 0.54 | 17.75 ± 0.40 | 17.79 ± 0.30 |
| MCHC (g/dL) | 34.86 ± 0.31 | 34.50 ± 0.37 | 34.69 ± 0.16 | 34.43 ± 0.36 |
| RDW (%) | 13.21 ± 0.78 | 12.85 ± 0.92 | 13.20 ± 1.00 | 12.78 ± 0.77 |
| Platelet (103/μL) | 1032.63 ± 115.25 | 1045.38 ± 106.94 | 1035.88 ± 62.60 | 980.38 ± 59.89 |
| MPV (fL) | 7.36 ± 0.44 | 7.35 ± 0.40 | 7.44 ± 0.37 | 7.64 ± 0.29 |
| WBC (103/μL) | 5.08 ± 1.21 | 4.78 ± 0.78 | 5.41 ± 1.11 | 5.52 ± 0.64 |
| Neutrophils (103/μL) | 1.17 ± 0.29 | 1.19 ± 0.32 | 1.31 ± 0.20 | 1.51 ± 0.33 |
| Lymphocytes (103/μL) | 3.73 ± 1.01 | 3.42 ± 0.97 | 3.89 ± 0.91 | 3.81 ± 0.53 |
| Monocytes (103/μL) | 0.08 ± 0.04 | 0.07 ± 0.02 | 0.09 ± 0.03 | 0.09 ± 0.02 |
| Eosinophils (103/μL) | 0.06 ± 0.02 | 0.06 ± 0.02 | 0.07 ± 0.01 | 0.06 ± 0.01 |
| Basophils (103/μL) | 0.01 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.01 | 0.01 ± 0.00 |
RBC, red blood cell count; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RDW, red cell distribution width; MPV, mean platelet volume; WBC, white blood cell count.
Values are expressed as means ± SD.
Table 4.
Hematological values of rats after 28-day recovery of Al2O3 nanoparticles
| Parameter | Dose (mg/m3) | |||
|---|---|---|---|---|
|
| ||||
| 0 | 0.2 | 1 | 5 | |
| No. of animals | 8 | 8 | 7 | 8 |
| RBC (106/μL) | 8.47 ± 0.28 | 8.49 ± 0.44 | 8.58 ± 0.20 | 8.49 ± 0.28 |
| Hemoglobin (g/dL) | 14.61 ± 0.31 | 14.79 ± 0.50 | 14.80 ± 0.26 | 14.71 ± 0.52 |
| Hematocrit (%) | 43.01 ± 0.92 | 42.78 ± 1.48 | 43.09 ± 0.65 | 42.83 ± 1.06 |
| MCV (fL) | 50.83 ± 1.43 | 50.45 ± 0.96 | 50.25 ± 1.30 | 50.46 ± 1.16 |
| MCH (pg) | 17.28 ± 0.53 | 17.44 ± 0.39 | 17.26 ± 0.55 | 17.33 ± 0.43 |
| MCHC (g/dL) | 33.98 ± 0.23 | 34.55 ± 0.23** | 34.34 ± 0.41 | 34.36 ± 0.63 |
| RDW (%) | 15.68 ± 1.28 | 15.69 ± 0.88 | 15.79 ± 1.30 | 15.68 ± 1.58 |
| Platelet (103/μL) | 1018.75 ± 67.21 | 1051.75 ± 127.74 | 994.75 ± 90.67 | 1004.25 ± 81.44 |
| MPV (fL) | 8.56 ± 0.57 | 8.15 ± 0.68 | 8.70 ± 0.44 | 8.28 ± 0.41 |
| WBC (103/μL) | 6.28 ± 0.96 | 5.22 ± 0.89 | 6.11 ± 0.69 | 6.26 ± 0.81 |
| Neutrophils (103/μL) | 1.62 ± 0.54 | 1.12 ± 0.20* | 1.43 ± 0.32 | 1.63 ± 0.16 |
| Lymphocytes (103/μL) | 4.32 ± 0.62 | 3.86 ± 0.87 | 4.39 ± 0.57 | 4.32 ± 0.78 |
| Monocytes (103/μL) | 0.16 ± 0.03 | 0.11 ± 0.04 | 0.13 ± 0.04 | 0.16 ± 0.06 |
| Eosinophils (103/μL) | 0.11 ± 0.03 | 0.08 ± 0.02 | 0.09 ± 0.03 | 0.08 ± 0.04 |
| Basophils (103/μL) | 0.01 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
RBC, red blood cell count; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RDW, red cell distribution width; MPV, mean platelet volume; WBC, white blood cell count.
Values are expressed as means ± SD.
Significantly different from vehicle control at p<0.05 and p<0.01.
Table 5.
Serum biochemical values of rats after 28-day exposure of Al2O3 nanoparticles
| Parameter | Dose (mg/m3) | |||
|---|---|---|---|---|
|
| ||||
| 0 | 0.2 | 1 | 5 | |
| No. of animals | 8 | 8 | 8 | 8 |
| AST (IU/L) | 105.06 ± 23.01 | 110.49 ± 43.87 | 100.30 ± 21.83 | 113.68 ± 23.94 |
| ALT (IU/L) | 51.41 ± 9.90 | 51.24 ± 9.05 | 46.46 ± 7.17 | 51.68 ± 6.35 |
| ALP (IU/L) | 971.38 ± 223.04 | 1110.54 ± 26.71 | 979.93 ± 87.44 | 1010.43 ± 20.96 |
| CPK (IU/L) | 533.18 ± 271.21 | 631.91 ± 220.95 | 782.96 ± 323.25 | 682.56 ± 266.52 |
| LDH (IU/L) | 1789.49 ± 942.80 | 2088.69 ± 838.93 | 2317.25 ± 957.19 | 2385.49 ± 1021.01 |
| TBil (mg/dL) | 0.17 ± 0.04 | 0.17 ± 0.02 | 0.16 ± 0.04 | 0.17 ± 0.03 |
| Glucose (mg/dL) | 142.20 ± 47.45 | 128.56 ± 12.83 | 130.38 ± 16.83 | 129.09 ± 18.48 |
| TCho (mg/dL) | 80.19 ± 40.16 | 68.93 ± 8.48 | 74.34 ± 10.43 | 68.41 ± 6.04 |
| Triglyceride (mg/dL) | 70.91 ± 42.38 | 48.49 ± 9.25 | 49.80 ± 14.73 | 46.36 ± 15.96 |
| TP (g/dL) | 5.89 ± 0.29 | 5.94 ± 0.13 | 5.93 ± 0.26 | 5.89 ± 0.15 |
| Albumin (g/dL) | 4.04 ± 0.27 | 4.11 ± 0.10 | 4.11 ± 0.08 | 4.19 ± 0.10 |
| BUN (mg/dL) | 15.46 ± 3.20 | 15.83 ± 1.49 | 14.99 ± 2.92 | 15.65 ± 2.16 |
| Creatinine (mg/dL) | 0.41 ± 0.04 | 0.40 ± 0.03 | 0.40 ± 0.02 | 0.41 ± 0.02 |
| IP (mg/dL) | 7.84 ± 0.55 | 7.98 ± 0.74 | 7.84 ± 0.94 | 8.01 ± 0.42 |
| Ca (mg/dL) | 9.19 ± 0.25 | 9.31 ± 0.24 | 9.35 ± 0.28 | 9.38 ± 0.28 |
AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; CPK, creatine phosphokinase; LDH, lactate dehydrogenase; TBil, total bilirubin; TCho, total cholesterol; TP, total protein; BUN, blood urea nitrogen; IP, inorganic phosphorus; Ca, calcium.
Values are expressed as means ± SD.
Table 6.
Serum biochemical values of rats after 28-day recovery Al2O3 nanoparticles
| Parameter | Dose (mg/m3) | |||
|---|---|---|---|---|
|
| ||||
| 0 | 0.2 | 1 | 5 | |
| No. of animals | 8 | 8 | 7 | 8 |
| AST (IU/L) | 100.78 ± 43.70 | 91.93 ± 9.36 | 95.56 ± 22.58 | 89.59 ± 21.93 |
| ALT (IU/L) | 60.80 ± 22.10 | 53.38 ± 7.35 | 52.61 ± 10.29 | 50.68 ± 6.70 |
| ALP (IU/L) | 647.04 ± 111.41 | 642.69 ± 92.87 | 624.91 ± 189.49 | 628.45 ± 116.61 |
| CPK (IU/L) | 560.05 ± 190.30 | 598.21 ± 219.25 | 546.59 ± 206.11 | 539.86 ± 257.62 |
| LDH (IU/L) | 1520.29 ± 615.41 | 1647.25 ± 486.74 | 1699.54 ± 528.25 | 1567.85 ± 738.63 |
| TBil (mg/dL) | 0.18 ± 0.02 | 0.18 ± 0.02 | 0.19 ± 0.02 | 0.17 ± 0.02 |
| Glucose (mg/dL) | 178.89 ± 30.84 | 184.49 ± 26.45 | 177.95 ± 24.11 | 183.06 ± 22.98 |
| TCho (mg/dL) | 80.98 ± 12.20 | 96.05 ± 6.32 | 78.71 ± 9.74 | 79.35 ± 9.37 |
| Triglyceride (mg/dL) | 103.83 ± 24.68 | 107.68 ± 32.74 | 97.73 ± 31.74 | 92.55 ± 24.60 |
| TP (g/dL) | 6.64 ± 0.20 | 6.51 ± 0.12 | 6.64 ± 0.22 | 6.65 ± 0.24 |
| Albumin (g/dL) | 4.23 ± 0.12 | 4.21 ± 0.11 | 4.27 ± 0.13 | 4.27 ± 0.17 |
| BUN (mg/dL) | 16.81 ± 2.60 | 18.95 ± 2.79 | 19.10 ± 2.43 | 18.03 ± 2.76 |
| Creatinine (mg/dL) | 0.48 ± 0.04 | 0.49 ± 0.03 | 0.49 ± 0.04 | 0.48 ± 0.03 |
| IP (mg/dL) | 7.39 ± 0.66 | 7.26 ± 0.48 | 7.08 ± 0.43 | 7.25 ± 0.48 |
| Ca (mg/dL) | 10.51 ± 0.30 | 10.49 ± 0.18 | 10.43 ± 0.18 | 10.44 ± 0.25 |
AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; CPK, creatine phosphokinase; LDH, lactate dehydrogenase; TBil, total bilirubin; TCho, total cholesterol; TP, total protein; BUN, blood urea nitrogen; IP, inorganic phosphorus; Ca, calcium.
Values are expressed as means ± SD.
Necropsy and organ weight
There were no treatment-related gross findings in all sacrificed animals. Compared with the control group, the absolute and relative lung weights showed a significant increase by 11.5 and 10.6% respectively in the 5 mg/m3 group during exposure (p < 0.01) (Table 7). However, compared with the control group, only the absolute kidney weight in the 1 mg/m3 group was significantly increased during recovery (p < 0.01) (Table 8).
Table 7.
Absolute and relative organ weights of rats after 28-day exposure of Al2O3 nanoparticles
| Parameter | Dose (mg/m3) | |||
|---|---|---|---|---|
|
| ||||
| 0 | 0.2 | 1 | 5 | |
| No. of animals | 8 | 8 | 8 | 8 |
| Body weight (g) | 331.07 ± 19.77 | 326.94 ± 24.48 | 333.43 ± 12.98 | 335.11 ± 12.24 |
| Lung | 1.829 ± 0.142 | 1.761 ± 0.119 | 1.839 ± 0.093 | 2.040 ± 0.073** |
| per body weight (%) | 0.615 ± 0.048 | 0.592 ± 0.040 | 0.619 ± 0.031 | 0.686 ± 0.024** |
| Liver (g) | 10.381 ± 1.034 | 9.783 ± 0.788 | 10.031 ± 0.899 | 9.822 ± 0.705 |
| per body weight (%) | 3.492 ± 0.348 | 3.291 ± 0.265 | 3.375 ± 0.302 | 3.304 ± 0.237 |
| Kidney | 2.172 ± 0.267 | 1.997 ± 0.182 | 2.022 ± 0.121 | 2.028 ± 0.137 |
| per body weight (%) | 0.731 ± 0.090 | 0.672 ± 0.061 | 0.680 ± 0.041 | 0.682 ± 0.046 |
| Spleen (g) | 0.602 ± 0.034 | 0.579 ± 0.044 | 0.623 ± 0.111 | 0.651 ± 0.046 |
| per body weight (%) | 0.202 ± 0.011 | 0.195 ± 0.015 | 0.210 ± 0.037 | 0.219 ± 0.016 |
| Brain (g) | 1.976 ± 0.074 | 2.012 ± 0.086 | 1.941 ± 0.067 | 2.053 ± 0.170 |
| per body weight (%) | 0.665 ± 0.025 | 0.677 ± 0.029 | 0.653 ± 0.023 | 0.691 ± 0.057 |
Values are expressed as means ± SD.
Significantly different from vehicle control at p<0.01.
Table 8.
Absolute and relative organ weights of rats after 28-day recovery Al2O3 nanoparticles
| Parameter | Dose (mg/m3)
|
|||
|---|---|---|---|---|
| 0 | 0.2 | 1 | 5 | |
| No. of animals | 8 | 8 | 7 | 8 |
| Body weight (g) | 475.87 ± 19.51 | 471.83 ± 21.29 | 466.94 ± 20.11 | 455.20 ± 17.99 |
| Lung | 2.236 ± 0.134 | 2.256 ± 0.212 | 2.159 ± 0.108 | 2.204 ± 0.118 |
| per body weight (%) | 0.470 ± 0.019 | 0.478 ± 0.042 | 0.463 ± 0.015 | 0.484 ± 0.023 |
| Liver (g) | 14.759 ± 1.476 | 14.457 ± 0.910 | 13.914 ± 1.384 | 13.338 ± 0.916 |
| per body weight (%) | 3.098 ± 0.240 | 3.062 ± 0.086 | 2.975 ± 0.188 | 2.932 ± 0.196 |
| Kidney | 2.591 ± 0.125 | 2.529 ± 0.148 | 2.750 ± 0.276** | 2.498 ± 0.154 |
| per body weight (%) | 0.544 ± 0.015 | 0.537 ± 0.042 | 0.589 ± 0.058 | 0.549 ± 0.025 |
| Spleen (g) | 0.819 ± 0.114 | 0.797 ± 0.099 | 0.770 ± 0.077 | 0.803 ± 0.103 |
| per body weight (%) | 0.172 ± 0.022 | 0.169 ± 0.018 | 0.165 ± 0.013 | 0.176 ± 0.022 |
| Brain (g) | 2.085 ± 0.069 | 2.079 ± 0.089 | 2.083 ± 0.071 | 2.072 ± 0.069 |
| per body weight (%) | 0.438 ± 0.017 | 0.441 ± 0.011 | 0.447 ± 0.022 | 0.456 ± 0.023 |
Values are expressed as means ± SD.
Significantly different from vehicle control at p<0.01.
Histopathology
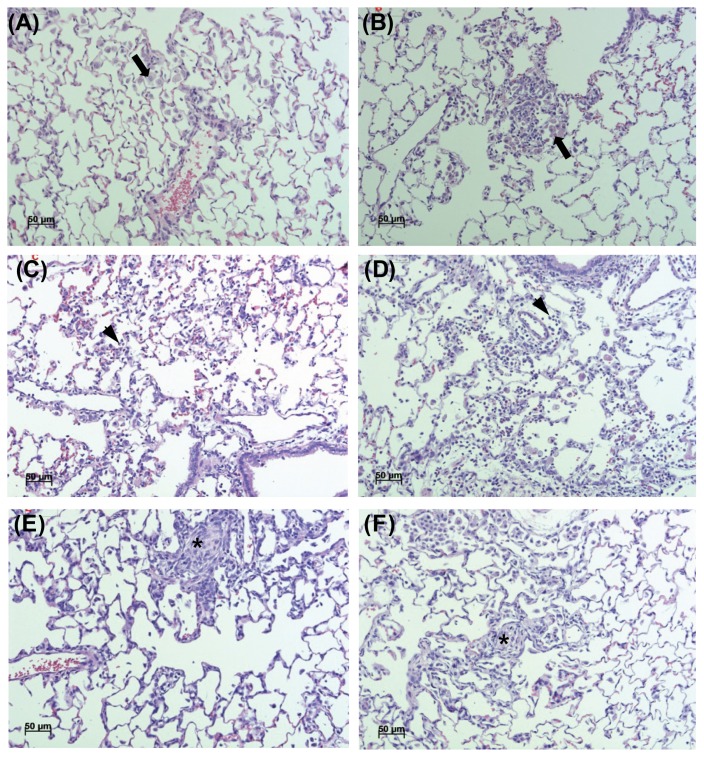
Results of the histopathological examination are presented in Table 9 and Fig. 3. Alveolar macrophage accumulation in the lungs was observed in 4 and 8 cases of the 5 mg/m3 group, during exposure and recovery respectively. Lesion severity increased during recovery, compared to that observed during exposure. No treatment-related lesions were observed in other organs.
Table 9.
Histopathologic findings of rat lungs treated with Al2O3 nanoparticles
| Finding (after 28-day exposure) | Grade | Dose (mg/m3) | |||
|---|---|---|---|---|---|
|
| |||||
| 0 | 0.2 | 1 | 5 | ||
| No. of animals | 8 | 8 | 8 | 8 | |
| Normal appearance | 8 | 5 | 8 | 3 | |
| Alveolar macrophage accumulation | ± | 0 | 1 | 0 | 4 |
| Inflammatory cells infiltration | ± | 0 | 1 | 0 | 1 |
| Granulomatous inflammation | ± | 0 | 1 | 0 | 0 |
|
| |||||
| Finding (after 28-day recovery) | Grade | Dose (mg/m3) | |||
|
| |||||
| 0 | 0.2 | 1 | 5 | ||
|
| |||||
| No. of animals | 8 | 8 | 8 | 8 | |
| Normal appearance | 8 | 6 | 6 | 0 | |
| Alveolar macrophage accumulation | ± | 0 | 1 | 0 | 0 |
| + | 0 | 0 | 0 | 8 | |
| Inflammatory cells infiltration | ± | 0 | 1 | 1 | 0 |
| Granulomatous inflammation | ± | 0 | 0 | 1 | 0 |
Grade: ±, minimal; +, slight.
Fig. 3.
Representative photographs of lung sections from the 5mg/m3 in exposure group (A), 5mg/m3 in recovery group (B), 1mg/m3 in exposure group (C), 1mg/m3 in recovery group (D), 0.2mg/m3 in exposure group (E) and 0.2mg/m3 in recovery group (F) stained with hematoxylin and eosin (200 ×). Alveolar macrophage accumulation (A, B; arrow), Inflammatory cells infiltration (C, D; arrowhead) and Granulomatous infiltration (E, F; asterisk) were shown.
Analysis of BALF
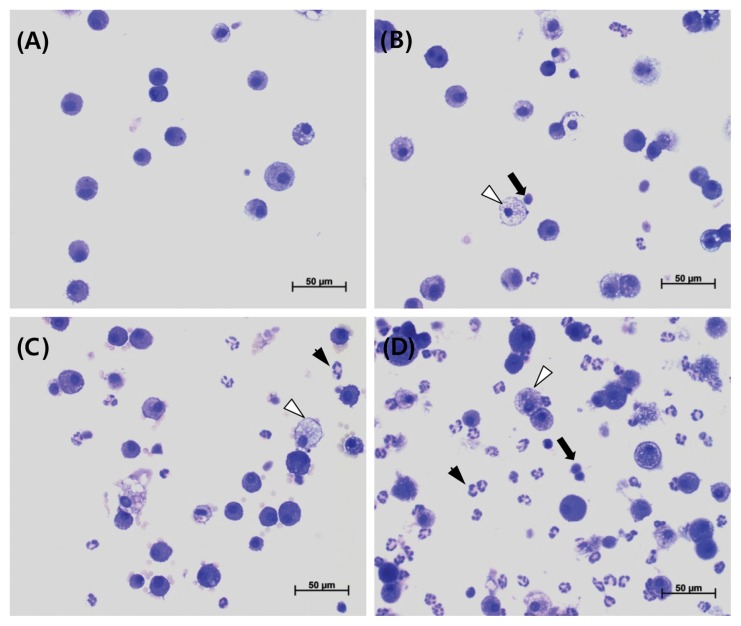
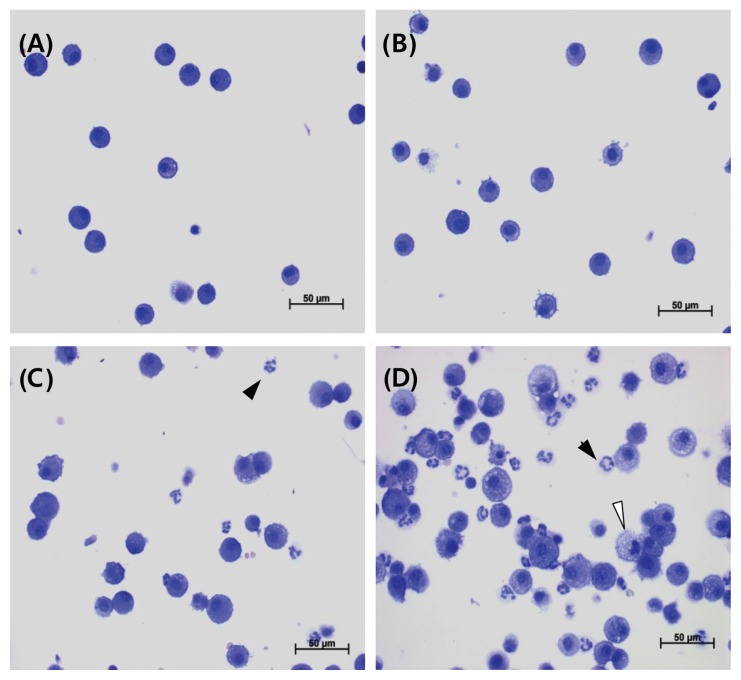
Results of BALF cellular analysis are presented in Table 10, Fig. 4 and 5. Compared with the control group, the total number of cells were significantly increased in the 1 and 5 mg/m3 groups during exposure, while polymorphonuclear leukocytes (PMNs) increased significantly in the 0.2, 1, and 5 mg/m3 groups. In addition, lymphocytes increased significantly in the 5 mg/m3 group during exposure. However, compared with the control group, only PMNs increased significantly in the 5 mg/m3 group during recovery.
Table 10.
Analysis of bronchoalveolar lavage fluid from rats treated with Al2O3 nanoparticles
| Parameter (after 28-day exposure) | Dose (mg/m3) | |||
|---|---|---|---|---|
|
| ||||
| 0 | 0.2 | 1 | 5 | |
| No. of animals | 8 | 8 | 8 | 8 |
| Total cells (103/μL) | 0.81 ± 0.26 | 0.91 ± 0.27 | 1.21 ± 0.28** | 2.53 ± 0.80** |
| Macrophages (103/μL) | 0.76 ± 0.24 | 0.82 ± 0.23 | 0.92 ± 0.32 | 0.70 ± 0.22 |
| Neutrophils (103/μL) | 0.02 ± 0.02 | 0.06 ± 0.04** | 0.25 ± 0.10** | 1.78 ± 0.65** |
| LDH (IU/L) | 22.03 ± 5.41 | 21.94 ± 5.47 | 34.96 ± 22.12 | 81.88 ± 37.02** |
| Albumin (mg/dL) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| IL-6 (pg/mL) | 126.4 ± 11.5 | 121.4 ± 10.2 | 128.9 ± 6.87 | 138.9 ± 6.0* |
| TNF-α (pg/mL) | 3.87 ± 2.71 | 2.92 ± 2.04 | 3.63 ± 1.70 | 9.26 ± 3.68** |
|
| ||||
| Parameter (after 28-day recovery) | Dose (mg/m3) | |||
|
| ||||
| 0 | 0.2 | 1 | 5 | |
|
| ||||
| No. of animals | 8 | 8 | 8 | 8 |
| Total cells (103/μL) | 1.28 ± 0.40 | 1.19 ± 0.32 | 1.29 ± 0.39 | 1.61 ± 0.27 |
| Macrophages (103/μL) | 1.19 ± 0.40 | 1.11 ± 0.32 | 1.15 ± 0.38 | 1.06 ± 0.25 |
| Neutrophils (103/μL) | 0.05 ± 0.04 | 0.05 ± 0.04 | 0.11 ± 0.11 | 0.51 ± 0.15** |
| LDH (IU/L) | 18.56 ± 8.19 | 19.94 ± 7.85 | 18.38 ± 9.00 | 38.28 ± 12.98** |
| Albumin (mg/dL) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| IL-6 (pg/mL) | 114.2 ± 10.0 | 110.9 ± 6.26 | 113.0 ± 5.94 | 121.4 ± 5.69* |
| TNF-α (pg/mL) | 3.02 ± 1.66 | 3.38 ± 1.22 | 2.42 ± 1.69 | 4.68 ± 1.76 |
LDH, lactate dehydrogenase; IL-6, interleukin 6; TNF-α, tumor necrosis factor-alpha.
Values are expressed as means ± SD.
Significantly different from vehicle control at p<0.05 and p<0.01.
Fig. 4.
Representative photographs of bronchoalveolar lavage fluids (BALF) from the control (A), 0.2mg/m3 (B), 1mg/m3 (C), and 5mg/m3 (D) in exposure groups stained with a Diff-Quick staining solution (400 ×). Lymphocyte infiltration (arrow), neutrophil infiltration (arrowhead), and morphological changes of alveolar macrophages (foamy; white arrowhead) were shown.
Fig. 5.
Representative photographs of bronchoalveolar lavage fluids (BALF) from the control (A), 0.2mg/m3 (B), 1mg/m3 (C), and 5mg/m3 (D) in recovery groups stained with a Diff-Quick staining solution (400 ×). Neutrophil infiltration (arrowhead), and morphological changes of alveolar macrophages (foamy; white arrowhead) were shown.
Results of the LDH, albumin, IL-6, and TNF-α levels in BALF supernatant are also presented in Table 10. Compared to the control group, LDH, IL-6, and TNF-α levels increased significantly in the 5 mg/m3 group during exposure. Similarly, LDH and IL-6 levels were significantly increased in the 5 mg/m3 group during recovery. Albumin was not detected in all groups during both exposure and recovery.
Oxidative stress analysis
There was no dose-dependent nature and/or statistical significance in catalase, GSH and TBARS detection (data not shown).
Aluminum content measurement
Aluminum content in the major organs and blood are presented in Table 11. Aluminum content in the lungs significantly increased in all treated groups during both exposure and recovery. However, it decreased during recovery by approximately 20, 14, and 19% in the 0.2, 1, and 5 mg/m3 groups, respectively. Aluminum content only increased significantly in the kidneys of the 0.2 mg/m3 group during exposure. No statistically significant changes were observed in the other groups.
Table 11.
Tissue content of aluminum from rats treated with Al2O3 nanoparticles
| Organ (after 28-day exposure) | Dose (mg/m3) | |||
|---|---|---|---|---|
|
| ||||
| 0 | 0.2 | 1 | 5 | |
| No. of animals examined | 8 | 8 | 8 | 8 |
| Lung (μg/g) | 0.01 ± 0.00 | 0.10 ± 0.02** | 0.59 ± 0.08** | 2.29 ± 0.24** |
| Brain (ng/g) | 6.63 ± 0.61 | 7.52 ± 1.89 | 7.02 ± 2.99 | 7.06 ± 0.94 |
| Liver (ng/g) | 4.67 ± 1.79 | 4.74 ± 0.92 | 5.09 ± 1.56 | 4.62 ± 1.05 |
| Spleen (ng/g) | 38.9 ± 16.4 | 36.0 ± 22.7 | 35.1 ± 4.12 | 39.0 ± 32.7 |
| Kidney (ng/g) | 4.22 ± 0.71 | 5.77 ± 0.74** | 5.11 ± 1.44 | 3.62 ± 0.38 |
| Whole blood (ng/g) | 4.13 ± 1.64 | 3.31 ± 4.02 | 3.80 ± 3.14 | 2.35 ± 0.49 |
|
| ||||
| Organ (after 28-day recovery) | Dose (mg/m3) | |||
|
| ||||
| 0 | 0.2 | 1 | 5 | |
|
| ||||
| No. of animals examined | 8 | 8 | 8 | 8 |
| Lung (μg/g) | 0.01 ± 0.00 | 0.08 ± 0.02** | 0.51 ± 0.11** | 1.85 ± 0.27** |
| Brain (ng/g) | 7.30 ± 2.12 | 8.62 ± 1.43 | 7.26 ± 2.28 | 5.92 ± 0.78 |
| Liver (ng/g) | 3.93 ± 0.69 | 4.51 ± 0.59 | 3.20 ± 1.13 | 5.70 ± 1.38 |
| Spleen (ng/g) | 35.9 ± 17.2 | 46.9 ± 6.72 | 28.8 ± 9.19 | 22.3 ± 3.13 |
| Kidney (ng/g) | 6.70 ± 1.53 | 9.40 ± 4.94 | 5.11 ± 2.75 | 5.24 ± 2.18 |
| Whole blood (ng/g) | 3.13 ± 2.44 | 2.41 ± 1.35 | 4.50 ± 4.22 | 3.08 ± 1.22 |
Values are expressed as means ± SD.
Significantly different from vehicle control at p<0.01.
DISCUSSION
As a result of their specific physiochemical properties, nanomaterial applications are increasing. Concerns regarding the health and environmental effects of these nanomaterials are consequently increasing, particularly for workers handling nanomaterials. Al2O3 NPs are among the most widely used nanomaterials (5); however, limited information is available regarding their risk identification and assessment, including inhalation toxicology data. In this study, we evaluated the inhalation toxicity of Al2O3 NPs using a nose-only inhalation system in SD rats. Exposure conditions to Al2O3 NPs were in accordance with the OECD test guidelines for 28-day inhalation toxicity studies (12), with exposure concentrations selected based on the results of a previous inhalation study of metal nanomaterials that included rare earth metals and Al2O3 (10,11). Male rats were preferentially used since men primarily work in environments prone to nanomaterial exposure.
There were no clinical symptoms or abnormal body weight changes related Al2O3 NP exposure during the experimental period. In addition, hematology and serum biochemistry results showed that there were no systemic effects related to the exposure to Al2O3 NPs. Kwon et al. showed similar results in SD rats, although the exposure to Al2O3 NPs was through intratracheal instillation (7). Although no systemic effects were observed in the present study after Al2O3 NP exposure, including body weight loss and clinical symptoms, the toxicological effects related to Al2O3 NPs exposure were mainly observed in the lungs. More specifically, this was reflected by an increase in the absolute and relative lung weights in the 5 mg/m3 group during exposure. This was in contrast to a previous study where intratracheal instillation of Al2O3 NPs induced no changes in organ weight (7).
In the BALF analysis, we observed a significant increase in total cell counts, PMNs, and lymphocytes during exposure, whereas only PMNs increased during recovery. Kwon et al. similarly reported an increase in the counts of total cells, PMNs, and lymphocytes after intratracheal instillation of Al2O3 NPs at 1, 20, and 40 mg/kg (7). The levels of LDH, IL-6, and TNF-α increased significantly in the 5 mg/m3 group during exposure; additionally, LDH and IL-6 concentrations increased significantly in the 5 mg/m3 group during recovery. By contrast, Kwon et al. (7) showed there were no significant changes in LDH, IL-6, and TNF-α levels in BALF, although they tended to increase in the middle and/or high dose-exposed groups. These opposing results might be attributed to the difference in the amount and/or particle size of Al2O3 NPs that reach deep into the lungs via inhalation compared to tracheal instillation.
Aluminum content in the major organs were determined after the 28-day exposure and subsequent 28-day recovery periods. Most of the inhaled aluminum was deposited in the lungs after the 28-day exposure, with a significant increase in the levels that showed a dose-dependent manner. However, aluminum was not significantly deposited in other organs, except for the kidneys in the 0.2 mg/m3 exposure group.
Zhang et al. (13) measured aluminum contents in the brain (cortex and midbrain) and lungs of mice following exposure to Al2O3 NPs for 28 consecutive days at a concentration of 0.5 mg/m3. Aluminum levels in the cortex and lungs were significantly increased; however, levels in the midbrain did not change. Direct comparison with aluminum levels measured in our study was not possible owing to the limited results of the previous study and different animal species being used. It is postulated that NPs may enter the bloodstream via the lungs, and aluminum might directly enter the brain from the nasal cavity, bypassing the systemic circulation; however, convincing evidence is lacking (1,14,15). Aluminum is poorly absorbed following inhalation, and approximately 1.5~2% of inhaled aluminum is absorbed; however, absorption efficiency is dependent on the chemical form and particle size. Aluminum binds to various ligands in the blood and thereby distributes to every organ, with the highest concentrations ultimately found in the bones and lungs. Absorbed aluminum is principally excreted in the urine and, to a lesser extent, in the bile (16).
Pathological findings showed marked alveolar macrophage accumulation in the lungs in the 5 mg/m3 exposure group. Importantly, the severity of these lesions was found to increase during recovery. This is in line with the results of our previous study, using nanosized metals, including lanthanum oxide and neodymium oxide (10,11). It is believed that this change is caused by a constantly stimulated immune response to foreign metals as a result of slow test material elimination. Nanomaterials could thereby damage the immune system and cause pathological changes (17).
NP toxicity may be induced by reactive oxygen species (ROS) generation, oxidative stress, DNA damage, inflammation, and cell death (3,18). In this study, we measured GSH, catalase, and TBARS in lung tissues; however, no Al2O3 NP-related changes were observed.
In this study, 28-day repeated inhalation exposure to 5 mg/m3 Al2O3 NPs in male rats increased lung weight and resulted in histopathological changes, including alveolar macrophage accumulation, increased total cell counts and neutrophils, as well as increased levels of IL-6, TNF-α, and LDH in BALF. After a 28-day recovery period, these changes generally recovered, except for the pathological changes. Under the present experimental conditions, the NOAEL of Al2O3 NPs in male rats was 1 mg/m3. Additionally, the target organ was the lung; however, further toxicity and kinetic studies are required for a precise assessment of the health risks.
Footnotes
CONFLICT OF INTEREST
Declaration of interests.
REFERENCES
- 1.Krewski D, Yokel RA, Nieboer E, Borchelt D, Cohen J, Harry J, Kacew S, Lindsay J, Mahfouz AM, Rondeau V. Human health. risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. J Toxicol Environ Health B Crit Rev. 2007;10:1–269. doi: 10.1080/10937400701597766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services Public Health Services Agency for Toxic Substances and Disease Registry. Aluminum. 2009:34–35. Available from: http://www.Atsdr.cdc.gov/pdns/pdfs/pdn_doc_22.pdf/
- 3.Li X, Zhang C, Zhang X, Wang S, Meng Q, Wu S, Yang H, Xia Y, Chen R. An acetyl-L-carnitine switch on mitochondrial dysfunction and rescue in the metabolomics study on aluminum oxide nanoparticles. Part Fibre Toxicol. 2016;13:4. doi: 10.1186/s12989-016-0115-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rai A, Park K, Zhou L, Zachariah MR. Understanding the mechanism of aluminium nanoparticle oxidation. Combust Theor Model. 2006;10:843–859. doi: 10.1080/13647830600800686. [DOI] [Google Scholar]
- 5.Prakash FA, Babu GJD, Lavanya M, Vidhya KS, Devasena T. Toxicity studies of aluminium oxide nanoparticles in cell lines. Int J Nanotechnol Appl. 2011;5:99–107. [Google Scholar]
- 6.Shirai T, Watanabe H, Fuji M, Takahashi M. Structural properties and surfaces characteristics on aluminum oxide powders. Annual Report of the Ceramics Research Laboratory Nagoya Institute of Technology. 2009;9:23–31. [Google Scholar]
- 7.Kwon JT, Seo GB, Lee MM, Kim HM, Shim IS, Jo EH, Kim PJ, Choi KH. Pulmonary toxicity assessment of aluminum oxide nanoparticles via nasal instillation exposure. J Environ Health Sci. 2013;39:48–55. [Google Scholar]
- 8.Jederlinic PJ, Abraham JL, Churg A, Himmelstein JS, Epler GR, Gaensier EA. Pulmonary fibrosis in aluminum oxide workers. Investigation of nine workers, with pathologic examination and microanalysis in three of them. Am Rev Respir Dis. 1990;142:1179–1184. doi: 10.1164/ajrccm/142.5.1179. [DOI] [PubMed] [Google Scholar]
- 9.Raj KJA, Viswanathan B. Effect of surface area, pore volume and particle size of P25 titania on the phase transformation of anatase to retile. Indian J Chem. 2009;48A:1378–1382. [Google Scholar]
- 10.Shin SH, Lim CH, Kim YS, Lee YH, Kim SH, Kim JC. Twenty-eight-day repeated inhalation toxicity study of nano-sized lanthanum oxide in male Sprague- Dawley rats. Environ Toxicol. 2017;32:1226–1240. doi: 10.1002/tox.22319. [DOI] [PubMed] [Google Scholar]
- 11.Kim YS, Lim CH, Shin SH, Kim JC. Twenty-eight-day repeated inhalation toxicity study of nanosized neodymium oxide in male Sprague-Dawley rats. Toxicol Res. 2017;33:239–253. doi: 10.5487/TR.2017.33.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.OECD. Subacute Inhalation Toxicity: 28-Day Study. OECD; Paris: 2009. Guidelines for Testing of Chemicals. Test Guideline 412. [Google Scholar]
- 13.Zhang X, Xu Y, Zhou L, Zhang C, Meng Q, Wu S, Wang S, Ding Z, Chen X, Li X, Chen R. sex-dependent depression-like behavior induced by respiratory administration of aluminum oxide nanoparticles. Int J Environ Res Public Health. 2015;12:15692–15705. doi: 10.3390/ijerph121215011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pauluhn J. Pulmonary toxicity and fate of agglomerated 10 and 40 nm aluminum oxyhydroxides following 4-week inhalation exposure of rats: toxic effects are determined by agglomerated, not primary particle size. Toxicol Sci. 2009;109:152–167. doi: 10.1093/toxsci/kfp046. [DOI] [PubMed] [Google Scholar]
- 15.Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arab J Chem. 2017 doi: 10.1016/j.arabjc.2017.05.011. [DOI] [Google Scholar]
- 16.ToxGuide™ for Aluminum. U.S. Department of Health and Human Services Public Health Service Agency for Toxic Substances and Disease Registry; Atlanta, GA: 2011. [Google Scholar]
- 17.Luo YH, Chang LW, Lin P. Metal-based nanoparticles and the immune system: activation, inflammation, and potential applications. Biomed Res Int. 2015;2015:143720. doi: 10.1155/2015/143720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khanna P, Ong C, Bay BH, Baeg GH. Nanotoxicity: an interplay of oxidative stress, inflammation and cell death. Nanomaterials. 2015;5:1163–1180. doi: 10.3390/nano5031163. [DOI] [PMC free article] [PubMed] [Google Scholar]