Abstract
Exposure to chemical agents is an inevitable consequence of modern society; some of these agents are hazardous to human health. The effects of chemical carcinogens are of great concern in many countries, and international organizations, such as the World Health Organization, have established guidelines for the regulation of these chemicals. Carcinogens are currently categorized into two classes, genotoxic and non-genotoxic carcinogens, which are subject to different regulatory policies. Genotoxic carcinogens are chemicals that exert carcinogenicity via the induction of mutations. Owing to their DNA interaction properties, there is thought to be no safe exposure threshold or dose. Genotoxic carcinogens are regulated under the assumption that they pose a cancer risk for humans, even at very low doses. In contrast, non-genotoxic carcinogens, which induce cancer through mechanisms other than mutations, such as hormonal effects, cytotoxicity, cell proliferation, or epigenetic changes, are thought to have a safe exposure threshold or dose; thus, their use in society is permitted unless the exposure or intake level would exceed the threshold. Genotoxicity assays are an important method to distinguish the two classes of carcinogens. However, some carcinogens have negative results in in vitro bacterial mutation assays, but yield positive results in the in vivo transgenic rodent gene mutation assay. Non-DNA damage, such as spindle poison or topoisomerase inhibition, often leads to positive results in cytogenetic genotoxicity assays such as the chromosome aberration assay or the micronucleus assay. Therefore, mechanistic considerations of tumor induction, based on the results of the genotoxicity assays, are necessary to distinguish genotoxic and non-genotoxic carcinogens. In this review, the concept of threshold of toxicological concern is introduced and the potential risk from multiple exposures to low doses of genotoxic carcinogens is also discussed.
Keywords: Genotoxic carcinogens, Non-genotoxic carcinogens, Threshold, Threshold of toxicological concern, TTC
INTRODUCTION
“The dose makes the poison” is a basic principle of toxicology. Coined by Paracelsus, who was a 15th century Swiss scientist, physician, alchemist, and mysterious thinker (https://en.wikipedia.org/wiki/The_dose_makes_the_poison), he is known as “the father of toxicology” because of this famous phrase. The adage means that any chemical can be poison if the dose is beyond a certain threshold and also that any poison can be non-toxic if the dose is below a certain threshold. Indeed, the aim of toxicology is to find the appropriate threshold or safe level of a chemical below which no hazardous effects to humans are thought to result (Fig. 1). For example, chemicals developed for food additives, pesticides, or veterinary drugs are all subject to toxicology assays before marketing; from these assays, the threshold level, that is, the acceptable daily intake (ADI), is determined by the authorities based on the no observed adverse effect level (NOAEL) and the safety factor, which is usually 100 (= 10 × 10), reflecting species difference between rodents and humans (10-fold) and individual variations in humans (10-fold) (1). The ADI is the daily intake level below which no adverse effects are estimated to occur, even if a person were to take the chemical for their entire life. The NOAEL is the highest dose in toxicological assays at which no significant adverse effects can be observed. The use of chemicals in society is permitted if the intake level is below the ADI. The concept underlying this risk management approach is exactly the principle established by Paracelsus: any poison can be non-toxic if the dose is below the appropriate threshold.
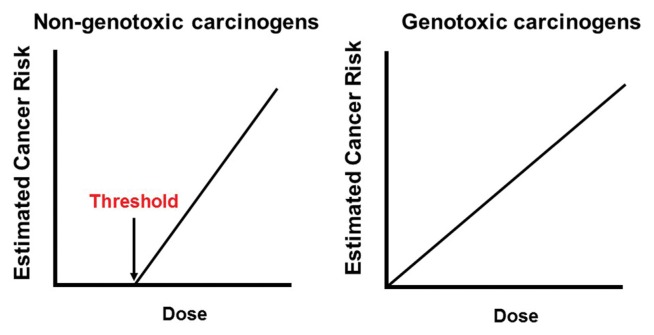
Fig. 1.
Models for dose-response curves of non-genotoxic and genotoxic carcinogens. Non-genotoxic carcinogens like as other toxic chemicals have threshold while genotoxic carcinogens have no threshold. Non-genotoxic carcinogens can be used in the society if the intake level is below the threshold. Genotoxic carcinogens are supposed to have carcinogenic risk even at very low doses. Therefore, genotoxic carcinogens are generally not be considered acceptable for use as food additives, pesticides or veterinary drugs.
The principle of Paracelsus cannot be applied to the regulation of genotoxic chemicals. Genotoxic chemicals are substances that interact with DNA and may subsequently induce mutations. Owing to their DNA interaction properties, genotoxic chemicals are not considered to have a safe threshold or dose (2–4). Therefore, they are expected to impose genotoxic and carcinogenic risks on humans, even at very low concentrations. This assumption is similar to the regulatory policy for radiation, which generally employs a linear non-threshold model (5). Genotoxic chemicals, like radiation, induce DNA damage and mutations that may lead to cancer; indeed, genotoxic chemicals used to be called “radiomimetic substances” owing to their DNA interaction properties (6) and it is therefore unsurprising that the regulatory policies are similar. Strict regulatory policies for genotoxic chemicals are globally accepted. The Environmental Health Criteria set by the World Health Organization (WHO) state that “substances that are both genotoxic and carcinogenic would generally not be considered acceptable for use as food additives, pesticides or veterinary drugs. For those substances that are genotoxic and carcinogenic, the traditional assumption is that there may not be a threshold dose and that some degree of risk may exist at any level of exposure” (7). Therefore, once a chemical is judged as genotoxic and carcinogenic, it will be banned for use as a food additive, pesticide, or veterinary drug. This is contrast to the policy for non-genotoxic carcinogens, which may be used in the market if the intake level is below the ADI (8). Thus, the ability to distinguish genotoxic and non-genotoxic is of critical importance in the regulation of chemicals.
WHAT ARE GENOTOXIC AND NON-GENOTOXIC CARCINOGENS?
The term “genotoxic carcinogens” was coined in the late 1980s based on the results of the United States National Toxicology Program (NTP) (9). In the program, chemicals were evaluated for their DNA reactivity, mutagenicity in Salmonella (Ames test), and carcinogenicity in rodents. Of the 222 chemicals tested, 115 were carcinogens; 71 of these 115 (62%) were DNA reactive (structure alert) positive and Salmonella (Ames test) positive. The remaining 44 (38%) were carcinogens, but were structure alert negative and Salmonella (Ames test) negative. The former group carcinogens was carcinogenic in rats and mice (trans-species carcinogens) and induced tumors in multiple organs in rodents. In contrast, the latter group of carcinogens was carcinogenic in either rats or mice (single-species carcinogens) and induced tumors in single organs, in particular in the liver of mice. The report clearly indicated that rodent carcinogens are not all equal and can be categorized into two classes: the former, genotoxic carcinogens, and the latter, non-genotoxic carcinogens (Table 1) (10). It is widely recognized that genotoxic carcinogens, such as benzo[a]pyrene and aflatoxin B1, induce tumors via DNA damage and mutations, whereas non-genotoxic carcinogens, such as phenobarbital, carbon tetrachloride, or diethylstilbestrol, induce tumors via mechanisms other than DNA damage, including cell proliferation, cytotoxicity, or hormonal effects (11–13). In practice, it is not easy to identify clear distinctions, because some genotoxicity assays, such as the chromosome aberration assay or the micronucleus assay, give positive results even in the absence of DNA damage (14–16). For example, aphidicolin, an inhibitor of DNA polymerases, and colchicine, a spindle poison, were shown to have a threshold to their clastogenicity (17–19). These chemicals do not interact with DNA, but instead inhibit functions of proteins involved in DNA replication or cell division. Therefore, it is important to consider the type of damage, either DNA damage or protein damage, which is responsible for the positive results in the genotoxicity assay.
Table 1.
Comparison of genotoxic and non-genotoxic carcinogens
| Genotoxic carcinogens | Non-genotoxic carcinogens |
|---|---|
| Carcinogens that directly interact with DNA | Carcinogens that indirectly affect structures of DNA or gene expression. They promote carcinogenesis through a variety of mechanisms, e.g., cell proliferation, cytotoxicity, hormonal effects or DNA methylation. |
| Ames test + Structural alert + | Ames test − Structural alert − |
| Carcinogenic in both rats and mice and carcinogenic in more than one organ | Carcinogenic in single species and single organ in rodents |
GENE MUTATION ASSAYS ARE CRITICAL TO DISTINGUISH GENOTOXIC AND NON-GENOTOXIC CARCINOGENS
The genotoxicity of chemicals is usually evaluated by multiple assays, including gene mutation assays and cytogenetic assays (Table 2). In vitro gene mutation assays include the bacterial reverse mutation assay (Ames test, the mammalian gene mutation assays, and the transgenic rodent gene mutation assay in vivo. These assays detect genotoxicity based on DNA damage. Positive chemicals in these assays can be considered as DNA-reactive genotoxic chemicals, which have no safe threshold. Conversely, cytogenetic assays, including the chromosome aberration assay in cultured mammalian cells or human lymphocytes in vitro and the micronucleus assay in vitro and in vivo, detect genotoxicity not only by DNA damage, but also by other mechanisms, such as topoisomerase inhibition, spindle poison, or excessive cytotoxicity (16,20–22). In general, Ames-positive and transgenic-positive chemicals can be regarded as DNA-reactive in vivo genotoxic chemicals. The importance of gene mutation assays in the assessment of carcinogenic risk at low doses is emphasized in the ICH M7 guideline (https://www.pmda.go.jp/files/000208234.pdf). The International Council for Harmonization (ICH) Technical Requirements for Pharmaceuticals for Human Use is an international organization for the establishment of guidelines for pharmaceuticals. M7 is the ICH guidelines for the assessment and control of DNA reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk. In Section 3 (general principles), it is stated that “the focus of this guideline is on DNA reactive substances that have a potential to directly cause DNA damage when present at low levels leading to mutations and therefore, potentially causing cancer. This type of mutagenic carcinogen is usually detected in a bacterial reverse mutation (mutagenicity) assay. Other types of genotoxicants that are non-mutagenic typically have threshold mechanisms and usually do not pose carcinogenic risk in humans at the level ordinarily present as impurities”. In Section 6 (hazard assessment elements), it is stated that “a positive bacterial mutagenicity result would warrant further hazard assessment and/or control measures. For instance, when levels of the impurity cannot be controlled at an appropriate acceptable limit, it is recommended that the impurity be tested in an in vivo gene mutation assay in order to understand the relevance of the bacterial mutagenicity assay result under in vivo conditions”. Thus, in vitro and in vivo gene mutation assays are of critical importance for the risk assessment of low doses of genotoxic chemicals, because chemicals with positive results in these assays should be considered to have no safe threshold.
Table 2.
Representative genotoxicity assays
| In vitro | In vivo | |
|---|---|---|
| Gene mutation assays | Bacterial reverse mutation assay (Ames test) (TG471) | Transgenic rodent gene mutation assay (TG488) |
| Mammalian gene mutation assay (TG476; 490) | ||
| Cytogenetic assays | Chromosome aberration assay (TG473) | Chromosome aberration assay (TG475) |
| Micronucleus assay (TG487) | Micronucleus assay (TG474) |
TG numbers represent numbers of test guidelines established by OECD (https://www.oecd-ilibrary.org).
Although it is neither gene mutation assay nor cytogenetic assay, OECD test guideline (TG489) has been established for in vivo comet assay, which detects DNA strand breaks.
IN VITRO GENE MUTATION ASSAY: BACTERIAL REVERSE MUTATION ASSAY
The bacterial reverse mutation assay is a representative in vitro gene mutation assays (https://www.oecd-ilibrary.org/docserver/9789264071247-en.pdf?expires=1529937092&id=id&accname=guest&checksum=7B88AFDD99C1C8A18CF725E5539CF272). In general, the assay uses four Salmonella typhimurium strains and one Escherichia coli strain to detect a variety of point mutations. This assay is called Ames test, because Dr. Bruce N. Ames developed it using Salmonella strains (23). It is a simple in vitro assay to determine to what extent histidine-dependent bacteria become independent by gene mutations induced by chemicals. In the case of E. coli, tryptophan-dependent bacteria become independent; because the phenotype reverts from histidine- or tryptophan-dependent to independent, this is called a reverse mutation assay. In practice, the bacterial culture is mixed with a test chemical and the mixture is incubated for two days on agar plates. If metabolic activation is needed, the 9,000 × g supernatant of liver homogenates of rats pretreated with inducers of drug metabolizing enzymes plus an NADPH-generating system (S9 mix) is added to the reaction mixture. After incubation, the number of revertant colonies is counted and the dose-response curves are produced. In the assay, different types of point mutations, such as base substitutions and frameshifts, are detected by using distinct bacterial tester strains. This assay detects only DNA reactive genotoxic chemicals; chemicals with a positive result in this assay usually have structural alert for reaction with DNA (24).
IN VIVO GENE MUTATION ASSAY: GPT DELTA TRANSGENIC MOUSE/RAT GENE MUTATION ASSAY
This assay can detect point mutations and deletions in various organs of rodents (https://www.oecd-ilibrary.org/docserver/9789264203907-en.pdf?expires=1529937241&id=id&accname=guest&checksum=3243A61DDEF495D57925 DB655E2F379F). The transgenic rodents have been established in C57BL/6 mice and Fisher 344, Sprague-Dawley, and Wistar Hannover rats (25). These transgenic rodents have reporter genes for mutations in all cells in all organs (26–28). After treatment of the rodents with test chemicals, the transgene, i.e., lambda EG10, is rescued as phage particles from various organs by in vitro packaging reactions and mutations are detected by infection of the rescued phages to E. coli strains. Point mutations and deletions can be detected by gpt and Spi− assay, respectively, with different bacterial strains. As this assay detects mutations in all organs of rodents, it is possible to examine the mutagenicity of the chemical carcinogens in the target organs for carcinogenesis. Approximately 20 chemicals, most of which are carcinogenic to rodents, have been examined by using gpt delta mice or rats to determine their food safety (29). The results showed that estragole, madder color, and methyleugenol yielded positive results in the transgenic assays and were therefore judged to be genotoxic carcinogens (30–32). In contrast, citrinin, flumequine, ginko biloba extract, and 3-monochloropropane-1,2-diol esters yielded negative results in the target organs for carcinogenicity and were therefore judged as non-genotoxic carcinogens (33–36). Therefore, gpt delta transgenic rodent gene mutation assays are able to effectively distinguish genotoxic and non-genotoxic carcinogens.
AMES-NEGATIVE, BUT TRANSGENIC ASSAY-POSITIVE CARCINOGENS: ARE THEY GENOTOXIC CARCINOGENS?
Although it appears simple to distinguish genotoxic and non-genotoxic carcinogens, the reality is more complex. Some rodent carcinogens yield negative results in the bacterial reverse mutation assay, but positive results in the gpt delta rodent gene mutation assay in the target organs for carcinogenesis. The following are examples for which the judgement of genotoxic or non-genotoxic carcinogens is difficult, even with in vitro and in vivo gene mutation assays (Table 3).
Table 3.
Examples of carcinogens that are non-mutagenic in vitro but mutagenic in vivo
| Chemical | Use or property | Carcinogenicity | In vitro mutagenicityc | In vivo mutagenicityd |
|---|---|---|---|---|
| Estragole | Fragrance | Positivea | Negative | Positivee |
| Leucomalachite green | Metabolite of malachite green, antifungal agent for fish | Positivea | Negative | Positivee |
| Dicyclanil | Insect growth regulator | Positivea | Negative | Positivee |
| Ochratoxin A | Mycotoxin | Positiveb | Negative | Positivef |
Liver tumors are induced in female mice.
Kidney tumors are induced in male and female rats.
Bacterial reverse mutation assay.
Transgenic gene mutation assay.
Gene mutations at the gpt or cII genes were induced in liver of female mice.
Deletion mutation was induced in outer medulla of kidney of male rats.
The first example is estragole, a fragrant herb, which induces liver tumors (hepatoma) in female mice (37). This chemical yields a negative result in the bacterial reverse mutation assay (38,39). To examine the genotoxicity in mice, male and female gpt delta mice were fed estragole for 13 weeks by gavage and micronucleus in bone marrow, gpt gene mutation status, and DNA adducts in liver were examined (40). Although the in vivo micronucleus assay was negative, gpt mutation frequency was clearly enhanced in liver in female mice. DNA adduct levels were also increased and higher in female than in male mice. Estragole is hydroxylated on its side chain and further activated by sulfotransferase (41). The activity of this enzyme is known to be higher in female mice than in male mice (42). The results indicate that estragole was a DNA-reactive genotoxic carcinogen.
The second example is leucomalachite green, a reductive metabolite of malachite green, which is an antifungal agent for fish (43). Leucomalachite green induces liver tumors in female mice (44). Malachite green and leucomalachite green yield negative results in the bacterial reverse mutation assay (45). However, it was reported by the US FDA that leucomalachite green induced gene mutations in the liver of female mice when administered in the diet for 16 weeks in Big Blue mice, another transgenic rodent model used for mutation detection (46). No mutagenicity was detected in liver of Big Blue rats (47). DNA adducts were also detected in the liver of female mice. Although malachite green did not induce tumors and genotoxicity in rats and mice, it induced DNA adducts in the liver of male rats and female mice (48). Therefore, the European Food Safety Authority (EFSA) concluded that malachite green and leucomalachite green should be regarded as genotoxic carcinogens (49); however, it remains to be clarified why leucomalachite green yields negative results in all in vitro genotoxicity assays, including the bacterial reverse mutation assay (45).
The third example is dicyclanil, which is used to regulate the growth of insects. It induces adenoma and adenocarcinoma in the liver of female mice (50,51). This chemical yields negative results in various genotoxicity assays, including in vitro assays such as the bacterial reverse mutation assay and in vivo assays such as the micronucleus assay and comet assay. Therefore, it was regarded as a nongenotoxic carcinogen (52). However, dicyclanil induces gpt gene mutations in the liver of female mice when administered in the diet for 13 weeks (51). No mutations were detected in the liver of male mice. 8-Hydroxy-guanine, an index of oxidative DNA damage, was increased in the liver of both male and female mice, but cell proliferation was increased only in female mice. Therefore, the induction of oxidative DNA damage and enhanced cell proliferation may be the reason for female-specific mutations induced by dicyclanil. However, it is unclear why this chemical is negative in bacterial reverse mutation assay and whether carcinogens that induce oxidative DNA damage via the generation of reactive oxygen species should be regarded as DNA reactive genotoxic carcinogens.
The fourth example is ochratoxin A, which is a mycotoxin that induces adenoma and adenocarcinoma in the kidney of rodents (53). It is regarded as a causative agent of Balkan epidemic disease in humans (54). Thus, ochratoxin A is classified into group 2B (possible human carcinogen) by International Agency for Research on Cancer (IARC) (55). Ochratoxin A yields a negative result in the bacterial reverse mutation assay, but a mixture of positive and negative results have been reported in chromosome aberration and micronucleus assays in vitro and in vivo (55). Hibi et al. reported that the frequency of mutation of Spi− mutant was significantly increased in the outer medulla of the kidney when gpt delta rats were fed ochratoxin A in their diet for 4 weeks (56). The outer medulla includes the target site for carcinogenesis, i.e., the S3 segment of proximal convoluted tubule. Interestingly, no increase in gpt mutant frequency was observed in the cortex or outer medulla. Because Spi− selection detects deletion mutations, increase in Spi− mutant frequency indicates that DNA strand breaks were induced in the target site for carcinogenesis (25,57). However, if DNA adducts were induced by ochratoxin A in the target site, gpt mutant frequency should have been increased in addition to Spi− mutant frequency. Therefore, ochratoxin A may inhibit functions of proteins involved in cell cycle or DNA repair and induce DNA strand breaks. Ochratoxin A may be a non-genotoxic carcinogen.
PRACTICAL THRESHOLDS OF GENOTOXIC CARCINOGENS
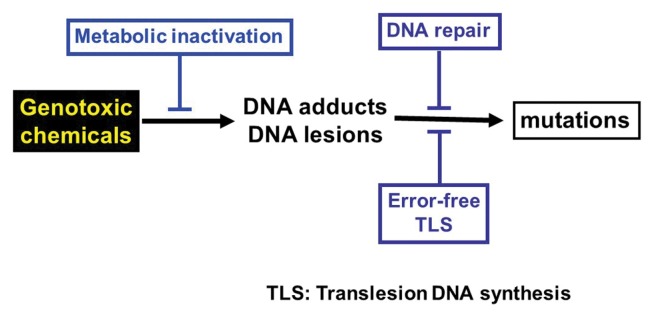
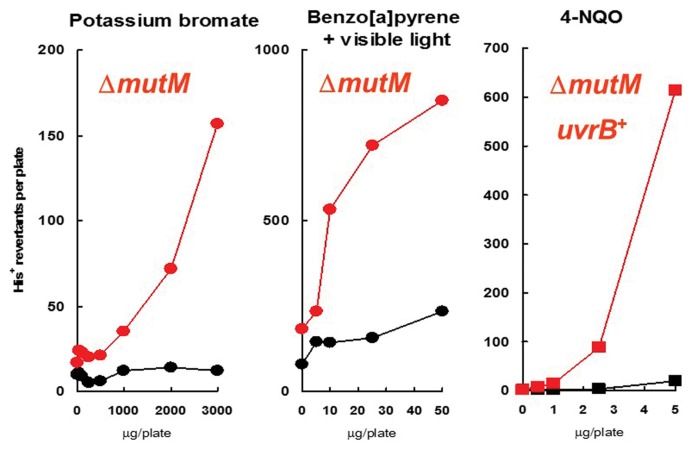
It was previously discussed the regulatory policy that states there are no safe exposure thresholds for genotoxic carcinogens. However, this policy was recently challenged by a number of experimental and theoretical approaches that claim that even DNA-reactive genotoxic carcinogens may have practical threshold for their action (58–61). Indeed, the consideration of the mechanisms through which a chemical induces mutation and cancer, there are several steps that may suppress the induction of mutation and cancer (62). Genotoxic compounds are metabolically activated to reactive intermediates that induce DNA adducts and DNA lesions; subsequently, the DNA lesions become mutations after DNA replication. To counteract this adverse pathway, humans and other organisms have self-defense mechanisms such as antioxidants, metabolic inactivation mechanisms, DNA repair or error-free translesion DNA synthesis (TLS) (Fig. 2). Detoxification mechanisms inactivate the genotoxic compounds, DNA repair removes the DNA adducts and error-free translesion synthesis incorporates the correct base opposite DNA lesion during DNA synthesis, thereby suppressing the induction of mutations. From mutations to cancer, there are other mechanisms, such as apoptosis, that suppress the induction of cancer. These self-defense mechanisms may constitute a practical threshold for genotoxic carcinogens. In an examination of this possibility, the DNA repair enzyme 8-hydroxyguanine DNA glycosylase encoded by the mutM gene in Salmonella typhimurium was disrupted (63). This enzyme repairs 8-hydroxyguanine in DNA and reduces G:C to T:A mutation. In fact, the enzyme-deficient strains exhibited a much greater sensitivity to the mutagenicity of oxidative mutagens than the enzyme-proficient strains (Fig. 3) (63,64). In particular, potassium bromate tested virtually negative for mutagenicity in the enzyme-proficient strain, whereas it exhibited high mutagenicity in the deficient strain. Therefore, 8-hydroxyguanine DNA glycosylase appears to be a constituent of practical thresholds for oxidative mutagens.
Fig. 2.
Self-defense mechanisms against genotoxic chemicals. Genotoxic chemicals may be inactivated by metabolic inactivation. When DNA adducts are formed, the adducts may be removed by DNA repair mechanisms. If the adducts remain in DNA, error-free translesion DNA synthesis (TLS) will incorporate correct dNTPs against the lesions, thereby suppressing induction of mutations.
Fig. 3.
Dose response curves of potassium bromate, benzo[a]pyrene plus visible light and 4-nitroquinoline-1-oxide (4-NQO). Closed black circles, Salmonella typhimurium TA1535; closed red circles, YG3001 (same as TA1535 but ΔmutM); closed black squares, TA1975 (same as TA1535 but uvrB+); closed red squares YG3003 (same as TA1975 but ΔmutM). When the mutagenicity of benzo[a]pyrene in the presence of visible light, plates were exposed to fluorescent light 15 W lamps at a distance of 30 cm during incubation at 37°C for two to three days. The data are from references (63,64).
THRESHOLD OF TOXICOLOGICAL CONCERN (TTC) OF GENOTOXIC CARCINOGENS
Another challenge to the regulatory policy is that DNA-reactive genotoxic carcinogens have no threshold is the concept of “threshold of toxicological concern” (TTC) or “threshold of regulation” (TOR) (65). Essentially, the underlying concept of TTC or TOR is that it is impossible to completely suppress the excess lifetime cancer risk associated with chemical exposure and that there is an increasing number of new chemicals for which there is insufficient toxicological information. Therefore, TTC or TOR are applied to prioritize the chemicals that need further toxicological evaluation. In 1995, US FDA adopted a TOR of 0.5 ppb for food contact materials (corresponding to 0.025 μg/kg body weight (bw)/day or 1.5 μg/person/day, based on 60 kg bw and combined food and drink daily consumption of 3 kg) if the chemical has no concern for DNA-reactive genotoxicity (66). In other words, the chemical can be used in the market without additional toxicity assays if the intake level is below 0.5 ppb or 1.5 μg/person/day and there is no concern that the chemical will have DNA-reactive genotoxicity. Later, ICH M7 guidance proposed a TTC for pharmaceutical impurities, in which an intake level below 1.5 μg/person/day does not increase excess lifetime cancer risk more than over 10−5, even when there is a concern that the impurity may have DNA-reactive genotoxicity (https://www.pmda.go.jp/files/000208234.pdf); however, the guidance indicates that highly potent DNA-reactive carcinogens, such as aflatoxinlike, azoxy- or N-nitroso-carcinogens, are outside of the application of the TTC approach. These exceptional chemicals are sometimes called the “cohort of concern” (COC) (67). Both the US FDA and ICH adopt the same value of 1.5 μg/person/day as the TOR and TTC; however, the former excludes DNA-reactive genotoxic chemicals while the latter includes them. The differences in the policy may depend on the usage of the chemicals. Food contact materials do not necessarily provide health benefits to consumers, and therefore the policy is more conservative or strict, whereas pharmaceuticals may be needed to maintain or improve health conditions of patients, even if the drugs contain some DNA-reactive impurities. In fact, EFSA/WHO proposed a level 10 times lower, i.e., 0.15 μg/person/day or 0.0025 μg/kg bw/day, than that proposed by ICH as a sufficiently protective TTC for DNA-reactive genotoxic chemicals (67,68). The COC chemicals are also excluded from the TTC approach. Currently, the use of the TTC approach has been established to regulate chemicals in several areas, such as food contact materials, food flavoring agents, and pharmaceutical impurities (65). A TTC approach for non-genotoxic carcinogens was also proposed (67,69). It should be noted, however, that the TTC approach is not an alternative to a chemical-specific risk assessment, but a screening tool to decide whether further toxicological evaluation is necessary for the chemical (68).
FUTURE CHALLENGE: RISK ESTIMATION OF COMBINED EXPOSURE OF GENOTOXIC CARCINOGENS AT LOW DOSES
Following the emergence of the TTC approach in several areas of chemical regulation, questions have been raised as to whether public is adequately protected from multiple exposure or intake of DNA-reactive genotoxic carcinogens at low doses. The current regulatory policy for chemicals is the evaluation of the genotoxic and carcinogenic risk individually. Moreover, TTC is not an absolute threshold and thus some low level of cancer risk, e.g., 10−5 or 10−6, exists, even below TTC. This is in contrast to the indication of an absolute threshold below which there is no risk on human health (19). Therefore, it is suspected that detectable carcinogenic risk may appear when people are exposed to multiple DNA-reactive genotoxic carcinogens, even below the TTC. It is reported that mutagenicity in Salmonella typhimurium strains was detectable when six DNA-reactive genotoxic carcinogens were combined at quite low doses (70). Each single carcinogen did no exhibit any detectable mutagenicity owing to the low dose. Although this is a simple additive effect, synergistic effects may occur, depending on the combination of chemicals. Although chemicals are regulated by different authorities depending on their intended use, e.g., food-related chemicals, industrial chemicals, air pollutants, pharmaceuticals and the impurities, simultaneous exposure to these chemicals is unavoidable. Currently, there is no effective approach to evaluate genotoxic and carcinogenic risk from exposure to low doses of multiple DNA-reactive genotoxic carcinogens. One approach for the regulation of the total carcinogenic risk on humans would be to establish weighted allocations for each class of chemicals, such as food-related chemicals, 50% (0.5 × 10−5); industrial chemicals, 10% (0.1 × 10−5); air pollutants, 10% (0.1 × 10−5); pharmaceuticals, including impurities, 20% (0.2 × 10−5), and others 10% (0.1 × 10−5). Then, the total carcinogenic risk would be less than 1 × 10−5, even when people are exposed to multiple genotoxic carcinogens. Risk assessment of multiple exposure to DNA-reactive genotoxic carcinogens below the TTC may be a challenge for regulatory genetic toxicology.
ACKNOWLEDGMENTS
This work was supported by the Japan Society for the Promotion of Science, grant number: JSPS KAKENHI Grant 26281029.
Abbreviations
- WHO
World Health Organization
- ADI
Acceptable daily intake
- NOAEL
No observable adverse effect level
- NTP
National Toxicology Program
- ICH
International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use
- S9 mix
9,000 × g supernatant of liver homogenates of rats pretreated with inducers of drug metabolizing enzymes plus NADPH-generating system
- EFSA
European Food Safety Authority
- IARC
International Agency for Research on Cancer
- TLS
Translesion DNA synthesis
- 4-NQO
4-Nitroquinoline-1-oxide
- TTC
Threshold of toxicological concern
- TOR
Threshold of regulation
- bw
Body weight
- COC
Cohort of concern.
Footnotes
CONFLICT OF INTEREST
There is no conflict of interest.
REFERENCES
- 1.The Food and Agricultural Organization of the United Nations and the World Health Organization (FAO/WHO) Chapter 5: dose-response assessment and derivation of health-based guidance values in Environmental Health Criteria 240. 2009. pp. 2–55. [Google Scholar]
- 2.Kirsch-Volders M, Aardema M, Elhajouji A. Concepts of threshold in mutagenesis and carcinogenesis. Mutat Res. 2000;464:3–11. doi: 10.1016/S1383-5718(99)00161-8. [DOI] [PubMed] [Google Scholar]
- 3.Nohmi T, Toyoda-Hokaiwado N, Yamada M, Masumura K, Honma M, Fukushima S. International symposium on genotoxic and carcinogenic thresholds. Genes Environ. 2008;30:101–107. doi: 10.3123/jemsge.30.101. [DOI] [Google Scholar]
- 4.Lovell DP. Dose-response and threshold-mediated mechanisms in mutagenesis: statistical models and study design. Mutat Res. 2000;464:87–95. doi: 10.1016/S1383-5718(99)00169-2. [DOI] [PubMed] [Google Scholar]
- 5.Boice JD., Jr The linear nonthreshold (LNT) model as used in radiation protection: an NCRP update. Int J Radiat Biol. 2017;93:1079–1092. doi: 10.1080/09553002.2017.1328750. [DOI] [PubMed] [Google Scholar]
- 6.Auerbach C. Radiomimetic substances. Radiat Res. 1958;9:33–47. doi: 10.2307/3570779. [DOI] [PubMed] [Google Scholar]
- 7.The Food and Agricultural Organization of the United Nations and the World Health Organization (FAO/WHO) Chapter 7: risk characterization in Environmental Health Criteria 240: Principles and Methods for Risk Assessment of Chemicals in Food. 2009. pp. 1–18. [Google Scholar]
- 8.Bolt HM. The concept of “practical thresholds” in the derivation of occupational exposure limits for carcinogens by the scientific committee on occupatinal exposure limits (SCOEL) of the European Union. Genes Environ. 2008;30:114–119. doi: 10.3123/jemsge.30.114. [DOI] [Google Scholar]
- 9.Ashby J, Tennant RW. Chemical structure, Salmonella mutagenicity and extent of carcinogenicity as indicators of genotoxic carcinogenesis among 222 chemicals tested in rodents by the U.S. NCI/NTP. Mutat Res. 1988;204:17–115. doi: 10.1016/0165-1218(88)90114-0. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi Y. Overview of genotoxic carcinogens and non-genotoxic carcinogens. Exp Toxicol Pathol. 1992;44:465–471. doi: 10.1016/S0940-2993(11)80159-4. [DOI] [PubMed] [Google Scholar]
- 11.MacGregor JT, Frotschl R, White PA, Crump KS, Eastmond DA, Fukushima S, Guerard M, Hayashi M, Soeteman-Hernandez LG, Johnson GE, Kasamatsu T, Levy DD, Morita T, Müller L, Schoeny R, Schuler MJ, Thybaud V. IWGT report on quantitative approaches to genotoxicity risk assessment II. Use of point-of-departure (PoD) metrics in defining acceptable exposure limits and assessing human risk. Mutat Res. 2015;783:66–78. doi: 10.1016/j.mrgentox.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Butterworth BE. Consideration of both genotoxic and nongenotoxic mechanisms in predicting carcinogenic potential. Mutat Res. 1990;239:117–132. doi: 10.1016/0165-1110(90)90033-8. [DOI] [PubMed] [Google Scholar]
- 13.Roberts RA, Goodman JI, Shertzer HG, Dalton TP, Farland WH. Rodent toxicity and nongenotoxic carcinogenesis: knowledge-based human risk assessment based on molecular mechanisms. Toxicol Mech Methods. 2003;13:21–29. doi: 10.1080/15376510309823. [DOI] [PubMed] [Google Scholar]
- 14.Ramirez T, Eastmond DA, Herrera LA. Nondisjunction events induced by albendazole in human cells. Mutat Res. 2007;626:191–195. doi: 10.1016/j.mrgentox.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Asanami S, Shimono K. The effect of hyperthermia on micronucleus induction by mutagens in mice. Mutat Res. 1999;446:149–154. doi: 10.1016/S1383-5718(99)00156-4. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong MJ, Gara JP, Gealy R, III, Greenwood SK, Hilliard CA, Laws GM, Galloway SM. Induction of chromosome aberrations in vitro by phenolphthalein: mechanistic studies. Mutat Res. 2000;457:15–30. doi: 10.1016/S0027-5107(00)00119-6. [DOI] [PubMed] [Google Scholar]
- 17.Galloway SM, Miller JE, Armstrong MJ, Bean CL, Skopek TR, Nichols WW. DNA synthesis inhibition as an indirect mechanism of chromosome aberrations: comparison of DNA-reactive and non-DNA-reactive clastogens. Mutat Res. 1998;400:169–186. doi: 10.1016/S0027-5107(98)00044-X. [DOI] [PubMed] [Google Scholar]
- 18.Elhajouji A, Van HP, Kirsch-Volders M. Indications for a threshold of chemically-induced aneuploidy in vitro in human lymphocytes. Environ Mol Mutagen. 1995;26:292–304. doi: 10.1002/em.2850260405. [DOI] [PubMed] [Google Scholar]
- 19.Kirsch-Volders M, Gonzalez L, Carmichael P, Kirkland D. Risk assessment of genotoxic mutagens with thresholds: a brief introduction. Mutat Res. 2009;678:72–75. doi: 10.1016/j.mrgentox.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi M. The micronucleus test-most widely used in vivo genotoxicity test. Genes Environ. 2016;38:18. doi: 10.1186/s41021-016-0044-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lynch A, Harvey J, Aylott M, Nicholas E, Burman M, Siddiqui A, Walker S, Rees R. Investigations into the concept of a threshold for topoisomerase inhibitor-induced clastogenicity. Mutagenesis. 2003;18:345–353. doi: 10.1093/mutage/geg003. [DOI] [PubMed] [Google Scholar]
- 22.Elhajouji A, Lukamowicz M, Cammerer Z, Kirsch-Volders M. Potential thresholds for genotoxic effects by micronucleus scoring. Mutagenesis. 2011;26:199–204. doi: 10.1093/mutage/geq089. [DOI] [PubMed] [Google Scholar]
- 23.Maron DM, Ames BN. Revised methods for the Salmonella mutagenicity test. Mutat Res. 1983;113:173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- 24.Ashby J, Tennant RW. Definitive relationships among chemical structure, carcinogenicity and mutagenicity for 301 chemicals tested by the U.S. NTP. Mutat Res. 1991;257:229–306. doi: 10.1016/0165-1110(91)90003-E. [DOI] [PubMed] [Google Scholar]
- 25.Nohmi T, Masumura K, Toyoda-Hokaiwado N. Transgenic rat models for mutagenesis and carcinogenesis. Genes Environ. 2017;39:11. doi: 10.1186/s41021-016-0072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nohmi T, Katoh M, Suzuki H, Matsui M, Yamada M, Watanabe M, Suzuki M, Horiya N, Ueda O, Shibuya T, Ikeda H, Sofuni T. A new transgenic mouse mutagenesis test system using Spi− and 6-thioguanine selections. Environ Mol Mutagen. 1996;28:465–470. doi: 10.1002/(SICI)1098-2280(1996)28:4<465::AID-EM24>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 27.Nohmi T, Suzuki T, Masumura K. Recent advances in the protocols of transgenic mouse mutation assays. Mutat Res. 2000;455:191–215. doi: 10.1016/S0027-5107(00)00077-4. [DOI] [PubMed] [Google Scholar]
- 28.Masumura K, Sakamoto Y, Kumita W, Honma M, Nishikawa A, Nohmi T. Genomic integration of lambda EG10 transgene in gpt delta transgenic rodents. Genes Environ. 2015;37:24. doi: 10.1186/s41021-015-0024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nohmi T. Past, present and future directions of gpt delta rodent gene mutation assays. Food Safety. 2015;4:1–13. doi: 10.14252/foodsafetyfscj.2015024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki Y, Umemura T, Hibi D, Inoue T, Jin M, Ishii Y, Sakai H, Nohmi T, Yanai T, Nishikawa A, Ogawa K. Possible involvement of genotoxic mechanisms in estragole-induced hepatocarcinogenesis in rats. Arch Toxicol. 2012;86:1593–1601. doi: 10.1007/s00204-012-0865-8. [DOI] [PubMed] [Google Scholar]
- 31.Ishii Y, Takasu S, Kuroda K, Matsushita K, Kijima A, Nohmi T, Ogawa K, Umemura T. Combined application of comprehensive analysis for DNA modification and reporter gene mutation assay to evaluate kidneys of gpt delta rats given madder color or its constituents. Anal Bioanal Chem. 2014;406:2467–2475. doi: 10.1007/s00216-014-7621-2. [DOI] [PubMed] [Google Scholar]
- 32.Jin M, Kijima A, Hibi D, Ishii Y, Takasu S, Matsushita K, Kuroda K, Nohmi T, Nishikawa A, Umemura T. In vivo genotoxicity of methyleugenol in gpt delta transgenic rats following medium-term exposure. Toxicol Sci. 2013;131:387–394. doi: 10.1093/toxsci/kfs294. [DOI] [PubMed] [Google Scholar]
- 33.Kuroda K, Ishii Y, Takasu S, Kijima A, Matsushita K, Watanabe M, Takahashi H, Sugita-Konishi Y, Sakai H, Yanai T, Nohmi T, Ogawa K, Umemura T. Cell cycle progression, but not genotoxic activity, mainly contributes to citrinin-induced renal carcinogenesis. Toxicology. 2013;311:216–224. doi: 10.1016/j.tox.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Maeda J, Kijima A, Inoue K, Ishii Y, Ichimura R, Takasu S, Kuroda K, Matsushita K, Kodama Y, Saito N, Umemura T, Yoshida M. In vivo genotoxicity of Ginkgo biloba extract in gpt delta mice and constitutive androstane receptor knockout mice. Toxicol Sci. 2014;140:298–306. doi: 10.1093/toxsci/kfu090. [DOI] [PubMed] [Google Scholar]
- 35.Onami S, Cho YM, Toyoda T, Horibata K, Ishii Y, Umemura T, Honma M, Nohmi T, Nishikawa A, Ogawa K. Absence of in vivo genotoxicity of 3-monochloropropane-1,2-diol and associated fatty acid esters in a 4-week comprehensive toxicity study using F344 gpt delta rats. Mutagenesis. 2014;29:295–302. doi: 10.1093/mutage/geu018. [DOI] [PubMed] [Google Scholar]
- 36.Kuroiwa Y, Umemura T, Nishikawa A, Kanki K, Ishii Y, Kodama Y, Masumura K, Nohmi T, Hirose M. Lack of in vivo mutagenicity and oxidative DNA damage by flumequine in the livers of gpt delta mice. Arch Toxicol. 2007;81:63–69. doi: 10.1007/s00204-006-0126-9. [DOI] [PubMed] [Google Scholar]
- 37.Wiseman RW, Miller EC, Miller JA, Liem A. Structure-activity studies of the hepatocarcinogenicities of alkenylbenzene derivatives related to estragole and safrole on administration to preweanling male C57BL/6J x C3H/HeJ F1 mice. Cancer Res. 1987;47:2275–2283. [PubMed] [Google Scholar]
- 38.Sekizawa J, Shibamoto T. Genotoxicity of safrole-related chemicals in microbial test systems. Mutat Res. 1982;101:127–140. doi: 10.1016/0165-1218(82)90003-9. [DOI] [PubMed] [Google Scholar]
- 39.Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K, Speck W. Salmonella mutagenicity tests: III. Results from the testing of 255 chemicals. Environ Mutagen. 1987;9(Suppl 9):1–109. doi: 10.1002/em.2860090602. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki Y, Umemura T, Ishii Y, Hibi D, Inoue T, Jin M, Sakai H, Kodama Y, Nohmi T, Yanai T, Nishikawa A, Ogawa K. Possible involvement of sulfotransferase 1A1 in estragole-induced DNA modification and carcinogenesis in the livers of female mice. Mutat Res. 2012;749:23–28. doi: 10.1016/j.mrgentox.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 41.Phillips DH, Miller JA, Miller EC, Adams B. Structures of the DNA adducts formed in mouse liver after administration of the proximate hepatocarcinogen 1′-hydroxyestragole. Cancer Res. 1981;41:176–186. [PubMed] [Google Scholar]
- 42.Alnouti Y, Klaassen CD. Tissue distribution and ontogeny of sulfotransferase enzymes in mice. Toxicol Sci. 2006;93:242–255. doi: 10.1093/toxsci/kfl050. [DOI] [PubMed] [Google Scholar]
- 43.Srivastava S, Sinha R, Roy D. Toxicological effects of malachite green. Aquat Toxicol. 2004;66:319–329. doi: 10.1016/j.aquatox.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 44.Culp SJ, Mellick PW, Trotter RW, Greenlees KJ, Kodell RL, Beland FA. Carcinogenicity of malachite green chloride and leucomalachite green in B6C3F1 mice and F344 rats. Food Chem Toxicol. 2006;44:1204–1212. doi: 10.1016/j.fct.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 45.Fessard V, Godard T, Huet S, Mourot A, Poul JM. Mutagenicity of malachite green and leucomalachite green in in vitro tests. J Appl Toxicol. 1999;19:421–430. doi: 10.1002/(SICI)1099-1263(199911/12)19:6<421::AID-JAT595>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 46.Mittelstaedt RA, Mei N, Webb PJ, Shaddock JG, Dobrovolsky VN, McGarrity LJ, Morris SM, Chen T, Beland FA, Greenlees KJ, Heflich RH. Genotoxicity of malachite green and leucomalachite green in female Big Blue B6C3F1 mice. Mutat Res. 2004;561:127–138. doi: 10.1016/j.mrgentox.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Manjanatha MG, Shelton SD, Bishop M, Shaddock JG, Dobrovolsky VN, Heflich RH, Webb PJ, Blankenship LR, Beland FA, Greenlees KJ, Culp SJ. Analysis of mutations and bone marrow micronuclei in Big Blue rats fed leucomalachite green. Mutat Res. 2004;547:5–18. doi: 10.1016/j.mrfmmm.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 48.Culp SJ, Blankenship LR, Kusewitt DF, Doerge DR, Mulligan LT, Beland FA. Toxicity and metabolism of malachite green and leucomalachite green during short-term feeding to Fischer 344 rats and B6C3F1 mice. Chem Biol Interact. 1999;122:153–170. doi: 10.1016/S0009-2797(99)00119-2. [DOI] [PubMed] [Google Scholar]
- 49.European Food Safety Authority (EFSA) Malachite Green in Food. EFSA J. 2016;14:1–80. [Google Scholar]
- 50.World Health Organization (WHO) Toxicological evaluation of certain veterinary drug residues in food, fifty-fourth meeting of the Joint FAO/WHO Expert Committee on Food Additives. WHO Food Additive Series. 2000;45:75–89. [Google Scholar]
- 51.Umemura T, Kuroiwa Y, Tasaki M, Okamura T, Ishii Y, Kodama Y, Nohmi T, Mitsumori K, Nishikawa A, Hirose M. Detection of oxidative DNA damage, cell proliferation and in vivo mutagenicity induced by dicyclanil, a non-genotoxic carcinogen, using gpt delta mice. Mutat Res. 2007;633:46–54. doi: 10.1016/j.mrgentox.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 52.Kasper P, Uno Y, Mauthe R, Asano N, Douglas G, Matthews E, Moore M, Mueller L, Nakajima M, Singer T, Speit G. Follow-up testing of rodent carcinogens not positive in the standard genotoxicity testing battery: IWGT workgroup report. Mutat Res. 2007;627:106–116. doi: 10.1016/j.mrgentox.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 53.National Toxicology Program (NTP) Toxicology and carcinogenesis studies of ochratoxin A (CAS No. 303-47-9) in F344/N rats (gavage studies) Natl Toxicol Program Tech Rep Ser. 1989;358:1–142. [PubMed] [Google Scholar]
- 54.Malir F, Ostry V, Pfohl-Leszkowicz A, Malir J, Toman J. Ochratoxin A: 50 years of research. Toxins (Basel) 2016;8:E191. doi: 10.3390/toxins8070191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.International Agency for Research on Cancer (IARC) Ochratoxin A. IARC Monogr Eval Carcinog Risks Hum. 1993;56:489–521. [PMC free article] [PubMed] [Google Scholar]
- 56.Hibi D, Suzuki Y, Ishii Y, Jin M, Watanabe M, Sugita-Konishi Y, Yanai T, Nohmi T, Nishikawa A, Umemura T. Site-specific in vivo mutagenicity in the kidney of gpt delta rats given a carcinogenic dose of ochratoxin A. Toxicol Sci. 2011;122:406–414. doi: 10.1093/toxsci/kfr139. [DOI] [PubMed] [Google Scholar]
- 57.Nohmi T, Suzuki M, Masumura K, Yamada M, Matsui K, Ueda O, Suzuki H, Katoh M, Ikeda H, Sofuni T. Spi− selection: An efficient method to detect gammaray-induced deletions in transgenic mice. Environ Mol Mutagen. 1999;34:9–15. doi: 10.1002/(SICI)1098-2280(1999)34:1<9::AID-EM2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 58.Fukushima S, Gi M, Kakehashi A, Wanibuchi H. Qualitative and quantitative assessments on low-dose carcinogenicity of genotoxic hepatocarcinogens: dose-response for key events in rat hapatocarcinogenesis. In: Nohmi T, Fukushima S, editors. Thresholds of Genotoxic Carcinogens: from Mechanisms to Regulation. Elsevier; 2016. pp. 1–17. [Google Scholar]
- 59.Fukushima S, Kinoshita A, Puatanachokchai R, Kushida M, Wanibuchi H, Morimura K. Hormesis and dose-response-mediated mechanisms in carcinogenesis: evidence for a threshold in carcinogenicity of non-genotoxic carcinogens. Carcinogenesis. 2005;26:1835–1845. doi: 10.1093/carcin/bgi160. [DOI] [PubMed] [Google Scholar]
- 60.Fukushima S, Wei M, Kakehashi A, Wanibuchi H. Threshold for genotoxic carcinogens: the central concern in carcinogenic risk assessment. Genes Environ. 2012;32:153–156. doi: 10.3123/jemsge.34.153. [DOI] [Google Scholar]
- 61.Jenkins GJ, Zair Z, Johnson GE, Doak SH. Genotoxic thresholds, DNA repair, and susceptibility in human populations. Toxicology. 2010;278:305–310. doi: 10.1016/j.tox.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 62.Nohmi T, Tsuzuki T. Possible mechanisms underlying genotoxic thresholds: DNA repair and translesion DNA synthesis. In: Nohmi T, Fukushima S, editors. Thresholds of Genotoxic Carcinogens: from Mechanisms to Regulation. Elsevier; 2016. pp. 49–66. [DOI] [Google Scholar]
- 63.Suzuki M, Matsui K, Yamada M, Kasai H, Sofuni T, Nohmi T. Construction of mutants of Salmonella typhimurium deficient in 8-hydroxyguanine DNA glycosylase and their sensitivities to oxidative mutagens and nitro compounds. Mutat Res. 1997;393:233–246. doi: 10.1016/S1383-5718(97)00108-3. [DOI] [PubMed] [Google Scholar]
- 64.Kim SR, Kokubo K, Matsui K, Yamada N, Kanke Y, Fukuoka M, Yamada M, Nohmi T. Light-dependent mutagenesis by benzo[a]pyrene is mediated via oxidative DNA damage. Environ Mol Mutagen. 2005;46:141–149. doi: 10.1002/em.20141. [DOI] [PubMed] [Google Scholar]
- 65.Boobis A, Brown P, Cronin MTD, Edwards J, Galli CL, Goodman J, Jacobs A, Kirkland D, Luijten M, Marsaux C, Martin M, Yang C, Hollnagel HM. Origin of the TTC values for compounds that are genotoxic and/or carcinogenic and an approach for their re-evaluation. Crit Rev Toxicol. 2017;47:705–727. doi: 10.1080/10408444.2017.1318822. [DOI] [PubMed] [Google Scholar]
- 66.Federal Register. Toxicological principles for the safety assessment of direct food additives and color additives used in food in Fed Regist. 1995. pp. 36582–36596. [Google Scholar]
- 67.Kroes R, Renwick AG, Cheeseman M, Kleiner J, Mangelsdorf I, Piersma A, Schilter B, Schlatter J, van SF, Vos JG, Würtzen G. Structure-based thresholds of toxicological concern (TTC): guidance for application to substances present at low levels in the diet. Food Chem Toxicol. 2004;42:65–83. doi: 10.1016/j.fct.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 68.Europena Food Safety Authority (EFSA) Review of the Threshold of Toxicological Concern (TTC) approach and development of new TTC decision tree. EFSA Support Publ. 2016;13:1–50. [Google Scholar]
- 69.Munro IC, Ford RA, Kennepohl E, Sprenger JG. Correlation of structural class with no-observed-effect levels: a proposal for establishing a threshold of concern. Food Chem Toxicol. 1996;34:829–867. doi: 10.1016/S0278-6915(96)00049-X. [DOI] [PubMed] [Google Scholar]
- 70.Ohta T. Mutagenic activity of a mixture of heterocyclic amines at doses below the biological threshold level of each. Genes Environ. 2006;28:181–184. doi: 10.3123/jemsge.28.181. [DOI] [Google Scholar]