Abstract
Background
Tooth loss is suggested to be associated with an increased risk of dementia in many studies. But the relationship between tooth loss and dementia is not yet fully understood. This systematic review and meta-analysis aimed to determine the relative effect of tooth loss on dementia risk.
Methods
An electronic search of PubMed, Scopus, Embase, and Web of Knowledge was conducted in March 2018 to identify relevant observational studies with the English language restriction. Studies were included if they assessed the relationship between tooth loss and risk of dementia. Study quality was detected by the modified Downs and Black scale. Odds risks (ORs) were pooled using a random-effects model in the crude model.
Results
The literature search initially yielded 1574 articles, and 21 observational studies published between 1994 and 2017 were finally included for the analyses. The crude results with random-effects model showed that patients with multiple tooth loss had higher incidence of dementia (OR 2.62, 95% CI 1.90–3.61, P < 0.001, I2 = 90.40%). The association remained noted when only adjusted results were pooled from 18 studies (OR 1.55, 95% CI 1.41–1.70, P = 0.13, I2 = 28.00%). Meta-regression analysis showed that study design explained about 16.52% of heterogeneity in the crude model. The overall quality rating scores of studies ranged from 11 to 16.
Conclusions
Findings from this review evidenced that tooth loss is positively associated with an increased risk of dementia in adults. Future well-designed longitudinal researches examining the direct and indirect relationship between tooth loss and dementia risk are encouraged.
Electronic supplementary material
The online version of this article (10.1186/s12888-018-1927-0) contains supplementary material, which is available to authorized users.
Keywords: Dementia, Cognitive impairment, Tooth loss, Risk assessment, Meta-analysis
Background
Dementia is characterized by cognitive and functional decline and neuropsychiatric symptoms caused by irreversible neurodegenerative diseases. The global population is aging at a rapid pace due to rising life expectance and over 47 million people live with dementia in 2016. The prevalence of dementia results in negative impacts on people’s life quality and economy according to the 2016 World Alzheimer Report [1]. To our knowledge, there is no effective anti-dementia drug available for the management of dementia. Therefore, it is in great need to identify modifiable risk factors for preventing cognitive impairment.
Tooth loss is prevalent in patients with dementia and it is a worldwide public health issue in older adults [2], impacting negatively on their quality of daily life, such as chewing, swallowing, and social life [3–5]. Evidence has shown that tooth loss is not only associated with oral health, but also with systemic health [6]. Recently, increasing studies have focused on the link between tooth loss and the risk of dementia [7–12]. There are several potential mechanisms by which tooth loss can negatively impact cognitive function. Periodontitis is one of the main causes of tooth loss, which is able to increased levels of pro-inflammatory mediators such as IL-1, IL-6 and TNF-α in the plasma, contributing to the aggravation of neuroinflammatory processes in brain and eventually resulting in cognitive decline [13–15]. Besides, masticatory disorder due to tooth loss can lead to poor nutrition, and reduce cerebral blood flow, which may be linked to memory deficits [9, 10]. It has been supported by several animal studies that tooth loss may induce decreased acetylcholine levels due to masticatory dysfunction, and lead to reductions in the number of pyramidal cells in the hippocampus, provoking cognitive dysfunction [16, 17].
A growing number of primary studies have demonstrated a close relationship between tooth loss and incidence of dementia, suggesting that tooth loss may be a modifiable risk factor for dementia [18–27]. However, this association is not noted in some studies [28–38]. To our knowledge, there are only two limited meta-analysis released by Shen et al [39] and Oh et al [40], exploring the relationship between tooth loss and cognitive impairment. In fact, some vital studies were not included without clear reasons, although Shen and colleagues have included observational studies from different study designs in the review. Moreover, the flow diagram of identification and selection process of studies could not be found in the analysis [39, 40]. Additionally, qualitative evaluation of selected studies and confounders for adjusted results of included studies were not demonstrated in the paper. As for the meta-analysis by Oh and colleagues, they intended to include cohort studies to prevent significant selection bias from cross-sectional studies [40]. However, one of the included studies is a cross-sectional design study, which was released by Luo et al [18]. Based on that, we therefore conducted a well-designed systematic review and meta-analysis of observational studies describing the association between tooth loss and the incidence of dementia in adults. We hope that our results can shed some light on the prevention of dementia in the future.
Methods
Search strategy
We systematically searched electronic databases, including PubMed, EMBASE, Scopus and Web of Science, to identify studies that analyzed the association between tooth loss and dementia in adults from inception to March 2018 with the English language restriction using the key terms: dementia, Alzheimer’s, mild cognitive impairment, cognitive impairment, cognitive decline, cognitive disorder, memory disorder, memory disorder, tooth loss, oral health and dental care. References of relevant papers were also screened for additional publications and we did not retrieve unpublished studies. Predefined data-collection worksheets were employed for the assessment of each included paper. Any disagreement among authors was resolved by discussion until a consensus was reached.
Inclusion/exclusion criteria
For inclusion in this analysis, eligible studies should define tooth loss as one of the exposure interests, while incidence of dementia as one of the outcome of interests, and present original data or an crude and/or adjusted effect size, such as odds ratio (OR), hazard ratio (HR), or risk ratio (RR) of dementia with their 95% confidence intervals (CIs), or enough data to quantify the association between tooth loss and dementia risk. Different study designs were included. Abstracts from conferences, letters to the editor and reviews were excluded in the overall analysis. Animal studies were also excluded in this analysis. Moreover, concerning the quality assessment criteria, studies with a quality score of less than 5 points were not considered.
Quality assessment
The quality of all selected studies was assessed using an adaptation of the Downs and Black criteria as described in previous systematic reviews [41–43]. From 27 original items in the checklist of the Downs and Black criteria, 17 were employed to accommodate the characteristics of observational studies, while other items specific for interventional randomization studies were removed. As recommended by Wehrmeister and colleagues [44], the total scores range from 0 to 18 points, given that each item scores one point, except for item 4 that can result in 0 (no), 1 (partially) and 2 (yes). Studies could be categorized with a quality score as: high chance of bias (0–5 points), moderate chance of bias (6–11 points) and low chance of bias (12–18 points) [41]. Two reviewers rated each study independently according to the above quality criteria, and discrepancies were discussed and resolved by consensus between referees.
Data extraction
We extracted data independently from each included study, using a standardized worksheet in particular concerning: name of first author, publication year, study region, study design, age, sample size, main exposures definition, crude effect size with their 95%CI, adjusted effect size with their 95%CI, and adjusted variables, follow-up time. We extracted the highest versus lowest effect size with their 95%CI of tooth loss number associated with dementia incidence for this analysis. The effect sizes with their 95%CI adjusted with the most confounders were extracted for the adjusted model [39]. Disagreements of methodology or result between investigators were solved by consensus.
Statistical analysis
The publications reported different measures of estimate effects including RR, OR and HR with their 95%CIs. Based on the assumption that the absolute risk of dementia was low and the person time of the exposed group was much smaller than that of the unexposed group, we did not make distinction between these size effects in this study. This way of pooling different measures of estimate effects has been used previously [45–49]. Meta-analyses were performed considering crude correlation between tooth loss and dementia risk and adjusted association between tooth loss and dementia risk. When various categories of tooth loss were shown, only the estimate comparing the most extreme categories was used for analysis as described in previous Meta-analyses [39, 41] Heterogeneity among studies was quantified using the Cochran’s Q test and chi-square (I2) test. Heterogeneity was considered statistically significant with P < 0.05 and random-effects model was used when heterogeneity was obvious (I2 > 50%) in this meta-analysis. Subgroup analyses and meta-regression were performed to explore the source of heterogeneity and it was conducted by the following subsets: study design (case-control or cohort or cross-sectional study), sample size, study region, and cognitive assessment. These approaches helped to identify whether the study characteristics mentioned above statistically affected estimate effects. We also assessed publication bias using both Begg-Mazumdar test and Egger’s regression test. When significant bias was found, we performed the trim and fill method to adjust for it. All analyses were completed with the Meta-analysis program software STATA 12.0 (StataCorp, College Station, TX, USA).
Results
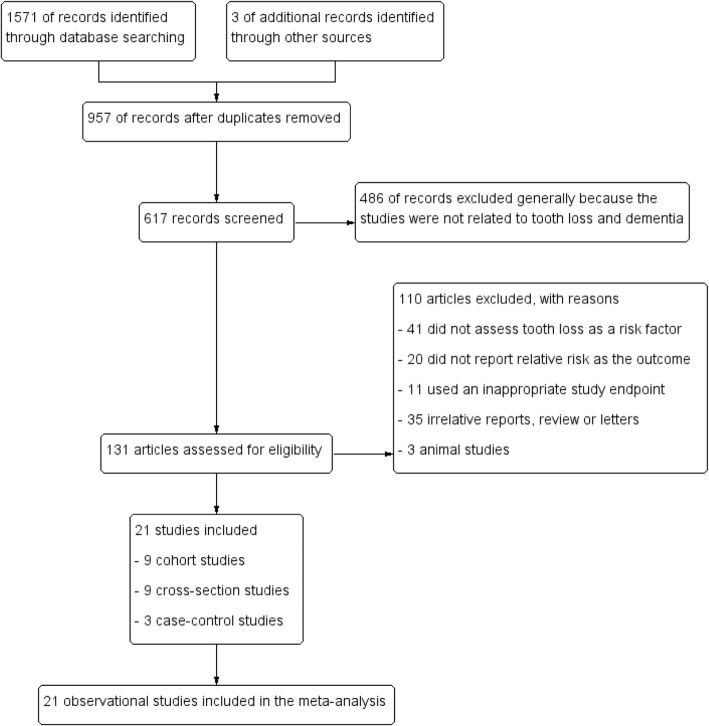
The selecting processes for eligible studies were shown in Fig. 1. The literature search initially yielded 1574 papers, and 957 studies were duplicated. Abstracts from conferences, letters to the editor and reviews were excluded. Articles only with animal experiments or with repetitive data were removed. In addition, studies failed to provide enough data to quantify the association between tooth loss and dementia risk were also excluded. Finally, 21 studies published between 1994 and 2017 were identified for this analysis. Among all the studies, there were nine cross-sectional studies, nine cohort studies and three case-control studies and all the included studies were published in English. The main characteristics of studies were described in Tables 1, 2 and 3. Among these studies, nine were carried out in Asia, six in Europe and three in America. The total quality rating scores of included studies ranged from 11 to 16.
Fig. 1.
Flow diagram of identification and selection process of studies
Table 1.
Summary of cross-sectional studies included in the meta-analysis
| Author /Year | Country | Sample size | Study design | Age, yr | Main exposure definition | Exposure cut-off point | Accessment of cognitive function | Effect size and crude association results with 95%CI highest vs. lowest category | Effect size and adjusted association results with 95%CI highest vs. lowest category | Adjustment | Quality scores |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Luo et al (2015) [18] | China | 3063 | Cross sectional | ≥60 | Number of teeth missing | 0–3, 4–6, 7–16, > 16 |
DSM-IV | 3.65 (2.75–4.86) | 1.56 (1.12–2.18) | Sex, age, education year, living alone, overweight, cigarette smoking, alcohol drinking, anxiety, depression, heart disease, hypertension, diabetes, and Apolipoprotein E-ε4 | 15 |
| Peres et al (2014) [19] | Brazil | 1705 | Cross sectional | ≥60 | Number of teeth present | ≥10, < 10, 0 |
MMSE | 6.40 (3.40–12.10) | 3.30 (1.20–9.30) | Sex, age, race, income, education, smoking, depression, diabetes, cardiova-scular disease, and hypertension | 14 |
| Nilsson et al (2014) [20] | Sweden | 1147 | Cross sectional | 60–96 | Number of teeth present | ≥20, 1–19, 0 |
MMSE | 9.20 (5.90–14.30) | 3.20 (1.90–53.00) | Age and education | 15 |
| Wang et al (2014) [28] | China | 930 | Cross sectional | ≥65 | Number of teeth present | ≥20, <20 |
MMSE | 1.54(1.13–2.10) | 1.30 (0.93–1.81) | Age, gender and life style habits | 14 |
| Park et al (2013) [21] | Korea | 438 | Cross sectional | ≥50 | Number of teeth missing | 0–5, 6–10, > 10 |
MMSE | 2.69(1.57–4.64) | 2.25 (1.26–4.02) | Age, gender, education, hypertension, diabetes, hyperlipidemia and current smoking | 13 |
| Saito et al (2013) [22] | Japan | 462 | Cross sectional | ≥60 | Number of teeth present | 22–32, 11–21, 0–10 |
MMSE | 27.33(3.62–206.21) | 20.21 (2.20–185.47) | Age, gender, education, smoking, alcohol intake, positive history of diseases, TMIG-IC score, and CES-D total score | 13 |
| Lexomboon et al (2012) [29] | Sweden | 557 | Cross sectional | ≥77 | Number of teeth missing | Multiple tooth loss | MMSE | 2.10 (1.35–3.25) | 1.36 (0.84–2.19) | Sex, age, and education | 12 |
| Okamoto et al (2010) [23] | Japan | 4061 | Cross sectional | ≥65 | Number of teeth present | 22–32, 11–21, 0–10 |
MMSE | – | 2.18 (1.51–3.14) | Depressive symptoms, age, sex, length of education, frequency of drinking, smoking habit, time spent walking every day, positive history of cancer and diabetes mellitus, and the levels of serum albumin, total cholesterol, and low-density lipoprotein cholesterol | 12 |
| Stewart et al (2007) [24] | England | 4032 | Cross sectional | ≥65 | Number of teeth present | > 0, 0 |
AMTS | 3.59 (2.36–5.47) | 2.61 (1.49–4.28) | Age, sex, education, sampling area, disability, and BMI | 12 |
Note: CI Confidence interval, AMTS Abbreviated Mental Test Score, TMIG-IC The Tokyo Metropolitan Institute of Gerontology Index of Competence, CES-D The Center for Epidemiologic studies depression scale, BMI Body mass index, MMSE Mini-mental status examination, DSM-IV The Diagnostic and Statistical Manual of Mental Disorders Fourth Edition
Table 2.
Summary of cohort studies included in the meta-analysis
| Author /Year | Country | Sample size | Study design | Age, yr | Main exposure definition | Exposure cut-off point | Accessment of cognitive function | Effect size and crude association results with 95%CI highest vs. lowest category | Effect size and adjusted association results with 95%CI highest vs. lowest category | Adjustment | Follow- up, yr | Quality scores |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Takeuchi et al (2017) [30] | Japan | 1566 | Cohort | ≥60 | Number of teeth present | ≥20, 10–19, 1–9, 0 |
DSM-III R | 3.83 (2.47–5.93) | 1.63 (0.95–2.80) | Sex, age, occupation, education, hypertension, diabetes mellitus, history of stroke, alcohol intake, tooth brushing frequency, regular visits to the dentist, and denture use. | 5 | 16 |
| Stewart et al (2015) [31] | Sweden | 697 | Cohort | 70–92 | Number of teeth present | ≥25, 21–24, 9–20, 0–8, |
DSM-III R | – | 1.62 (0.84–3.11) | Age, education, social class, and vascular risk factors | 37 | 13 |
| Batty et al (2013) [30] | 20 Countries | 11,140 | Cohort | 55–88 | Number of teeth present | ≥22, 1–21, 0 |
MMSE | – | 1.48 (1.24–1.78) | Age, sex, socio-economic CVD risk factors, treatment allocation and ethnicity | 5 | 14 |
| Yamamoto et al (2012) [32] | Japan | 4425 | Cohort | ≥65 | Number of teeth present | ≥20, ≤19, 0 |
Standardized questionnaire | 3.42 (1.05–11.08) | 1.41 (0.42–4.70) | Age, adjusted household income, BMI, present illness, alcohol consumption, exercise, and forgetfulness | 4 | 15 |
| Paganini-Hill et al (2012) [33] | USA | 5468 | Cohort | 52–105 | Number of teeth present | 26–32, 16–25, 1–15, 0 |
MMSE | 0.84 (0.67–1.06) | – | – | 18 | 13 |
| Arrivé et al (2011) [34] | France | 405 | Cohort. | 66–80 | Number of teeth missing | < 11 ≥11 |
DSM-III R | 1.35 (0.81–2.25) | – | – | 15 | 12 |
| Kim et al. (2007) [35] | Korea | 686 | Cohort | ≥65 | Number of teeth present | ≥28 25–27, 15–24, 1–14, 0 |
DSM-IV | 1.38 (1.12–1.69) | 1.26 (1.00–1.59) | Age, gender and education, reported diet, vascular disease/risk, BMI and MAC, albumin and cholesterol | 2.4 | 14 |
| Stein et al (2007) [26] | USA | 101 | Cohort | 75–98 | Number of teeth present | 10–28, 0–9 |
MMSE | 2.69 (1.07–6.73) | 2.20 (1.10–4.50) | Age, education, and apolipoprotein E4 allele | 12 | 13 |
| Shimazakil et al (2001) [36] | Japan | 517 | Cohort study | ≥65 | Number of teeth present | >20, 1–19, 0 |
Historical diagnosis information from medical records | 5.20 (2.00–13.10) | 2.40 (0.90–6.50) | Age, and classification of institution, physical health status, and cerebrovascular disorder | 6 | 13 |
Notes: BMI Body mass index, CI Confidence interval, CVD Cardiovascular disease, DSM-IV The Diagnostic and Statistical Manual of Mental Disorders Fourth Edition, DSM-III R Diagnostic and Statistical Manual of Mental Disorders Third Edition, Revised, MAC Mid arm circumference, MMSE The Mini-Mental State Examination
Table 3.
Summary of case-control studies included in the meta-analysis
| Author / Year | Country | Sample size | Study design | Age, yr | Main exposure definition | Exposure cut-off point | Accessment of cognitive function | Effect size and crude association results with 95%CI highest vs. lowest category | Effect size and adjusted association results with 95%CI highest vs. lowest category | Adjustment | Quality scores |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gil-Montoya et al (2015) [37] | Spain | 409 | Case-control | > 50 | Number of teeth present | 20–32, 10–19, 1–9 |
DSM-IV | 1.76 (1.05–2.95) | 1.25 (0.67–2.36) | Age, sex, clinical attachment loss, oral hygiene habits, and hyperlipidemia | 13 |
| Gatz et al (2006) [27] | Sweden | 3373 | Case-control | 59–107 | Number of teeth missing | All, Half, Has all teeth |
Clinical diagnostic evaluations for dementia | 1.74 (1.35–2.24) | 1.49 (1.14–1.95) | Age, sex, education, mentally stimulating activities, physical exercise, parents’ social class, short adult height | 12 |
| Kondo et al (1994) [38] | Japan | 180 | Case-control | 43–89 | Number of teeth missing | More than half of the teeth, Total denture with no own teeth | DSM-III R | 1.90 (1.00–3.60) | – | – | 11 |
Notes: DSM-IV The Diagnostic and Statistical Manual of Mental Disorder, Fourth Edition, DSM-III R Diagnostic and Statistical Manual of Mental Disorders Third Edition, Revised
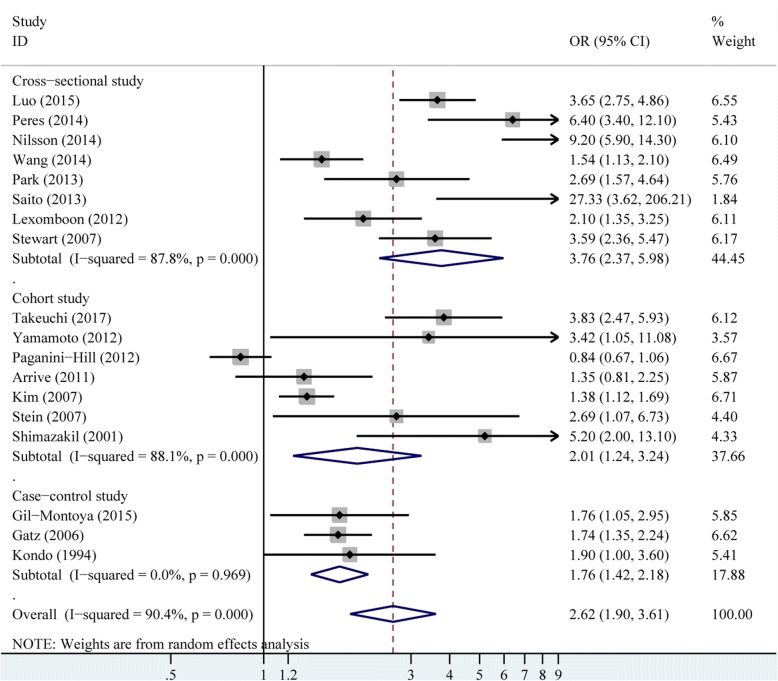
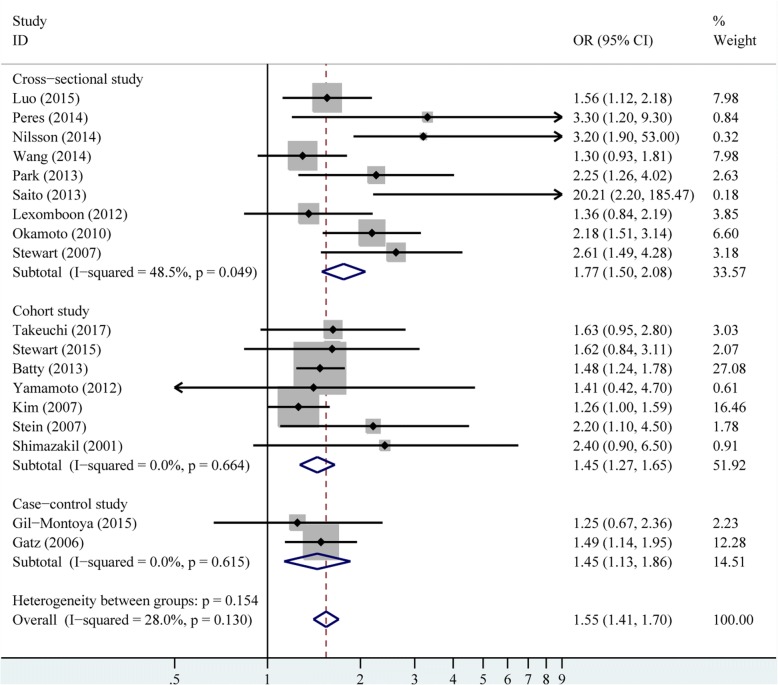
There were 18 studies provided crude estimates for the risk of dementia. The pooled crude results revealed that patients with fewer tooth remaining had higher incidence of dementia (OR 2.62, 95% CI 1.90–3.61), with significant heterogeneity among these studies (P < 0.001, I2 = 90.40%), as shown in Fig. 2. The random-effects model was used for the crude results. The heterogeneity was also explored by subgroup and meta-regression analysis for the crude model (Table 4). The study design and sample size explained about 16.52% and 6.90% of the heterogeneity, respectively. There were 18 studies presenting adjusted estimates for the risk of dementia. The adjusted results remained significant when only adjusted results were pooled (OR 1.55, 95% CI 1.41–1.70), without obvious heterogeneity (P = 0.13, I2 = 28.00%; Fig. 3).
Fig. 2.
Pooled effect of crude results of tooth loss on dementia risk
Table 4.
Random-effect meta-analyses of tooth loss and dementia risk by subgroup and meta-regression analyses
| Studies with crude results | ||||
|---|---|---|---|---|
| Number of estimates | Pooled OR and 95% CI | P-value | % heterogeneity explained | |
| Study design | 16.52 | |||
| Cross-sectional | 8 | 3.76 (2.37–5.98) | < 0.001 | |
| Cohort | 7 | 2.10 (1.24–3.24) | < 0.001 | |
| Case-control | 3 | 1.76 (1.42–2.18) | 0.969 | |
| Sample size | 6.90 | |||
| >1000 | 8 | 3.26 (1.79–5.93) | < 0.001 | |
| <1000 | 10 | 1.95 (1.51–2.52) | 0.008 | |
| Study region | 0 | |||
| Asia | 9 | 2.73 (1.83–4.07) | < 0.001 | |
| Europe | 6 | 2.57 (1.50–4.41) | < 0.001 | |
| America | 3 | 2.38 (0.57–9.91) | < 0.001 | |
| Cognitive assessment | 0 | |||
| MMSE | 8 | 3.12 (1.58–6.18) | < 0.001 | |
| Others | 10 | 2.38 (1.73–3.27) | < 0.001 | |
| Total | 18 | 2.62 (1.90–3.61) | < 0.001 | – |
Fig. 3.
Pooled effect of adjusted results of tooth loss on dementia risk
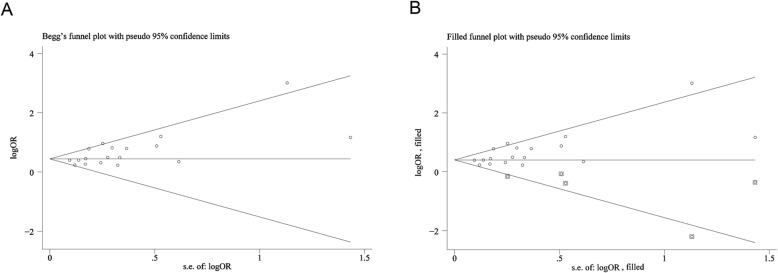
Both the Begg-Mazumdar test (P = 0.11) and Egger’s regression test (P = 0.07) showed no significant evidence of publication bias for all included studies in the crude model (Additional file 1: Figure S1 A-B). Although the Begg-Mazumdar test showed not statistically significance (P = 0.15), the Egger’s regression test (P = 0.01) revealed significant publication bias in the adjusted mode (Additional file 1: Figure S1 C-D). Therefore, the trim and fill method was conducted as a sensitivity analysis by imputing hypothetical negative unpublished studies conservatively to mirror the positive studies that cause the funnel plot asymmetry [50–52]. The symmetrical funnel plot appeared with the imputed studies and the pooled analysis remained significant, incorporating the hypothetical studies in the adjusted model (OR 1.50; 95% CI 1.36–1.64; P < 0.001; Fig. 4).
Fig. 4.
Funnel plots without and with Trim and Fill. a Begg’s funnel plot with pseudo 95% CIs of the adjusted model. b Filled funnel plot with pseudo 95% CIs of the adjusted model
Discussion
Findings from this well-designed meta-analysis of 21 observational studies add to the accumulating evidence that tooth loss is a risk factor for dementia. Results from the crude model showed an overall 162% increase in dementia risk in adults, comparing individuals with high number of tooth loss to those with low number of tooth loss. We also observed an overall 55% increase in dementia occurrence risk in the adjusted model.
In the subgroup analysis by study design, the results remained significant in both the crude model and the adjusted model. However, it is possible to observe that in the crude model the association was not noted in European studies in the subgroup analysis by study region, while it was significant in Asia studies and American studies. These findings could be partially explained by the difference of healthcare systems and dental care access among different countries as described in the previous study [40]. Indeed, the great needs for dental care have been unmet in older adult population in many countries [9].
There is no known effective management for dementia and oral diseases are pretty common worldwide, particularly among older adults. Both dementia and tooth loss can result in significant impacts on people’s quality of life. Our findings have highlighted that adults living with higher number of tooth loss may have higher risk of dementia. In the general population, a general lack of knowledge of the importance of oral health partially account for the prevalence of tooth loss. Given the importance of tooth loss in the incidence risk of cognitive decline, oral health knowledge education programs and medical insurance policies are in urgent need among older adults population [9]. Oral health care and oral hygiene education are encouraged for both patients and their caregivers. Importantly, clinicians should be aware of this association, and oral examination should be a part of comprehensive assessments for those with high risk of dementia. Timely intervention of tooth loss may infuse new hopes for decreasing the incidence of dementia.
This study is not free of limitations. Firstly, we included cross-sectional studies in this analysis. In the light of such limitation, we conducted subgroup analysis by study design and the relationship remained significant in cohort studies, cross-sectional studies, and case-control studies. Secondly, different cognitive assessments were administered to determine participants’ cognitive function and various categories of the number of tooth loss were shown in studies. Finally, there was significant heterogeneity across studies in the crude model and publication bias in the adjusted model. Therefore, we used a random-effects model throughout to incorporate heterogeneity into the current analysis and we further identified possible sources of heterogeneity through meta-regression analyses. Additionally, the trim and fill analysis showed that the overall imputation did not alter the general results, indicating the results were robust to the possibility of unpublished negative studies. Regardless of the limitations, our review presents strengths that should be pondered. To the best of authors’ knowledge, this is the first well-designed systematic review with meta-analysis revealing both the crude and adjusted association between tooth loss and risk of dementia occurrence in adults. Secondly, the included studies from different settings demonstrate that the association between tooth loss and dementia risk is a global concern. Thirdly, the large number of sample size included in this analysis decreased the sampling error to a great extent.
Conclusions
This review provides valuable evidence for the positive association between tooth loss and increased risk of dementia in adults. The association remained significant in both the crude and adjusted models. These findings may implicate clinically on improving oral health and cognitive function. However, considering the inherent limitations of the included studies, further well-designed longitudinal studies exploring the direct and indirect relationship between tooth loss and dementia are urgently needed for a more definitive conclusion.
Additional file
Figure S1. Begg’s funnel plots and Egger’s publication bias plots. (A-B) Begg’s funnel plot and Egger’s publication bias plot of the unadjusted model, respectively. (C-D) Begg’s funnel plot and Egger’s publication bias plot of the adjusted models, respectively. (TIF 117 kb).
Acknowledgements
Not applicable.
Funding
This study was supported financially by Guangzhou Science and Technology Program key projects (No. 201604020100), Guangdong Science and Technology Program (No. 2017A020211017) and Supporting Foundation of Sun Yat-sen University (No. 17ykjc17). The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication. The funding bodies of this study had no role in the study design, collection, analysis, and interpretation of data, or writing of the manuscript.
Availability of data and materials
All data that have been used are reported in the manuscript.
Abbreviations
- AMTS
Abbreviated mental test score
- BMI
Body mass index
- CES-D
The Center for epidemiologic studies depression scale
- CI
Confidence interval
- CVD
Cardiovascular disease
- DSM-III R
Diagnostic and Statistical Manual of Mental Disorders Third Edition, Revised
- DSM-IV
The Diagnostic and Statistical Manual of Mental Disorders Fourth Edition
- HR
Hazard ratio
- IL-1
Interleukin-1
- IL-6
Interleukin-6
- MAC
Mid arm circumference
- MMSE
Mini-Mental State Examination
- OR
Odds ratio
- RR
Risk ratio
- TNF-α
Tumor necrosis factor –α
- TMIG-IC
The Tokyo Metropolitan Institute of Gerontology Index of Competence
Authors’ contributions
Study design: JL, WLF, and MJJ. Literature searching and initial screening of records: WLF, MJJ, BBG, and YMW. Abstract and article screening for eligibility: SNF, WL, and YQZ. Data extraction and risk of bias assessments: WLF and MJJ. Data analysis: YX, SWL, and YL. Manuscript preparation: WLF. Manuscript editing: JL, BBG, MJJ, and SHX. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Wen-li Fang, Email: fangwl@mail2.sysu.edu.cn.
Mu-jun Jiang, Email: 459602153@qq.com.
Bei-bei Gu, Email: beibeigu719@163.com.
Ying-mei Wei, Email: whm_51@163.com.
Sheng-nuo Fan, Email: arnoldvan@163.com.
Wang Liao, Email: liaow7@mail2.sysu.edu.cn.
Yu-qiu Zheng, Email: zhengyq6@mail2.sysu.edu.cn.
Shao-wei Liao, Email: liaoshaowei2015@163.com.
Ying Xiong, Email: xyzjy09154013@163.com.
Yi Li, Email: liyi_lynn@126.com.
Song-hua Xiao, Email: xiaosh2017@163.com.
Jun Liu, Email: docliujun@hotmail.com.
References
- 1.Prince M, Comas-herrera A, Knapp M, Guerchet M, Karagiannidou M. World Alzheimer report 2016: improving healthcare for people living with dementia: coverage, quality and costs now and in the future. Alzheimer’s Dis Int. 2016; https://www.alz.co.uk/research/world-report-2016.
- 2.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al., Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2197–23. [DOI] [PubMed]
- 3.Furuta M, Yamashita Y. Oral health and swallowing problems. Curr Phys Med Rehabil Rep. 2013;1:216–222. doi: 10.1007/s40141-013-0026-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerritsen AE, Allen PF, Witter DJ, Bronkhorst EM, Creugers NH. Tooth loss and oral health-related quality of life: a systematic review and meta-analysis. Health Qual Life Outcomes. 2010;8:126. doi: 10.1186/1477-7525-8-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Musacchio E, Perissinotto E, Binotto P, Sartori L, Silvanetto F, Zambon S, et al. Tooth loss in the elderly and its association with nutritional status, socio-economic and lifestyle factors. Acta Odontol Scand. 2007;65:78–86. doi: 10.1080/00016350601058069. [DOI] [PubMed] [Google Scholar]
- 6.Marcenes W, Kassebaum NJ, Bernabe E, Flaxman A, Naghavi M, Lopez A, et al. Global burden of oral conditions in 1990–2010: a systematic analysis. J Dent Res. 92:592–7. [DOI] [PMC free article] [PubMed]
- 7.Seraj Z, Al-Najjar D, Akl M, Aladle N, Altijani Y, Zaki A, et al. The effect of number of teeth and chewing ability on cognitive function of elderly in UAE: a pilot study. Int J Dent. 2017;2017:5732748. doi: 10.1155/2017/5732748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao W, Wang X, Wang X, Cai Y, Luan Q. Association of Cognitive Function with tooth loss and mitochondrial variation in adult subjects: a community-based study in Beijing, China. Oral Dis. 2016;22:697–702. doi: 10.1111/odi.12529. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Xu H, Pan W, Wu B. Association between tooth loss and cognitive decline: a 13-year longitudinal study of Chinese older adults. PLoS One. 2017;12:e0171404. doi: 10.1371/journal.pone.0171404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsakos G, Watt RG, Rouxel PL, De OC, Demakakos P. Tooth loss associated with physical and cognitive decline in older adults. J Am Geriatr Soc. 2015;63:91–99. doi: 10.1111/jgs.13190. [DOI] [PubMed] [Google Scholar]
- 11.Mummolo S, Ortu E, Necozione S, Monaco A, Marzo G. Relationship between mastication and cognitive function in elderly in L'Aquila. Int J Clin Exp Med. 2014;7:1040–1046. [PMC free article] [PubMed] [Google Scholar]
- 12.Kaye EK, Valencia A, Baba N, Spiro A, 3rd, Dietrich T, Garcia RI. Tooth loss and periodontal disease predict poor cognitive function in older men. J Am Geriatr Soc. 2010;58:713–718. doi: 10.1111/j.1532-5415.2010.02788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beck JD, Sharp T, Koch GG, Offenbacher S. A 5-year study of attachment loss and tooth loss in community-dwelling older adults. J Periodontal Res. 1997;32:516–523. doi: 10.1111/j.1600-0765.1997.tb00567.x. [DOI] [PubMed] [Google Scholar]
- 14.Blasko I, Grubeck-Loebenstein B. Role of the immune system in the pathogenesis, prevention and treatment of Alzheimer’s disease. Drugs Aging. 2003;20:101–113. doi: 10.2165/00002512-200320020-00002. [DOI] [PubMed] [Google Scholar]
- 15.Rogers J. The inflammatory response in alzheimer's disease. J Periodontol. 2008;79:1535–1543. doi: 10.1902/jop.2008.080171. [DOI] [PubMed] [Google Scholar]
- 16.Makiura T, Ikeda Y, Hirai T, Terasawa H, Hamaue N, Minami M. Influence of diet and occlusal support on learning memory in rats behavioral and biochemical studies. Res Commun Mol Pathol Pharmacol. 2000;107:269–277. [PubMed] [Google Scholar]
- 17.Oue H, Miyamoto Y, Okada S, Koretake K, Jung CG, Michikawa M, et al. Tooth loss induces memory impairment and neuronal cell loss in APP transgenic mice. Behav Brain Res. 2013;252:318–325. doi: 10.1016/j.bbr.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 18.Luo J, Wu B, Zhao Q, Guo Q, Meng H, Yu L, et al. Association between tooth loss and cognitive function among 3063 Chinese older adults: a community-based study. PLoS One. 2015;10:e0120986. doi: 10.1371/journal.pone.0120986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peres MA, Bastos JL, Watt RG, Xavier AJ, Barbato PR, D'Orsi E. Tooth loss is associated with severe cognitive impairment among older people: findings from a population-based study in Brazil. Aging Ment Health. 2014;19:876–884. doi: 10.1080/13607863.2014.977770. [DOI] [PubMed] [Google Scholar]
- 20.Nilsson H, Berglund J, Renvert S. Tooth loss and cognitive functions among older adults. Acta Odontol Scand. 2014;72:639–644. doi: 10.3109/00016357.2014.882983. [DOI] [PubMed] [Google Scholar]
- 21.Park H, Suk SH, Cheong JS, Lee HS, Chang H, Do SY, et al. Tooth loss may predict poor cognitive function in community-dwelling adults without dementia or stroke: the PRESENT project. J Korean Med Sci. 2013;28:1518–1521. doi: 10.3346/jkms.2013.28.10.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saito Y, Sugawara N, Yasuifurukori N, Takahashi I, Nakaji S, Kimura H. Cognitive function and number of teeth in a community-dwelling population in Japan. Ann Gen Psychiatr. 2013;12:1–6. doi: 10.1186/1744-859X-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okamoto N, Morikawa M, Okamoto K, Habu N, Iwamoto J, Tomioka K, et al. Relationship of tooth loss to mild memory impairment and cognitive impairment-findings from the Fujiwara-Kyo study. Behav Brain Funct. 2010;6:77. doi: 10.1186/1744-9081-6-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stewart R, Hirani V. Dental health and cognitive impairment in an English national survey population. J Am Geriatr Soc. 2007;55:1410–1414. doi: 10.1111/j.1532-5415.2007.01298.x. [DOI] [PubMed] [Google Scholar]
- 25.Batty G, Li Q, Huxley R, Zoungas S, Taylor BA, Neal B, et al. Oral disease in relation to future risk of dementia and cognitive decline: prospective cohort study based on the action in diabetes and vascular disease: Preterax and Diamicron modified-release controlled evaluation (ADVANCE) trial. Eur Psychiat. 2013;28:49–52. doi: 10.1016/j.eurpsy.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stein PS, Desrosiers M, Donegan SJ, Yepes JF, Kryscio RJ. Tooth loss, dementia and neuropathology in the Nun study. J Am Dent Assoc. 2007;138:1314–1322. doi: 10.14219/jada.archive.2007.0046. [DOI] [PubMed] [Google Scholar]
- 27.Gatz M, Mortimer JA, Fratiglioni L, Johansson B, Berg S, Reynolds CA, et al. Potentially modifiable risk factors for dementia in identical twins. Alzheimers Dement. 2006;2:110–117. doi: 10.1016/j.jalz.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Wang TF, Chen YY, Liou YM, Chou C. Investigating tooth loss and associated factors among older Taiwanese adults. Arch Gerontol Geri. 2014;58:446–453. doi: 10.1016/j.archger.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Lexomboon D, Trulsson M, Wårdh I, Parker MG. Chewing ability and tooth loss: association with cognitive impairment in an elderly population study. J Am Geriatr Soc. 2012;60:1951–1956. doi: 10.1111/j.1532-5415.2012.04154.x. [DOI] [PubMed] [Google Scholar]
- 30.Takeuchi K, Ohara T, Furuta M, Takeshita T, Shibata Y, Hata J, et al. Tooth loss and risk of dementia in the community: the Hisayama study. J Am Geriatr Soc. 2017;65(5):e95–e100. doi: 10.1111/jgs.14791. [DOI] [PubMed] [Google Scholar]
- 31.Stewart R, Stenman U, Hakeberg M, Hagglin C, Gustafson D, Skoog I. Associations between oral health and risk of dementia in a 37-year follow-up study: the prospective population study of women in Gothenburg. J Am Geriatr Soc. 2015;63:100–105. doi: 10.1111/jgs.13194. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto T, Kondo K, Hirai H, Nakade M, Aida J, Hirata Y. Association between self-reported dental health status and onset of dementia: a 4-year prospective cohort study of older Japanese adults from the Aichi Gerontological evaluation study (AGES) project. Psychosom Med. 2012;74:241–248. doi: 10.1097/PSY.0b013e318246dffb. [DOI] [PubMed] [Google Scholar]
- 33.Paganini-Hill A, White SC, Atchison KA. Dentition, dental health habits, and dementia: the leisure world cohort study. J Am Geriatr Soc. 2012;60:1556–1563. doi: 10.1111/j.1532-5415.2012.04064.x. [DOI] [PubMed] [Google Scholar]
- 34.Arrivé E, Letenneur L, Matharan F, Laporte C, Helmer C, Barberger-Gateau P, et al. Oral health condition of French elderly and risk of dementia: a longitudinal cohort study: a longitudinal cohort study. Community Dent Oral Epidemiol. 2011;40:230–238. doi: 10.1111/j.1600-0528.2011.00650.x. [DOI] [PubMed] [Google Scholar]
- 35.Kim JM, Stewart R, Prince M, Kim SW, Yang SJ, Shin IS, et al. Dental health, nutritional status and recent-onset dementia in a Korean community population. Int J Geriatr Psychiatry. 2007;22:850–855. doi: 10.1002/gps.1750. [DOI] [PubMed] [Google Scholar]
- 36.Shimazaki Y, Soh I, Saito T, Yamashita Y, Koga T, Miyazaki H, et al. Influence of dentition status on physical disability, mental impairment, and mortality in institutionalized elderly people. J Dent Res. 2001;80(1):340–345. doi: 10.1177/00220345010800010801. [DOI] [PubMed] [Google Scholar]
- 37.Gil-Montoya JA, Sanchezlara I, Carneropardo C, Fornieles F, Montes J, Vilchez R, et al. Is periodontitis a risk factor for cognitive impairment and dementia? a case-control study. J Periodontol. 2015;86:244–253. doi: 10.1902/jop.2014.140340. [DOI] [PubMed] [Google Scholar]
- 38.Kondo K, Niino M, Shido K. A case-control study of Alzheimer's disease in Japan-significance of life-styles. Dementia. 1994;5:314–326. doi: 10.1159/000106741. [DOI] [PubMed] [Google Scholar]
- 39.Shen T, Lv J, Wang L, Wang W, Zhang D. Association between tooth loss and dementia among older people: a meta-analysis. Int J Geriatr Psychiatry. 2015;31:953–955. doi: 10.1002/gps.4396. [DOI] [PubMed] [Google Scholar]
- 40.Oh B, Han DH, Han KT, Liu XB, Ukken J, Chang C, et al. Association between residual teeth number in later life and incidence of dementia: a systematic review and meta-analysis. BMC Geriatr. 2018;18:48. doi: 10.1186/s12877-018-0729-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seerig LM, Nascimento GG, Peres MA, Horta B, Demarco FF. Tooth loss in adults and income: systematic review and meta-analysis. J Dent. 2015;43:1051–1059. doi: 10.1016/j.jdent.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 42.Hajduk AM, Kiefe CI, Person SD, Gore JG, Saczynski JS. Cognitive change in heart failure: a systematic review. Circ Cardiovasc Qual Outcomes. 2013;6:451–460. doi: 10.1161/CIRCOUTCOMES.113.000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Placzek H, Madoff LC. The use of immunization registry-based data in vaccine effectiveness studies. Vaccine. 2011;29:399–411. doi: 10.1016/j.vaccine.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 44.Wehrmeister FC, Menezes AM, Muniz LC, Martinez-Mesa J, Domingues MR, Horta BL. Waist circumference and pulmonary function: a systematic review and meta-analysis. Syst Rev. 2012;1:55. doi: 10.1186/2046-4053-1-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ. 1997;315:1533–1537. doi: 10.1136/bmj.315.7121.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Li Y, Li X, Zhang S, Zhao J, Zhu X, et al. Head injury as a risk factor for dementia and Alzheimer's disease: a systematic review and meta-analysis of 32 observational studies. PLoS One. 2017;12:e0169650. doi: 10.1371/journal.pone.0169650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anstey KJ, von Sanden C, Salim A, O’Kearney R. Smoking as a risk factor for dementia and cognitive decline: a meta-analysis of prospective studies. Am J Epidemiol. 2007;166:367–378. doi: 10.1093/aje/kwm116. [DOI] [PubMed] [Google Scholar]
- 48.Bajaj A, Driver JA, Schernhammer ES. Parkinson’s disease and cancer risk: a systematic review and meta-analysis. Cancer Causes Control. 2010;21:697–707. doi: 10.1007/s10552-009-9497-6. [DOI] [PubMed] [Google Scholar]
- 49.Power MC, Weuve J, Gagne JJ, Mcqueen MB, Viswanathan A, Blacker D. The association between blood pressure and incident Alzheimer disease: a systematic review and meta-analysis. Epidemiology. 2011;22:646–659. doi: 10.1097/EDE.0b013e31822708b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao QF, Tan L, Wang HF, Jiang T, Tan MS, Tan L, et al. The prevalence of neuropsychiatric symptoms in Alzheimer's disease: systematic review and meta-analysis. J Affect Disord. 2016;190:264–271. doi: 10.1016/j.jad.2015.09.069. [DOI] [PubMed] [Google Scholar]
- 51.Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2007;298:2654–2664. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]
- 52.Duval S, Tweedie R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Begg’s funnel plots and Egger’s publication bias plots. (A-B) Begg’s funnel plot and Egger’s publication bias plot of the unadjusted model, respectively. (C-D) Begg’s funnel plot and Egger’s publication bias plot of the adjusted models, respectively. (TIF 117 kb).
Data Availability Statement
All data that have been used are reported in the manuscript.