Abstract
Background
Follistatin (FST), an activin-binding protein, inhibits activin action by interfering with activin binding to its receptor. The prognostic value of FST has been studied in various cancers. However, these studies rarely focus on lung cancer. In our study, we investigated the relationship between serum FST levels and lung cancer with histologic types, TNM staging, and recurrence.
Methods
A total of 150 serum samples were collected, including 91 from patients with SCLC or NSCLC, 22 from patients with benign lung diseases, and 37 from healthy subjects. Enzyme-linked immunosorbent assay was used to determine serum FST levels in healthy subjects, patients with benign lung diseases and patients with lung cancers.
Results
Serum FST levels in patients with LADC, SCC, LASC, LCLC, and SCLC were much higher than those in healthy subjects and in patients with lung benign disease. A ROC curve was constructed for differentiating the lung cancer from the healthy subjects and benign lung diseases. The results indicated that the area under the ROC curve (AUC) was 0.971 and 0.728 respectively. According to TNM staging, serum FST level increased significantly in patients with stage III and IV of LADC. Moreover, serum FST expression were increased in LADC patients with different TNM category. Furthermore, we found that a higher expression of serum FST was correlated with recurrence in LADC patients.
Conclusions
The serum FST levels gradually increased with the rise of TNM staging and category in lung cancer patients. These data suggest that serum FST levels not only can be used in auxiliary diagnosis for lung cancer but also might be associated with the disease progression and metastasis of lung cancers.
Keywords: Follistatin, Small cell lung cancer, Non-small cell lung cancer, Histological types
Background
Lung cancer is a worldwide health problem, with more than 1.8 million new cases and almost 1.6 million deaths estimated in 2012 [1, 2]. Inadequate early diagnosis is one of the major reasons for the fast-growing incidence of lung cancer in recent years, which is especially common in developing countries. Thus, exploiting new diagnostic methods is essential for extending the survival of patients with lung cancer.
Tumor biomarkers, which highly express in tumors tissues, are major indicators in auxiliary diagnosis for tumor. So far, several tumor biomarkers have been applied to the clinical diagnosis, such as AFP in hepatocellular carcinoma, CEA, and CA19-9 in colorectal carcinoma and CA125 in ovarian carcinoma [3–8]. The biomarkers closely correlating with lung cancer mainly include NSE, CEA, CA19-9, CYFRA21, SCCA and PROGRP, which the specificity and susceptibility account for 20–62% [9–11]. Since tumor biomarkers in the blood can be quickly and easily obtained in a noninvasive manner, the development of potential blood-based markers will be helpful for early diagnosis of lung cancer, monitoring of disease status, development of targeted therapies, evaluation of response to therapy and survival.
Follistatin (FST), a single chain glycoprotein, is originally isolated from the follicular fluid of ovary, which can suppress follicle-stimulating hormone (FSH) secretion from anterior pituitary cells and participate in various physiological and pathological processes [12–15]. FST widely exists in gonads and extragonadal tissues, peripheral blood and cell culture supernatant [16–19]. Serum FST levels were correlated not only with pregnancy but also with various solid tumors, including gonadal cancer, gastric cancer, hepatocellular carcinoma, basal cell carcinoma, and melanoma [20–23]. The recent studies have reported that FST was aberrantly expressed in human lung adenocarcinoma cells, suggesting that FST might be a potential biomarker for diagnosis of lung adenocarcinoma [22, 24]. However, it remains unclear whether serum FST expression is associated with lung cancer patients with different histological types, TNM staging, tumor progression, and recurrence.
In this study, we firstly investigated the association of serum FST levels with patients in two broad histological subtypes of lung cancers: small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), and NSCLC was subdivided into lung adenocarcinoma (LADC), squamous cell carcinoma (SCC), lung adenosquamous cell carcinoma (LASC) and large cell lung cancer (LCLC). Next, we assessed FST expression in LADC according to TNM staging and category. Finally, we analyzed the relationship between serum FST expression and recurrence in LADC patients.
Materials and methods
Patients and healthy subjects
The subjects were chosen from both the patients with lung cancer (LC) and the patients with benign lung diseases (BLD) admitted into Tianjin Medical University Cancer Institute & Hospital between October 2014 and December 2016. All diseases were verified by pathological and cytological diagnoses. Tumor node metastasis (TNM) staging were based on the Criteria of Lung Cancer Staging from the 8th edition of the Union for International Cancer Control (UICC) and American Joint Committee on Cancer (AJCC) Cancer Staging Manual [25–27]. The healthy subjects (HSs) were selected randomly from the Physical Examination Center of Tianjin Medical University Cancer Institute & Hospital. We collected the clinical data of all the subjects, including name, age, serology, imaging (ultrasound, CT, MRI, etc.), pathology, etc. The patients with benign lung disease excluded malignant tumors, and the health subject’s imaging excluded lung disease. All subjects excluded autoimmune diseases, cardiovascular diseases, severe liver and kidney diseases, blood diseases, infectious diseases, and other malignant tumors.
Serum sample processing
Peripheral blood was collected from each patient under an empty belly in the morning of the second day after hospitalization according to the previously described methods [21]. The serum was obtained by centrifuging at 1500g for 10 min at 4 °C then stored at − 80 °C 200 μL/tube separately. The control serum samples were similarly collected from the healthy subjects in the morning on the day of their routine examination.
ELISA for serum FST
Serum FST levels in patients with LADC, SCC, LASC, LCLC and SCLC were measured by using ELISA kits (R&D Systems, Minneapolis, USA) according to the manufacturer’s instructions. Absorbance at 450 nm was measured and the serum FST levels were calculated based on the standard curve.
Statistical methods
Data were expressed as the mean ± standard deviation and the differences among groups were compared via ANOVA. A value of P < 0.05 was considered statistically significant. The diagnostic performance of FST was evaluated by nonparametric receiver operating characteristic (ROC) curves, sensitivity, specificity, the area under the ROC curve (AUC), and with 95% confidence intervals (CI). The cut-off value of FST was calculated by Youden’s index, the peak point of ‘sensitivity + specificity − 1’, according to all points of a ROC curve, and served as a standard for choosing the most suitable cut-off value. Data analyses were performed by SPSS 16.0 for Windows (SPSS Inc, Chicago, IL, USA).
Results
Clinical characteristics of healthy subjects and patients
A total of 150 serum samples were collected, including 91 patients with lung cancer, 22 benign lung diseases patients with pulmonary tuberculosis or fibroma and 37 healthy subjects. The histological type of lung cancer was identified by a pathological expert using H&E staining. TNM staging was based on the eighth TNM staging system (8-TNM) [25–27]. Characteristics of healthy subjects, patients with benign lung diseases and patients with lung cancer were presented in Table 1. Table 2 showed the association of serum FST levels with gender and age, we found that serum FST levels had no significant correlation with gender and age. As LADC ranks first in the incidence of lung cancers [28], the category and staging of TNM in the patients with LADC were independently shown in Table 3.
Table 1.
Characteristics of patients with lung cancer (LC), patients with benign lung diseases (BLD) and healthy subjects (HSs)
| Total (n) | Gender | Age | |||
|---|---|---|---|---|---|
| Male (n) | Female (n) | < 60 years | ≥ 60 years | ||
| Healthy subjects | 37 | 25 | 12 | 26 | 11 |
| Benign lung diseases | 22 | 10 | 12 | 18 | 4 |
| Lung cancer | 91 | 63 | 28 | 44 | 47 |
| NSCLC | 67 | 43 | 24 | 32 | 35 |
| LADC | 33 | 16 | 17 | 17 | 16 |
| SCC | 29 | 23 | 6 | 12 | 17 |
| LASC | 2 | 2 | 0 | 0 | 2 |
| LCLC | 3 | 2 | 1 | 2 | 1 |
| SCLC | 24 | 20 | 4 | 13 | 11 |
| Total | 150 | 98 | 52 | 88 | 62 |
Table 2.
Characteristics of serum FST levels in subjects by gender and age
| Total (n) | Mean ± SD, pg/mL (male) | Mean ± SD, pg/mL (female) | Mean ± SD, pg/mL (< 60 years) | Mean ± SD, pg/mL (≥ 60 years) | |
|---|---|---|---|---|---|
| Healthy subjects | 37 | 780.89 ± 122.37 | 716.76 ± 85.24 | 746.93 ± 83.80 | 787.52 ± 159.68 |
| Benign lung diseases | 22 | 1194.48 ± 320.23 | 1251.53 ± 323.74 | 1207.00 ± 322.37 | 1309.28 ± 314.66 |
| Lung cancer | 91 | 1548.90 ± 388.54 | 1580.00 ± 360.68 | 1555.88 ± 402.03 | 1560.88 ± 359.06 |
Serum FST levels were not correlated with gender and age
Table 3.
Category and staging of LADC according to 8-TNM
| Total (n) | Gender | Age | |||
|---|---|---|---|---|---|
| Male (n) | Female (n) | < 60 years | ≥ 60 years | ||
| TNM category | |||||
| T | |||||
| T1 | 11 | 4 | 7 | 7 | 4 |
| T2 | 17 | 10 | 7 | 9 | 8 |
| T3 | 2 | 1 | 1 | 0 | 2 |
| T4 | 3 | 1 | 2 | 1 | 2 |
| N | |||||
| N0 | 17 | 10 | 7 | 7 | 10 |
| N1 | 2 | 1 | 1 | 1 | 1 |
| N2 | 12 | 4 | 8 | 9 | 3 |
| N3 | 2 | 1 | 1 | 0 | 2 |
| M | |||||
| M0 | 26 | 14 | 12 | 13 | 13 |
| M1 | 7 | 2 | 5 | 4 | 3 |
| TNM staging | |||||
| I | 14 | 9 | 5 | 7 | 7 |
| II | 5 | 2 | 3 | 2 | 3 |
| III | 7 | 3 | 4 | 4 | 3 |
| IV | 7 | 2 | 5 | 4 | 3 |
Serum levels of FST in lung cancer patients with different histological types
We firstly investigated the association between serum FST expression and the patients with lung cancer. We found that serum FST levels in patients with lung cancer were significantly higher as compared to HSs (P < 0.0001) and BLD (P < 0.001; Fig. 1).
Fig. 1.
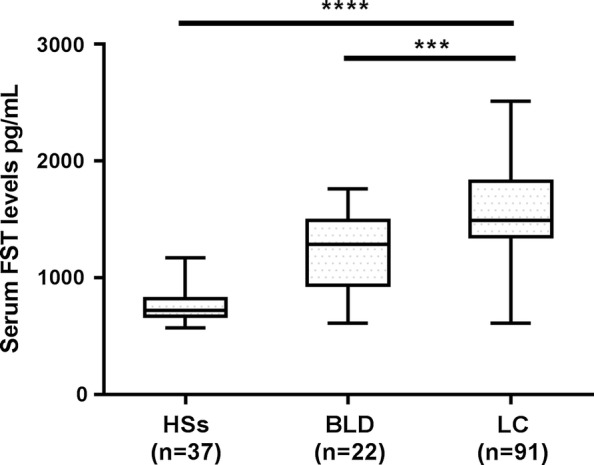
Serum FST levels in patients with lung cancer as compared with the healthy subjects and the patients with benign lung diseases. Serum FST levels (pg/mL) in the healthy subjects (HSs) (n = 37), patients with benign lung diseases (BLD) (n = 22) and patients with lung cancer (LC) (n = 91). Asterisks indicate values that are significantly different compared to that in the healthy group (***P < 0.001, ****P < 0.0001)
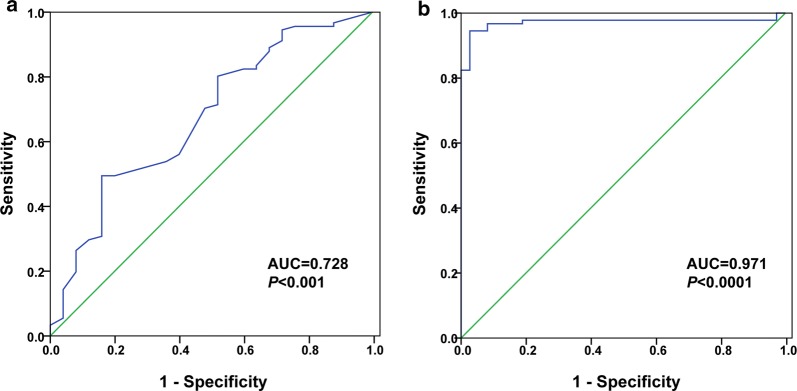
ROC curves and the area under the curve (AUC) were used to assess the performance of the serum FST level as a biomarker for lung cancer diagnosis. The results showed that the AUC was 0.728 (95% confidence interval 0.636–0.807; P < 0.001, to see Fig. 2) in differentiating LC patients from BLD, with the optimal cut-off value of 1509.55 pg/mL. The AUC was 0.971 (95% confidence interval 0.926–0.993; P < 0.0001, to see Fig. 2) in differentiating LC patients from HSs, with the optimal cut-off value of 970.74 pg/mL.
Fig. 2.
The diagnostic power of FST for lung cancer (n = 91) against the healthy subject (n = 37) and patients with benign lung disease (n = 22). a Power of FST in differentiating LC patients from BLD. Optimal cutoff value, where the sum of sensitivity and specificity was maximum, were 1509.55 pg/mL. b Power of FST in differentiating LC patients from HSs. The optimal cutoff values were 970.74 pg/mL. ROC, receiver operating characteristic; AUC, area under the curve
We next calculated the sensitivity and specificity of serum FST level in patients with different histological types of lung cancer using 1509.55 pg/mL and 970.74 pg/mL as the cut-off value respectively. We found that there were significant differences in patients with LC patients as compared to HSs and BLD. In contrast, no significant differences were found among different histological types of lung cancer (Table 4).
Table 4.
Serum FST levels in healthy subjects and patients with benign diseases or lung cancer
| Subjects | n | FST mean ± SD (range), pg/mL | LC patients vs BLD patients | BLD/LC patients vs HSs | ||
|---|---|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | |||
| Healthy subjects | 37 | 760.09 ± 115.65 (570.88–1170.24) | – | – | – | – |
| Benign lung diseases | 22 | 1225.60 ± 323.40 (608.36–1763.76)# | – | – | 68.18% | 97.30% |
| Lung cancer | 91 | 1558.47 ± 380.45 (608.36–2513.85)#** | 45.45% | 90.91% | 94.51% | 97.30% |
| LADC | 33 | 1526.71 ± 305.68 (877.29–2241.09)#** | 45.45% | 90.91% | 96.97% | 97.30% |
| SCC | 29 | 1569.30 ± 411.57 (608.36–2377.47)#** | 44.83% | 90.91% | 93.10% | 97.30% |
| LASC | 2 | 1559.17 ± 136.38 (1422.81–1695.57) | 50% | 90.91% | 100.00% | 97.30% |
| LCLC | 3 | 1877.41 ± 362.26 (1422.81–2309.28) | 66.67% | 90.91% | 100.00% | 97.30% |
| SCLC | 24 | 1549.11 ± 428.23 (608.36–2513.85)#** | 58.33% | 90.91% | 91.67% | 97.30% |
#P < 0.01 compared with healthy group; * P < 0.05, ** P < 0.01, compared with benign disease group
Taken together, these results suggested that expression of serum FST seemed likely to have a potential diagnostic value in patients with lung cancer.
Serum levels of FST in patients with LADC according to TNM staging
Since LADC is the most common histological type of lung cancer with a classical TNM staging [28], we further evaluated serum FST expression in patients with LADC according to the TNM staging. As shown in Table 5, serum FST levels significantly increased in all stage LADC, especially in stage III and IV patients as compared with HSs, BLD and the patients with I–II stage.
Table 5.
Serum FST levels in patients with LADC according to TNM staging
| n | Mean ± SD, pg/mL | Minimum–maximum, pg/mL | |
|---|---|---|---|
| Healthy subjects | 37 | 760.09 ± 115.65 | 570.88–1170.24 |
| Benign diseases | 22 | 1225.60 ± 323.40# | 608.36–1763.76 |
| LADC | 33 | ||
| I | 14 | 1404.69 ± 245.02# | 877.29–1900.14 |
| II | 5 | 1409.18 ± 199.51# | 1150.05–1763.76 |
| III | 7 | 1578.68 ± 276.90#* | 1150.05–2104.71 |
| IV | 7 | 1802.73 ± 311.12#**S | 1150.05–2241.09 |
#P < 0.01 compared with the healthy group; * P < 0.05, ** P < 0.01, compared with benign disease group; SP < 0.05, compared with I and II stage group
Serum levels of FST in patients with LADC according to T category
Serum FST levels were evaluated in patients with LADC according to T category in 8-TNM staging system. The results showed that serum FST levels were significantly increased in patients with T1 and T2 subgroups of LADC (T3 and T4 subgroups had not carried out the statistical analysis because of only 2–3 samples), compared with those in the healthy subject group and lung benign disease group, but there were no significant differences among T categories (Table 6).
Table 6.
Serum FST levels in patients with LADC according to T category
| n | Mean ± SD, pg/mL | Minimum–maximum, pg/mL | |
|---|---|---|---|
| Healthy subjects | 37 | 760.09 ± 115.65 | 570.88–1170.24 |
| Benign diseases | 22 | 1225.60 ± 323.40# | 608.36–1763.76 |
| LADC | 33 | ||
| T1 | 11 | 1492.72 ± 378.65#* | 877.29–2241.09 |
| T2 | 17 | 1474.96 ± 237.98#* | 1150.05–1900.14 |
| T3 | 2 | 1797.86 ± 170.47 | 1627.38–1968.33 |
| T4 | 3 | 1763.76 ± 192.87 | 1491.00–1900.14 |
#P < 0.01 compared with the healthy group; * P < 0.05, ** P < 0.01, compared with benign disease group
Serum levels of FST in patients with LADC according to N category
Similarly, serum FST levels were examined in patients with LADC classified as N category in 8-TNM staging system. Serum FST expression was increased in patients with different N category of LADC, and especially, significantly higher in patients with N0 and N2 category (N1 and N3 subgroups were not analyzed because of the above reasons), compared with those in healthy subject group, lung benign disease group (Table 7).
Table 7.
Serum FST levels in patients with LADC according to N category
| n | Mean ± SD, pg/mL | Minimum–maximum, pg/mL | |
|---|---|---|---|
| Healthy subjects | 37 | 760.09 ± 115.65 | 570.88–1170.24 |
| Benign diseases | 22 | 1225.60 ± 323.40# | 608.36–1763.76 |
| LADC | 33 | ||
| N0 | 17 | 1375.80 ± 214.15# | 877.29–1763.76 |
| N1 | 2 | 1525.10 ± 102.28 | 1422.81–1627.38 |
| N2 | 12 | 1672.84 ± 329.78#**S | 1150.05–2241.09 |
| N3 | 2 | 1934.24 ± 34.09 | 1900.14–1968.33 |
#P < 0.01 compared with the healthy group; * P < 0.05, ** P < 0.01, compared with benign disease group
SP < 0.05 compared with group N0
Serum levels of FST in patients with LADC according to M category
Furthermore, we assessed serum FST levels in patients with LADC classified as M category in 8-TNM staging system. A higher level of serum FST expression was found in patients with M0 and M1 category of LADC, and especially, significantly increase in patients with the M1 category, compared with those in the healthy subject group, lung benign disease group and patients with M0 group of LADC (Table 8).
Table 8.
Serum FST levels in patients with LADC according to M category
| n | Mean ± SD, pg/mL | Minimum–maximum, pg/mL | |
|---|---|---|---|
| Healthy subjects | 37 | 760.09 ± 115.65 | 570.88–1170.24 |
| Benign diseases | 22 | 1225.60 ± 323.40# | 608.36–1763.76 |
| LADC | 33 | ||
| M0 | 26 | 1452.39 ± 257.88#* | 877.29–2104.71 |
| M1 | 7 | 1802.73 ± 311.12#**S | 1150.05–2241.09 |
#P < 0.01 compared with the healthy group; * P < 0.05, ** P < 0.01, compared with benign disease group; S P < 0.05, compared with the M0 group
Serum FST levels in patients with recurrent lung cancer
Finally, serum FST levels were evaluated in patients with recurrent lung cancer. The result showed that in the diagnosed patients with recurrent, serum FST levels are much higher than those in the healthy subject group (Table 9).
Table 9.
Serum FST levels in patients with recurrent lung cancer
| N | Mean ± SD, pg/mL | Minimum–maximum, pg/mL | |
|---|---|---|---|
| Healthy subjects | 37 | 760.09 ± 115.65 | 570.88–1170.24 |
| Recurrent lung cancer group | 28 | 1209.16 ± 312.20# | 601.36–2613.85 |
#P < 0.01 compared with the healthy group
Discussion
Lung cancer is the most frequent cancer diagnosed and the leading cause of mortality in the world. Reductions in lung cancer mortality can be attained through treatment, especially if the disease is diagnosed at a stage where curative therapy is possible. Thus, it is urgent for finding new molecular biomarkers for early diagnosis of lung cancer, monitoring of disease status, development of targeted therapies, evaluation of response to therapy and survival [3].
FST is a monomeric, cysteine-rich polypeptide which suppresses pituitary FSH release in a similar manner to inhibin [13, 29]. Subsequent discovers indicate that this molecule can also play a variety of roles in several reproductive and nonreproductive systems as potent tissue regulators in the gonad, pituitary gland, pregnancy membranes, vasculature, and liver [30]. Recent studies suggest that FST, as a stress responsive protein, plays a protective role under a variety of stresses [31]. In addition, inactivation of hepatic FST may contribute to improve glucose tolerance and alleviate hyperglycemia [32, 33].
Accumulating evidence indicates that FST has been implicated in the development and progression of solid tumours [23]. Overexpression of FST was found in several human tumors, including gastric cancer [34], ovarian cancer [35], prostate cancer [36], basal cell carcinoma [37] and hepatocellular carcinoma [38]. Several recent studies revealed the closed relationship between FST and breast cancer, one of these studies by Zabkiewicz et al. showed that FST overexpression appears to promote breast cancer in vitro proliferation and reduce invasiveness [39–41]. Furthermore, FST plays also a role in angiogenesis and metastasis of solid tumours. The effect of FST on tumour angiogenesis seems to be complex, some observations in both lung- and liver-derived tumours are strongly suggestive of FST inhibiting tumour angiogenesis [42], but other evidence shows FST may have a promotory effect on tumour angiogenesis [43, 44]. Additionally, some studies support the role of FST in controlling tumor metastasis [40, 42, 45, 46]. Very recently, Seachrist et al. [47] found FST is a metastasis suppressor in a mouse model of HER2-positive breast cancer.
FST has shown strong promise as a diagnostic or prognostic marker for solid tumours. Some studies reported that serum FST levels were significantly increased in patients with ovarian cancer [21], hepatocellular cancer [48], and breast cancer [39, 49]. In the previous study, we have reported serum FST overexpression in lung adenocarcinoma [22]. However, the prognostic value of FST in the serum of lung patients with different types, TNM staging, and recurrent lung cancer remains poorly investigated.
In this study, we firstly examined serum FST levels in lung cancer patients with different histological types. We found that serum FST levels in patient group with lung cancer were significantly higher than those in the healthy control group and lung benign disease group. ROC analysis revealed that when compared LC with HSs and BLD, the serum FST levels provided a diagnosis efficacy with AUC of 0.971 and 0.728 respectively, indicating that serum FST seemed likely to have a potential role in lung cancer diagnosis. Since LADC ranks first in the incidence of lung cancers with all histological types and the studies on individualized LADC treatments are gradually intensified, we further observed the correlation of serum FST levels with LADC according to the TNM staging. Our data showed that serum FST levels had a much higher expression in patients with stage III and IV LADC. Simultaneously, our results also showed that serum FST levels increased significantly in patients with different T category of LADC, but there was no significant difference among the T categories. Moreover, serum FST levels were also elevated in patients with LADC according to N and M categories. Notably, we found that serum FST was elevated in the diagnosed patients with recurrent as compared to the healthy subjects. Taken together, the above results indicated that serum FST levels modestly reflected the disease progression and metastasis of lung cancers.
To date, the potential mechanism for an increase of serum FST levels in human carcinogenesis is not clear, a possible mechanism of FST overexpression may represent a unique strategy of tumors to overcome the inhibitory action of activin by decreasing its local bioavailability [50].
Although our data has shown a close relationship between FST expression and lung cancer with different histologic types, TNM staging and disease recurrence after surgery, thus suggesting the potential of FST as a biomarker for lung cancer diagnosis, we are aware that the sample size in this cohort is rather small, which limits the power of multivariate analyses, therefore, further validation by larger scale prospective trials is needed. Another limitation of our study is the use of one testing methodology, i.e., serum FST levels measurement by ELISA, it needs to be further corroborated by optimal tissue-based analysis of FST expression in lung cancer tissues. Furthermore, although rigorous screening in this experiment has been performed, future study needs to take into consideration of the possibility that the patients were previously treated, because local inflammatory response to therapy could also contribute to an increases of serum FST levels.
Conclusions
In summary, a significant increase of serum FST levels in the patients with lung cancer and those with recurrent lung cancer is closely related to the clinical staging of tumors. Therefore, determination of serum FST levels not only can be used in the auxiliary clinical diagnosis of lung cancer but also might be associated with tumor progression and metastasis.
Authors’ contributions
PZ, YR, and FC collected the data; PZ and JX performed the statistical analysis; PZ and XZ conceived and designed this study. PZ, JX, and XZ wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank the researchers and study participants for their contributions.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The data and materials of this work are all included in this published study.
Consent for publication
All authors are responsible for the submission of this article and accept the conditions of submission.
Ethics approval and consent to participate
The study was approved by The Ethics Committee of Tianjin Medical University Cancer Institute & Hospital, Tianjin, China.
Funding
This work was supported by the Research Project of Tianjin Medical University (No. 2014KYM04); the Natural Science Foundation of Tianjin (13JCZDJC30000); and the National Natural Science Foundation of China (No. 31101039, 31771093 and 31370891).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- FST
follistatin
- FSH
follicle-stimulating hormone
- AFP
alpha-fetoprotein
- CEA
carcinoembryonic antigen
- CA19-9
cancer antigen 19-9
- CA125
cancer antigen 125
- NSE
neuron-specific enolase
- CYFRA21
cytokeratin fragment 21
- TNM
tumor node metastasis
- LC
lung cancer
- BLD
benign lung diseases
- HSs
healthy subjects
- SCLC
small cell lung cancer
- NSCLC
non-small cell lung cancer
- LADC
lung adenocarcinoma
- SCC
squamous cell carcinoma
- LASC
lung adenosquamous cell carcinoma
- LCLC
large cell lung cancer
- ROC
receiver operating characteristic
- AUC
area under the curve
- CI
95% confidence intervals
- ELISA
enzyme-linked immunosorbent assay
- CT
computed tomography
- 8-TNM
eighth TNM staging system
- UICC
International Cancer Control
- AJCC
American Joint Committee on Cancer
Contributor Information
Pengyu Zhang, Email: pengyuzhang99@sina.com.
Yingxin Ruan, Email: yingxinruan@sina.com.
Jun Xiao, Email: xiaojun_ligno@yahoo.com.
Fangfang Chen, Email: cff@jlu.edu.cn.
Xuejun Zhang, Email: xjzhimmunology@163.com.
References
- 1.Hoffman PC, Mauer AM, Vokes EE. Lung cancer. Lancet. 2000;355(9202):479–485. doi: 10.1016/S0140-6736(00)82038-3. [DOI] [PubMed] [Google Scholar]
- 2.Field JK, Oudkerk M, Pedersen JH, Duffy SW. Prospects for population screening and diagnosis of lung cancer. Lancet. 2013;382(9893):732–741. doi: 10.1016/S0140-6736(13)61614-1. [DOI] [PubMed] [Google Scholar]
- 3.Abu Hassan SO, Nielsen DL, Tuxen MK, Petersen PH, Soletormos G. Performance of seven criteria to assess CA125 increments among ovarian cancer patients monitored during first-line chemotherapy and the post-therapy follow-up period. Future Sci OA. 2017;3(3):FSO216. doi: 10.4155/fsoa-2017-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Battaglia A, Fossati M, Buzzonetti A, Scambia G, Fattorossi A. A robust immune system conditions the response to abagovomab (anti-idiotypic monoclonal antibody mimicking the CA125 protein) vaccination in ovarian cancer patients. Immunol Lett. 2017;191:35–39. doi: 10.1016/j.imlet.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Kline JB, Kennedy RP, Albone E, Chao Q, Fernando S, McDonough JM, Rybinski K, Wang W, Somers EB, Schweizer C, et al. Tumor antigen CA125 suppresses antibody-dependent cellular cytotoxicity (ADCC) via direct antibody binding and suppressed Fc-gamma receptor engagement. Oncotarget. 2017;8(32):52045–52060. doi: 10.18632/oncotarget.19090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stojkovic Lalosevic M, Stankovic S, Stojkovic M, Markovic V, Dimitrijevic I, Lalosevic J, Petrovic J, Brankovic M, Pavlovic Markovic A, Krivokapic Z. Can preoperative CEA and CA19-9 serum concentrations suggest metastatic disease in colorectal cancer patients? Hell J Nucl Med. 2017;20(1):41–45. doi: 10.1967/s002449910505. [DOI] [PubMed] [Google Scholar]
- 7.Qin QF, Weng J, Xu GX, Chen CM, Jia CK. Combination of serum tumor markers dickkopf-1, DCP and AFP for the diagnosis of primary hepatocellular carcinoma. Asian Pac J Trop Med. 2017;10(4):409–413. doi: 10.1016/j.apjtm.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Zhang C, Wang Z, Yang Z, Wang M, Li S, Li Y, Zhang R, Xiong Z, Wei Z, Shen J, et al. Phase I escalating-dose trial of CAR-T therapy targeting CEA(+) metastatic colorectal cancers. Mol Ther. 2017;25(5):1248–1258. doi: 10.1016/j.ymthe.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spinazzi A, Soresi E, Boffi R, Nonis A, Noseda A, Cobelli S, Scanni A. Evaluation of neuron-specific enolase, tissue polypeptide antigen, and carcinoembryonic antigen as markers for staging and monitoring response to therapy of lung cancer. Cancer Detect Prev. 1994;18(3):209–220. [PubMed] [Google Scholar]
- 10.Cabrera-Alarcon JL, Carrillo-Vico A, Santotoribio JD, Leon-Justel A, Sanchez-Gil R, Gonzalez-Castro A, Guerrero JM. CYFRA 21-1 as a tool for distant metastasis detection in lung cancer. Clin Lab. 2011;57(11–12):1011–1014. [PubMed] [Google Scholar]
- 11.Romero-Ventosa EY, Blanco-Prieto S, Gonzalez-Pineiro AL, Rodriguez-Berrocal FJ, Pineiro-Corrales G, Paez de la Cadena M. Pretreatment levels of the serum biomarkers CEA, CYFRA 21-1, SCC and the soluble EGFR and its ligands EGF, TGF-alpha, HB-EGF in the prediction of outcome in erlotinib treated non-small-cell lung cancer patients. SpringerPlus. 2015;4:171. doi: 10.1186/s40064-015-0891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robertson DM, Klein R, de Vos FL, McLachlan RI, Wettenhall RE, Hearn MT, Burger HG, de Kretser DM. The isolation of polypeptides with FSH suppressing activity from bovine follicular fluid which are structurally different to inhibin. Biochem Biophys Res Commun. 1987;149(2):744–749. doi: 10.1016/0006-291X(87)90430-X. [DOI] [PubMed] [Google Scholar]
- 13.Ueno N, Ling N, Ying SY, Esch F, Shimasaki S, Guillemin R. Isolation and partial characterization of follistatin: a single-chain Mr 35,000 monomeric protein that inhibits the release of follicle-stimulating hormone. Proc Natl Acad Sci USA. 1987;84(23):8282–8286. doi: 10.1073/pnas.84.23.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicolas N, Muir JA, Hayward S, Chen JL, Stanton PG, Gregorevic P, de Kretser DM, Loveland KL, Bhushan S, Meinhardt A, et al. Induction of experimental autoimmune orchitis in mice: responses to elevated circulating levels of the activin-binding protein, follistatin. Reproduction. 2017;154(3):193–205. doi: 10.1530/REP-17-0010. [DOI] [PubMed] [Google Scholar]
- 15.Kawao N, Morita H, Obata K, Tatsumi K, Kaji H. Role of follistatin in muscle and bone alterations induced by gravity change in mice. J Cell Physiol. 2018;233(2):1191–1201. doi: 10.1002/jcp.25986. [DOI] [PubMed] [Google Scholar]
- 16.Michel U, Albiston A, Findlay JK. Rat follistatin: gonadal and extragonadal expression and evidence for alternative splicing. Biochem Biophys Res Commun. 1990;173(1):401–407. doi: 10.1016/S0006-291X(05)81072-1. [DOI] [PubMed] [Google Scholar]
- 17.Kogawa K, Ogawa K, Hayashi Y, Nakamura T, Titani K, Sugino H. Immunohistochemical localization of follistatin in rat tissues. Endocrinol Japonica. 1991;38(4):383–391. doi: 10.1507/endocrj1954.38.383. [DOI] [PubMed] [Google Scholar]
- 18.Liu ZH, Shintani Y, Sakamoto Y, Harada K, Zhang CY, Fujinaka Y, Abe M, Goto T, Saito S. Effects of LHRH, FSH and activin A on follistatin secretion from cultured rat anterior pituitary cells. Endocr J. 1996;43(3):321–327. doi: 10.1507/endocrj.43.321. [DOI] [PubMed] [Google Scholar]
- 19.Seder CW, Arndt AT, Jordano L, Basu S, Fhied CL, Sayidine S, Chmielewski GW, Gallo K, Liptay MJ, Borgia JA. Serum biomarkers may prognosticate recurrence in node-negative, non-small cell lung cancers less than 4 centimeters. Ann Thorac Surg. 2017;104(5):1637–1643. doi: 10.1016/j.athoracsur.2017.06.036. [DOI] [PubMed] [Google Scholar]
- 20.Wakatsuki M, Shintani Y, Abe M, Liu ZH, Shitsukawa K, Saito S. Immunoradiometric assay for follistatin: serum immunoreactive follistatin levels in normal adults and pregnant women. J Clin Endocrinol Metab. 1996;81(2):630–634. doi: 10.1210/jcem.81.2.8636280. [DOI] [PubMed] [Google Scholar]
- 21.Ren P, Chen FF, Liu HY, Cui XL, Sun Y, Guan JL, Liu ZH, Liu JG, Wang YN. High serum levels of follistatin in patients with ovarian cancer. J Int Med Res. 2012;40(3):877–886. doi: 10.1177/147323001204000306. [DOI] [PubMed] [Google Scholar]
- 22.Chen F, Ren P, Feng Y, Liu H, Sun Y, Liu Z, Ge J, Cui X. Follistatin is a novel biomarker for lung adenocarcinoma in humans. PLoS ONE. 2014;9(10):e111398. doi: 10.1371/journal.pone.0111398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi L, Resaul J, Owen S, Ye L, Jiang WG. Clinical and therapeutic implications of follistatin in solid tumours. Cancer Genom Proteom. 2016;13(6):425–435. doi: 10.21873/cgp.20005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoda MA, Rozsas A, Lang E, Klikovits T, Lohinai Z, Torok S, Berta J, Bendek M, Berger W, Hegedus B, et al. High circulating activin A level is associated with tumor progression and predicts poor prognosis in lung adenocarcinoma. Oncotarget. 2016;7(12):13388–13399. doi: 10.18632/oncotarget.7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 26.Choi HS, Jeong BK, Jeong H, Lee YH, Ha IB, Song JH, Kang KM. Application of the new 8th TNM staging system for non-small cell lung cancer: treated with curative concurrent chemoradiotherapy. Radiat Oncol. 2017;12(1):122. doi: 10.1186/s13014-017-0848-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kay FU, Kandathil A, Batra K, Saboo SS, Abbara S, Rajiah P. Revisions to the tumor, node, metastasis staging of lung cancer (8(th) edition): rationale, radiologic findings and clinical implications. World J Radiol. 2017;9(6):269–279. doi: 10.4329/wjr.v9.i6.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saito M, Shiraishi K, Kunitoh H, Takenoshita S, Yokota J, Kohno T. Gene aberrations for precision medicine against lung adenocarcinoma. Cancer Sci. 2016;107(6):713–720. doi: 10.1111/cas.12941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esch FS, Shimasaki S, Mercado M, Cooksey K, Ling N, Ying S, Ueno N, Guillemin R. Structural characterization of follistatin: a novel follicle-stimulating hormone release-inhibiting polypeptide from the gonad. Mol Endocrinol. 1987;1(11):849–855. doi: 10.1210/mend-1-11-849. [DOI] [PubMed] [Google Scholar]
- 30.Phillips DJ, de Kretser DM. Follistatin: a multifunctional regulatory protein. Front Neuroendocrinol. 1998;19(4):287–322. doi: 10.1006/frne.1998.0169. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, Liu K, Han B, Xu Z, Gao X. The emerging role of follistatin under stresses and its implications in diseases. Gene. 2018;639:111–116. doi: 10.1016/j.gene.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 32.Tao R, Wang C, Stohr O, Qiu W, Hu Y, Miao J, Dong XC, Leng S, Stefater M, Stylopoulos N, et al. Inactivating hepatic follistatin alleviates hyperglycemia. Nat Med. 2018;24(7):1058–1069. doi: 10.1038/s41591-018-0048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leong I. Follistatin inactivation improves glucose tolerance. Nat Rev Endocrinol. 2018;14(8):439. doi: 10.1038/s41574-018-0052-y. [DOI] [PubMed] [Google Scholar]
- 34.Uchida T, Wada K, Akamatsu T, Yonezawa M, Noguchi H, Mizoguchi A, Kasuga M, Sakamoto C. A novel epidermal growth factor-like molecule containing two follistatin modules stimulates tyrosine phosphorylation of erbB-4 in MKN28 gastric cancer cells. Biochem Biophys Res Commun. 1999;266(2):593–602. doi: 10.1006/bbrc.1999.1873. [DOI] [PubMed] [Google Scholar]
- 35.Matsumura N, Huang Z, Mori S, Baba T, Fujii S, Konishi I, Iversen ES, Berchuck A, Murphy SK. Epigenetic suppression of the TGF-beta pathway revealed by transcriptome profiling in ovarian cancer. Genome Res. 2011;21(1):74–82. doi: 10.1101/gr.108803.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lall RK, Syed DN, Adhami VM, Khan MI, Mukhtar H. Dietary polyphenols in prevention and treatment of prostate cancer. Int J Mol Sci. 2015;16(2):3350–3376. doi: 10.3390/ijms16023350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim HC, Sohng SH, Shin DH, Choi JS, Bae YK. Immunohistochemical expression of cytokeratin 15, cytokeratin 19, follistatin, and Bmi-1 in basal cell carcinoma. Int J Dermatol. 2016;55(1):36–44. doi: 10.1111/ijd.12771. [DOI] [PubMed] [Google Scholar]
- 38.Zhai W, Lim TK, Zhang T, Phang ST, Tiang Z, Guan P, Ng MH, Lim JQ, Yao F, Li Z, et al. The spatial organization of intra-tumour heterogeneity and evolutionary trajectories of metastases in hepatocellular carcinoma. Nat Commun. 2017;8:4565. doi: 10.1038/ncomms14565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zabkiewicz C, Resaul J, Hargest R, Jiang WG, Ye L. Increased expression of follistatin in breast cancer reduces invasiveness and clinically correlates with better survival. Cancer Genom Proteom. 2017;14(4):241–251. doi: 10.21873/cgp.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Resaul J, Sun P-H, Mason M, Jiang WG, Ye L. Potential role of follistatin in breast cancer progression and bone metastasis. Anticancer Res. 2015;35(7):4328. [Google Scholar]
- 41.Couto HL, Dela Cruz C, Buzelin MA, Toppa NH, Wainstein AJ, Reis FM. Follistatin expression in human invasive breast tumors: pathologic and clinical associations. Appl Immunohistochem Mol Morphol. 2018;26(2):108–112. doi: 10.1097/PAI.0000000000000385. [DOI] [PubMed] [Google Scholar]
- 42.Ogino H, Yano S, Kakiuchi S, Muguruma H, Ikuta K, Hanibuchi M, Uehara H, Tsuchida K, Sugino H, Sone S. Follistatin suppresses the production of experimental multiple-organ metastasis by small cell lung cancer cells in natural killer cell-depleted SCID mice. Clin Cancer Res. 2008;14(3):660–667. doi: 10.1158/1078-0432.CCR-07-1221. [DOI] [PubMed] [Google Scholar]
- 43.Kozian DH, Ziche M, Augustin HG. The activin-binding protein follistatin regulates autocrine endothelial cell activity and induces angiogenesis. Lab Invest. 1997;76(2):267–276. [PubMed] [Google Scholar]
- 44.Krneta J, Kroll J, Alves F, Prahst C, Sananbenesi F, Dullin C, Kimmina S, Phillips DJ, Augustin HG. Dissociation of angiogenesis and tumorigenesis in follistatin- and activin-expressing tumors. Cancer Res. 2006;66(11):5686–5695. doi: 10.1158/0008-5472.CAN-05-3821. [DOI] [PubMed] [Google Scholar]
- 45.Ogino H, Yano S, Kakiuchi S, Muguruma H, Ikuta K, Doljinsuren T, Tsuchida K, Sugino H, Sone S. Follistatin, an activin-binding protein, suppresses the production of experimental multiple-organ metastasis by small cell lung cancer cells in NK-cell depleted SCID mice. Clin Exp Metastasis. 2007;24(4):297. [Google Scholar]
- 46.Talmadge JE. Follistatin as an inhibitor of experimental metastasis. Clin Cancer Res. 2008;14(3):624–626. doi: 10.1158/1078-0432.CCR-07-2216. [DOI] [PubMed] [Google Scholar]
- 47.Seachrist DD, Sizemore ST, Johnson E, Abdul-Karim FW, Weber Bonk KL, Keri RA. Follistatin is a metastasis suppressor in a mouse model of HER2-positive breast cancer. Breast Cancer Res. 2017;19(1):66. doi: 10.1186/s13058-017-0857-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tomoda T, Nouso K, Miyahara K, Kobayashi S, Kinugasa H, Toyosawa J, Hagihara H, Kuwaki K, Onishi H, Nakamura S, et al. Prognotic impact of serum follistatin in patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 2013;28(8):1391–1396. doi: 10.1111/jgh.12167. [DOI] [PubMed] [Google Scholar]
- 49.Mange A, Dimitrakopoulos L, Soosaipillai A, Coopman P, Diamandis EP, Solassol J. An integrated cell line-based discovery strategy identified follistatin and kallikrein 6 as serum biomarker candidates of breast carcinoma. J Proteom. 2016;142:114–121. doi: 10.1016/j.jprot.2016.04.050. [DOI] [PubMed] [Google Scholar]
- 50.Rossmanith W, Chabicovsky M, Grasl-Kraupp B, Peter B, Schausberger E, Schulte-Hermann R. Follistatin overexpression in rodent liver tumors: a possible mechanism to overcome activin growth control. Mol Carcinog. 2002;35(1):1–5. doi: 10.1002/mc.10068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and materials of this work are all included in this published study.