Abstract
Supplemental oxygen (O2) therapy in patients with chest pain has been a cornerstone in the treatment of suspected myocardial infarction (MI). Recent randomized controlled trials have, however, shown that supplemental O2 therapy has no positive nor negative effects on cardiovascular functions, mortality, morbidity or pain in normoxic patients with suspected MI and foremost patients with ST Elevation Myocardial Infarction (STEMI). O2 therapy in normoxic STEMI patients should therefore be omitted. More studies are needed in discussing hemodynamically unstable STEMI patients, as well as patients with non-STEMI, unstable angina and other emergency conditions.
Keywords: Oxygen, Oxygen therapy, ST elevation myocardial infarction, STEMI, Physiology, Pathology, Emergency medicine
Main text
ST Elevation Myocardial Infarction (STEMI) is the most serious manifestation of Acute Coronary Syndrome (ACS) and is by the Fourth Universal Definition of Myocardial Infarction deemed to be a Type I myocardial infarction, caused by an acute atherothrombotic coronary artery disease (CAD) [1]. The consequence of CAD is a partial or complete occlusion of a coronary artery thus contributing to the termination of oxygen (O2) supply to the myocardium, giving rise to ischemia [1, 2].
Because of its seriousness, prompt and rapid diagnosis and treatment is of high importance to reduce both mortality and morbidity [3]. The most important treatment in STEMI is Percutaneous Coronary Intervention (PCI), which should be performed as soon as possible after that the condition is diagnosed [4–6].
International guidelines also emphasize on treatment with dual antiplatelets in the emergency phase before the start of the PCI [4–6]. Previous guidelines also recommended the immediate administration of O2 to patients with diagnosed or suspected ACS, without any respect to the blood O2 saturation [7–10]. In discussing current guidelines, only the 2017 ESC Guidelines for the management of patients with STEMI [5] states that O2 should not routinely be administrated to patients with STEMI, and that only those with a blood O2 saturation < 90% or PaO2 < 60 mmHg should receive O2 therapy.
With an overwhelming scientific evidence that O2 therapy has no positive (nor negative) effects in patients with STEMI, all international, regional and local guidelines should be updated and omit O2 therapy in normoxic (≥ 90%) STEMI patients.
Oxygen therapy
The history of O2 as a medicine dates back to 1775 when the British chemist Joseph Priestly discovered O2 and stated that it could be used as a medicine [11]. It was, however, in 1900 that the first publication on the role of O2 therapy in patients with chest pain was published. It was a short letter by Dr. Charles Steele, in which he deemed that O2 therapy had relieved chest pains in one single patient he believed to have angina [12]. Ever since this letter by Dr. Steele, several studies have tried to answer how supplemental O2 therapy in both healthy and ill patients affect their cardiovascular system.
The rationale behind O2 therapy has been that by adding O2 to the patient’s blood, the myocardium can be oxygenated, which in turn will contribute to a diminished ischemic area and infarct size, thereby minimizing the risk for lethal arrhythmias [13, 14]. Studies on canines [14–16] have given some support to this theory, showing that O2 therapy decreases infarct size and ischemia in these animals. A recent study on swine, however, showed that hyperoxemia can aggravate and worsen myocardial ischemia [17].
In healthy individuals, experimental studies have shown that hyperoxemia because of supplemental O2 therapy, may contribute to negative cardiovascular effects like a decrease in coronary blood flow, arterial vasoconstriction, diminished cardiac output, an increase in the systemic vascular resistance as well as impaired blood flow to organs and tissues [18–26].
In patients with suspected as well as confirmed myocardial infarction, the role of O2 were for a long time highly inconclusive. Our knowledge gap in this matter was not because of lack of studies, but rather because of the poor methodologies used in these studies. Ever since 1900, several studies have been published on the role of O2 in patients with chest pain, coronary artery disease, cardiac failure as well as suspected and confirmed myocardial infarction. All of them have unfortunately had serious limitations and have therefore not been able to correctly answer the question of how O2 therapy affects the cardiovascular system in both healthy patients and patients with myocardial infarction and cardiac failure. The studies have either been case studies or small reports including only a few patients, thus not being generalizable, or small studies [18–20, 27–43]. Furthermore, the vast majority of the studies was conducted in the pre-PCI era and even before Troponin was used as an important part in the diagnosis of myocardial infarction.
Because of the above limitations, a Cochrane report from 2013 [44] called for randomized controlled trials to once for all answer the question about which role supplemental O2 therapy should have in patients with chest pain and suspected myocardial infarction. The authors of the report stated that “A definitive randomised controlled trial is urgently required […].”
Randomized controlled trials
Before the Cochrane reports call for a definitive randomized controlled trial (RCT) in 2013, there were already four RCTs on the role of supplemental O2 in patients with myocardial infarction; Rawles et al. from 1976 [45], Wilson et al. from 1997 [42], Ukholkina et al. from 2005 [46] and Ranchord et al. from 2012 [47].
The two first studies were conducted in the pre-PCI era. While Wilson et al. [42] found no significant differences between the patients with myocardial infarction randomized to supplemental O2 or air, Rawles et al. [45] showed that patients with myocardial infarction receiving supplemental O2, had a larger infarct size as measured with serum aspartate aminotransferase.
The study by Ukholkina et al. [46] is the only randomized study showing a positive effect of supplemental O2 therapy in patients with myocardial infarction. The study is thus highly biased because of a limited methodology [44].
Rancord et al. [47] have a sound methodology, and should be considered the first modern RCT on the role of supplemental O2 in patients with STEMI. The authors found no significant differences between the two arms (supplemental O2 vs titrated O2) with regard to infarct size as measured by cardiac Troponin T, as well as cardiac MRI (CMRI) close to one month after inclusion.
After the Cochrane report from, three more RCTs have been published discussing the role of supplemental O2 therapy in myocardial infarction; the AVOID study [48, 49], the SOCCER study [50–53] and the DETO2X study [54–57].
The main publication of the AVOID study was conducted by Stub et al. [48] in which 441 STEMI patients were randomized to supplemental O2 therapy or air. Even though the study found no significant difference in infarct size as measured by cardiac Troponin, a subset of the patients undergoing CMRI after six months, showed that those randomized to supplemental O2 therapy, had a larger infarct size as measured in absolute mass but not in percent of the left ventricle. A sub study [49] of the AVOID trial showed later that patients randomized to the O2 arm, had significantly higher cardiac Troponin rates than those randomized to the air arm.
The SOCCER study was conducted in Sweden by Khoshnood et al. and aimed to evaluate the effects of supplemental O2 in normoxic first-time STEMI patients accepted for PCI. Patients were randomized to either supplemental O2 therapy or air. All patients underwent CMRI, while only a subset of patients underwent echocardiography. Their chest pain was scored and assessed prehospital and in-hospital with the Visual Analog Scale (VAS) [50]. Ninety-four patients underwent CMRI which showed no significant difference between the two arms in discussing infarct size, myocardium at risk and myocardial salvage index [51]. Of the 87 patients undergoing echocardiography, no significant differences could be measured between the two arms in discussing left ventricular ejection fraction and wall motion score index [52]. In a recently published sub study, 111 patients were assessed in regard to chest pain to evaluate the analgesic effect of O2 therapy. Those randomized to the supplemental O2 group had significantly higher median VAS and also received significantly higher amounts of morphine. The study could not show that supplemental O2 diminished chest pain [53].
The DETO2X study was also conducted in Sweden. The main publication by Hofmann et al. included more than 6000 patients and evaluated the one-year-all-cause mortality in normoxic patients with suspected myocardial infarction randomized to supplemental O2 therapy or ambient air. The study found no significant differences between the two arms in regard to mortality nor morbidity [55]. A sub study on patients with only STEMI (n = 2807) did not show any significant differences between the two arms in regard to one-year all-cause mortality, or morbidity like myocardial infarction and cardiogenic shock [56]. In a recent published DETO2X sub study by Sparv et al. [57] on the analgesic effect of supplemental O2 therapy in patients with suspected myocardial infarction, there were no significant differences between the two arms in regard to pain nor the amount of morphine and sedatives received during PCI.
Table 1 summarizes all the RCTs.
Table 1.
A summary of the randomized controlled trials studying the effects of O2 therapy in patients with suspected or confirmed myocardial infarctions
| Author (Year) | Study Design | Outcome | Limitations |
|---|---|---|---|
| Rawles et al. [45] (1976) | Double blind. Inclusion: Suspected MI. Patients randomized to O2 or air. | IS increased in patients treated with O2 as measured by AST. No significant differences were shown between the arms in discussing mortality, malignant arrythmias and use of analgesics. | Only those with suspected MI was included,why it is uncertain how many who in fact did had a MI. The study was conducted pre-PCI era. IS was measured by AST. No description of how the randomization sequence was conducted. |
| Wilson et al. [42] (1997) | Open label. Inclusion: Confirmed MI. Patients randomized to O2 or air. |
No significant differences were shown between the arms in discussing arrhythmias as well as ST segment changes in the ECG. | The study was conducted pre-PCI era. IS was measured by AST. 16% of those initially included, fell out and was thus not analyzed in the final analysis cohort. |
| Ukholkina et al. [46] (2005) | Open label. Inclusion: Confirmed MI. Patients randomized to O2 or air. | MaR, IS and arrhythmias were significantly lower in the O2 group. | The randomization process is unclear. Many have been excluded without any discussion. IS was measured by CKMB and through ECG mapping. |
| Ranchord et al. [47] (2012) | Open label. Inclusion: STEMI/LBBB. Patients randomized to O2 or titrated O2. | No significant differences between the two arms in discussing IS as measured with cTn and MRI, as well as 30-day mortality. | Data is lacking for a considerable amount of the patients in regard to mortality. MRI was performed in a subgroup of patients surviving more than 30 days, thus giving rise to a possible selection bias. |
| Stub et al. [48] (2015) | Open label. Inclusion: STEMI. Patients randomized to O2 or air. | Patients in the O2 group had a significantly higher mean peak CK but not cTn, increased IS as measured with MRI, and a higher rate of arrythmias as well as recurrent MI. | CK is not specific for MI. MRI was conducted in only some patients, thus giving rise to a possible selection bias. MRI showed increased IS measured in grams of the LV, but not as a percentage of the LV. |
| Nehme et al. [49] (2016) | Sub study. The main study was conducted by Stub et al. (2015). | For every 100 L of O2 given to a patient, both cTnI as well as CK, increased with 1.4% and 1.2% respectively. | See limitations for Sub et al. (2015). A little over 8% of the patients were excluded since they had no cTnI measurements. |
| Khoshnood et al. [51] (2017) | Single blind. Inclusion: STEMI. Patients randomized to O2 or air. | No significant differences between the two groups in discussing MSI, MaR and IS. | MRI was conducted in only some patients, thus giving rise to a possible selection bias. |
| Khoshnood et al. [52] (2017) | Sub study. The main study was conducted by Khoshnood et al. (2017; ref. 44). | No significant differences between the groups in discussing WMSI, LVEF as well as NT-proBNP. | A considerable number of patients were excluded because they, among others, denied participation after that they were initially included. This may be a source of bias. |
| Khoshnood et al. [53] (2018) | Sub study. The main study was conducted by Khoshnood et al. (2017; ref. 44). | Before the randomization, patients in the O2 group had a significantly higher VAS and also received significantly more morphine. No significant differences between the two groups in regard to VAS at the start of the PCI or median VAS decrease from randomization to PCI. |
A considerable amount of the patients missed VAS rates and were therefore excluded. This may be a source of bias. |
| Hoffman et al. [55] (2017) | Open label. Inclusion: Suspected MI. Patients randomized to O2 or air. | No significant differences between the groups on all-cause mortality at 1 year. | The study may have been underpowered. |
| Hoffman et al. [56] (2018) | Sub study. The main study was conducted by Hoffman et al. (2017). | No significant differences between the groups in discussing all-cause mortality at 1 year, or adverse cardiac events like MI rehospitalization or cardiogenic chock. | See limitations for Hoffman et al. (2017). |
| Sparv et al. [57] (2018) | Sub study. The main study was conducted by Hoffman et al. (2017). | No significant differences between the groups in discussing analgesic effect, or the use of both sedatives and opiates during PCI. | Some of the included patients received opiates in the ambulance, why it may have decreased pain at the PCI. |
AMI Acute Myocardial Infarction, AST Aspartate Transaminase, CK Creatine Kinase, CKMB Creatine kinase-MB, cTn Cardiac Troponin, cTnI Cardiac Troponin I, ECG Electrocardiogram, IS Infarct Size, LBBB Left Bundle Branch Block, LV Left Ventricle, LVEF Left Ventricular Ejection Fraction, MaR Myocardium at Risk, MI Myocardial Infarction, MRI Magnetic Resonance Imaging, MSI Myocardial Salvage Index, O2 Oxygen, PCI Percutaneous Coronary Intervention, STEMI ST Elevation Myocardial Infarction, VAS Visual Analog Scale, WMSI Wall Motion Score Index
Omit supplemental O2 therapy in STEMI
The above RCTs clearly show that O2 therapy has so positive nor negative cardiovascular effects, when used in normoxic patients with STEMI both prehospital and in-hospital. Two recent reviews and meta-analysis on the role of supplemental O2 therapy in acute myocardial infarction, showed also no benefit of using O2 therapy in these patients [58, 59].
In discussing supplemental O2 therapy in normoxic STEMI patients, the evidences are clear and consistent, why all guidelines must be reformed to state that supplemental O2 therapy in these patients should be omitted. It is, however, of high importance to point that patients diagnosed with STEMI, and who have a low blood oxygen saturation, should receive supplemental O2. It is the routine use of O2 therapy, with no respect to blood oxygen saturation, that should be omitted (Fig. 1). With this said, it is important to point out that the RCTs presented above does also have some limitations as the majority of them have had a small cohort, and the focus have been stable and normoxic STEMI patients. These limitations might reduce the generalizability of the studies. More studies are therefore needed in discussing supplemental O2 therapy in hemodynamic unstable STEMI patients, patients with non-STEMI as well as unstable angina. This is especiCally of importance since some studies argue that supplemental O2 therapy administrated to acutely ill patients can be toxic and increase mortality and morbidity [60, 61].
Fig. 1.
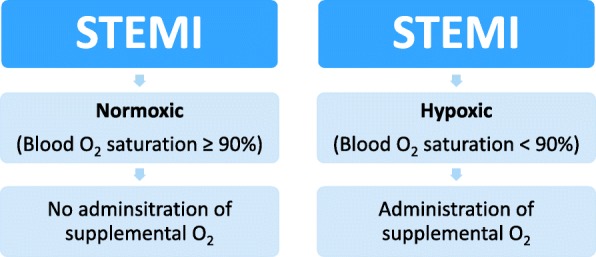
Suggestion on how to manage patients with ST Elevation Myocardial Infarction (STEMI) both in a prehospital setting and in-hospital
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Abbreviations
- ACS
Acute Coronary Syndrome
- CAD
Coronary Artery Disease
- CMRI
Cardiac Magnetic Resonance Imaging
- MI
Myocardial Infarction
- O2
Oxygen
- PCI
Percutaneous Coronary Intervention
- RCT
Randomized Controlled Trials
- STEMI
ST Elevation Myocardial Infarction
- VAS
Visual Analog Scale
Author’s contribution
The author read and approved the final manuscript.
Authors’ information
Dr. Ardavan Khoshnood is a senior resident in Emergency Medicine, and holds a PhD in Clinical Medicine, Emergency Medicine. He is a lecturer at the Medical Faculty at Lund University, and also serves as an Associate Editor for the BMC Emergency Medicine.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Dr. Khoshnood have authored several articles on the role of supplemental O2 therapy in patients with myocardial infarction and STEMI. Dr. Khoshnood is also an Associate Editor at the BMC Emergency Medicine.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD, Group ESCSD. Fourth universal definition of myocardial infarction (2018). Eur Heart J. 2018:ehy462. [DOI] [PubMed]
- 2.Heusch G, Gersh BJ. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: a continual challenge. Eur Heart J. 2017;38(11):774–784. doi: 10.1093/eurheartj/ehw224. [DOI] [PubMed] [Google Scholar]
- 3.Scholz KH, Maier SKG, Maier LS, Lengenfelder B, Jacobshagen C, Jung J, Fleischmann C, Werner GS, Olbrich HG, Ott R, et al. Impact of treatment delay on mortality in ST-segment elevation myocardial infarction (STEMI) patients presenting with and without haemodynamic instability: results from the German prospective, multicentre FITT-STEMI trial. Eur Heart J. 2018;39(13):1065–1074. doi: 10.1093/eurheartj/ehy004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Gara Patrick T., Kushner Frederick G., Ascheim Deborah D., Casey Donald E., Chung Mina K., de Lemos James A., Ettinger Steven M., Fang James C., Fesmire Francis M., Franklin Barry A., Granger Christopher B., Krumholz Harlan M., Linderbaum Jane A., Morrow David A., Newby L. Kristin, Ornato Joseph P., Ou Narith, Radford Martha J., Tamis-Holland Jacqueline E., Tommaso Carl L., Tracy Cynthia M., Woo Y. Joseph, Zhao David X. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: Executive Summary. Journal of the American College of Cardiology. 2013;61(4):485–510. doi: 10.1016/j.jacc.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 5.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevationThe task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39(2):119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 6.Excellence NIfHaC . NICE guideline (CG167) 2013. Myocardial infarction with ST-segment elevation: acute management. [Google Scholar]
- 7.Arntz H-R, Bossaert L, Filippatos GS. European resuscitation council guidelines for resuscitation 2005. Resuscitation. 2005;67:S87–S96. doi: 10.1016/j.resuscitation.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Pollack CV, Jr, Diercks DB, Roe MT, Peterson ED. 2004 American College of Cardiology/American Heart Association guidelines for the Management of Patients with ST-elevation myocardial infarction: implications for emergency department practice. Ann Emerg Med. 2005;45(4):363–376. doi: 10.1016/j.annemergmed.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Nolan JP, Deakin CD, Soar J, Böttiger BW, Smith G. European resuscitation council guidelines for resuscitation 2005. Resuscitation. 2005;67:S39–S86. doi: 10.1016/j.resuscitation.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Chavey WE, Fesmire FM, Hochman JS, Levin TN, et al. ACC/AHA 2007 guidelines for the Management of Patients with Unstable Angina/non–ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (writing committee to revise the 2002 guidelines for the Management of Patients with Unstable Angina/non–ST-elevation myocardial infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50(7):e1–e157. doi: 10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Priestly J. Experiments and observations on different kinds of air. London: St. Paul’s Church-Yard; 1775. [Google Scholar]
- 12.Steele C. Severe angina pectoris relieved by oxygen inhalations. The British Med J. 1900:1568–8.
- 13.Madias J, Hood W., Jr Reduction of precordial ST-segment elevation in patients with anterior myocardial infarction by oxygen breathing. Circulation. 1976;53(3 Suppl):I198. [PubMed] [Google Scholar]
- 14.Maroko PR, Radvany P, Braunwald E, Hale SL. Reduction of infarct size by oxygen inhalation following acute coronary occlusion. Circulation. 1975;52(3):360–368. doi: 10.1161/01.CIR.52.3.360. [DOI] [PubMed] [Google Scholar]
- 15.Sayen J, Sheldon W, Horwitz O, Kuo PT, Peirce G, Zinsser HF, Mead J. Studies of coronary disease in the experimental animal. II. Polarographic determinations of local oxygen availability in the dog's left ventricle during coronary occlusion and pure oxygen breathing. J Clin Invest. 1951;30(9):932–940. doi: 10.1172/JCI102514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly RF, Hursey TL, Parrillo JE, Schaer GL. Effect of 100% oxygen administration on infarct size and left ventricular function in a canine model of myocardial infarction and reperfusion. Am Heart J. 1995;130(5):957–965. doi: 10.1016/0002-8703(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 17.Guensch DP, Fischer K, Shie N, Lebel J, Friedrich MG. Hyperoxia exacerbates myocardial ischemia in the presence of acute coronary artery stenosis in swine. Circ Cardiovasc Interv. 2015;8(10):e002928. doi: 10.1161/CIRCINTERVENTIONS.115.002928. [DOI] [PubMed] [Google Scholar]
- 18.Milone SD, Newton GE, Parker JD. Hemodynamic and biochemical effects of 100% oxygen breathing in humans. Can J Physiol Pharmacol. 1999;77(2):124–130. doi: 10.1139/y99-010. [DOI] [PubMed] [Google Scholar]
- 19.Waring WS, Thomson AJ, Adwani SH, Rosseel AJ, Potter JF, Webb DJ, Maxwell SR. Cardiovascular effects of acute oxygen administration in healthy adults. J Cardiovasc Pharmacol. 2003;42(2):245–250. doi: 10.1097/00005344-200308000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Rousseau A, Bak Z, Janerot-Sjöberg B, Sjöberg F. Acute hyperoxaemia-induced effects on regional blood flow, oxygen consumption and central circulation in man. Acta Physiol Scand. 2005;183(3):231–240. doi: 10.1111/j.1365-201X.2005.01405.x. [DOI] [PubMed] [Google Scholar]
- 21.Bergofsky EH, Bertun P. Response of regional circulations to hyperoxia. J Appl Physiol. 1966;21(2):567–572. doi: 10.1152/jappl.1966.21.2.567. [DOI] [PubMed] [Google Scholar]
- 22.Kenmure AC, Murdoch WR, Hutton I, Cameron AJ. Hemodynamic effects of oxygen at 1 and 2 Ata pressure in healthy subjects. J Appl Physiol. 1972;32(2):223–226. doi: 10.1152/jappl.1972.32.2.223. [DOI] [PubMed] [Google Scholar]
- 23.Thomson AJ, Drummond GB, Waring WS, Webb DJ, Maxwell SR. Effects of short-term isocapnic hyperoxia and hypoxia on cardiovascular function. J Appl Physiol. 2006;101(3):809–816. doi: 10.1152/japplphysiol.01185.2005. [DOI] [PubMed] [Google Scholar]
- 24.Whitehorn W, Edelmann A, Hitchcock FA. The cardiovascular responses to the breathing of 100 per cent oxygen at normal barometric pressure. Am J Physiol-Legacy Content. 1946;146(1):61–65. doi: 10.1152/ajplegacy.1946.146.1.61. [DOI] [PubMed] [Google Scholar]
- 25.Bodetoft S, Carlsson M, Arheden H, Ekelund U. Effects of oxygen inhalation on cardiac output, coronary blood flow and oxygen delivery in healthy individuals, assessed with MRI. Eur J Emerg Med. 2011;18(1):25–30. doi: 10.1097/MEJ.0b013e32833a295e. [DOI] [PubMed] [Google Scholar]
- 26.Eggers G, Jr, Paley H, Leonard J, Warren J. Hemodynamic responses to oxygen breathing in man. J Appl Physiol. 1962;17(1):75–79. doi: 10.1152/jappl.1962.17.1.75. [DOI] [Google Scholar]
- 27.Levy RL, Barach AL. The therapeutic use of oxygen in coronary thrombosis. J Am Med Assoc. 1930;94(18):1363–1365. doi: 10.1001/jama.1930.02710440001001. [DOI] [Google Scholar]
- 28.EW B. Oxygen in high concentrations for relief of pain: in coronary thrombosis and severe angina pectoris. J Am Med Assoc. 1940;114(16):1512–1514. [Google Scholar]
- 29.HI R, FD R, CF N. One hundred per cent oxygen in the treatment of acute myocardial infarction and severe angina pectoris. J Am Med Assoc. 1950;144(5):373–375. doi: 10.1001/jama.1950.02920050013003. [DOI] [PubMed] [Google Scholar]
- 30.Neill WA. Effects of arterial hypoxemia and hyperoxia on oxygen availability for myocardial metabolism: patients with and without coronary heart disease. Am J Cardiol. 1969;24(2):166–171. doi: 10.1016/0002-9149(69)90399-3. [DOI] [PubMed] [Google Scholar]
- 31.Haque WA, Boehmer J, Clemson BS, Leuenberger UA, Silber DH, Sinoway LI. Hemodynamic effects of supplemental oxygen administration in congestive heart failure. J Am Coll Cardiol. 1996;27(2):353–357. doi: 10.1016/0735-1097(95)00474-2. [DOI] [PubMed] [Google Scholar]
- 32.McNulty PH, King N, Scott S, Hartman G, McCann J, Kozak M, Chambers CE, Demers LM, Sinoway LI. Effects of supplemental oxygen administration on coronary blood flow in patients undergoing cardiac catheterization. Am J Phys Heart Circ Phys. 2005;288(3):H1057–H1062. doi: 10.1152/ajpheart.00625.2004. [DOI] [PubMed] [Google Scholar]
- 33.Saadjian A, Paganelli F, Levy S. Hemodynamic response to oxygen Administration in Chronic Heart Failure: role of Chemoreflexes. J Cardiovasc Pharmacol. 1999;33(1):144–150. doi: 10.1097/00005344-199901000-00022. [DOI] [PubMed] [Google Scholar]
- 34.Daly WJ, Behnke RH. Hemodynamic consequences of oxygen breathing in left ventricular failure. Circulation. 1963;27(2):252–256. doi: 10.1161/01.CIR.27.2.252. [DOI] [PubMed] [Google Scholar]
- 35.Mak S, Azevedo ER, Liu PP, Newton GE. Effect of hyperoxia on left ventricular function and filling pressures in patients with and without congestive heart failure. Chest. 2001;120(2):467–473. doi: 10.1378/chest.120.2.467. [DOI] [PubMed] [Google Scholar]
- 36.Bourassa MG, Campeau L, Bois MA, Rico O. The effects of inhalation of 100 per cent oxygen on myocardial lactate metabolism in coronary heart disease. Am J Cardiol. 1969;24(2):172–177. doi: 10.1016/0002-9149(69)90400-7. [DOI] [PubMed] [Google Scholar]
- 37.Foster GL, Casten GG, Reeves T, Hurst wSADC. The effects of oxygen breathing in patients with acute myocardial infarction. Cardiovasc Res. 1969;3(2):179–189. doi: 10.1093/cvr/3.2.179. [DOI] [PubMed] [Google Scholar]
- 38.Ganz W, Donoso R, Marcus H, Swan H. Coronary hemodynamics and myocardial oxygen metabolism during oxygen breathing in patients with and without coronary artery disease. Circulation. 1972;45(4):763–768. doi: 10.1161/01.CIR.45.4.763. [DOI] [PubMed] [Google Scholar]
- 39.Kenmure A, Murdoch W, Beattie A, Marshall J, Cameron A. Circulatory and metabolic effects of oxygen in myocardial infarction. Br Med J. 1968;4(5627):360–364. doi: 10.1136/bmj.4.5627.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stone GW, Martin JL, de Boer M-J, Margheri M, Bramucci E, Blankenship JC, Metzger DC, Gibbons RJ, Lindsay BS, Weiner BH. Effect of supersaturated oxygen delivery on infarct size after percutaneous coronary intervention in acute myocardial infarction. Circ Cardiovasc Interv. 2009;2(5):366–375. doi: 10.1161/CIRCINTERVENTIONS.108.840066. [DOI] [PubMed] [Google Scholar]
- 41.Lancaster R, McNicol M. Oxygen therapy in myocardial infarction. Postgrad Med J. 1967;43(505):706. doi: 10.1136/pgmj.43.505.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson A, Channer K. Hypoxaemia and supplemental oxygen therapy in the first 24 hours after myocardial infarction: the role of pulse oximetry. J R Coll Physicians Lond. 1997;31(6):657–661. [PMC free article] [PubMed] [Google Scholar]
- 43.Madias JE, Madias NE, Hood WB. Precordial ST-segment mapping. 2. Effects of oxygen inhalation on ischemic injury in patients with acute myocardial infarction. Circulation. 1976;53(3):411–417. doi: 10.1161/01.CIR.53.3.411. [DOI] [PubMed] [Google Scholar]
- 44.Cabello JB, Burls A, Emparanza JI, Bayliss S, Quinn T. Oxygen therapy for acute myocardial infarction. The Cochrane database of systematic reviews. 2013;2013(8):CD007160. doi: 10.1002/14651858.CD007160.pub3. [DOI] [PubMed] [Google Scholar]
- 45.Rawles J, Kenmure A. Controlled trial of oxygen in uncomplicated myocardial infarction. Br Med J. 1976;1(6018):1121–1123. doi: 10.1136/bmj.1.6018.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ukholkina GBKI, Kuchkina NV, Grendo EP, Gofman I. Oxygen therapy in combination with endovascular reperfusion during the first hours of acute myocardial infarction: clinical and laboratory findings. Int J Interv Cardioangiology. 2005;9:45–51. [Google Scholar]
- 47.Ranchord AM, Argyle R, Beynon R, Perrin K, Sharma V, Weatherall M, Simmonds M, Heatlie G, Brooks N, Beasley R. High-concentration versus titrated oxygen therapy in ST-elevation myocardial infarction: a pilot randomized controlled trial. Am Heart J. 2012;163(2):168–175. doi: 10.1016/j.ahj.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 48.Stub D, Smith K, Bernard S, Nehme Z, Stephenson M, Bray JE, Cameron P, Barger B, Ellims AH, Taylor AJ: Air versus oxygen in ST-segment elevation myocardial infarction. Circulation 2015:CIRCULATIONAHA. 114.014494. [DOI] [PubMed]
- 49.Nehme Z, Stub D, Bernard S, Stephenson M, Bray JE, Cameron P, Meredith IT, Barger B, Ellims AH, Taylor AJ. Effect of supplemental oxygen exposure on myocardial injury in ST-elevation myocardial infarction. Heart. 2016; heartjnl-2015-308636. [DOI] [PubMed]
- 50.Khoshnood A, Carlsson M, Akbarzadeh M, Bhiladvala P, Roijer A, Bodetoft S, Höglund P, Zughaft D, Todorova L, Erlinge D. The effects of oxygen therapy on myocardial salvage in ST elevation myocardial infarction treated with acute percutaneous coronary intervention: the supplemental oxygen in catheterized coronary emergency reperfusion (SOCCER) study. Cardiology. 2015;132(1):16–21. doi: 10.1159/000398786. [DOI] [PubMed] [Google Scholar]
- 51.Khoshnood A, Carlsson M, Akbarzadeh M, Bhiladvala P, Roijer A, Nordlund D, Höglund P, Zughaft D, Todorova L, Mokhtari A. Effect of oxygen therapy on myocardial salvage in ST elevation myocardial infarction: the randomized SOCCER trial. Eur J Emerg Med. 2018;25(2):78–84. doi: 10.1097/MEJ.0000000000000431. [DOI] [PubMed] [Google Scholar]
- 52.Khoshnood A, Akbarzadeh M, Roijer A, Meurling C, Carlsson M, Bhiladvala P, Höglund P, Sparv D, Todorova L, Mokhtari A. Effects of oxygen therapy on wall-motion score index in patients with ST elevation myocardial infarction—the randomized SOCCER trial. Echocardiography. 2017;34(8):1130–1137. doi: 10.1111/echo.13599. [DOI] [PubMed] [Google Scholar]
- 53.Khoshnood A, Akbarzadeh M, Carlsson M, Sparv D, Bhiladvala P, Mokhtari A, Erlinge D, Ekelund U. Effect of oxygen therapy on chest pain in patients with ST elevation myocardial infarction: results from the randomized SOCCER trial. Scand Cardiovasc J. 2018;52(2):69–73. doi: 10.1080/14017431.2018.1439183. [DOI] [PubMed] [Google Scholar]
- 54.Hofmann R, James SK, Svensson L, Witt N, Frick M, Lindahl B, Östlund O, Ekelund U, Erlinge D, Herlitz J. DETermination of the role of OXygen in suspected acute myocardial infarction trial. Am Heart J. 2014;167(3):322–328. doi: 10.1016/j.ahj.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 55.Hofmann R, James SK, Jernberg T, Lindahl B, Erlinge D, Witt N, Arefalk G, Frick M, Alfredsson J, Nilsson L, et al. Oxygen therapy in suspected acute myocardial infarction. N Engl J Med. 2017;377(13):1240–1249. doi: 10.1056/NEJMoa1706222. [DOI] [PubMed] [Google Scholar]
- 56.Hofmann R, Witt N, Lagerqvist B, Jernberg T, Lindahl B, Erlinge D, Herlitz J, Alfredsson J, Linder R, Omerovic E, et al. Oxygen therapy in ST-elevation myocardial infarction. Eur Heart J. 2018;39(29):2730–2739. doi: 10.1093/eurheartj/ehy326. [DOI] [PubMed] [Google Scholar]
- 57.Sparv D, Hofmann R, Gunnarsson A, James S, Hedberg C, Lauermann J, Torild P, Omerovic E, Bergström K, Haugen E et al: The analgesic effect of oxygen in suspected acute myocardial infarction. A Substudy of the DETO2X-AMI Trial 2018, 11(16):1590–1597. [DOI] [PubMed]
- 58.Abuzaid A, Fabrizio C, Felpel K, Al Ashry HS, Ranjan P, Elbadawi A, Mohamed AH, Barssoum K, Elgendy IY. Oxygen therapy in patients with acute myocardial infarction: a systemic review and meta-analysis. Am J Med. 2018;131(6):693–701. doi: 10.1016/j.amjmed.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 59.Sepehrvand N, James SK, Stub D, Khoshnood A, Ezekowitz JA, Hofmann R. Effects of supplemental oxygen therapy in patients with suspected acute myocardial infarction: a meta-analysis of randomised clinical trials. Heart. 2018. [DOI] [PubMed]
- 60.Girardis M, Busani S, Damiani E, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the oxygen-icu randomized clinical trial. JAMA. 2016;316(15):1583–1589. doi: 10.1001/jama.2016.11993. [DOI] [PubMed] [Google Scholar]
- 61.Chu DK, Kim LHY, Young PJ, Zamiri N, Almenawer SA, Jaeschke R, Szczeklik W, Schünemann HJ, Neary JD, Alhazzani W. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391(10131):1693–1705. doi: 10.1016/S0140-6736(18)30479-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


