Abstract
We evaluated, by means of a non-invasive procedure based on MRI, the masticatory muscular microstructure in a 55-year-old-female patient affected by bruxism. The patient underwent MR examination before and after 1 month of splint therapy, when she mentioned the complete disappearance of all symptoms. By means of diffusion tensor imaging we observed changes at microstructural level of masticatory muscular complex. We conclude that diffusion tensor imaging may be a useful instrument both to perform panoramic reconstruction of the masticatory muscle complex and to investigate microstructural modifications related to the pain relief in bruxism.
Introduction
Bruxism has been defined as a repetitive jaw muscle activity characterized by clenching or grinding of teeth as well as by bracing or thrusting of the mandible.1–3 It is often misdiagnosed, and can cause dental wear, jaw muscle pain and fatigue, temporal or chronic headaches with neck irradiation.4
Several therapies, both medical and mechanical, have been proposed in order to treat bruxism, with different results.3 In order to objectively evaluate the effect of mechanical therapy on masticatory muscle on patients affected by bruxism, surface electromyography is commonly used.5 Unfortunately this technique is not able to provide information about each single component of masticatory muscle complex, and is usually adopted only for masseter muscle analysis.5
Herein we evaluated the masticatory muscular microstructure in a patient affected by bruxism, pre- and post-splint therapy. To this end, we adopted a MRI non-invasive procedure, called diffusion tensor imaging (DTI) tractography. By means of DTI tractography we assessed differences in muscular microstructure.
Methods and materials
Clinical evaluation
For this study we chose a 55-year-old-female referring chronic headaches with cervical irradiation, which has been lasting for 6 years. She reported previous medical treatments with analgesics and physiotherapy for several years. None of them were able to solve her chronic pain. Moreover, she referred a sense of pain and constriction mostly present in the early morning subsequent to a regular night sleep. Based on clinical history and dental wear on oral cavity inspection, the suspect of bruxism was raised. The patient refused to undergo polysomnography examination and surface electromyography. A full cover smooth splint on the superior arch was then built and dressed by the patient during the regular night sleep for 30 days. After 1 week of therapy, the patient reported a complete recovery from all symptoms. Written informed consent for the case to be published (incl. images, case history and data) was obtained from the patient for publication.
MR acquisition parameters
The subject underwent head and masticatory MRI with a 1.5T MR scanner (Ingenia, Philips Healthcare, Best, Netherlands) before undergoing splint therapy. After 1 month treatmentthe patient referred the complete disappearance of any symptom; subsequently, MRI scans were acquired again. The second MRI examination was performed to assess if DTI was able to detect any microstructural change which may be linked to pain relief. MRI protocol consisted in a 3D-T1 weighted fast field echo sequence, with repetition time (TR) of 25 ms, echo time (TE) of 4.6 ms, reconstruction matrix 256 × 256 and voxel size 1 × 1 × 1 mm, covering from the vertex to an axial plane through the body of C5. In addition, high resolution T1 weighted turbo spin echo (TSE) images were acquired in true sagittal and coronal planes of the pterygoid muscles, covering from 1 cm above the nasion to C5 level on axial planes, with TR of 500 ms, TE 9 ms, reconstruction matrix 512 × 512, slice thickness 2.5 mm and no interslice gap.
Spatial measures of length, width and height were performed on T1 weighted TSE images.
DTI sequence was acquired in the axial plane, with the same coverage of high resolution T1 weighted TSE. Following parameters were adopted: TR of 4320 ms, TE of 90 ms, reconstruction matrix 128 × 128, voxel size 2 × 2 × 2 mm; 32 unique diffusion encoding gradient directions (b = 1000 s mm–2) and one volume with no diffusion encoding (b = 0 s mm–2) were gathered.
MRI protocol lasted less than 20 min. During acquisitions, the subject laid in a comfortable supine position with a postural position of jaw, without any masticatory or talking or clenching movement. The subject was asleep for the whole MR examination time. A visual check for movement artifact on images was performed and the examination was evaluated suitable for the study, since no movement artifacts were present.
As recently described by St-Jean et al6 in order to improve image quality and analysis reliability, a denoising procedure was applied on all DTI data before the evaluation.
MR fiber tracking parameters
Linear co-registration of MRI images was performed using Statistical Parameter Mapping tool (Wellcome Trust Centre for Neuroimagin, Univeristy College of London - SPM12 - www.fil.ion.ucl.ac.uk/spm), running on the scientific computing software called Matlab [MathWorks (R)], release R2013a (https://www.mathworks.com/products/matlab.html). To this end, fractional anisotropy (FA) maps were extracted from DTI datasets;7 subsequently, T1 weighted images and FA maps were co-registered using tools described above. DTI tractography consists in following the most likely water diffusion pathway based on Diffusion Tensors estimated. diffusion tensor estimation, FA map extraction and deterministic tractography were performed using DSI Studio (http://dsi-studio.labsolver.org).7 The following parameters were adopted: fractional anisotropy threshold as termination index 0.19, angular threshold 80°, step size 0.98 mm with no smoothing, minimum fiber length 6 mm. A single seed approach was used in order to start fiber tracking in both directions. Manual regions of interest (ROIs) were drawn on DTI images to select the area where the muscle is larger, in order to increase the number of reconstructed muscle fibers. ROIs were moreover checked on coregistered T1 weighted images. Three separate ROIs were selected: medial pterygoid muscle (MPM), lateral pterygoid muscle (LPM) and masseter muscle (MM). We decided to exclude temporal muscles from DTI evaluation since it was only partially included in the field of view of the MR images: the field of view indeed included only its insertion on medial and anterior aspect of coronoid process of mandible.
Manual segmentation, slice by slice, of mandible was also performed on T1 weighted MRI images in order to have a better three-dimensional (3D) rendering visualization, as well as to ease the evaluation of spatial relationship of masticatory muscles and their morphology. Mandibular volume was set as a region of avoidance (ROA) to avoid any unreliable reconstructions going through this region.
MR fiber tracking 3D rendering
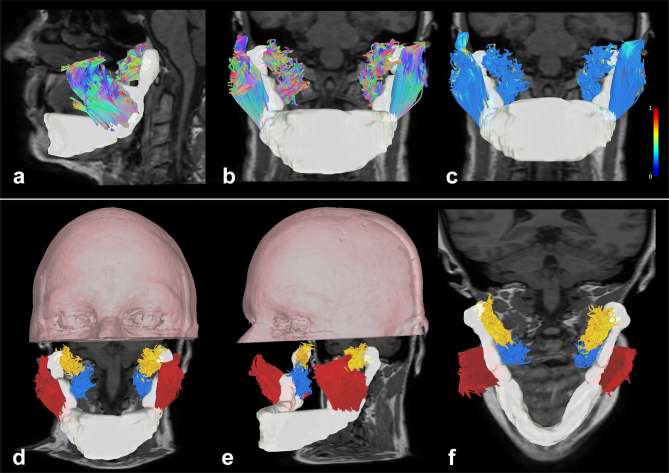
Muscle tract colors were set following the color-coded tractography convention of Pajevic and Pierpaoli for white matter fibers, with the blue color representing fibers oriented craniocaudally, the red color representing fibers oriented latero-laterally and the green color representing fibers oriented anteroposteriorly.8 A whole set of intermediate color was automatically selected from these primary colors for intermediate fiber muscle orientation (Figure 1a,b). To better discriminate muscles, an arbitrary color coding was given to all fibers belonging to a single muscle(Figure 1d–f). For each muscle group, we extracted and averaged the following diffusion tensor parameters: FA, mean diffusivity (MD), axial diffusivity (AD) and radial diffusivity (RD). FA maps were furthermore adopted for visualization purposes to highlight fiber muscle diffusivity characteristics (Figure 1c). This procedure gives us the opportunity to investigate locally FA variability. Eventually, the percentage of variation of DTI parameters was calculated to assess the amount of alterations after 1 month of therapy.
Figure 1.
Tractographic reconstructions of masticatory muscular complex. (a, b) Sagittal and frontal view of the masticatory muscular complex with a directional color coding representation, overimposed on a T1 weighted image (the mandible is reconstructed as a white volume). (c) Frontal view of the masticatory muscular complex with a local index of FA color coding representation overimposed on a T1 weighted image (lateral scale represents FA values from 0 to 1). (d–f) Volume rendering of the upper portion of the head of the patient with tractographic reconstructions of masticatory muscular complex (axial and coronal T1 weighted images are underlaid). Arbitrary colors were given to fibers: masseter muscles are represented in red, lateral pterygoid muscles are represented in yellow and medial pterygoid muscles are represented in blue.
Results
DTI tractography was able to reconstruct each component of the masticatory complex on both sides and in both pre- and post-treatment MRI examinations. The volume rendering representation of masticatory muscles did not show shape differences. No differences in length, width or height were observed on LPM, MPM and MM muscles.
Visual inspection of FA local variation in fiber muscles did not show any intramuscular abnormal peak, whereas a slight FA increase was detected in correspondence of muscular insertion.
The patient, after 1 month of splint therapy, referred the complete disappearance of any symptom. Comparison of DTI parameters pre- and post-mechanical treatment showed FA decrement and MD, AD and RD increment in each analyzed muscle. In particular, there was a mean FA decrement of 14.05% in MMs (left −20.26%; right −7.84%), 15.15% in LPMs (left −17.60%; right −12.71%) and 1.17% in MPMs (left −0.53%; right −1.81%). Average MD increased of 14.5% in MMs (left +24.80%; right +4.20%), 18.67% in LPMs (left +22.52%; right +14.83%), and 4.88% in MPMs (left +7.89%; right +1.86%). DTI parameter variations are reported in detail in Table 1.
Table 1.
Pre- and post-splint therapy diffusion tensor parameter values
| Masseter muscle | Lateral pterygoid muscle | Medial pterygoid muscle | |||||
| Left | Right | Left | Right | Left | Right | ||
| FA | Pre | 0.385 ± 0.17 | 0.395 ± 0.17 | 0.392 ± 0.12 | 0.362 ± 0.10 | 0.380 ± 0.11 | 0.386 ± 0.80 |
| Post | 0.307 ± 0.11 | 0.364 ± 0.14 | 0.323 ± 0.10 | 0.316 ± 0.09 | 0.378 ± 0.12 | 0.379 ± 0.12 | |
| Variation | ↓ 20.26 | ↓ 7.84 | ↓ 17.60 | ↓ 12.71 | ↓ 0.53 | ↓ 1.81 | |
| MD | Pre | 0.915 ± 0.41 | 0.927 ± 0.42 | 0.937 ± 0.29 | 0.998 ± 0.25 | 0.824 ± 0.19 | 0.913 ± 0.20 |
| Post | 1.142 ± 0.30 | 0.966 ± 0.31 | 1.148 ± 0.32 | 1.146 ± 0.29 | 0.889 ± 0.25 | 0.930 ± 0.25 | |
| Variation | ↑ 24.80 | ↑ 4.20 | ↑ 22.52 | ↑ 14.83 | ↑ 7.89 | ↑ 1.86 | |
| AD | Pre | 1.193 ± 0.45 | 1.215 ± 0.47 | 1.252 ± 0.33 | 1.330 ± 0.30 | 1.096 ± 0.23 | 1.206 ± 0.22 |
| Post | 1.449 ± 0.34 | 1.266 ± 0.34 | 1.467 ± 0.36 | 1.464 ± 0.32 | 1.185 ± 0.28 | 1.215 ± 0.28 | |
| Variation | ↑ 21.45 | ↑ 4.20 | ↑17.17 | ↑ 10.07 | ↑ 8.12 | ↑ 0.75 | |
| RD | Pre | 0.776 ± 0.39 | 0.783 ± 0.39 | 0.780 ± 0.18 | 0.831 ± 0.19 | 0.688 ± 0.28 | 0.767 ± 0.24 |
| Post | 0.989 ± 0.29 | 0.816 ± 0.30 | 0.989 ± 0.24 | 0.988 ± 0.24 | 0.740 ± 0.30 | 0.787 ± 0.28 | |
| Variation | ↑ 27.45 | ↑ 4.21 | ↑ 26.80 | ↑ 18.89 | ↑ 7.56 | ↑ 2.61 | |
AD, axial diffusivity; FA, fractional anisotropy; LPM, lateral pterygoid muscle; MD, mean diffusivity; MM, Masseter muscle; MPM, medial pterygoid muscle; Pre, pre splint therapy; Post, post splint therapy; RD, radial diffusivity; SD, standard deviation.
FA, MD, AD and RD values, ± SD are evaluated as mean of all tracts for each muscle. Percentage variation (variation) is represented in bold as increment (↑) and decrement (↓) values of diffusion tensor parameters between pre and post splint therapy.
Discussion
Bruxism has been estimated in 5–8% of the adult population with any documented sex differences.4 Patients with an anxious habit seem to have a higher risk to develop bruxism, although the pathogenesis is still unclear.9 The main treatments available are sleep hygiene measures combined with relaxation techniques, pharmacological therapy, contingent electrical stimulation and splint therapy.3, 10,11 Splint therapy is a mechanical treatment which cover the whole dental arch and focuses on avoidance of dental wear and muscle spasticity, which cause typical headache affecting patients suffering of bruxism.10
In this study, we investigated masticatory muscles microstructure changes in bruxism by means of DTI parameters and DTI tractography, evaluated before and after 1 month of mechanical treatment. The aim of the study was to evaluate whether it was possible to detect any microstructural alteration after mechanical therapy and how this latter may influence masticatory muscle complex. Our results showed variations in diffusion parameters of MPM, LPM and MM muscles, when comparing pre- and post-treatment MRI datasets.
Notably, the complete regression of subject’s symptoms was accompanied by a corresponding variation of diffusion metrics. On the contrary, muscles morphology, namely width, height and length, did not show any change.
DTI tractography is a technique which permits the non-invasive evaluation of microstructural as well as macroscopic properties of masticatory muscles.12–20 The use of isotropic high resolution T1 images allowed image reformatting in arbitrary planes, making the selection of ROIs and the evaluation of masticatory muscle shapes easier to perform if compared to the use of DWI b0 images only.
To the best of our knowledge, this is the first study which have evaluated the mechanical treatment outcome on masticatory muscles by means of diffusion tensor parameters.
From a physiological point of view, in muscular contraction reduction in muscular fiber length and increase of fiber muscular diameter usually occur. During contraction, fibers diameter increment may cause a bigger amount of fibers to insist over the same MR voxel; this in turn may affect diffusion tensor estimation resulting in FA increment. FA is a measure describing the amount of water molecules diffusion asymmetric displacement.21 High FA values correspond to a cigar shape of the diffusion tensor ellipsoid indicating a prominent diffusion directionality, whereas low FA values indicate the lack of a precise water molecules diffusion directionality.
In our study, mechanical therapy was able to decrease muscle contraction of masticatory complex, which was demonstrated by chronic pain and headaches disappearing. The longitudinal diffusion tensor evaluation showed FA decrease in each muscle evaluated, and similarly MD, AD and RD increment (Table 1). These changes in diffusion tensor parameters are probably related to the decrement of masticatory muscle chronic contraction, which may have influenced changes in muscular microstructure.
Several studies showed how muscular contraction and relaxation may result in heterogeneous change of diffusion parameters.13–20 Schwenzer et al reported how the passive shortening of leg muscles may cause a FA decrement with a consensual MD increment and how stretching muscles have an inverse effect on diffusion parameters.19 On the other hand, Liu et al12 detected FA increment and MD decrement, when comparing active clenching and rest position of LPM inside an MR scanner. In our study, we performed MR scans in neutral postural position with no active clenching. Moreover, gender differences have been observed.13–20 It is important to remark that our analyses are based on a single patient affected by bruxism. For this reason case-control studies involving longitudinal evaluations are needed in order to confirm and validate our results.
Conclusion
DTI tractography, a non-invasive technique based on MRI is able to easily reconstruct the masticatory muscle complex in order to assess its morphology as well as its microstructural properties. By means of diffusion tensor parameters were able to detect post-treatment changes in fiber microstructure in a patient affected by chronic bruxism.
Footnotes
Consent: Written informed consent for the case to be published (including images, case history and data) was obtained from the patient for publication.
REFERENCES
- 1.Lobbezoo F, Ahlberg J, Glaros AG, Kato T, Koyano K, Lavigne GJ, et al. . Bruxism defined and graded: an international consensus. J Oral Rehabil 2013; 40: 2–4. doi: 10.1111/joor.12011 [DOI] [PubMed] [Google Scholar]
- 2.Manfredini D, Lobbezoo F. Role of psychosocial factors in the etiology of bruxism. J Orofac Pain 2009; 23: 153–66. [PubMed] [Google Scholar]
- 3.Guaita M, Högl B. Current treatments of Bruxism. Curr Treat Options Neurol 2016; 18: 10. doi: 10.1007/s11940-016-0396-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dharmadhikari S, Romito LM, Dzemidzic M, Dydak U, Xu J, Bodkin CL, et al. . GABA and glutamate levels in occlusal splint-wearing males with possible bruxism. Arch Oral Biol 2015; 60: 1021–9. doi: 10.1016/j.archoralbio.2015.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Testa M, Geri T, Gizzi L, Falla D. High-density EMG reveals novel evidence of altered masseter muscle activity during symmetrical and asymmetrical bilateral jaw clenching tasks in people with chronic nonspecific neck pain. Clin J Pain 2017; 33: 148–59. doi: 10.1097/AJP.0000000000000381 [DOI] [PubMed] [Google Scholar]
- 6.St-Jean S, Coupé P, Descoteaux M. Non local spatial and angular matching: enabling higher spatial resolution diffusion MRI datasets through adaptive denoising. Med Image Anal 2016; 32: 115–30. doi: 10.1016/j.media.2016.02.010 [DOI] [PubMed] [Google Scholar]
- 7.Yeh FC, Verstynen TD, Wang Y, Fernández-Miranda JC, Tseng WY. Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS One 2013; 8: e80713. doi: 10.1371/journal.pone.0080713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pajevic S, Pierpaoli C. Color schemes to represent the orientation of anisotropic tissues from diffusion tensor data: application to white matter fiber tract mapping in the human brain. Magn Reson Med 1999; 42: 526–40. doi: [DOI] [PubMed] [Google Scholar]
- 9.Winocur E, Gavish A, Voikovitch M, Emodi-Perlman A, Eli I. Drugs and bruxism: a critical review. J Orofac Pain 2003; 17: 99–111. [PubMed] [Google Scholar]
- 10.Ramar K, Dort LC, Katz SG, Lettieri CJ, Harrod CG, Thomas SM, et al. . Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015. J Clin Sleep Med 2015; 11: 773–827. doi: 10.5664/jcsm.4858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shim YJ, Lee MK, Kato T, Park HU, Heo K, Kim ST. Effects of botulinum toxin on jaw motor events during sleep in sleep bruxism patients: a polysomnographic evaluation. J Clin Sleep Med 2014; 10: 291–8. doi: 10.5664/jcsm.3532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu S, Wang M, Ai T, Wang Q, Wang R, Chen W, et al. . In vivo morphological and functional evaluation of the lateral pterygoid muscle: a diffusion tensor imaging study. Br J Radiol 2016; 10: 20160041. doi: 10.1259/bjr.20160041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galbán CJ, Maderwald S, Uffmann K, de Greiff A, Ladd ME. Diffusive sensitivity to muscle architecture: a magnetic resonance diffusion tensor imaging study of the human calf. Eur J Appl Physiol 2004; 93: 253–62. doi: 10.1007/s00421-004-1186-2 [DOI] [PubMed] [Google Scholar]
- 14.Sinha S, Sinha U, Edgerton VR. In vivo diffusion tensor imaging of the human calf muscle. J Magn Reson Imaging 2006; 24: 182–90. doi: 10.1002/jmri.20593 [DOI] [PubMed] [Google Scholar]
- 15.Budzik JF, Le Thuc V, Demondion X, Morel M, Chechin D, Cotten A. In vivo MR tractography of thigh muscles using diffusion imaging: initial results. Eur Radiol 2007; 17: 3079–85. doi: 10.1007/s00330-007-0713-z [DOI] [PubMed] [Google Scholar]
- 16.Karampinos DC, King KF, Sutton BP, Georgiadis JG. In vivo study of cross-sectional skeletal muscle fiber asymmetry with diffusion-weighted MRI. Conf Proc IEEE Eng Med Biol Soc 2007; 2007: 327–30. doi: 10.1109/IEMBS.2007.4352290 [DOI] [PubMed] [Google Scholar]
- 17.Galbán CJ, Maderwald S, Uffmann K, Ladd ME. A diffusion tensor imaging analysis of gender differences in water diffusivity within human skeletal muscle. NMR Biomed 2005; 18: 489–98. doi: 10.1002/nbm.975 [DOI] [PubMed] [Google Scholar]
- 18.Galbán CJ, Maderwald S, Stock F, Ladd ME. Age-related changes in skeletal muscle as detected by diffusion tensor magnetic resonance imaging. J Gerontol A Biol Sci Med Sci 2007; 62: 453–8. doi: 10.1093/gerona/62.4.453 [DOI] [PubMed] [Google Scholar]
- 19.Schwenzer NF, Steidle G, Martirosian P, Schraml C, Springer F, Claussen CD, et al. . Diffusion tensor imaging of the human calf muscle: distinct changes in fractional anisotropy and mean diffusion due to passive muscle shortening and stretching. NMR Biomed 2009; 22: 1047–1053. doi: 10.1002/nbm.1409 [DOI] [PubMed] [Google Scholar]
- 20.Bolsterlee B, D'Souza A, Gandevia SC, Herbert RD. How does passive lengthening change the architecture of the human medial gastrocnemius muscle? J Appl Physiol 2017; 122: 727–38. doi: 10.1152/japplphysiol.00976.2016 [DOI] [PubMed] [Google Scholar]
- 21.Mukherjee P, Berman JI, Chung SW, Hess CP, Henry RG. Diffusion tensor MR imaging and fiber tractography: theoretic underpinnings. AJNR Am J Neuroradiol 2008; 29: 632–41. doi: 10.3174/ajnr.A1051 [DOI] [PMC free article] [PubMed] [Google Scholar]