Abstract
Objectives:
This study aims to answer whether acoustic radiation force impulse imaging (ARFI) can reasonably be employed in initial examination and follow-up during therapy in patients with sialolithiasis, one of the most common non-malignant disorders of the salivary glands.
Methods:
Mechanical tissue properties of affected and contralateral healthy salivary glands were analyzed by ARFI in 129 patients with sialolithiasis. In different subgroup analyses, ARFI shear wave velocity values were compared between healthy and diseased submandibular or parotid glands, salivary glands with calculi exhibiting different sizes, as well as before and after therapy. The patients’ symptoms were evaluated by a standardized questionnaire. The t-test (2 groups) or the One-way ANOVA test (>2 groups) was used for the estimation of stochastic probability in intergroup comparisons.
Results:
Submandibular or parotid glands affected by sialolithiasis were found to exhibit significant lower ARFI values as compared to the healthy contralateral glands in the same individuals. ARFI values in submandibular glands with a single calculus of more than 5 mm in diameter or with multiple calculi as well as in parotid glands with calculi exhibiting diameters of more than 5 mm were significantly higher as compared to the respective healthy contralateral glands. No significant differences in ARFI values of affected salivary glands were detected between patients with low or high symptom perception.
Conclusions:
ARFI provides an easy, quick and reliable diagnostic tool for the objective assessment of disease severity and progression in patients with sialolithiasis that can simply be implemented in pre-existing ultrasound protocols.
Introduction
Obstructive sialadenitis is the most common non-malignant disorder of the salivary glands. Caused by calculi, duct stenosis, foreign bodies, or anatomic variations of the duct system, this pathology leads to painful periprandial swelling and recurrent bacterial infections of the affected gland. As a consequence of the retention of saliva and ascending infections, remodeling processes in the glands' parenchyma occur, which may ultimately result in tissue fibrosis. With appropriate treatment, however, injured salivary glands are able to recover.1–4
Sialolithiasis represents the predominant cause of obstructive salivary gland disease. Post-mortem studies revealed a prevalence of 0.115% of calculi in salivary glands in the human population. Sialolithiasis affects the submandibular gland in more than 80% and the parotid gland in about 10% of cases. In contrast to the rare intraparenchymal calculi, the vast majority of "stones" is located in the distal third of the duct or at the hilum of the gland. Aetiopathogenic factors of lithogenesis include obstruction, reduction of the salivary flow rate, dehydration and changes in the salivary pH, which is associated with impaired crystalloid solubility.1–4 In addition, calculi formation has been suggested to be supported by retrograde migration of foods, bacteria, or foreign bodies from the oral cavity into the duct system.5 Minimal-invasive surgical techniques such as endoscopy-based methods, extracorporal shock-wave lithotripsy (ESWL) and transoral duct splitting are now standard for the treatment of obstructive salivary gland disease thereby alleviating the need for more invasive measures such as gland resection.1–4
Elastography is a technical approach that has been proven to be useful for the evaluation of malignant tumors, thyroid pathologies, or liver fibrosis.6–12 Acoustic Radiation Force Impulse Imaging (ARFI) represents a novel ultrasound elastography technology that can be integrated into conventional real-time ultrasound equipment and thereby be performed along with B-mode imaging. In ARFI, short duration acoustic pulses are emitted into the tissue under investigation. Subsequently, the impulses induce localized micro-shifts within the tissue, which allow transverse waves to spread centrifugally from the center of excitation. The shear wave velocity, which can be measured using ultrasound waves, provides an indication of the tissues elasticity. Using ARFI, we have recently demonstrated that healthy parotid glands exhibit a lower tissue elasticity as compared to healthy submandibular glands.13 In the present study, we sought to evaluate the employment of this technique in both the initial examination as well as the therapy follow-up of patients suffering from sialolithiasis.
Methods and materials
Study design and study population
This retrospective study was performed at the Department of Otorhinolaryngology, Head and Neck Surgery, Grosshadern Medical Center, Munich, Germany. The study protocol was approved by the local ethics committee and written informed consent was obtained from all patients.
Between 2010 and 2013, patients with present diseases affecting one or more salivary glands were recruited by the employees of the Department of Otorhinolaryngology and underwent a routine ultrasound examination. Subsequently, patients diagnosed with sialolithiasis were referred for elastography and had to complete a standardized questionnaire on their complaints. Ultrasound and elastography examinations as well as the completion of a standardized questionnaire by the patient were repeated after the end of successful therapy (defined by the sonographically/endoscopically validated disappearance of the calculus).
Patients with recurrent swelling of their submandibular or parotid glands and ultrasound findings documenting one or more calculi in one or more of their salivary glands were included in the study. For elastography analyses, five regions of interest were chosen in each of the parotid and submandibular glands.
Exclusion criteria
There were no exclusion criteria because the elastography measurement is considered to be a safe standard ultrasound technique.
Ultrasound imaging
Conventional ultrasound examinations were performed on a Siemens ACUSON S 2000 (Siemens Medical Systems, Erlangen, Germany) using a linear 9 MHz multifrequency transducer. The examination consisted of a conventional B-scan followed by color-coded duplex sonography. The color gain mode was employed only to the extent necessary to avoid obscuring artifacts. Additionally, automatic image gain optimization [TEQ (tissue equalization technology)] was performed. For elastography analyses, elastography ARFI software was used to measure the velocity of shear waves.
The “Virtual Touch TM Tissue Quantification” allows for the tracking of a shear wave within the region of interest as it travels perpendicularly to the transmitted longitudinal push pulse. “Time to Peak analysis” allows for the computation of a numerical value of the shear wave velocity obtained over the region of interest, which is expressed in meters per second (m/s). The stiffer the tissue, the greater the shear wave velocity.14 Thus, measurements provide quantitative information about tissue elasticity. The ultrasound device features a high performance processor and allows the documentation of dynamic image sequences in cine mode by a digital frame buffer.
Data and statistical analysis
Data analysis from digitally stored video sequence data sets was performed using a statistical software package (SigmaStat for Windows, Jandel Scientific, Erkrath, Germany). After testing for normality of data (Shapiro-Wilk test), the t-test (2 groups) or the One-way ANOVA test (>2 groups) was used for the estimation of stochastic probability in intergroup comparisons. Mean values and standard deviations are given. p values < 0.05 were considered significant.
Results
Study population
129 Caucasian patients with sialolithiasis, consisting of 64 males and 65 females, were included in our study. The mean age of the patients was 44.8 years with a range of 14 to 84 years (male mean 46.0 years, range 14 to 84 years; female mean 43.5 years, range 14 to 81 years). Of these cases, 106 patients suffered from sialolithiasis of the submandibular gland, consisting of 54 males and 52 females, and 23 patients suffered from sialolithiasis of the parotid gland, consisting of 11 males and 12 females. The mean age of the patients with sialolithiasis of the submandibular gland was 42.7 years, ranging from 14 to 84 years (male mean 45.3 years; female mean 40.0 years). The sialolith was located in the right submandibular gland in 50 patients and in the left submandibular gland in 56 patients. The mean age of the patients with sialolithiasis of the parotid gland was 54.0 years, ranging from 31 to 76 years (male mean 49.6 years; female mean 57.8 years). The sialolith was located in the right parotid gland in 9 patients and in the left parotid gland in 14 patients.
Effect of sialolithiasis on tissue elasticity
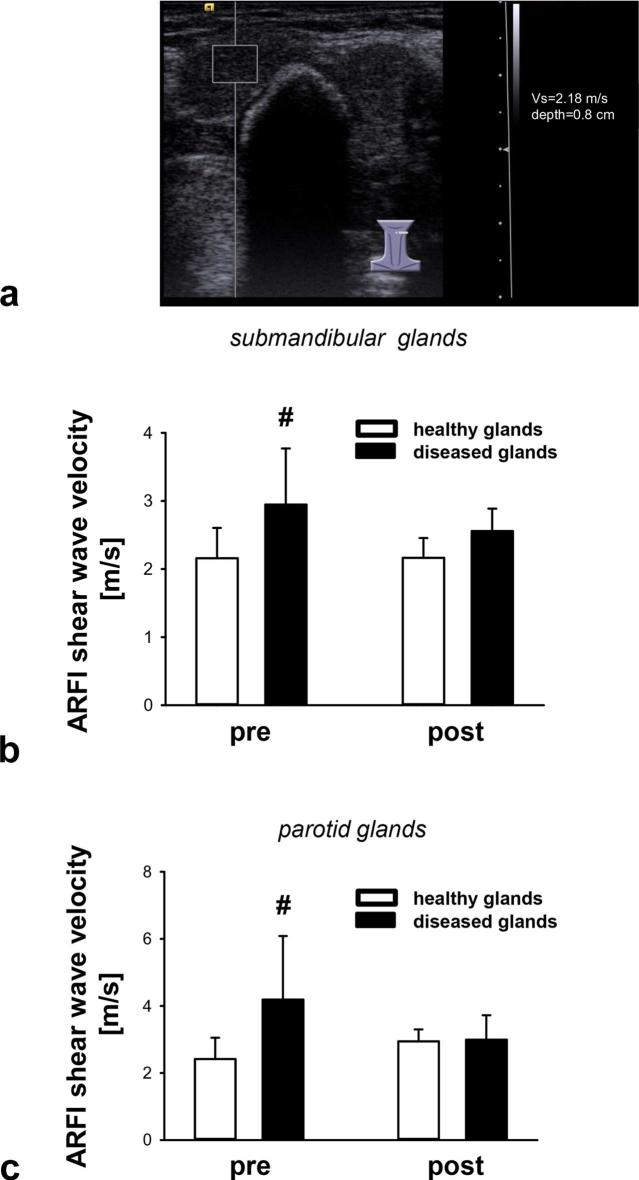
As a measure of tissue elasticity, ARFI analyses were performed in individuals with unilateral sialolithiasis of their salivary glands (Figure 1a). The ARFI values obtained in healthy glands were comparable to those described in previously published observations.15, 16 Submandibular glands with evidence of calculi exhibited significantly higher ARFI shear wave velocity values compared to healthy contralateral submandibular glands in the same individuals, indicating lower tissue elasticity/more stiffness (Figure 1b). Similarly, ARFI shear wave velocity values of parotid glands affected by sialolithiasis were significantly higher compared to healthy contralateral parotid glands (Figure 1c) (Supplementary table 1).
Figure 1.
Effect of successful therapy on tissue elasticity of salivary glands in patients with sialolithiasis. A representative B-mode scan image of a calculus in the left submandibular gland is shown, the region of interest on the left hand side in the image indicates the region of tissue elasticity measurement (a). Panels show results for submandibular (b) or parotid (c) glands before (pre) and after (post) successful therapy (mean ± SD for n = 7–25 per group; #p ≤ 0.05 vs healthy gland). ARFI, acoustic radiation force impulse imaging.
Effect of calculus diameter on tissue elasticity
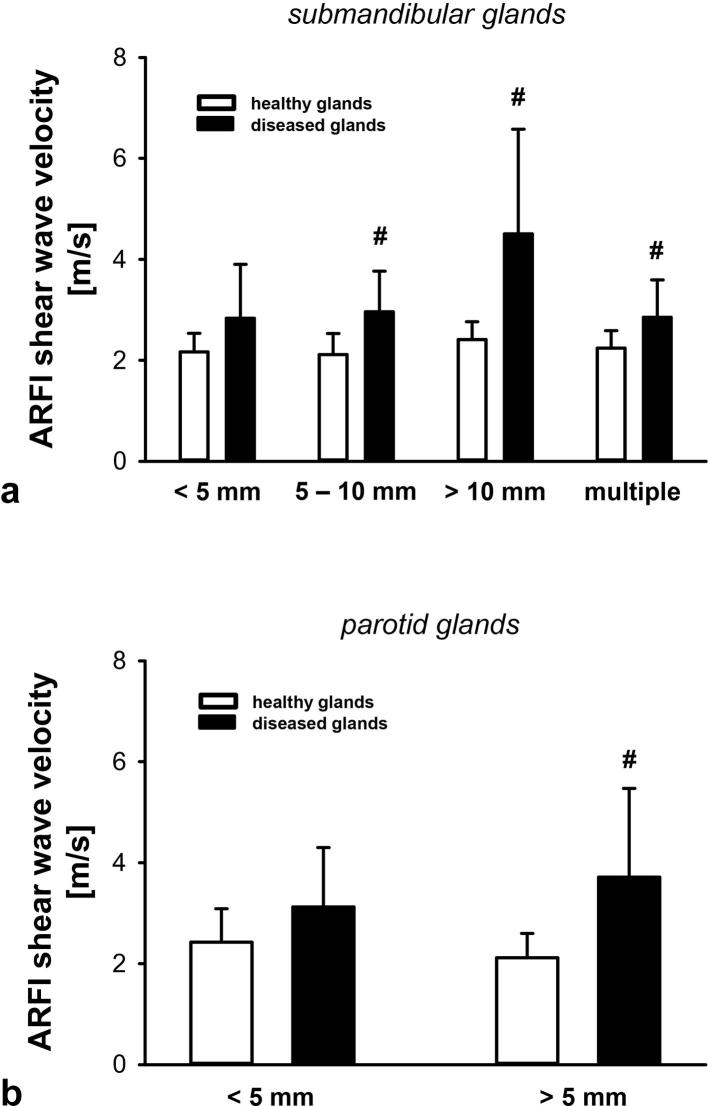
The effect of the calculus’ diameter on tissue elasticity of affected salivary glands was characterized in a subgroup analysis. ARFI shear wave velocity values in submandibular glands with a single calculus exhibiting a diameter of more than 5 mm or with multiple calculi were significantly higher compared to healthy contralateral submandibular glands (Figure 2a). Likewise, ARFI shear wave velocity values in parotid glands with calculi exhibiting diameters of more than 5 mm were significantly higher compared to healthy contralateral parotid glands (Figure 2b) (Supplementary table 1).
Figure 2.
Effect of calculus size on tissue elasticity of salivary glands in patients with sialolithiasis. Panels show results for submandibular (a) or parotid (b) glands with a single calculus of different sizes or multiple calculi (mean ± SD for n = 8–39 per group; #p ≤ 0.05 vs healthy gland).
Effect of successful therapy on tissue elasticity of diseased glands
Furthermore, the effect of successful therapy (ESWL or surgical/endoscopic extraction) on tissue elasticity of diseased glands was determined after the complete removal of the calculus. After successful therapy, ARFI shear wave velocity values in submandibular glands decreased to values not significantly different from unaffected contralateral glands (Figure 1b). Similar results were obtained for the treatment of affected parotid glands (Figure 1c).
Association of subjective parameters and tissue elasticity measurements
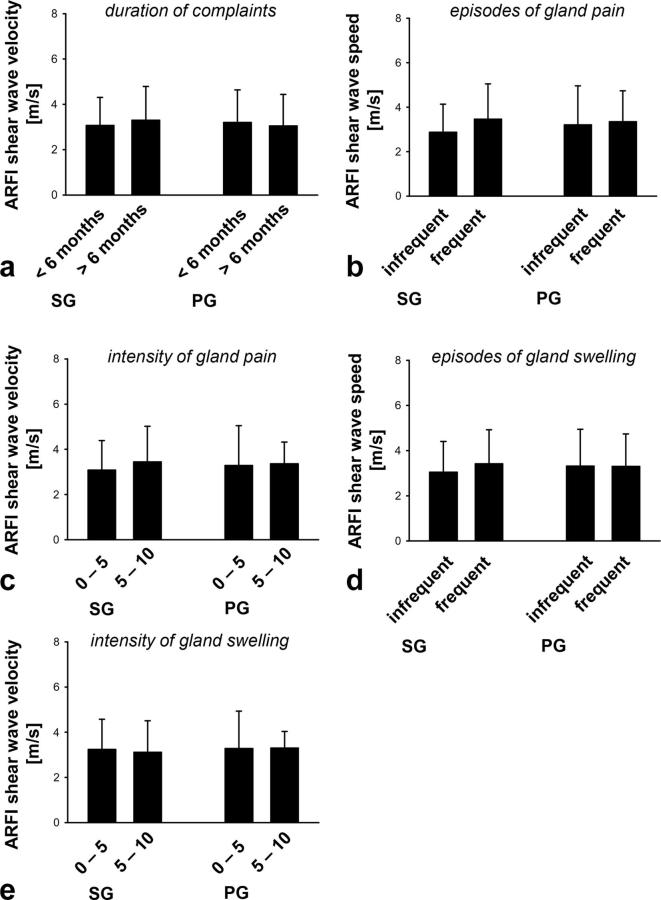
Symptoms of the individuals with sialolithiasis of their salivary glands were assessed using a standardized questionnaire. No significant differences in ARFI shear wave velocity values of affected salivary glands were detected between patients with short (<6 months) or long (>6 months) duration of complaints (Figure 3a), infrequent (<50) or frequent (>50) episodes (Figure 3b), low (visual analogue scale (VAS) 0–5) or high (VAS 6–10) intensity of gland pain (Figure 3c), infrequent (<50) or frequent (>50) episodes (Figure 3d), or low (VAS 0–5) or high (VAS 6–10) intensities of gland swelling (Figure 3e).
Figure 3.
Association of the patient’s complaints with tissue elasticity of salivary glands in patients with sialolithiasis. Panels show results for diseased SG and PG glands in individuals with: varying durations of the complaints (a), varying numbers of episodes (b) or intensities (VAS values) of subjective gland pain (c), varying numbers of episodes (d) or intensities (VAS values) of subjective gland swelling (e) (mean ± SD for n = 9–42 per group). ARFI, acoustic radiation force impulse imaging; PG, parotid gland; SD, standard deviation; SG, submandibular gland, VAS, visual analogue scale.
Discussion
In chronic obstructive sialadenitis, the affected parenchyma can become edematous and fibrotic, ultimately leading to the loss of the gland’s secretory function. Due to emerging knowledge about the salivary glands’ potential to recover after removal of obstructions in their duct system, the treatment of sialolithiasis shifted in the past decades from ablative surgical techniques to minimal-invasive therapeutical strategies.1–4 In order to evaluate the effect of these therapeutic modalities on the recovery of the salivary glands’ parenchyma, different technical means including scintigraphic, sialographic and echostructural analyses as well as questionnaire-based approaches have been employed – all with more or less unsatisfying results regarding their usefulness in clinical routine.17–20 Recently, ARFI has been implemented in the diagnostic work-up of different pathological entities such as malignant tumors, thyroid pathologies, or liver fibrosis.6–12 We have shown that this technical approach also serves as a valid method to determine the tissue elasticity of salivary glands.13 Consequently, ARFI might be useful as a clinical routine to objectively measure structural alterations in the parenchyma of salivary glands in patients with sialolithiasis.
Based on our previous findings,13 we routinely employed ARFI elastography in the initial examination and the follow-up during therapy of patients with sialolithiasis. In the first visit of our patients, submandibular glands with evidence of one or more calculi were found to exhibit a lower tissue elasticity compared to healthy contralateral submandibular glands in the same individual. Similarly, parotid glands affected by sialolithiasis were “stiffer” than the healthy contralateral parotid glands, thereby extending previously published preliminary data.13 Since these elastographic analyses were now integrated into our standard ultrasound examination protocol and usually took less than a minute to perform on a patient, our studies collectively indicate that ARFI provides a quick, easy and reliable diagnostic instrument to quantitatively assess the structural tissue status of salivary glands affected by sialolithiasis.
In this context, it is worth noting that submandibular glands with a single calculus exhibiting a diameter of more than 5 mm or multiple calculi were significantly “stiffer” compared to healthy contralateral submandibular glands. Similarly, parotid glands with a calculus of more than 5 mm in diameter demonstrated a significantly reduced tissue elasticity compared to parotid glands with a “stone” of less than 5 mm in diameter. These findings might be related to the anatomical organization of the glands’ duct systems as well as the spatial distribution of calculi in patients with sialolithiasis: Collected in the smallest ductuli, the salivary gland’s secretion products are drained into the duct of Wharton/Stenon through a tree of duct segments with increasing diameters. As larger calculi tend to be deposited in larger duct segments,21 the volume of parechyma affected by retention of saliva gradually increases with higher calculus diameters, potentially leading to tissue remodeling processes in broader parts of the diseased salivary gland. The overall tissue elasticity of the affected gland consequently decreases with higher calculi diameters. Hence, these data suggest that ARFI allows the quantitative assessment of disease severity in patients with sialolithiasis.
In addition to the initial patient examination, we also sought to evaluate the effect of our therapeutic interventions on the salivary glands’ tissue elasticity in follow-up visits. Here, we show that ESWL or surgical/endoscopic interventions not only lead to the removal of calculi, but also facilitate the recovery of the diseased glands as indicated by decreased ARFI shear wave velocity values. Thus, ARFI also appears to be useful for monitoring the patient’s benefit of therapy regarding tissue recovery. Obtaining ARFI data might therefore particularly be valuable for identifying patients with dysfunctional salivary glands despite successful removal of calculi which should be considered for surgical excision, although we are not able to provide a histopathological correlation to our elastography findings.
In the initial patient visit and in the follow-up examinations, our patients complete a standardized questionnaire on their complaints. Interestingly enough, we were not able to detect any correlation between the extent of the patients’ symptoms and our objective tissue elastography results. This might be explained by the patients’ highly individual and diverse symptom perception emphasizing the use of objective measures such as ARFI elastography in diagnosis and follow-up of patients with sialolithiasis.
Conclusions
The results of our study demonstrate that ARFI elastography is a valuable diagnostic tool for assessing disease severity and progression in patients with sialolithiasis. This method is easy to use, not time-consuming, and can be easily and seamlessly integrated into pre-existing ultrasound examination protocols.
Footnotes
Acknowledgments: This study is part of the doctoral thesis of T.V.
REFERENCES
- 1.Koch M, Zenk J, Iro H, Witt RL, Iro H, Koch M. Algorithms for treatment of salivary gland obstructions. Otolaryngol Clin North Am 2009; 42: 1173–92. doi: 10.1016/j.otc.2009.08.002 [DOI] [PubMed] [Google Scholar]
- 2.Rahmati R, Gillespie MB, Eisele DW. Is sialendoscopy an effective treatment for obstructive salivary gland disease? Laryngoscope 2013; 123: 1828–9. doi: 10.1002/lary.23958 [DOI] [PubMed] [Google Scholar]
- 3.Thomas BL, Brown JE, McGurk M. Salivary gland disease. Front Oral Biol 2010; 14: 129–46. doi: 10.1159/000313711 [DOI] [PubMed] [Google Scholar]
- 4.Witt RL, Iro H, Koch M, McGurk M, Nahlieli O, Zenk J. Minimally invasive options for salivary calculi. Laryngoscope 2012; 122: 1306–11. doi: 10.1002/lary.23272 [DOI] [PubMed] [Google Scholar]
- 5.Marchal F, Kurt AM, Dulguerov P, Lehmann W. Retrograde theory in sialolithiasis formation. Arch Otolaryngol Head Neck Surg 2001; 127: 66–8. doi: 10.1001/archotol.127.1.66 [DOI] [PubMed] [Google Scholar]
- 6.Balleyguier C, Ciolovan L, Ammari S, Canale S, Sethom S, Al Rouhbane R, et al. . Breast elastography: the technical process and its applications. Diagn Interv Imaging 2013; 94: 503–13PubMed PMID. doi: 10.1016/j.diii.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 7.Cantisani V, Lodise P, Grazhdani H, Mancuso E, Maggini E, Di Rocco G, et al. . Ultrasound elastography in the evaluation of thyroid pathology. Current status. Eur J Radiol 2014; 83: 420–8. doi: 10.1016/j.ejrad.2013.05.008 [DOI] [PubMed] [Google Scholar]
- 8.Horster S, Mandel P, Zachoval R, Clevert DA. Comparing acoustic radiation force impulse imaging to transient elastography to assess liver stiffness in healthy volunteers with and without valsalva manoeuvre. Clin Hemorheol Microcirc 2010; 46: 159–68. doi: 10.3233/CH-2010-1342 [DOI] [PubMed] [Google Scholar]
- 9.Sporea I, Sirli R, Popescu A, Danilă M. Acoustic radiation force impulse (ARFI)-a new modality for the evaluation of liver fibrosis. Med Ultrason 2010; 12: 26–31. [PubMed] [Google Scholar]
- 10.D'Anastasi M, Schneevoigt BS, Trottmann M, Crispin A, Stief C, Reiser MF, et al. . Acoustic radiation force impulse imaging of the testes: a preliminary experience. Clin Hemorheol Microcirc 2011; 49: 105–14. doi: 10.3233/CH-2011-1461 [DOI] [PubMed] [Google Scholar]
- 11.Trottmann M, Marcon J, D’Anastasi M, Bruce MF, Stief CG, Reiser MF, et al. . Shear-wave elastography of the testis in the healthy man - determination of standard values. Clin Hemorheol Microcirc 2016; 62: 273–81. doi: 10.3233/CH-162046 [DOI] [PubMed] [Google Scholar]
- 12.Trottmann M, Marcon J, D’Anastasi M, Karl A, Stief CG, Reiser M, et al. . The role of VTIQ as a new tissue strain analytics measurement technique in testicular lesions. Clin Hemorheol Microcirc 2014; 58: 195–209. doi: 10.3233/CH-141904 [DOI] [PubMed] [Google Scholar]
- 13.Zengel P, Schrötzlmair F, Schwarz F, Paprottka P, Kramer M, Berghaus A, et al. . Elastography: a new diagnostic tool for evaluation of obstructive diseases of the salivary glands; primary results. Clin Hemorheol Microcirc 2012; 50: 91–9. doi: 10.3233/CH-2011-1446 [DOI] [PubMed] [Google Scholar]
- 14.Nightingale K, McAleavey S, Trahey G. Shear-wave generation using acoustic radiation force: in vivo and ex vivo results. Ultrasound Med Biol 2003; 29: 1715–23PubMed PMID. doi: 10.1016/j.ultrasmedbio.2003.08.008 [DOI] [PubMed] [Google Scholar]
- 15.Hofauer B, Mansour N, Heiser C, Gahleitner C, Thuermel K, Bas M, et al. . Sonoelastographic modalities in the evaluation of salivary gland characteristics in Sjögren's syndrome. Ultrasound Med Biol 2016; 42: 2130–9. doi: 10.1016/j.ultrasmedbio.2016.04.011 [DOI] [PubMed] [Google Scholar]
- 16.Zhang S, Zhu J, Zhang X, He J, Li J. Assessment of the stiffness of major salivary glands in primary Sjögren’s syndrome through quantitative acoustic radiation force impulse imaging. Ultrasound Med Biol 2016; 42: 645–53. doi: 10.1016/j.ultrasmedbio.2015.11.009 [DOI] [PubMed] [Google Scholar]
- 17.Anjos DA, Etchebehere EC, Santos AO, Lima MC, Ramos CD, Paula RB, et al. . Normal values of [99mTc]pertechnetate uptake and excretion fraction by major salivary glands. Nucl Med Commun 2006; 27: 395–403. doi: 10.1097/01.mnm.0000202864.52046.b1 [DOI] [PubMed] [Google Scholar]
- 18.Hermann GA, Vivino FB, Shnier D, Krumm RP, Mayrin V, Shore JB. Variability of quantitative scintigraphic salivary indices in normal subjects. J Nucl Med 1998; 39: 1260–3. [PubMed] [Google Scholar]
- 19.Yoshimura Y, Morishita T, Sugihara T. Salivary gland function after sialolithiasis: scintigraphic examination of submandibular glands with 99mTc-pertechnetate. J Oral Maxillofac Surg 1989; 47: 704–10. doi: 10.1016/S0278-2391(89)80009-6 [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Escudier M, Brown J, Capaccio P, Pignataro L, McGurk M. Long-term outcome after intraoral removal of large submandibular gland calculi. Laryngoscope 2010; 120: 964–6. doi: 10.1002/lary.20839 [DOI] [PubMed] [Google Scholar]
- 21.Nahlieli O, Bar T, Shacham R, Eliav E, Hecht-Nakar L. Management of chronic recurrent parotitis: current therapy. J Oral Maxillofac Surg 2004; 62: 1150–5PubMed PMID. doi: 10.1016/j.joms.2004.05.116 [DOI] [PubMed] [Google Scholar]