Summary
Background
Gestational diabetes and gestational hypertensive disorders are associated with offspring obesity. The role of maternal adiposity in these associations remains unclear. We aimed to evaluate if these pregnancy complications affect the odds of offspring obesity independently of maternal obesity.
Methods
In this individual participant data meta-analysis of 160757 mother-offspring pairs from 34 prospective European or North-American pregnancy/birth cohorts, we assessed the associations of gestational diabetes, gestational hypertension and pre-eclampsia with childhood BMI and the odds of overweight and obesity throughout childhood. We explored to what extent any association was explained by maternal pre/early-pregnancy BMI.
Findings
Gestational diabetes was associated with a higher odds of overweight throughout childhood (Odds Ratio (OR) 1·59 (95% Confidence Intervals (CI) 1·36, 1·86); OR 1·41 (95% CI 1·26, 1·57); OR 1·32 (95% CI 0·99, 1·78) for early-, mid- and late-childhood, respectively) when compared to uncomplicated pregnancies. These associations attenuated towards the null following adjustment for maternal BMI. Likewise, gestational hypertension was associated with a higher odds of overweight throughout childhood, with the strongest association in late-childhood (OR 1·49 (95% CI 1·30, 1·70)), when compared to uncomplicated pregnancies. Additional adjustment for maternal BMI largely explained these associations. Pre-eclampsia was associated with a lower BMI in early-childhood only (difference in BMI-SDS - 0.05 SDS (95% CI - 0·09, - 0·01)), when compared to uncomplicated pregnancies. This association strengthened upon additional adjustment for maternal BMI.
Interpretation
Our results suggest that lowering maternal risk of gestational diabetes and hypertensive disorders is unlikely to have a direct impact on childhood obesity. Preventive strategies for reducing childhood obesity should focus on maternal BMI rather than on pregnancy complications.
Funding
This work was supported by the European Union's Horizon 2020 research and innovation programme under grant agreement 733206 (LifeCycle Project).
Introduction
Gestational diabetes and gestational hypertensive disorders are among the most common pregnancy complications, affecting 10-25% and 2-4% of all pregnancies, respectively.1,2 These complications are major risk factors for maternal and fetal morbidity and mortality.1–3 Overall, 12% of maternal deaths are attributable to gestational hypertensive disorders, whereas up to 10% of adverse fetal outcomes, such as delivering a large size for gestational age infant are attributable to gestational diabetes.4–6 Fetal exposure to hyperglycemia due to gestational diabetes or altered utero-placental perfusion due to gestational hypertension and pre-eclampsia affect fetal nutrient supply.7,8 Alterations in the fetal supply line may influence fetal development, and trigger developmental adaptations in adipose tissue, neuroendocrine and metabolic function, which could predispose offspring to adiposity in later life.9
Maternal obesity is a risk factor for gestational diabetes and gestational hypertensive disorders, and is also associated with an increased risk of obesity in offspring.10 It is not clear whether gestational diabetes and gestational hypertensive disorders affect the risk of offspring obesity independently of the risk conferred by maternal obesity. Previous studies have shown that diabetes during pregnancy is associated with an increased risk of offspring obesity and higher fat mass levels, independent of maternal socio-demographic and lifestyle characteristics.11–17 Inconsistent findings were reported for the specific role of maternal obesity in these associations. Prospective cohort studies have assessed the relations of gestational hypertensive disorders with offspring blood pressure, but only few have also assessed the relation with offspring adiposity.18–20 A UK study reported that children of women who had either gestational hypertension or pre-eclampsia had higher risk of obesity at 9 years, whereas an Australian study found similar associations only in term born young adults of mothers with gestational hypertension, but not with pre-eclampsia.18,19 For the development of new preventive strategies focused on reducing childhood obesity, insight into the effects of gestational diabetes and gestational hypertensive disorders, independent of maternal obesity, on offspring obesity is needed.
Using individual participant data (IPD) from 160757 mother-offspring pairs, we assessed the associations of maternal gestational diabetes, gestational hypertension and pre-eclampsia with the odds of offspring overweight and obesity throughout childhood. We further explored whether any observed association was independent of maternal pre-/early-pregnancy BMI.
Methods
Inclusion criteria and participating cohorts
We used data from an existing international collaboration on maternal obesity and childhood outcomes. Pregnancy and birth cohort studies were eligible for inclusion in this international collaboration if they were able to provide IPD on mothers with singleton live-born children born from 1989 onwards, and had information available on maternal pre/early-pregnancy BMI and birth weight and/or childhood BMI. We invited 50 cohorts from Europe, North America and Oceania identified from existing collaborations on childhood health (EarlyNutrition Project, CHICOS Project, www.birthcohorts.net; last accessed July 2014), of which 38 agreed to participate and provided data on 274174 singleton births. For this study, we only included cohorts that were able to provide IPD on maternal gestational diabetes, gestational hypertension or pre-eclampsia, and childhood BMI obtained at least once between age 2 and 17·9 years. This resulted in 160757 mothers-offspring pairs available for analysis (Supplemental Figure S1). Anonymized datasets were stored on a single central secured data server with access for the main analysts (BPG, SS). All studies were approved by their local institutional review boards. Additional ethics approval was not required to perform this IPD meta-analysis.
Pregnancy complications and pre/early-pregnancy BMI
Information on gestational diabetes, gestational hypertension, pre-eclampsia and maternal BMI was obtained from medical records, through research assessments or was self-reported (cohort-specific information is shown in Supplemental Table S1). Where possible we used maternal pre-pregnancy BMI (<18·5, 18·5 - <25, 25 - <30, ≥30 kg/m2). Five cohorts (including 6,513 participants) did not have information about pre-pregnancy BMI but obtained BMI in early pregnancy (all assessed before or at 20 weeks of gestation).
Childhood BMI
Data on childhood weight and height were mostly obtained through direct research assessments, with a small number of studies abstracting information about weight and height, or BMI, from medical records, report by parents/caregivers or self-reported (cohort-specific information is provided in Supplemental Table S1). We grouped BMI based on the child’s age at assessment into early-childhood (2·0–4·9 years), mid-childhood (5·0–9·9 years), and late-childhood (10·0–17·9 years). The age intervals, which correspond roughly to preschoolers, school-age children and adolescents, respectively, were predefined based on data availability and data for each period were provided by the cohorts. If studies had multiple repeated measurements within the same age period, we used data collected at the oldest age. We calculated sex- and age-adjusted standard deviation scores (SDS) of childhood BMI using WHO reference growth charts (Growth Analyzer 4·0, Dutch Growth Research Foundation).21–24 Childhood underweight, normal weight, overweight and obesity (further referred as overweight) were defined based on the age- and sex- specific WHO criteria.21,22
Covariates
Covariates were mostly obtained by questionnaires and provided by cohorts as categorical variables (cohort-specific information is shown in Supplemental Table S1 and S2). To allow handling of missing data, the continuous covariates were categorized. As potential confounders other than maternal pre-pregnancy BMI, we considered maternal age (defined based on data availability: <25·0 years, 25-29·9 years, 30-34·9 years, ≥35·0 years), educational level (low, medium, high), ethnicity (European/White, non-European/non-White), parity (nulliparous, multiparous), smoking during pregnancy (yes, no), and offspring’s sex. We did not adjust the primary analyses for offspring birth weight and gestational age at delivery, as these birth characteristics are likely to be mediators on the causal pathway and adjustment might introduce bias.25
Statistical analysis
We assessed the percentage of childhood overweight in early, mid and late childhood for each combined maternal BMI and pregnancy complication group. We applied multilevel mixed effects models, taking into account clustering of participants within cohorts, to analyze simultaneously IPD from all cohorts.26 Our models were defined assuming a random intercept at cohort level, which allowed for differences in the intercepts between cohorts. First, we used multilevel linear mixed effects models to examine the associations of gestational diabetes, gestational hypertension or pre-eclampsia with BMI SDS in early-, mid-, and late-childhood. Second, we used multilevel binary logistic mixed effects models to examine the associations of these pregnancy complications with the odds of childhood underweight, and overweight in the same age windows. Participants exposed to either gestational hypertension or pre-eclampsia were compared to those with none of these conditions, irrespective of their gestational diabetes status. Similarly, those exposed to gestational diabetes were compared to those with no gestational diabetes, irrespective of whether or not they had gestational hypertension or pre-eclampsia. For all analyses, we constructed an unadjusted model (Basic model), a model adjusted for offspring’s sex, maternal age, educational level, ethnicity, parity and smoking during pregnancy (Lifestyle characteristics model), and a model additionally adjusted for maternal pre/early-pregnancy BMI (Maternal BMI model). For the associations of pregnancy complications with the odds of childhood underweight, only the basic model was applied due to insufficient sample size. Based on findings from previous studies and clinical relevance, we tested potential interactions between each pregnancy complication and (i) offspring´s sex and (ii) maternal BMI, in their associations with childhood BMI.27–29 Since no consistent significant interactions were observed, no further stratified analyses were performed. To prevent exclusion of non-complete cases, we used missing values in the covariates as an additional group (percentage missings per cohort given in Supplemental Table S2). We did not include information for a cohort for a specific categorical covariate, if information for this variable was available for less than 50% of the cohort sample. Due to the strategy used to handle missing data, the confounding factors were included in the models as categorical covariates. However, in an analysis using complete cases of maternal pre-pregnancy BMI and age, similar associations were observed when adjusting for them as continuous or categorical covariates (Supplemental Table S3). As sensitivity analysis, we performed 2-stage random effects meta-analyses and tested for heterogeneity between cohorts using the I2.26 Analyses were undertaken using the Statistical Package of Social Sciences version 21.0 for Windows (SPSS Inc, Chicago, IL, USA) and Review Manager ((RevMan) Version [5.3.5]. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) software.
Role of the funding source
This work was supported by the European Union's Horizon 2020 research and innovation programme under grant agreement 733206 (LifeCycle Project). The funding source had no role in the study design, data collection, analysis, interpretation, and in the writing of the report. BPG and SS had access to the raw data. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Results
Response and subject characteristics
Out of 50 cohorts identified and invited, 38 agreed to participate and 34 were included in these analyses. Only 4 cohorts (113417 participants) were excluded due to missing data on exposures and/or outcomes.
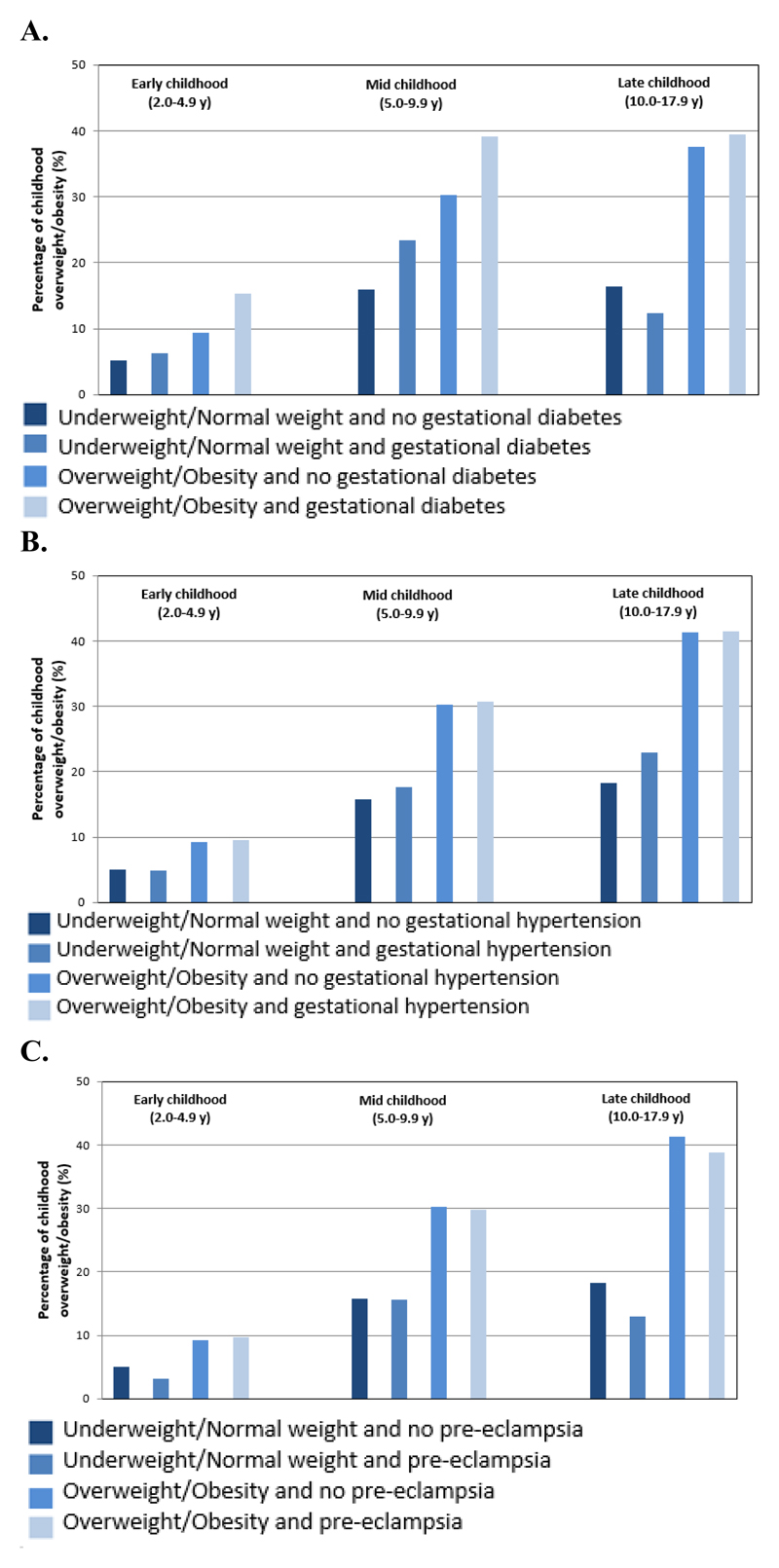
Table 1 shows the characteristics of participants of each cohort. Out of a total of 160757 mothers, 2618 (1·7%) had gestational diabetes, 9755 (6·5%) had gestational hypertension, and 4836 (3·3%) had pre-eclampsia. 30927 (19·7%) of the mothers were overweight and 12467 (7·9%) obese. Among offspring 5606 (6·6%), 24254 (20·1%) and 3699 (21·3 %) were overweight in early-, mid-, and late-childhood, respectively. Cohort-specific information on childhood age at assessment and BMI is shown in Supplemental Table S4. Figure 1 shows the percentages of childhood overweight according to their mothers pre-pregnancy BMI category and presence or absence of each pregnancy complication. The highest percentages of childhood overweight were observed in children whose mothers were overweight or obese at the start of pregnancy, independent of whether their mothers had gestational diabetes or gestational hypertensive disorders. A small increase in the percentage of childhood overweight was observed by the presence of gestational diabetes in both normal weight and overweight or obese mothers, whereas no differences were observed by presence or absence of gestational hypertensive disorders.
Table 1.
| Cohort name, number of participants, birth years (country) |
Gestational diabetes |
Gestational hypertension | Pre-eclampsia | Early childhood (2·0-4·9y) | Mid childhood (5·0-9·9y) | Late childhood (10·0-17·9y) | |||
|---|---|---|---|---|---|---|---|---|---|
| Body mass index (SDS) | Overweight and obesity | Body mass index (SDS) | Overweight and obesity | Body mass index (SDS) | Overweight and obesity | ||||
| ABCD, n=5512, 2003-2004 (The Netherlands) | 120 (2·2) | 1045 (19·0) | 233 (4·2) | 0·3 (-1·5, 2·4) | 213 (4·5) | 0·1 (-1·7, 2·4) | 768 (17·1) | NA | NA |
| ALSPAC, n=9041, 1991-1992 (United Kingdom) | 77 (0·9) | 1282 (14·5) | 170 (1·9) | 0·6 (-1·0, 2·5) | 77 (6·5) | 0·2 (-1·6, 2·7) | 2113 (26·4) | 0·2 (-1·9, 2·5) | 2063 (26·4) |
| AOB/F, n=1672, 2008-2010 (Canada) |
71 (4·2) | 118 (7·1) | 104 (6·2) | 0·2 (-2·3, 2·7) | 96 (5·7) | NA | NA | NA | NA |
| BAMSE, n=3329, 1994-1996 (Sweden) |
56 (1·7) | NA | 55 (1·7) | 0·6 (-0·9, 2·5) | 187 (6·5) | 0·5 (-1·2, 2·6) | 799 (31·2) | 0·1 (-1·7, 2·0) | 425 (16·9) |
| BIB, n=983, 2007-2010 (United Kingdom) | 100 (10·2) | 51 (5·3) | 13 (1·4) | 0·5 (-1·4, 2·6) | 74 (7·5) | NA | NA | NA | NA |
| Co.N.ER, n=528, 2004-2005 (Italy) |
14 (2·7) | 20 (3·8) | 12 (2·3) | 0·3 (-2·3, 2·9) | 47 (9·7) | 0·7 (-1·3, 2·9) | 102 (35·5) | NA | NA |
| DNBC, n=40349, 1996-2002 (Denmark) |
273 (0·7) | 1878 (4·7) | 1380 (3·4) | NA | NA | 0 (-1·9, 2·1) | 6304 (15·6) | NA | NA |
| EDEN, n=1361, 2003-2005 (France) |
92 (6·8) | 67 (4·9) | 30 (2·2) | 0·3 (-1·4, 2·0) | 27 (2·2) | 0 (-1·5, 2·0) | 147 (12·9) | NA | NA |
| FCOU, n=2332, 1993-1996 (Ukraine) |
5 (0·2) | 367 (15·7) | 148 (6·3) | 0·5 (-1·9, 3·1) | 140 (10·6) | 0 (-2·0, 2·0) | 124 (12·6) | -0·1 (-2·0, 1·8) | 75 (8·9) |
| GASPII, n=570, 2003-2004 (Italy) |
25 (4·4) | 28 (4·9) | 5 (0·9) | 0·7 (-1·1, 2·9) | 52 (9·7) | 0·7 (-1·4, 2·7) | 172 (37·1) | NA | NA |
| GECKO Drenthe, n=2119, 2006-2007 (The Netherlands) |
72 (3·4) | 209 (11·0) | 46 (2·4) | NA | NA | 0·4 (-1·2, 2·4) | 465 (21·9) | NA | NA |
| GENESIS, n=2143, 2003-2004 (Greece) |
30 (1·4) | NA | NA | 0·8 (-1·2, 3·6) | 297 (14·6) | 1·0 (-1·5, 3·9) | 45 (42·1) | NA | NA |
| GENERATION R, n=7550, 2002-2006 (The Netherlands) |
80 (1·1) | 274 (4·0) | 149 (2·3) | 0·3 (-1·5, 2·5) | 220 (5·1) | 0·3 (-1·5, 2·7) | 1849 (27·4) | 0·4 (-1·5, 2·6) | 160 (30·0) |
| GENERATION XXI, n=5921, 2005-2006 (Portugal) |
390 (6·6) | 135 (2·3) | 90 (1·5) | 0·5 (-1·3, 3·1) | 485 (10·4) | 0·6 (-1·4, 3·2) | 2015 (38·0) | NA | NA |
| GINIplus, n=2313, 1995-1998 (Germany) |
61 (2·6) | NA | NA | 0·1 (-1·7, 2·0) | 53 (2·4) | 0 (-1·8, 1·9) | 227 (10·6) | 0 (-1·9, 2·1) | 365 (16·0) |
| HUMIS, n=970, 2003-2008 (Norway) |
5 (0·5) | 37 (3·8) | 70 (7·2) | 0·3 (-1·8, 2·4) | 53 (6·0) | 0·0 (-2·0, 2·3) | 63 (17·6) | NA | NA |
| INMA, n=1933, 1997-2008 (Spain) |
191 (11·3) | 58 (3·0) | 4 (0·9) | 0·5 (-1·2, 2·8) | 143 (8·2) | 0·6 (-1·4, 3·3) | 503 (37·6) | 0·3 (-1·6, 2·5) | 79 (25·3) |
| KOALA, n=2061, 2000-2002 (The Netherlands) | 21 (1·0) | 72 (3·5) | 26 (1·3) | -0·1 (-2·0, 1·9) | 17 (1·7) | -0·2 (-2·2, 1·8) | 199 (11·3) | -0·2 (-2·1, 2·2) | 19 (18·1) |
| Krakow Cohort, n=424, 2000-2003 (Poland) |
18 (4·2) | 19 (4·5) | 0 (0·0) | 0 (-2·2, 2·3) | 11 (4·1) | 0·2 (-1·8, 2·6) | 90 (26·5) | NA | NA |
| LISAplus, n=1584, 1997-1999 (Germany) |
58 (3·7) | NA | NA | 0 (-1·7, 1·9) | 33 (2·3) | -0·1 (-1·8, 1·8) | 140 (9·9) | 0 (1·8, 2·1) | 236 (15·2) |
| MoBa, n=55008, 1999-2009 (Norway) |
418 (0·8) | 3131 (5·7) | 2023 (3·7) | 0·4 (-1·8, 2·5) | 2456 (6·1) | 0·1 (-2·0, 2·3) | 6793 (19·5) | NA | NA |
| NINFEAb, n=1726, 2005-2010 (Italy) |
132 (7·7) | 136 (7·9) | 44 (2·6) | 0·1 (-2·3, 2·5) | 86 (5·1) | 0 (-2·2, 2·4) | 90 (21·2) | NA | NA |
| PÉLAGIE, n=738, 2002-2005 (France) |
21 (2·8) | 24 (3·3) | 8 (1·1) | 0·1 (-1·8, 1·9) | 15 (2·0) | NA | NA | NA | NA |
| PIAMA, n=1815, 1996-1997 (The Netherlands) | 19 (1·0) | 179 (9·9) | 46 (2·5) | 0·7 (-1·2, 2·5) | 78 (8·9) | 0·1 (-1·6, 2·4) | 325 (20·0) | -0·2 (-1·7, 1·8) | 77 (10·0) |
| Piccolipiù, n=687, 2011-2015 (Italy) |
69 (10·1) | 24 (3·5) | 6 (0·9) | 0·3 (-2·1, 2·5) | 40 (5·8) | NA | NA | NA | NA |
| Project Viva, n=1389, 1999-2002 (United States) |
64 (4·7) | 85 (6·3) | 46 (3·4) | 0·7 (-1·0, 2·7) | 87 (7·1) | 0·4 (-1·4, 3·0) | 328 (30·8) | 0·4 (-1·5, 3·7) | 8 (25·8) |
| REPRO_PL, n=291, 2007-2011 (Poland) |
13 (4·5) | 17 (5·8) | 0 (0·0) | 0·3 (-2·2, 2·5) | 19 (6·9) | 0·6 (-1·5, 3·6) | 19 (38·8) | NA | NA |
| RHEA, n=740, 2007-2008 (Greece) |
60 (8·8) | 35 (5·2) | 5 (0·7) | 0·6 (-1·1, 3·6) | 91 (12·3) | NA | NA | NA | NA |
| ROLO, n=283, 2007-2011 (Ireland) |
10 (3·5) | NA | NA | 0·2 (-1·7, 2·6) | 19 (6·7) | NA | NA | NA | NA |
| SCOPE BASELINE, n=1046, 2008-2011 (Ireland) | NA | 129 (12·3) | 35 (3·3) | 0·6 (-1·0, 2·3) | 62 (5·9) | NA | NA | NA | NA |
| SEATON, n=872, 1998-1999 (United Kingdom) | NA | 119 (13·9) | 16 (2·1) | 0·7 (-0·9, 2·7) | 36 (8·1) | 0·6 (-1·1, 2·8) | 55 (19·8) | 0·4 (-1·6, 2·6) | 192 (32·9) |
| Slovak PCB study, n=524, 2002-2004 (Slovakia) | 3 (0·6) | 54 (12·4) | NA | 1·9 (-2·4, 5·3) | 222 (48·2) | 0·3 (-1·7, 3·3) | 123 (31·0) | NA | NA |
| STEPS, n=297, 2008-2010 (Finland) | 20 (6·7) | NA | NA | 0·5 (-1·2, 2·2) | 13 (4·4) | NA | NA | NA | NA |
| SWS, n=2646, 1998-2007 (United Kingdom) | 30 (1·1) | 162 (6·1) | 72 (2·7) | 0·5 (-1·3, 2·6) | 157 (6·2) | 0·2 (-1·5, 2·5) | 396 (22·0) | NA | NA |
Values are expressed as medians (95% range) or numbers of subjects (valid %). NA, not available.
Subset of participants with follow-up completed at 4 years of child’s age by the time of data transfer (March 2015)
Figure 1.
Percentages of childhood overweight according to maternal pre-pregnancy BMI category and presence or absence of A. gestational diabetes, B. gestational hypertension, and C. pre-eclampsia.
Gestational diabetes and childhood BMI
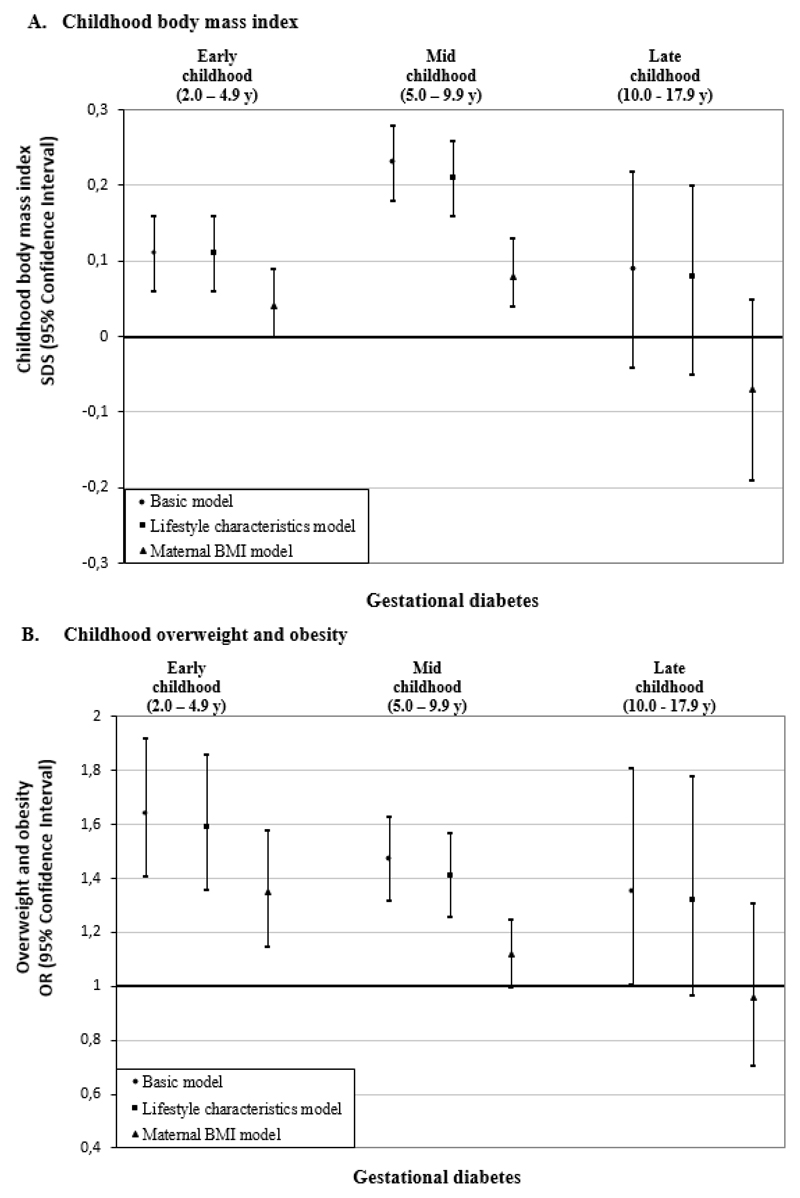
Figure 2A shows that in basic and lifestyle characteristics models, gestational diabetes was associated with a higher offspring BMI throughout childhood (differences in BMI-SDS in lifestyle characteristics models: 0·11 SDS (95% Confidence Intervals (CI) 0·06, 0·16); 0·21 SDS (95% CI 0·16, 0·26); 0·08 SDS (95% CI -0·04, 0·21) in early-, mid- and late-childhood, respectively), as compared to uncomplicated pregnancies. After additional adjustment for maternal BMI, these associations largely attenuated towards the null. Similarly, gestational diabetes was associated with increased odds of overweight throughout childhood in basic and lifestyle characteristics models, with the strongest association in early-childhood (Odds Ratio (OR) 1·59 (95% CI 1·36, 1·86) (Figure 2B). Adjustment for maternal BMI largely attenuated the effect estimates towards the null. Only the association of gestational diabetes with the odds of early childhood overweight remained significant (OR 1·35 (95% CI 1·15; 1·58)). Gestational diabetes tended to be associated with a lower odds of underweight throughout childhood in basic model (Supplemental Table S5). Additional adjustment for gestational hypertension and pre-eclampsia did not affect the observed associations of gestational diabetes with childhood outcomes (Supplemental Table S6). Although no statistical interaction was present, as sensitivity analyses, we have explored the associations of gestational diabetes with childhood BMI, stratified by maternal BMI groups and found similar associations across groups (Supplemental Table S7). Similar associations of gestational diabetes with childhood BMI were also observed when adjusting for pre/early-pregnancy or pre-pregnancy BMI (Supplemental Table S8).
Figure 2.
Associations of gestational diabetes with offspring BMI outcomes in early, mid, and late childhood.a
aValues are regression coefficients (95% confidence intervals) from multilevel linear mixed effects models and odds ratios (95% confidence intervals) from multilevel binary logistic models that reflect differences in early childhood (2·0 to 4·9 years), mid childhood (5·0 to 9·9 years) and late childhood (10·0 to 17·9 years) BMI SDS and risk of overweight and obesity, respectively, for children born to mothers with gestational diabetes, as compared with the reference group (children born to mothers with an uncomplicated pregnancy). Lifestyle characteristics models are adjusted for offspring’s sex, maternal age, educational level, ethnicity, parity, and smoking during pregnancy. Maternal BMI models are additionally adjusted for maternal pre/early-pregnancy BMI.
Gestational hypertensive disorders and childhood BMI
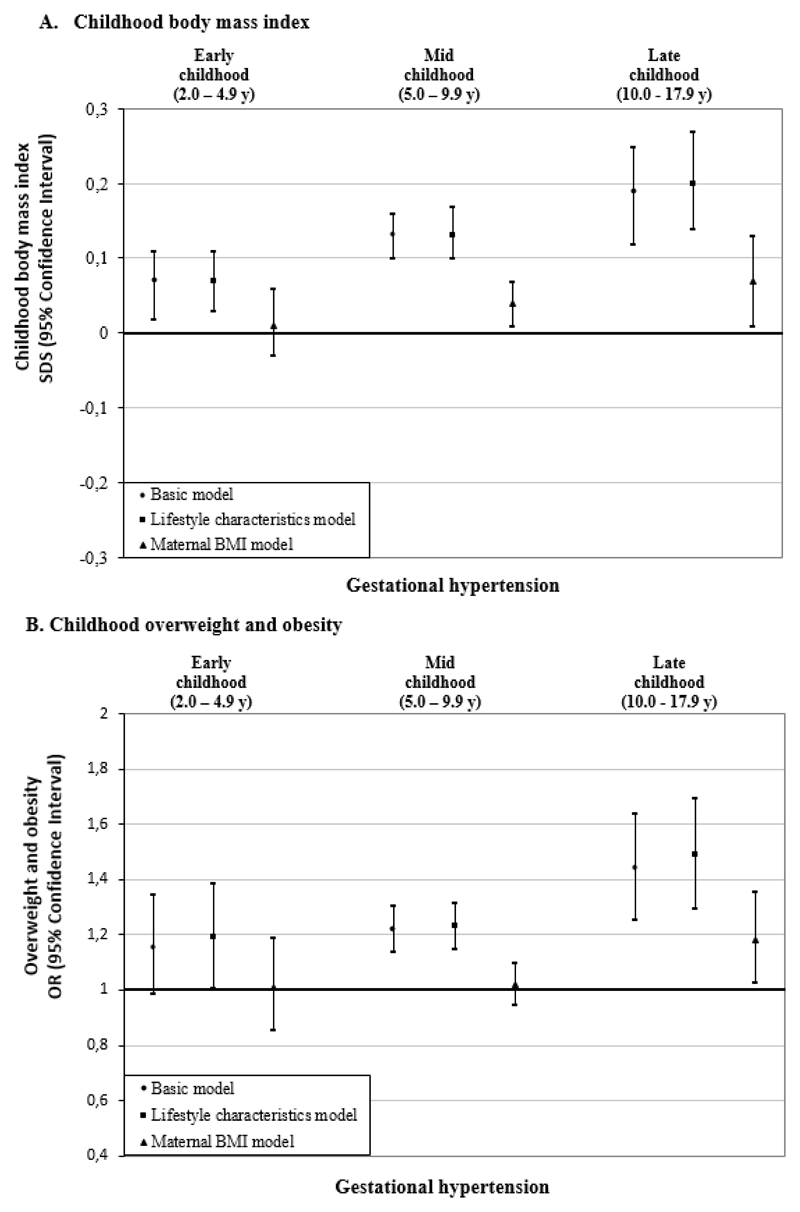
Figure 3A shows that, as compared to uncomplicated pregnancies, gestational hypertension was associated with a higher BMI throughout childhood in basic and lifestyle characteristics models (differences in BMI-SDS in lifestyle characteristics models: 0·07 SDS (95% CI 0·03, 0·11); 0·13 SDS (95% CI 0·10, 0·17); 0·20 SDS (95% CI 0·14, 0·27) in early-, mid- and late-childhood, respectively). These associations were partly explained by maternal BMI (difference in BMI-SDS: 0·01 SDS (95% CI - 0·03, 0·06); 0·04 SDS (95% CI 0·01, 0·07); 0·07 SDS (95% CI 0·01, 0·13) in early-, mid- and late-childhood, respectively). Gestational hypertension was also associated with higher odds of overweight throughout childhood. The strongest association was observed for late-childhood (OR 1·49 (95% CI 1·30, 1·70)). Additional adjustment for maternal BMI largely attenuated these associations (Figure 3B). In the basic model, gestational hypertension was associated with a lower odds of childhood underweight at all ages (Supplemental Table S5).
Figure 3.
Associations of gestational hypertension with offspring BMI outcomes in early, mid, and late childhood.a
aValues are regression coefficients (95% confidence intervals) from multilevel linear mixed effects models and odds ratios (95% confidence intervals) from multilevel binary logistic models that reflect differences in early childhood (2·0 to 4·9 years), mid childhood (5·0 to 9·9 years) and late childhood (10·0 to 17·9 years) BMI SDS and risk of overweight and obesity, respectively, for children born to mothers with gestational hypertension, as compared with the reference group (children born to mothers with an uncomplicated pregnancy). Lifestyle characteristics models are adjusted for offspring’s sex, maternal age, educational level, ethnicity, parity, and smoking during pregnancy. Maternal BMI models are additionally adjusted for maternal pre/early-pregnancy BMI.
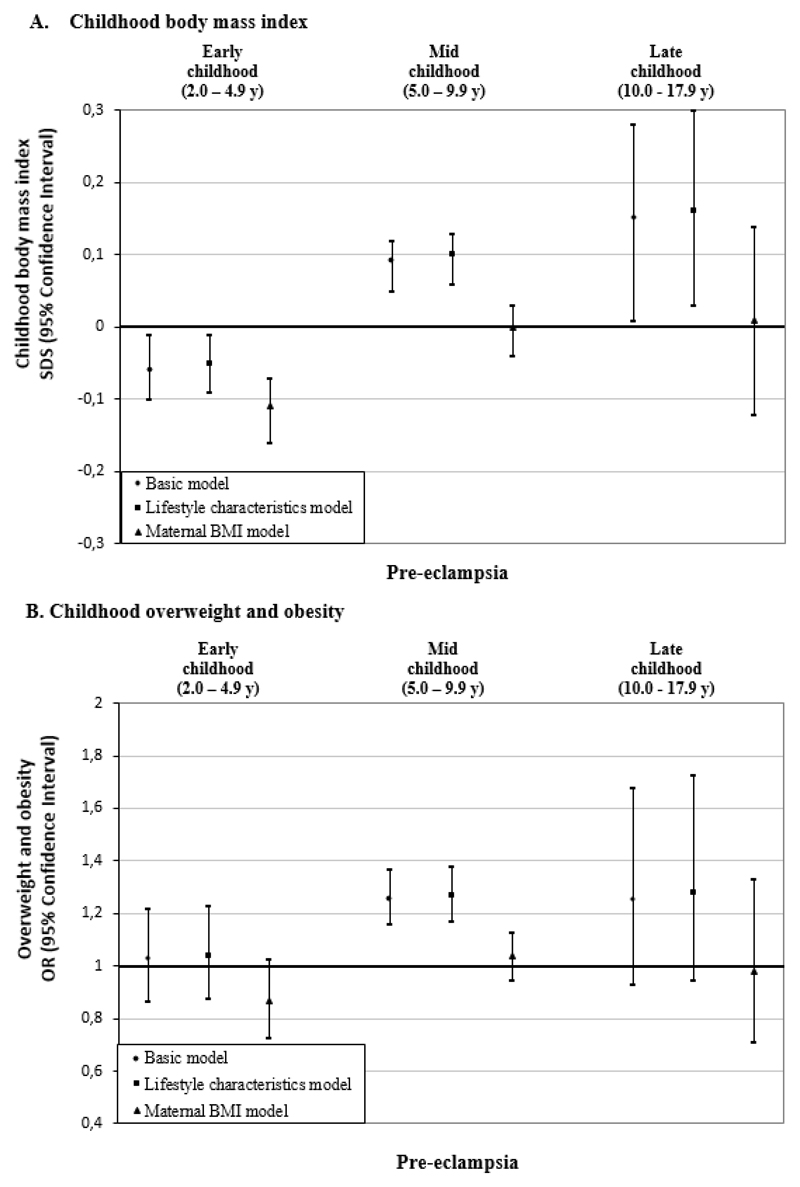
Pre-eclampsia was associated with a lower BMI in early-childhood in both basic and lifestyle characteristics models (difference in BMI-SDS in lifestyle characteristics model: - 0.05 SDS (95% CI - 0·09, - 0·01)) (Figure 4A). This inverse association strengthened upon additional adjustment for maternal BMI. In contrast, we observed a positive association of pre-eclampsia with BMI in mid- and late-childhood in basic and lifestyle characteristics models (differences in BMI-SDS in lifestyle characteristics models: 0·10 SDS (95% CI 0·06, 0·13); 0·16 SDS (95% CI 0·03, 0·30) in mid- and late-childhood, respectively). These associations fully attenuated after additional adjustment for maternal BMI. The associations of pre-eclampsia with overweight in mid- and late-childhood also fully attenuated after adjustment for maternal BMI (Figure 4B). In the basic model, pre-eclampsia was associated with a higher odds of underweight in early-childhood only (Supplemental Table S5). Similar associations between both gestational hypertension and pre-eclampsia with childhood outcomes were observed when adjusting for pre/early-pregnancy or pre-pregnancy BMI (Supplemental Table S8) and when additionally adjusted for gestational diabetes (Supplemental Table S6).
Figure 4.
Associations of pre-eclampsia with offspring BMI outcomes in early, mid, and late childhood.a
aValues are regression coefficients (95% confidence intervals) from multilevel linear mixed effects models and odds ratios (95% confidence intervals) from multilevel binary logistic models that reflect differences in early childhood (2·0 to 4·9 years), mid childhood (5·0 to 9·9 years) and late childhood (10·0 to 17·9 years) BMI SDS and risk of overweight and obesity, respectively, for children born to mothers with pre-eclampsia, as compared with the reference group (children born to mothers with an uncomplicated pregnancy). Lifestyle characteristics models are adjusted for offspring’s sex, maternal age, educational level, ethnicity, parity, and smoking during pregnancy. Maternal BMI models are additionally adjusted for maternal pre/early-pregnancy BMI.
2-stage meta-analyses
Analyses performed with 2-stage meta-analyses were consistent with those obtained from our main (1-stage) meta-analyses (Supplemental Figures S2-10). We did not observe substantial heterogeneity between the cohorts in any association that we assessed (I2 range 0 to 40%, with 6 out of the 9 meta-analyses having I2 <25%; Supplemental Figures S2-10).
Discussion
Results from our IPD analysis of 160757 mothers and children from Europe and North America demonstrated that children born to mothers with gestational diabetes and gestational hypertension had a higher BMI and higher odds of being overweight throughout childhood, whereas pre-eclampsia was associated with a lower BMI in early-childhood. These associations were of small magnitude and largely explained by maternal pre/early-pregnancy BMI.
Interpretation of main findings
Gestational diabetes and gestational hypertensive disorders affect substantial numbers of pregnancies and are associated with adverse maternal and fetal pregnancy outcomes.1–3 Gestational diabetes may lead to fetal overnutrition as a result of maternal hyperglycemia during pregnancy.7 Gestational hypertension and pre-eclampsia are related to placental dysfunction, which may lead to impaired fetal nutrient supply.8,30 Both conditions may subsequently induce permanent changes in offspring body composition, neuroendocrine systems and metabolic functions, which predispose offspring to an increased risk of obesity in later life.9 Our aim was to explore if gestational diabetes and gestational hypertensive disorders affect the risk of offspring obesity independently of the risk conferred by maternal obesity. Disentangling the independent role of these maternal pregnancy complications on childhood obesity risk is important for development of future childhood obesity prevention strategies.
Results from two systematic reviews of 12 and 9 published cohort studies suggested that maternal diabetes during pregnancy was associated with a higher offspring BMI. These associations were no longer present in single studies that adjusted for maternal pre-pregnancy BMI.14,31 Recently, several prospective observational studies reported inconsistent findings for the association of gestational diabetes with offspring BMI after adjustment for maternal BMI.11–13,15–17,32–34 An older prospective cohort study of 280866 Swedish men recruited from 1973 to 1988, suggested that diabetes during pregnancy, including gestational diabetes and pre-existing diabetes, was associated with a higher BMI at the age of 18 years. This association was independent of maternal BMI.17 In contrast, we observed that children of mothers with gestational diabetes had a higher BMI throughout childhood, but this association was largely explained by maternal pre/early-pregnancy BMI. This different finding might be explained by methodological differences between our IPD meta-analysis and the Swedish Study, as the Swedish study was focused on men only, measured offspring BMI in adulthood, and does not reflect contemporary clinical practice which involves newer screening and treatment strategies. We observed the strongest association of gestational diabetes with offspring obesity in early-childhood. This may be explained by tracking of birth size.35,36 Consistent with our findings, treatment of gestational diabetes, defined as a fasting glucose <95 mg/dL and two of three timed measurements that exceeded established thresholds, was found to be beneficial for neonatal outcomes, but did not influence offspring obesity risk at age 5-10 years in a multicenter randomized controlled trial.37 Using the diagnosis of gestational diabetes may insufficiently reflect glycemic status during pregnancy. Maternal glucose levels below current diagnostic criteria of diabetes, are linearly and positively associated with adverse perinatal outcomes.35,38 However, several studies showed that maternal gestational glucose was not associated with offspring BMI in early childhood, after adjustment for maternal BMI.39,40 Thus, our findings strongly suggest that the association of gestational diabetes with higher BMI in the offspring is largely explained by maternal BMI.
Few studies have examined the association of gestational hypertensive disorders with childhood adiposity. Data from a UK prospective cohort study of 6343 mother-offspring pairs found a positive association of gestational hypertension with childhood adiposity at age 9, which attenuated after adjustment for parental BMI.18 This study also found inverse associations of pre-eclampsia with offspring lean mass and adiposity at age 9 after adjustment for parental BMI.18 In contrast, an Australian cohort study of 1151 mother-offspring pairs born at term showed that offspring of mothers with gestational hypertension had higher risk of overweight at 20 years, independently of maternal BMI.19 No association of pre-eclampsia with offspring BMI was present.19 We observed that gestational hypertension was associated with a higher BMI and odds of overweight throughout childhood, but this association was also largely explained by maternal BMI. Pre-eclampsia was associated with a lower BMI in early-childhood, and this inverse association was stronger after adjustment for maternal BMI. In later childhood, pre-eclampsia was associated with a higher childhood BMI, but this association was no longer present after adjustment for maternal BMI. This might be explained by a smaller size at birth among children of mothers with pre-eclampsia due to placental dysfunction and fetal growth restriction.41 These children might experience accelerated growth in infancy and early-childhood, leading to higher BMI in mid- and late-childhood.42 Further studies using detailed assessment of maternal blood pressure and placental function, which provides details on disease severity, may provide more insight into the effects of pre-eclampsia on childhood adiposity development.
Our observations are important from an etiological and preventive perspective. The associations of gestational diabetes and gestational hypertensive disorders with offspring obesity were of limited clinical importance and largely dependent on maternal BMI. Interventions to reduce the risk of pregnancy complications or improve the effectiveness of their treatment may be important in relation to a range of problems for the mother and child, but they are unlikely to influence directly the development of obesity in offspring.
Strengths and limitations
Major strengths of our study are the large sample of contemporary populations reflecting current diagnosis and treatment policies and the use of IPD from a wide selection of existing studies to reduce the risk of publication bias. We were able to adjust for multiple confounders, with a particular focus on maternal pre-pregnancy BMI. Out of 50 cohorts identified and invited, 38 agreed to participate and 34 were included in these analyses. Only 4 cohorts (113417 participants) were excluded due to missing data on exposures and/or outcomes. Bias in our findings is unlikely since the reasons for not participating are not related to the research question of the study but rather to study design or follow-up. Although we have included cohorts from early 1990´s onwards, the study period does not seem to influence our findings since we observed low heterogeneity between all cohorts in the associations assessed. We included data from cohort studies from Europe and North America, so our findings are mainly applicable for Western populations. The inclusion of data from other regions could have led to large differences in maternal and childhood obesity prevalences, treatment of pregnancy complications and ethnic and sociodemographic characteristics, complicating or limiting the possibility to meta-analyse the data. Further studies are needed to assess these associations among populations from low- and middle-income countries, and to explore potential differences among women from different ethnic backgrounds known to be at higher risk to develop pregnancy complications. Within some cohorts, women might have participated with multiple singleton pregnancies. We were unable to account for within-mother clustering, as this data was not available within our IPD meta-analysis. However, as this only reflects a very small number of women, we consider it unlikely that this has affected our results. Our observed associations are applicable for singleton pregnancies. As important differences in placental development and function, fetal nutrient supply and growth are present among twin pregnancies, further studies are needed to explore whether these findings are also present among twin pregnancies. We are aware that our study cannot overcome potential limitations of individual studies in terms of their design and conduct, differences in measurements and definitions of exposure and outcome data, variation in missing data and loss to follow up. Some cohorts relied on self-reporting to obtain information on pregnancy complications. We were not able to perform sensitivity analyses based on the methods of data collection since some cohorts have collected data on pregnancy complications using more than one method. Misclassification of women, if present, might have led to attenuation of the associations. The prevalence of gestational diabetes in our sample was relatively low and varied substantially between cohorts, which might suggest underascertainment of this condition, differences in screening protocols between cohorts and a selection towards a relatively healthy study population. This might affect the generalizability of our findings. We have no information on how any of the pregnancy complications were treated, which might also differ per cohort. Effective treatment that reduced circulating glucose and blood pressure during pregnancy may lead to weaker associations of these conditions with offspring obesity. Given that our IPD meta-analysis includes contemporary cohorts reflecting contemporary screening and treatment protocols, it seems likely that a large number of women received treatment for the pregnancy complication. Self-reported maternal pre-pregnancy BMI might also be a source of bias. However, a systematic review showed that reporting error did not bias associations between pregnancy-related weight and birth outcomes43, suggesting that bias due to self-reported maternal BMI is unlikely in our results. If pre-pregnancy BMI was not available, we used early-pregnancy BMI, which might have overestimated BMI as a result of gestational weight gain. However, similar results were observed when restricting the analyses to the participants with information on pre-pregnancy BMI. Most cohorts have relied on measured childhood weight and height and therefore bias based on self-reported weight and height is unlikely. We did not perform mediation analyses, which we consider beyond the scope of this manuscript. However, based on our findings, a mediating role of pregnancy complications in the associations of maternal BMI with childhood BMI seems unlikely. Further studies are needed to explore which pregnancy, birth, genetic or lifestyle characteristics mediate the associations of maternal BMI with childhood BMI. Missing values of covariates were used as an additional group, which might not be optimal, but is a method commonly used in large IPD meta-analyses.44 Finally, due to the observational design, residual confounding cannot be excluded.
Conclusions
The associations of gestational diabetes, gestational hypertension and pre-eclampsia with childhood obesity are largely explained by maternal pre/early-pregnancy BMI. These findings from a large international IPD meta-analysis of contemporary cohorts are important for future public health strategies on childhood obesity. Interventions focused on prevention or treatment of these pregnancy complications, though important for other maternal and fetal pregnancy outcomes, are unlikely to have a direct impact on offspring BMI.
Supplementary Material
Research in Context.
Evidence before this study
We searched Medline (through PubMed) up to January 2018 in order to identify relevant cohort studies as well as systematic reviews that focused on the associations of gestational diabetes and hypertensive disorders of pregnancy with offspring obesity and the role of maternal obesity in these associations. Our search was a combination of the key words [free text and MeSH (Medical Subject Headings) terms] related to our exposures (gestational diabetes OR gestational hypertension OR pre-eclampsia) and outcomes (overweight OR obesity OR body mass index OR adiposity). We were able to identify several systematic reviews and some prospective cohort studies, that varied in terms of methodological quality. Gestational diabetes and gestational hypertensive disorders are common pregnancy complications associated with increased risks of perinatal mortality and morbidity, and seem to influence the risk of obesity in the offspring. Maternal obesity is a major risk factor for common pregnancy complications, and is also associated with an increased risk of obesity in the offspring. However, it is not clear whether gestational diabetes and gestational hypertensive disorders affect the risk of offspring obesity independently of the risk conferred by maternal obesity.
Added value of this study
We performed an individual participant data meta-analysis of 160757 mother-offspring pairs from 34 prospective European or North-American contemporary pregnancy/birth cohorts. We observed that children born to mothers with gestational diabetes and gestational hypertension had a higher BMI and higher odds of being overweight throughout childhood. Pre-eclampsia was associated with a lower BMI in early-childhood. All associations were largely explained by maternal pre/early-pregnancy BMI. Our study strongly suggests that the previously observed associations of common pregnancy complications with higher offspring BMI are largely explained by maternal BMI.
Implications of all the available evidence
Lowering maternal risk of gestational diabetes, gestational hypertension and pre-eclampsia, though important in relation to maternal and fetal pregnancy outcomes, is unlikely to have a direct impact on offspring obesity among Western women receiving contemporary medical care. Preventive strategies for reducing childhood obesity should focus on maternal BMI rather than on pregnancy complications.
Acknowledgments
The authors gratefully acknowledge participants from all cohorts involved in this study.
Footnotes
MOCO Study Group
Bernadeta Patro Golab, Susana Santos, Ellis Voerman, Henrique Barros, Anna Bergström, Marie-Aline Charles, Leda Chatzi, Cécile Chevrier, George P. Chrousos, Eva Corpeleijn, Nathalie Costet, Sarah Crozier, Graham Devereux, Merete Eggesbø, Sandra Ekström, Maria Pia Fantini, Sara Farchi, Francesco Forastiere, Vagelis Georgiu, Keith M. Godfrey, Davide Gori, Wojciech Hanke, Irva Hertz-Picciotto, Barbara Heude, Daniel Hryhorczuk, Hazel Inskip, Jesus Ibarluzea, Louise C. Kenny, Leanne K. Küpers, Hanna Lagström, Irina Lehmann, Virissa Lenters, Sabrina Llop, Per Magnus, Renata Majewska, Johanna Mäkelä, Yannis Manios, Fionnuala M. McAuliffe, Sheila W. McDonald, John Mehegan, Monique Mommers, Camilla S. Morgen, George Moschonis, Deirdre Murray, Carol Ní Chaoimh, Ellen A. Nøhr, Anne-Marie Nybo Andersen, Emily Oken, Adriëtte J.J.M. Oostvogels, Agnieszka Pac, Eleni Papadopoulou, Costanza Pizzi, Kinga Polanska, Daniela Porta, Lorenzo Richiardi, Sheryl L. Rifas-Shiman, Franca Rusconi, Ana C. Santos, Henriette A. Smit, Thorkild I.A. Sørensen, Marie Standl, Camilla Stoltenberg, Jordi Sunyer, Michelle Taylor, Elisabeth Thiering, Carel Thijs, Maties Torrent, Suzanne C. Tough, Tomas Trnovec, Steve Turner, Lenie van Rossem, Andrea von Berg, Martine Vrijheid, Tanja Vrijkotte, Jane West, John Wright, Oleksandr Zvinchuk, Debbie A. Lawlor, Vincent W.V. Jaddoe, Romy Gaillard
Conflict of Interest Disclosures
Bernadeta Patro Golab received a research training fellowship grant from the Nestle Nutrition Institute.
Debbie A. Lawlor has received support from Roche Diagnostics and Medtronic in relation to biomarker research that is not related to the research presented in this paper. The other authors declared no conflicts of interest.
Author Contributions
BPG and SS had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: BPG, SS, VJ, RG
Analysis and interpretation of data: BPG, SS, EV, VJ, RG
Drafting of the manuscript: BPG, SS, DL, VJ, RG
Critical revision of the manuscript for important intellectual content: All authors
References
- 1.Magee LA, von Dadelszen P, Stones W, Mathai M, editors. The FIGO Textbook of Pregnancy Hypertension: An evidence-based guide to monitoring, prevention and management. London, UK: The Global Library of Women’s Medicine; 2016. [Google Scholar]
- 2.World Health Organization. Global report on diabetes. [Accessed December 1, 2016]; http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf.
- 3.Wagner SJ, Barac S, Garovic VD. Hypertensive pregnancy disorders: current concepts. J Clin Hypertens (Greenwich) 2007;9(7):560–6. doi: 10.1111/j.1524-6175.2007.06695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirst JE, Villar J, Victora CG, Papageorghiou AT, Finkton D, Barros FC, et al. The antepartum stillbirth syndrome: risk factors and pregnancy conditions identified from the INTERGROWTH-21(st) Project. BJOG : an international journal of obstetrics and gynaecology. 2018;125(9):1145–53. doi: 10.1111/1471-0528.14463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization Collaboration. The world health report: make every mother and child count. Department of Reproductive Health and Research, WHO; 2005. [Accessed June 2018]. www.who.int/whr/2005/en/index.html. [Google Scholar]
- 6.Casey BM, Lucas MJ, McIntire DD, Leveno KJ. Pregnancy outcomes in women with gestational diabetes compared with the general obstetric population. Obstetrics and gynecology. 1997;90(6):869–73. doi: 10.1016/s0029-7844(97)00542-5. [DOI] [PubMed] [Google Scholar]
- 7.Fraser A, Lawlor DA. Long-term health outcomes in offspring born to women with diabetes in pregnancy. Curr Diab Rep. 2014;14(5):489. doi: 10.1007/s11892-014-0489-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herzog EM, Eggink AJ, Reijnierse A, Kerkhof MA, de Krijger RR, Roks AJ, et al. Impact of early- and late-onset preeclampsia on features of placental and newborn vascular health. Placenta. 2017;49:72–9. doi: 10.1016/j.placenta.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 9.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359(1):61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godfrey KM, Reynolds RM, Prescott SL, Nyirenda M, Jaddoe VW, Eriksson JG, et al. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017;5(1):53–64. doi: 10.1016/S2213-8587(16)30107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nehring I, Chmitorz A, Reulen H, von Kries R, Ensenauer R. Gestational diabetes predicts the risk of childhood overweight and abdominal circumference independent of maternal obesity. Diabet Med. 2013;30(12):1449–56. doi: 10.1111/dme.12286. [DOI] [PubMed] [Google Scholar]
- 12.Zhao YL, Ma RM, Lao TT, Chen Z, Du MY, Liang K, et al. Maternal gestational diabetes mellitus and overweight and obesity in offspring: a study in Chinese children. J Dev Orig Health Dis. 2015;6(6):479–84. doi: 10.1017/S2040174415007205. [DOI] [PubMed] [Google Scholar]
- 13.Crume TL, Ogden L, Daniels S, Hamman RF, Norris JM, Dabelea D. The impact of in utero exposure to diabetes on childhood body mass index growth trajectories: the EPOCH study. J Pediatr. 2011;158(6):941–6. doi: 10.1016/j.jpeds.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim SY, England JL, Sharma JA, Njoroge T. Gestational diabetes mellitus and risk of childhood overweight and obesity in offspring: a systematic review. Exp Diabetes Res. doi: 10.1155/2011/541308. [published online September 22, 2011] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel S, Fraser A, Davey Smith G, Lindsay RS, Sattar N, Nelson SM, et al. Associations of gestational diabetes, existing diabetes, and glycosuria with offspring obesity and cardiometabolic outcomes. Diabetes Care. 2012;35(1):63–71. doi: 10.2337/dc11-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pham MT, Brubaker K, Pruett K, Caughey AB. Risk of childhood obesity in the toddler offspring of mothers with gestational diabetes. Obstet Gynecol. 2013;121(5):976–82. doi: 10.1097/AOG.0b013e31828bf70d. [DOI] [PubMed] [Google Scholar]
- 17.Lawlor DA, Lichtenstein P, Langstrom N. Association of maternal diabetes mellitus in pregnancy with offspring adiposity into early adulthood: sibling study in a prospective cohort of 280,866 men from 248,293 families. Circulation. 2011;123(3):258–65. doi: 10.1161/CIRCULATIONAHA.110.980169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geelhoed JJ, Fraser A, Tilling K, Benfield L, Davey Smith G, Sattar N, et al. Preeclampsia and gestational hypertension are associated with childhood blood pressure independently of family adiposity measures: the Avon Longitudinal Study of Parents and Children. Circulation. 2010;122(12):1192–9. doi: 10.1161/CIRCULATIONAHA.110.936674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis EF, Lewandowski AJ, Aye C, Williamson W, Boardman H, Huang RC, et al. Clinical cardiovascular risk during young adulthood in offspring of hypertensive pregnancies: insights from a 20-year prospective follow-up birth cohort. BMJ Open. 2015;5(6):e008136. doi: 10.1136/bmjopen-2015-008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis EF, Lazdam M, Lewandowski AJ, Worton SA, Kelly B, Kenworthy Y, et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics. 2012;129(6):e1552–61. doi: 10.1542/peds.2011-3093. [DOI] [PubMed] [Google Scholar]
- 21.de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85(9):660–7. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. Training Course on Child Growth Assessemnt. WHO Child Growth Standards. [Accessed June 18, 2017]; http://www.who.int/childgrowth/training/module_c_interpreting_indicators.pdf?ua=1.
- 24.World Health Organization. Growth reference 5-19 years. [Accessed June 18, 2017]; http://www.who.int/growthref/who2007_bmi_for_age/en/
- 25.Pearce N, Lawlor DA. Causal inference-so much more than statistics. Int J Epidemiol. 2016;45(6):1895–903. doi: 10.1093/ije/dyw328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Debray TP, Moons KG, Abo-Zaid GM, Koffijberg H, Riley RD. Individual participant data meta-analysis for a binary outcome: one-stage or two-stage? PLoS One. 2013;8(4):e60650. doi: 10.1371/journal.pone.0060650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogland B, Vatten LJ, Romundstad PR, Nilsen ST, Forman MR. Pubertal anthropometry in sons and daughters of women with preeclamptic or normotensive pregnancies. Archives of disease in childhood. 2009;94(11):855–9. doi: 10.1136/adc.2008.150870. [DOI] [PubMed] [Google Scholar]
- 28.Le Moullec N, Fianu A, Maillard O, Chazelle E, Naty N, Schneebeli C, et al. Sexual dimorphism in the association between gestational diabetes mellitus and overweight in offspring at 5-7 years: The OBEGEST cohort study. PloS one. 2018;13(4):e0195531. doi: 10.1371/journal.pone.0195531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Catalano PM, McIntyre HD, Cruickshank JK, McCance DR, Dyer AR, Metzger BE, et al. The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes care. 2012;35(4):780–6. doi: 10.2337/dc11-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan T, Zhang T, Han Z. Placental vascularization alterations in hypertensive disorders complicating pregnancy (HDCP) and small for gestational age with HDCP using three-dimensional power doppler in a prospective case control study. BMC Pregnancy Childbirth. 2015;15:240. doi: 10.1186/s12884-015-0666-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Philipps LH, Santhakumaran S, Gale C, Prior E, Logan KM, Hyde MJ, et al. The diabetic pregnancy and offspring BMI in childhood: a systematic review and meta-analysis. Diabetologia. 2011;54(8):1957–66. doi: 10.1007/s00125-011-2180-y. [DOI] [PubMed] [Google Scholar]
- 32.Bider-Canfield Z, Martinez MP, Wang X, Yu W, Bautista MP, Brookey J, et al. Maternal obesity, gestational diabetes, breastfeeding and childhood overweight at age 2 years. Pediatr Obes. 2017;12(2):171–8. doi: 10.1111/ijpo.12125. [DOI] [PubMed] [Google Scholar]
- 33.West NA, Crume TL, Maligie MA, Dabelea D. Cardiovascular risk factors in children exposed to maternal diabetes in utero. Diabetologia. 2011;54(3):504–7. doi: 10.1007/s00125-010-2008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ouyang F, Parker MG, Luo ZC, Wang X, Zhang HJ, Jiang F, et al. Maternal BMI, gestational diabetes, and weight gain in relation to childhood obesity: The mediation effect of placental weight. Obesity (Silver Spring) 2016;24(4):938–46. doi: 10.1002/oby.21416. [DOI] [PubMed] [Google Scholar]
- 35.Tyrrell J, Richmond RC, Palmer TM, Feenstra B, Rangarajan J, Metrustry S, et al. Genetic Evidence for Causal Relationships Between Maternal Obesity-Related Traits and Birth Weight. JAMA. 2016;315(11):1129–40. doi: 10.1001/jama.2016.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dode MA, Santos IS, Gonzalez DA. Anthropometry from birth to 24 months among offspring of women with gestational diabetes: 2004 Pelotas Birth Cohort. J Dev Orig Health Dis. 2011;2(3):144–51. doi: 10.1017/S2040174410000619. [DOI] [PubMed] [Google Scholar]
- 37.Landon MB, Rice MM, Varner MW, Casey BM, Reddy UM, Wapner RJ, et al. Mild gestational diabetes mellitus and long-term child health. Diabetes Care. 2015;38(3):445–52. doi: 10.2337/dc14-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farrar D, Simmonds M, Bryant M, Sheldon TA, Tuffnell D, Golder S, et al. Hyperglycaemia and risk of adverse perinatal outcomes: systematic review and meta-analysis. BMJ. 2016;354:i4694. doi: 10.1136/bmj.i4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pettitt DJ, McKenna S, McLaughlin C, Patterson CC, Hadden DR, McCance DR. Maternal glucose at 28 weeks of gestation is not associated with obesity in 2-year-old offspring: the Belfast Hyperglycemia and Adverse Pregnancy Outcome (HAPO) family study. Diabetes Care. 2010;33(6):1219–23. doi: 10.2337/dc09-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aris IM, Soh SE, Tint MT, Saw SM, Rajadurai VS, Godfrey KM, et al. Associations of gestational glycemia and prepregnancy adiposity with offspring growth and adiposity in an Asian population. Am J Clin Nutr. 2015;102(5):1104–12. doi: 10.3945/ajcn.115.117614. [DOI] [PubMed] [Google Scholar]
- 41.Odegard RA, Vatten LJ, Nilsen ST, Salvesen KA, Austgulen R. Preeclampsia and fetal growth. Obstet Gynecol. 2000;96(6):950–5. [PubMed] [Google Scholar]
- 42.Gishti O, Gaillard R, Manniesing R, Abrahamse-Berkeveld M, van der Beek EM, Heppe DH, et al. Fetal and infant growth patterns associated with total and abdominal fat distribution in school-age children. The Journal of clinical endocrinology and metabolism. 2014;99(7):2557–66. doi: 10.1210/jc.2013-4345. [DOI] [PubMed] [Google Scholar]
- 43.Headen I, Cohen AK, Mujahid M, Abrams B. The accuracy of self-reported pregnancy-related weight: a systematic review. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2017;18(3):350–69. doi: 10.1111/obr.12486. [DOI] [PubMed] [Google Scholar]
- 44.Sonnenschein-van der Voort AM, Arends LR, de Jongste JC, Annesi-Maesano I, Arshad SH, Barros H, et al. Preterm birth, infant weight gain, and childhood asthma risk: a meta-analysis of 147,000 European children. The Journal of allergy and clinical immunology. 2014;133(5):1317–29. doi: 10.1016/j.jaci.2013.12.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.