Abstract
OBJECTIVES:
Racial differences in susceptibility and progression of pancreatitis have been reported in epidemiologic studies using administrative or retrospective data. There has been little study, however, on the clinical profile, causes, and outcome of chronic pancreatitis (CP) in black patients.
METHODS:
We analyzed data on black patients with CP prospectively enrolled in the multicenter North American Pancreatitis Studies from 26 US centers during the years 2000–2014. CP was defined by definitive evidence on imaging studies or histology. Information on demographics, etiology, risk factors, disease phenotype, treatment, and perceived effectiveness was obtained from responses to detailed questionnaires completed by both patients and physicians.
RESULTS:
Of the 1,159 patients enrolled, 248 (21%) were black. When compared with whites, blacks were significantly more likely to be male (60.9 vs. 53%), ever (88.2 vs. 71.8%), or current smokers (64.2 vs. 45.9%), or have a physician-defined alcohol etiology (77 vs. 41.9%). There was no overall difference in the duration of CP although for alcoholic CP, blacks had a longer duration of disease (8.6 vs. 6.97 years; P=0.02). Blacks were also significantly more likely to have advanced changes on pancreatic morphology (calcifications (63.3 vs. 55.2%), atrophy (43.2 vs. 34.6%), pancreatic ductal stricture or dilatation (72.6 vs. 65.5%) or common bile duct stricture (18.6 vs. 8.2%)) and function (endocrine insufficiency 39.9 vs. 30.2%). Moreover, the prevalence of any (94.7 vs. 83%), constant (62.6 vs. 51%), and severe (78.4 vs. 65.8%) pain and disability (35.1 vs. 21.4%) were significantly higher in blacks. Observed differences were in part related to variances in etiology and duration of disease. No differences in medical or endoscopic treatments were seen between races although prior cholecystectomy (31.1 vs. 19%) was more common in white patients.
CONCLUSIONS:
Differences were observed between blacks and whites in the underlying cause, morphologic expression, and pain characteristics of CP, which in part are explained by the underlying risk factor(s) with alcohol and tobacco being much more frequent in black patients as well as disease duration.
INTRODUCTION
Chronic pancreatitis (CP) is a chronic irreversible inflammatory process with many underlying etiologies, treatments, and outcomes. While relatively uncommon, the disease results in significant health-care expenditures with aggregate costs of over 150 million dollars yearly (1). The cardinal manifestation is abdominal pain that is typically chronic, characteristically severe and unremitting, and associated with significant morbidity leading to a severely reduced quality of life (2,3). Recent studies have expanded the causes of CP to genetic causes as well as to the important role of tobacco use (4–6). The majority of these studies, however, have focused on Caucasians, typically males.
Several observations, including our own, have highlighted racial differences in the epidemiology of pancreatitis. Specifically, the risk of hospitalization for pancreatitis in black subjects has been noted to be 2–3 times greater when compared with whites (7–10), black patients with pancreatitis have a higher likelihood of alcohol etiology (11), and after an episode of acute pancreatitis (AP), they are noted to have a greater risk for readmissions (12), as well as higher rates of AP on CP and extrapancreatic manifestations of AP (13). Unfortunately, many of these studies are retrospective, or, have used administrative data, which are limited by lack of validation or detailed information on disease phenotype and outcomes. Although the importance of these data cannot be discounted, studies with large number of blacks with patient level data are needed to characterize the clinical profile, causes, treatment, and outcome of CP in this subgroup, and to determine whether and how they differ from the large body of knowledge in white subjects. Such information is critical to perform more focused evaluation, plan treatments, and provide individualized counseling in black patients with CP.
In a series of multicenter studies conducted by the North American Pancreatitis Study Group from 2000 to 2014, 248 African-American (AA) patients with CP have been prospectively ascertained with deep phenotyping and collection of data on environmental exposures, clinical symptoms including pain experience, and treatments delivered. The aim of our study was to define the clinical profile, causes, and outcome of CP in black patients, and to compare them with over 900 white subjects enrolled in these studies. Through this analysis, we hoped to explore potential pathophysiologic differences to better our understanding of CP in this demographic group.
METHODS
North American Pancreatitis Study 2 studies
Between 2000 and 2014, the North American Pancreatitis Study group conducted three sequential studies (North American Pancreatitis Study 2 (NAPS2), NAPS2 continuation and validation study (NAPS2-CV), and NAPS2 Ancillary study (NAPS2-AS)) to prospectively ascertain patients with recurrent acute pancreatitis (RAP), CP, and controls (related, family, unrelated) subjects with an overarching goal to understand the role of genetic and environmental factors in the susceptibility and progression of pancreatitis (Supplementary Table 1). Detailed methods of the original NAPS2 and NAPS2-CV studies have been published (14,15). Since the proportion of blacks in these two studies was few (7 and 8% respectively), the NAPS2-AS study was undertaken to specifically recruit AA subjects. Two centers (Johns Hopkins and University of Chicago) with a higher volume of black subjects were asked to participate in the NAPS-AS study, and thus they only recruited black subjects. Overall, 27 centers participated in at least one of the NAPS2 studies, of which one center only contributed control subjects. At the end of enrollment in 2014, the number of RAP, CP, and control subjects enrolled in the NAPS2 studies was 569, 1,195 and 1,109, respectively, and had clinical data for analysis. The study was approved by the Institutional Review Board at each participating center and all patients provided informed consent before enrollment.
Patient cohort for the current study
The present study included 248 black and 911 white subjects with CP enrolled in the NAPS2 studies that also had clinical data available for analysis. Supplementary Table 1 shows detailed information on the number of participating centers and CP patients enrolled in individual NAPS2 studies. CP was defined by the presence of definitive changes on imaging studies—computerized tomography scan, magnetic resonance imaging/magnetic resonance cholangiopancreatography, endoscopic retrograde cholangiopancreatography (Cambridge classification), or endoscopic ultrasound (presence of 5 or more findings or presence of calcifications) or histology. Disease duration was defined as age at enrollment minus earliest age at first attack of pancreatitis or the development of symptoms compatible with CP according to physician reporting.
Questionnaires
The NAPS2 studies used two sets of questionnaires to collect detailed information from study subjects (administered by a trained study coordinator) and the enrolling physicians. As reported previously, patient questionnaires collected information on demographics, personal and family history, exposure to alcohol and tobacco, pain experience, quality of life, and medication use. The enrolling physician provided information on disease phenotype including information on history and age at first episode of AP, age at onset of symptoms and diagnosis of CP, pain patterns, exocrine and endocrine insufficiency, the most likely etiology, TIGAR-O risk factors (16), findings on imaging studies, treatments tried, and their perceived effectiveness. Based on experience gained from the initial NAPS2 study, some data elements in the questionnaires used for the NAPS2-CV study were modified, as previously described (15). The NAPS2-AS study used questionnaires similar to those of the NAPS2-CV study.
In the original NAPS2 study, patients were asked to choose from five patterns of descriptions that best characterized their pain experience. No leading question was used before asking the pain pattern (i.e., do you have pain from pancreatitis?). A subset of CP patients in this study (126/540, 23%) did not complete the question on pain pattern. Due to this, it is difficult to know whether they had “no pain” or their pain experience did not fit into the five predefined categories (2). In the NAPS2-CV and NAPS2-AS studies, a leading question was given (Have you had abdominal pain from pancreatitis in the past year?). Patients who responded “yes” to this question were then asked to choose from one of the five patterns of pain description that best characterizes their pain experience. To appropriately compare the presence of pain and the pain pattern, we limited analyses for the presence of pain, pain patterns, and pain medication use to only the NAPS2-CV and NAPS2-AS studies.
Data elements
For this study, we analyzed data on demographic factors, age of onset of symptoms, diagnosis, and enrollment, body mass index (BMI) (maximum during lifetime and at the time of enrollment), physician-defined etiology, TIGAR-O risk factors identified by the enrolling physician, presence and pattern of pain in the year preceding enrollment as reported by the patient, use of pain medication as identified by the enrolling physician, morphological changes in the pancreas on imaging studies, exocrine and endocrine insufficiency, treatments used, and their perceived effectiveness according to the enrolling physician.
Statistics
Descriptive analyses are presented as proportions for categorical data and median and interquartile range for continuous data. Bivariate comparisons were performed based on race using Fischer’s exact test for categorical data and Student’s t-test for continuous data. A priori data were analyzed both before and after stratification by physician-defined etiology. We used logistic regression to control for center, age, and sex for our primary observed associations. Separate models were fit including all centers and only the top three recruiting centers as sensitivity analysis. To evaluate whether the observed associations differed by center, we added an interaction term between race and center. We excluded centers with only 1 or 0 black or white patients from the models with the interaction term.
Data analysis was performed using SAS version 9.4 (Cary, NC). Two-tailed P values <0.05 were considered as statistically significant. Owing to the exploratory nature of this study, no adjustments were made for multiple comparisons.
RESULTS
Demographic factors and disease onset
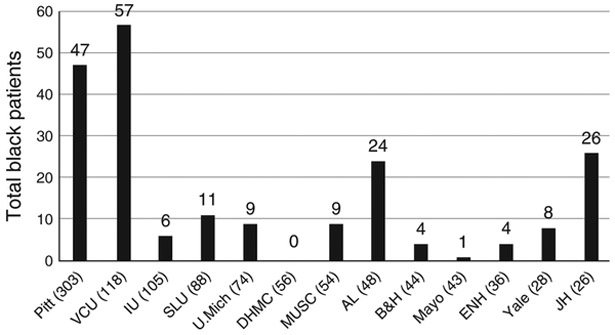
Over the study period, 1,159 white or black CP patients were enrolled into the NAPS2 studies, of whom 248 (21%) were black. The percentage of black patients by study centers that enrolled ≥25 CP patients is shown in Figure 1. Johns Hopkins participated only in the NAPS2-AS study, so all enrollees from this center were black. Among other centers, the proportion of black patients ranged from <5% (Dartmouth-Hitchcock, Lebanon, NH and Mayo Clinic, Jacksonville, FL) to 45–50% (Virginia Commonwealth University, Richmond, VA and University of Alabama, Birmingham, AL). Baseline demographics of the cohort by race are provided in Table 1. As shown, when compared with whites, black patients were significantly more likely to be male (60.9 vs. 53%). The median age of black and white subjects was similar at 53.3 and 51.5 years, respectively. However, their age distribution at the time of enrollment differed from whites, in that two-thirds of black subjects were middle aged (i.e., 44–65 years) as compared with a broader distribution of age groups among whites. The age at onset of pancreatitis symptoms, age at diagnosis, and a history of AP were similar in the two races. BMI at the time of enrollment was significantly lower in blacks when compared with whites (22.9 vs. 24.1). Blacks were more likely to have ever smoked (88.2 vs. 71.8%) and two-thirds of them were current smokers at the time of enrollment. Interestingly, the amount of tobacco use was significantly lower in blacks when compared with whites. Among ever-smokers who reported their amount of smoking, whites were 1.8 times more likely to be heavy smokers (i.e., ≥1 p.p.d.) when compared with blacks (RR 1.84, P<0.05).
Figure 1.
Proportion of black patients with CP among the NAPS2 centers that enrolled 25 or more patients. Enrolled patients by center. Numbers in parentheses on the X axis represent patients enrolled from each center. AL, University of Alabama; B&H, Brigham & Women’s Hospital; CP, chronic pancreatitis; DHMC, Dartmouth-Hitchcock Medical Center; ENH, Evanston Northwestern Healthcare; IU, Indiana University; JH, Johns Hopkins Medical University; Mayo, Mayo Clinic Jacksonville; MUSC, Medical University of South Carolina; NAPS2, North American Pancreatitis Study 2; Pitt, University of Pittsburgh; SLU, St. Louis University; U Mich, University of Michigan; VCU, Virginia Commonwealth University; Yale, Griffin Hospital, Yale affiliate.
Table 1.
Demographics by race among subjects with chronic pancreatitis
| Demographics | Black (N=248) | White (N=911) |
|---|---|---|
| Age at enrollment, median (Q1–Q3) | 53.3 (45.7–57.7) | 51.5 (40.9–62.3) |
| Age distribution at enrollment; N (%)* | ||
| <25 | 3 (1.2) | 51 (5.6) |
| 25–<45 | 56 (22.6) | 269 (29.5) |
| 45–<65 | 162 (65.3) | 407 (44.7) |
| 65+ | 27 (10.9) | 184 (20.2) |
| Age at diagnosis, median (Q1–Q3)a | 48.0 (39.0–54.0) | 48.0 (38.0–59.0) |
| Age at symptoms, median (Q1–Q3)b | 46.0 (37.0–54.0) | 47.0 (37.0–58.0) |
| Gender distribution; N %)* | ||
| Male | 151 (60.9) | 483 (53.0) |
| Female | 97 (39.1) | 428 (47.0) |
| BMI at enrollment, median (Q1–Q3)c* | 22.9 (19.7–26.6) | 24.1 (21.2–27.8) |
| BMI maximum, median (Q1–Q3)d | 27.7 (24.4–33.5) | 28.8 (25.1–32.9) |
| BMI difference (BMI max–BMI at enrollment) | 4.8 | 4.7 |
| History of acute pancreatitise; N (%) |
141 (65.9) | 586 (67.5) |
| Smoking statusf* | ||
| Never | 29 (11.8) | 256 (28.2) |
| Past | 59 (24.0) | 235 (25.9) |
| Current | 158 (64.2) | 417 (45.9) |
| Smoking—Packs per dayf* | ||
| Never | 29 (12.1) | 256 (29.7) |
| Less than 1 p.p.d. | 145 (60.4) | 256 (29.7) |
| 1 or more p.p.d. | 66 (27.5) | 349 (40.5) |
BMI, body mass index.
P value <0.05. Proportions/medians shown are based on effective numbers.
Missing data: 92 patients.
Missing data: 86 patients.
Missing data: 11 patients.
Missing data: 86 patients.
77 patients.
Missing data: 58 patients.
Cause of pancreatitis
As shown in Tables 2 and 3, physician-defined etiology and risk factors between the races were significantly different. Physicians identified alcohol as the etiology of CP in the majority of blacks ((77 vs. 42%; RR 1.84, P<0.05)). In contrast, other etiologies including genetic (10.2 vs. 1.6%), obstructive (7.9 vs. 2.4%), idiopathic (27.8 vs. 12.9%), and autoimmune (2.6 vs. 0%) were identified by physicians significantly more often in whites.
Table 2.
Distribution of risk factors by race according to the etiologic classification
| Etiologic classification:N (%)a (overall P value <0.001) |
Black (N=248) | White (N=910) |
|---|---|---|
| Alcohol* | 191 (77.0) | 381 (41.9) |
| Genetic* | 4 (1.6) | 93 (10.2) |
| Idiopathic* | 32 (12.9) | 253 (27.8) |
| Obstructive* | 6 (2.4) | 72 (7.9) |
| Autoimmune* | 0 (0.0) | 24 (2.6) |
| Hyperlipidemia | 2 (0.8) | 13 (1.4) |
| Gallstone | 1 (0.4) | 9 (1.0) |
| Medications | 0 (0.0) | 1 (0.1) |
| Others | 12 (4.8) | 64 (7.0) |
P value <0.05.
Missing data: 1 patient; proportions shown are based on effective numbers.
Table 3.
Distribution of risk factors by race according to the TIGAR-O classification
| TIGAR-O factors, N (%) | Black (N=248) | White (N=911) |
|---|---|---|
| Toxic-metabolic | ||
| Alcohol* | 198 (79.8) | 401 (44.0) |
| Tobacco* | 182 (73.4) | 395 (43.4) |
| Hyperlipidemia | 18 (7.3) | 98 (10.8) |
| Hypercalcemia | 1 (0.4) | 8(0.9) |
| Medications | 6 (2.4) | 24 (2.6) |
| Chronic renal failure* | 8(3.2) | 11 (1.2) |
| Toxins* | 2 (0.8) | 0 (0.0) |
| Idiopathic* | 37 (14.9) | 345 (37.9) |
| Genetic* | 6 (2.4) | 93 (10.2) |
| Autoimmune | ||
| Autoimmune pancreatitis* | 0 (0.0) | 27 (3.0) |
| Autoimmune-associated diseases |
2 (0.8) | 27 (3.0) |
| RAP and SAP associated CP | 8(3.2) | 55 (6.0) |
| Obstructive* | 25 (10.1) | 189 (20.8) |
| Pancreas divisum* | 12 (4.8) | 93 (10.2) |
| Sphincter of Oddi* | 4 (1.6) | 52 (5.7) |
| Post-trauma stricture | 0 (0.0) | 6 (0.7) |
| Duct obstruction* | 6 (2.4) | 51 (5.6) |
| Others (CV/AA only) | 5 (2.6) | 24 (5.3) |
| Miscellaneous (CV/AS only) | 19 (10.0) | 46 (10.2) |
AA, African-American; AS, NAPS2-AS study (North American Pancreatitis Study 2-Ancillary Study—which enrolled only African-American patients); CP, chronic pancreatitis; CV, NAPS2-CV study (North American Pancreatitis Study 2-Continuation and Validation Study); RAP, recurrent acute pancreatitis; SAP, severe acute pancreatitis; TIGAR-O, toxic-metabolic, idiopathic, genetic, autoimmune, recurrent/severe acute pancreatitis, obstructive.
P value <0.05.
Based upon the TIGAR-O classification, physicians identified tobacco as a risk factor more often in blacks than in whites (73.4 vs. 43.4%). To evaluate whether this effect was due to a higher prevalence of smoking in blacks, we limited this analysis only to subjects who were self-reported smokers. Interestingly, among these subjects also, physicians identified tobacco as a risk factor in a higher fraction of blacks when compared with whites (82.7 vs. 59.4%). Similar to etiology, obstructive causes were significantly different between the groups with obstruction of some type reported as a risk factor in 20.8% of whites as compared with 10.1% of blacks (P<0.05). Pancreas divisum was listed in 10.2% of whites as compared with 4.8% of blacks. Likewise, sphincter of Oddi dysfunction was attributed in 5.7% of whites as compared with 1.6% of blacks.
When data were analyzed after further stratification by sex, alcohol and tobacco smoking remained the most frequently identified causes and risk factors for CP in both black men and women, and these were significantly different from white men and women, respectively (data not shown). To determine whether the prevalence and intensity of smoking based on race was limited to CP patients, we evaluated the prevalence of smoking in controls enrolled in the NAPS2 studies. The prevalence of ever smoking was similar (50 vs. 51%), current smoking was higher (31 vs. 20%), heavy smoking (>=1 ppd) overall was lower (8 vs. 23%) in black controls when compared with white controls.
However, when comparing controls vs. CP patients, the relative risk of being a heavy smoker overall was higher (3.43 vs. 1.76 times) among blacks CP vs. controls (27.5 vs. 8%) when compared with whites CP vs. controls (40.5 vs. 23%). These findings suggest that being a heavy smoker is a stronger risk factor for CP in the black population compared with the white population.
To address the effect of center on the primary observations (alcohol, tobacco), we have performed logistic regression analyses where we used the status of etiology or primary risk factors under investigation as the outcome variable (physician-defined alcohol etiology; patient reported heavy or very heavy drinking; physician identification of tobacco as risk factor; patient reported smoking status—yes vs. no) and center as one of the covariates. We tested the interaction between race and center in these regression models. These models were applied to data from all centers with at least two white and two black patients recruited (15/26 centers) and data from the top three centers as sensitivity analysis. In these analyses, the interaction terms between centers and race were not statistically significant (range of P values: 0.054–0.979), suggesting that the observed association between race and alcohol and tobacco was not related to centers.
Because the interaction term in these models was not significant we dropped the interaction term and included all 26 centers in the “all centers” model in the results below (Table 4). We found that the odds for black patients for alcohol exposure or etiology, and tobacco exposure or risk factor was significantly higher when compared with white patients, controlling for center, age, and sex.
Table 4.
Logistic regression analyses to assess the effect of center on the association between race and alcohol and tobacco among patients with CP
| Outcome | OR (95% CI)—Black vs. White CP patients | |
|---|---|---|
| All centers | Top 3 centers | |
| Physician-defined alcohol etiology (yes) | 4.31 (2.92–6.35) | 4.35 (2.57–7.36) |
| Physician-identified tobacco as risk factor (yes) | 2.54 (1.75–3.67) | 2.97 (1.79–4.92) |
| Self-reported heavy–very heavy drinker (yes) | 2.62 (1.82–3.78) | 2.50 (1.53–4.09) |
| Self-reported ever smoking (yes) | 2.97 (1.80–4.90) | 4.16 (1.98–8.77) |
CI, confidence interval; CP, chronic pancreatitis; OR, odds ratio. All models were controlled for age, sex, and center. Joint test of interaction terms between center and race was not statistically significant (range of P values: 0.054–0.979) for any of the models.
Top 3 centers—University of Pittsburgh, Virginia Commonwealth University, and University of Alabama.
Morphology, exocrine, and endocrine insufficiency
Many significant differences were noted in the morphological appearance of the pancreas in blacks when compared with whites on imaging studies. Overall, blacks had a significantly higher prevalence (P<0.05) of calcifications (63.3 vs. 55.2%), pancreatic atrophy (43.2 vs. 34.6%), pancreatic ductal strictures or dilatation (72.6 vs. 65.5%), pancreatic duct irregularities (52.4 vs. 24.2%), common bile duct dilatation (24.6 vs. 13.7%), or stricture (18.6 vs. 8.2%) when compared with whites (Table 5). The prevalence of each of these morphological findings was also significantly higher in black patients with alcoholic pancreatitis, suggesting that these findings were attributable to differences between the morphologic abnormalities in black vs. white patients with alcoholic CP. After stratification by alcohol etiology, blacks with non-alcoholic etiologies were significantly less likely to have calcifications (28.8 vs. 50.4%) and had a higher prevalence of pancreatic duct irregularities (45.6 vs. 24%) when compared with whites, with no difference noted for other morphological features.
Table 5.
Morphological features, exocrine and endocrine insufficiency, and disability by race among subjects with chronic pancreatitis
| N (%) | All (N=1,159) |
Alcohol (N=572) |
Other (N=587) |
|||
|---|---|---|---|---|---|---|
| Black (N=248) | White (N=911) | Black (N=191) | White (N=381) | Black (N=57) | White (N=530) | |
| Calcifications | 157 (63.3)* | 503 (55.2) | 140 (73.3)* | 236 (61.9) | 17 (29.8) | 267 (50.4)* |
| Pseudocysts | 86 (34.7) | 258 (28.3) | 75 (39.3) | 153 (40.2) | 11 (19.3) | 105 (19.8) |
| Pancreatic atrophy | 107 (43.2)* | 315 (34.6) | 90 (47.1)* | 134 (35.2) | 17 (29.8) | 181 (34.2) |
| Pancreatic duct stricture or dilation | 180 (72.6)* | 597 (65.5) | 140 (73.3) | 251 (65.9) | 40 (70.2) | 346 (65.3) |
| Pancreatic duct irregularities | 130 (52.4)* | 220 (24.2) | 104 (54.5)* | 93 (24.4) | 26 (45.6)* | 127 (24.0) |
| Common bile duct stricture | 46 (18.6)* | 75 (8.2) | 43 (22.5)* | 46 (12.1) | 3 (5.3) | 29 (5.5) |
| Common bile duct dilatation | 61 (24.6)* | 125 (13.7) | 54 (28.3)* | 69 (18.1) | 7 (12.3) | 56 (10.6) |
| Exocrine insufficiency | 100 (40.3) | 325 (35.7) | 79 (41.4) | 148 (38.9) | 21 (36.8) | 177 (33.4) |
| Endocrine insufficiency | 99 (39.9)* | 275 (30.2) | 75 (39.3)* | 118 (31.0) | 24 (42.1) | 157 (29.6) |
| Disability | 87 (35.1)* | 195 (21.4) | 69 (36.1)* | 97 (25.5) | 18 (31.6)* | 98 (18.5) |
P value <0.05.
Similar to morphological changes, blacks were more likely to have endocrine insufficiency (35.1 vs. 21.4%, P<0.04), a difference that continued to be seen after stratification in blacks with alcoholic CP but not with other etiologies. No differences were observed in the prevalence of exocrine insufficiency between the races.
These differences in part were explained by longer duration of disease in blacks when compared with whites, which showed a trend for all patients (8.21±8.28 vs. 7.25±7.62, P=0.10), and significant results for alcoholic CP (8.6±8.41 vs. 6.97±7.07, P=0.02).
Pain, pain patterns, narcotic use, and disability
Overall, in the NAPS2-CV and NAPS2-AS studies, 556/643 (86.5%) patients reported having pain related to CP in the year preceding enrollment. Important differences were detected in pain experience based on race. Black patients were significantly (P<0.05) more likely to report any pain (94.7 vs. 83%), constant pain (62.6 vs. 51%), and severe pain (78.4 vs. 65.8%) when compared with whites. These differences persisted after stratification of patients by etiology (Table 6). Roughly, ~80% patients who reported having pain in the preceding year were on pain medications (non-narcotic or narcotic). The proportion of patients with pain using any pain medication or narcotic medications was statistically higher in blacks, overall and after stratification by alcohol etiology. Greater than one-third of black patients reported being disabled due to CP, which was significantly higher when compared with whites (21%, RR 1.64, P<0.05), irrespective of etiology.
Table 6.
Pain patterns and pain medication use by race
| CV/AA only | All (N=643) |
Alcohol (N=340) |
Other (N=303) |
|||
|---|---|---|---|---|---|---|
| Black (N=190) | White (N=453) | Black (N=150) | White (N=190) | Black (N=40) | White (N=263) | |
| Pain frequency: N (%) | ||||||
| None | 10 (5.3) | 77 (17.0)* | 8 (5.3) | 31 (16.3)* | 2 (5.0) | 46 (17.5) |
| Intermittent | 61 (32.1) | 145 (32.0) | 49 (32.7) | 52 (27.4) | 12 (30.0) | 93 (35.4) |
| Constant | 119 (62.6)* | 231 (51.0) | 93 (62.0)* | 107 (56.3) | 26 (65.0)* | 124 (47.2) |
| Severity of pain: N (%) | ||||||
| None | 10 (5.3) | 77 (17.0)* | 8 (5.3) | 31 (16.3)* | 2 (5.0) | 46 (17.5) |
| Mild-moderate | 31 (16.3) | 78 (17.2) | 25 (16.7) | 25 (13.2) | 6 (15.0) | 53 (20.2) |
| Severe | 149 (78.4)* | 298 (65.8) | 117 (78.0) | 134 (70.5) | 2 (80.0)* | 164 (62.4) |
| Pain medicationsa: N (%) | 149 (79.3)* | 300 (66.5) | 118 (79.7)* | 129 (67.9) | 31 (77.5) | 171 (65.5) |
| Non-narcotic | 16 (8.5) | 33 (7.3) | 12 (8.1) | 10 (5.3) | 4 (10.0) | 23 (8.8) |
| Intermittent narcotic | 52 (27.7) | 109 (24.2) | 43 (29.1)* | 36 (19.0) | 9 (22.5) | 73 (28.0) |
| Constant narcotic | 81 (43.1) | 158 (35.0) | 63 (42.6) | 83 (43.7) | 18 (45.0)* | 75 (28.7) |
AA, African-American; CV, NAPS2-CV study (North American Pancreatitis Study 2-Continuation and Validation Study).
P value <0.05.
Missing data: 2 Patients. Proportions shown are based on effective numbers.
Analysis was also performed stratifying patients based upon a median duration of disease (less or greater than 4.55 years). This analysis demonstrated persistence of racial differences for many of these morphological features as well as pain. Specifically, among patients with a duration of disease >4.55 years, blacks were still statistically more likely than whites to have calcifications (P<0.001), pseudocyst at anytime (P=005), atrophy (P=0.03), and diabetes (0.02). Prevalence of ductal irregularities and CBD strictures (P<0.05 for both) was significantly more common in blacks irrespective of disease duration, while ductal dilatation or strictures and CBD dilatation were significantly more common in blacks with disease duration of <4.55 years. The prevalence of disability increases in both races with disease duration, but was significantly higher in blacks when compared with whites for disease duration <4.55 years (27.9 vs. 16.5%, P=0.007) and >4.55 years (41.9 vs. 26.7%, P=0.002). Differences in the prevalence of pain persisted among between races for no pain (P=0.007) and severe pain (P=0.006) for disease duration <4.55 years, and for constant pain (P=0.005) for duration of >4.55 years.
Therapies
The use of medical, endoscopic, or surgical therapies was not different between the two races, overall and after stratification of alcohol etiology (Table 7), except for cholecystectomy that had been performed in a higher percentage of white patients when compared with blacks (31.1 vs. 19%, P<0.05). The majority of patients were taking oral pancreatic enzyme replacement therapy (66.8%). Celiac nerve block was infrequently used across all patients. Also, endoscopic therapy overall was used in 50% of patients and surgical treatment other than cholecystectomy had been undertaken in 20% of patients.
Table 7.
Therapies tried by race among subjects with complications of chronic pancreatitis
| All (N=1,159) |
Alcohol (N=572) |
Other (N=587) |
||||
|---|---|---|---|---|---|---|
| Black (N=248) | White (N=911) | Black (N=191) | White (N=381) | Black (N=57) | White (N=530) | |
| Medical therapies: N(%) | ||||||
| Pancreatic enzymes | 153 (61.7) | 621 (68.2) | 114 (59.7) | 256 (67.2) | 39 (68.4) | 365 (68.9) |
| Vitamins/antioxidants | 49 (19.8) | 185 (20.3) | 37 (19.4) | 76 (19.9) | 12 (21.1) | 109 (20.6) |
| Celiac nerve block | 8 (3.2) | 51 (5.6) | 5 (2.6) | 24 (6.3) | 3 (5.3) | 27 (5.1) |
| Endoscopic therapies: N(%) | ||||||
| Biliary or pancreatic sphincterotomy | 85 (34.3) | 372 (40.8) | 59 (30.9) | 120 (31.5) | 26 (45.6) | 252 (47.6) |
| Bile duct stenting | 34 (13.7) | 111 (12.2) | 29 (15.2) | 48 (12.6) | 5 (8.8) | 63 (11.9) |
| Pancreatic duct stenting | 72 (29.0) | 295 (32.4) | 56 (29.3) | 113 (29.7) | 16 (28.1) | 182 (34.3) |
| Pancreatic duct stone removal | 34 (13.7) | 114 (12.5) | 30 (15.7) | 48 (12.6) | 4 (7.0) | 66 (12.5) |
| Surgical therapies: N(%) | ||||||
| Cholecystectomy (CV/AA only) | 36 (19.0) | 141 (31.1)* | 26 (17.3) | 42 (22.1) | 10 (25.0) | 99 (37.6) |
| Partial/complete pancreatectomy | 22 (8.9) | 109 (12.0) | 13 (6.8) | 39 (10.2) | 9 (15.8) | 70 (13.2) |
| Drainage surgery | 17 (6.9) | 73 (8.0) | 14 (7.3) | 37 (9.7) | 3 (5.3) | 36 (6.8) |
| Cyst/pseudocyst operation | 14 (5.7) | 54 (5.9) | 12 (6.3) | 33 (8.7) | 2 (3.5) | 21 (4.0) |
AA, African-American; CV, NAPS2-CV study (North American Pancreatitis Study 2-Continuation and Validation Study).
P value <0.05.
DISCUSSION
This is the first comprehensive study of the clinical profile of CP in a well-phenotyped prospectively ascertained cohort of black patients in the United States. We found that when compared with whites, black patients were almost twice as likely to be diagnosed to have CP due to alcohol or smoking; more likely to have morphologic abnormalities of the pancreas; more likely to have, and describe their pain as constant or severe; have a higher prevalence of endocrine insufficiency; and have a greater degree of disease-related disability. No clinically important differences were observed in treatment based on race other than higher frequency of cholecystectomy among white patients. Some of these racial differences could be explained by the higher prevalence of environmental exposures in black CP patients.
We confirm prior reports of a higher prevalence of physician-defined alcohol etiology, and cigarette smoking in black patients. In fact, physicians identified alcohol as the risk factor for CP in as many as 80%, tobacco smoking in 73%, and either of these two habits in 87% of all black patients. As an independent measure of alcohol exposure, we evaluated drinking categories based on self-reported data from patients and found that black patients had a significantly higher prevalence of very heavy or heavy drinking when compared with white patients (69 vs. 41%, P<0.05). A higher prevalence of alcohol etiology in blacks cannot be explained by the established genetic susceptibility factors (PRSS1, CTRC, SPINK1, and CFTR), as these have not been shown to increase the risk of alcoholic pancreatitis (6). In two recent genome-wide association studies, polymorphisms at the claudin locus were found to increase the risk of alcoholic pancreatitis—but, both of these studies were limited to Caucasian patients of European descent. Whether this finding is merely a reflection of a higher prevalence of alcohol consumption in black patients or is a result of their increased susceptibility to pancreatitis from alcohol exposure deserves further evaluation with focused genetic studies. Black patients also had a higher prevalence of ever and current smoking, which are now accepted as a risk factor for susceptibility to and progression of pancreatitis. Interestingly, the intensity of smoking was greater among whites, an observation that has also been noted in the general population (17). The difference we detected between blacks and whites regarding heavy smoking also suggests this to be a greater risk factor for CP in blacks.
Explanation for the morphologic differences identified between the two races is speculative. These differences were explained in part by underlying cause and duration of disease in stratified analysis. Calcifications were more common in blacks than in white patients both overall and in those with alcohol etiology. Alcohol-induced CP is characteristically associated with calcifications (18). Prior studies suggest that smoking is commonly associated with pancreatic calcifications (19,20) and with continued smoking once the diagnosis of CP is made, these pancreatic calcifications typically progress (21). Whether the combination of tobacco and alcohol or some other genetic factor(s) has a role in the greater rate of calcifications and other morphological findings we identified among blacks is unknown. Given that both alcohol (22) and smoking (23) adversely affect CFTR localization and CFTR mislocalization results in formation of pancreatic stone formation (24), the increased levels of alcohol and smoking in black patients, compared with white patients may be a potential explanation for their increased prevalence of calcifications. Black patients also had a higher prevalence of endocrine insufficiency. The explanation of this finding is likely multifactorial and includes a higher prevalence of alcohol and tobacco exposure, more advanced morphological findings, and a higher risk of diabetes in blacks.
The most important symptom of CP is abdominal pain, which strongly impacts quality of life (2). These data demonstrate differences among the races in the presence, character, and quality of pain. Black patients were almost twice as likely to have their pain described as constant and only 5% had no abdominal pain. Severe pain was much more likely in blacks than in whites and perhaps this is related to underlying causes of alcohol and tobacco. Prior studies have not shown differences in pain response based upon race per se (25). Alcohol-induced CP may be linked to greater degrees of pain. Further work would be required to differentiate any differences in pain response in CP based upon race.
Several limitations of our study deserve mention. Our study was a cross-sectional study and thus long-term follow-up to evaluate for differences over time and mortality could not be performed. Other issues related to outcome including access to and use of outpatient or inpatient resources before diagnosis and until enrollment were not captured. Health-care access is important as it is clearly recognized that early alcohol and perhaps smoking cessation counseling can prevent recurrent pancreatitis and potential progression to CP (26,27). Potential disparities in health-care access based on race will need to be further explored given the significantly higher rates of alcohol and tobacco use as well as disability in black compared with white CP patients. We also found a discrepancy between patient reported and physician reported alcohol use more often in black than in white patients. Whether this represents some inherent bias regarding cause by physicians is unknown or is an important finding requires further study. Our data were generated primarily from academic medical centers and thus may not be applicable to community patients. Our study focused on patients with a definite diagnosis of CP. Therefore, we were unable to evaluate whether there are racial differences in the evaluation of patients who present with symptoms suggestive of CP.
In summary, we identified relevant and significant differences among black and white patients with CP. Alcohol and tobacco use were far more likely in black than in white patients and these were also associated with greater rates of morphologic abnormalities, more constant and severe pain, endocrine insufficiency as well as disability. Additional research will be required to determine whether any greater genetic susceptibility to alcohol and/or tobacco induced disease and other features of CP is present based upon race.
Supplementary Material
Study Highlights
WHAT IS CURRENT KNOWLEDGE
-
✓
Chronic pancreatitis (CP) results in significant morbidity and health-care expenditures.
-
✓
Alcohol and tobacco use are the most common causes of CP.
-
✓
Causes and outcomes based upon race have not been well studied.
WHAT IS NEW HERE
-
✓
As compared with whites, blacks were almost twice as likely to have alcohol as a cause of disease.
-
✓
Advanced morphological abnormalities including calcifications, atrophy, and ductal strictures were significantly more common in blacks than in whites.
-
✓
Abdominal pain was more common, more severe, and more likely to result in disability in blacks as compared with whites.
ACKNOWLEDGMENTS
The following physicians also contributed patients to this study: Robert H. Hawes, Medical University of South Carolina, Charleston, SC; James DiSario, University of Utah, Salt Lake City, UT; Simon K. Lo, MD, Cedars-Sinai Medical Center, University of California, Los Angeles; Mark T. DeMeo, MD, Rush University Medical Center, Chicago, Illinois; William M. Steinberg, MD, Washington Hospital Center, Washington, DC; Michael L. Kochman, MD, University of Pennsylvania, Philadelphia; Babak Etemad, MD, Ochsner Medical Center, New Orleans, LA; Wahid Wassef, MD, University of Massachusetts, Worcester, MA. We also acknowledge contributions from Michal O’Connell, PhD (data management), Emil Bauer (Data entry), Elizabeth D. Kennard, PhD, Ye Tian, PhD, Stephen R. Wisniewski, PhD (Data management and analyses), Kim Stello, Danielle Dwyer (laboratory and biosample management). The study was supported by R01DK061451 (DCW), R01 DK077906 (DY) and UL1 RR024153 and UL1TR000005 (PI—Steven E Reis, MD).
Financial support: The study was supported by R01DK061451 (DCW), R01 DK077906 (DY), and UL1 RR024153 and UL1TR000005 (PI—Steven E Reis, MD).
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/ajg
Guarantor of the article: C. Mel Wilcox, MD, MSPH.
Potential competing interests: Dr Whitcomb is an inventor of intellectual property that is licensed to Ambry Genetics, which has been evaluated in this study. He also has an ownership interest in Ambry Genetics.
REFERENCES
- 1.Peery AF, Crockett SD, Barritt AS et al. Burden of gastrointestinal, liver and pancreatic diseases in the United States. Gastroenterology 2015;149:1731–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mullady DK, Yadav D, Amann ST et al. for the NAPS2 Consortium. Type of pain, pain-associated complications, quality of life, disability and resource utilization in chronic pancreatitis: a prospective cohort study. Gut 2011;60:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amann ST, Yadav D, Barmada MM et al. Physical and mental quality of life in chronic pancreatitis. A case-control study from the North American Pancreatitis Study 2 Cohort. Pancreas 2013;42:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yadav D, Hawes RH, Brand RE et al. Alcohol consumption, cigarette smoking, and the risk of recurrent acute and chronic pancreatitis. Arch Intern Med 2009;169:1035–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fjeld K, Wiess FU, Lasher D et al. A recombined allele of the lipase gene CEL and its pseudogene CELP confers susceptibility to chronic pancreatitis. Nat Genet 2015;47:518–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitcomb DC, LaRusch J, Krasinskas AM et al. Common genetic variants in the CLDN2 and PRSS1-PRSS2 loci alter risk for alcohol-related and sporadic pancreatitis. Nat Genet. 2012;44:1349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang AL, Vadhavkar S, Singh G et al. Epidemiology of alcohol related liver and pancreatic disease in the United States. Arch Intern Med 2008;168:649–56. [DOI] [PubMed] [Google Scholar]
- 8.Yadav D, Muddana V, O’Connell M. Hospitalizations for chronic pancreatitis in Allegheny County, Pennsylvania, USA. Pancreatology 2011;11:546–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yadav D, O’Connell M, Papachristou GI. Natural history following the first attack of acute pancreatitis. Am J Gastroenterol. 2012;107:1096–103. [DOI] [PubMed] [Google Scholar]
- 10.Lowenfels AB, Maisonneuve P, Grover H et al. Racial factors and the risk of chronic pancreatitis. Am J Gastroenterol 1999;94:790–4. [DOI] [PubMed] [Google Scholar]
- 11.Cote GA, Yadav D, Slivka A et al. North American Pancreatitis Study Group. Alcohol and smoking as risk factors in an epidemiology study of patients with chronic pancreatitis. Clin Gastroenterol Hepatol 2011;9:266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yadav D, Lee E, Papachristou GI et al. A population-based evaluation of readmissions after first hospitalization for acute pancreatitis. Pancreas 2014;43:630–7. [DOI] [PubMed] [Google Scholar]
- 13.Akhtar AJ, Shaheen M. Extrapancreatic manifestations of acute pancreatitis in African-American and Hispanic patients. Pancreas 2004;29:291–7. [DOI] [PubMed] [Google Scholar]
- 14.Whitcomb DC, Yadav D, Adams S et al. Multicenter approach to recurrent acute and chronic pancreatitis in the United States: the North American Pancreatitis Study 2 (NAPS2). Pancreatology 2008;8:520–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilcox CM, Yadav D, Ye T et al. Chronic pancreatitis pain pattern and severity are independent of abdominal imaging findings. Clin Gastroenterol Hepatol 2015;13:552–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology 2001;120:682–707. [DOI] [PubMed] [Google Scholar]
- 17.American Lung Association, Research and Program Services; Epidemiology and Statistics Unit. Trends in Tobacco Use, 2011; http://www.lung.org/assets/documents/research/tobacco-trend-report.pdf.
- 18.Ammann RW, Heitz PU, Kloppel G. Course of alcoholic chronic pancreatitis: a prospective clinicomorphological long-term study. Gastroenterology 1996;111:224–31. [DOI] [PubMed] [Google Scholar]
- 19.Maisonneuve P, Frulloni L, Müllhaupt B et al. Impact of smoking on patients with idiopathic chronic pancreatitis. Pancreas 2006;33:163–8. [DOI] [PubMed] [Google Scholar]
- 20.Luaces-Regueira M, Iglesias-Garcia J, Lindkvist B et al. Smoking as a risk factor for complications in chronic pancreatitis. Pancreas 2014;43:275–80. [DOI] [PubMed] [Google Scholar]
- 21.Maisonneuve P, Lowenfels AB, Müllhaupt B et al. Cigarette smoking accelerates progression of alcoholic chronic pancreatitis. Gut 2005;54:510–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maleth J, Balazs A, Pallagi P et al. Alcohol disrupts levels and function of the cystic fibrosis transmembranes conductance regulator to promote development of pancreatitis. Gastroenterology 2015;148:427–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raju SV, Jackson PL, Courville CA et al. Cigarette smoke induces systemic defects in cystic fibrosis transmembrane conductance regulator function. Am J Respir Crit Care Med 2013;188:1321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ko SB, Azuma S, Yoshikawa T et al. Molecular mechanism of pancreatic stone formation in chronic pancreatitis. Front Physiol 2012;3:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cruz-Almeida Y, Riley JL 3rd, Fillingim RB. Experimental pain phenotype profiles in a racially and ethnically diverse sample of healthy adults. Pain Med 2013;14:1708–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nordback I, Pelli H, Lappalainen-Lehto R et al. The recurrence of acute alcohol-associated pancreatitis can be reduced: a randomized controlled trial. Gastroenterology 2009;136:848–55. [DOI] [PubMed] [Google Scholar]
- 27.Nikkola J, Raty S, Laukkarinen J et al. Abstinence after first acute alcohol-associated pancreatitis protects against recurrent pancreatitis and minimizes the risk of pancreatic dysfunction. Alcohol Alcohol 2013;48:483–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.