Antibiotics prevent and treat infections, yet also perturb microbial-host ecosystems with potential detriments to the host1. They injure indigenous gut microbiota leading to dysbiosis, a prevalent and substantial problem in hematopoietic cell transplantation (HCT). Gut barrier damage, nutritional changes, recurrent and prolonged healthcare facility and personnel contact, and graft-versus-host disease (GVHD) further exacerbate dysbiosis. Possible approaches to prevent and correct dysbiosis range from microbiota protective protocols with strict antibiotic stewardship, nutritional interventions, or microbiota restoration strategies such as fecal microbiota transplantation2.
Generalizability of microbiota research from one center to others may depend on center-specific antibiotic practice patterns. The type of antibiotics and timing of their initiation or de-escalation can influence major transplant outcomes. For example, earlier (pre-HCT) exposure to broad-spectrum antibiotics has been associated with a loss of commensal Clostridiales and higher transplant-related mortality (TRM) compared to later (post-HCT) or no exposure3. Knowledge about center-specific antibiotic practice patterns can guide the design of multi-center studies and interpretation of their results. To detail antibacterial antibiotic practices, we surveyed Blood and Marrow Transplant Clinical Trials Network (BMT CTN) centers for their current antibiotic practice patterns in autologous (auto) and allogeneic (allo) HCT. The survey was created in Google Forms and explored three characteristics of antibacterial antibiotic uses (initiation, duration, and specific types) in four settings: pre-engraftment prophylaxis, prophylaxis in acute GVHD, prophylaxis in chronic GVHD, and empiric treatment of neutropenic fever (Supplementary Figure 1). The survey was approved by the BMT CTN which also provided a list of principal investigators (PIs) for 100 CTN centers and their emails. One author (DJW) emailed the form to the PIs. In case more than one PI was listed for a given center, the first PI on the list was selected to receive the survey request. If the invitation bounced back due to invalid email, the next PI on the list was selected. Recipients were given 14 days to electronically respond, with one reminder email sent after 12 days if no reply had been received.
The survey was emailed to all 100 centers, 41 of which responded with a completed survey. The responding centers included 12 of the 20 Core Clinical Centers and 9 of the additional 18 Consortium Centers. The median (range) number of allogeneic HCTs performed between 2015 and 2017 in the responding centers was 188 (28–1061). The following patterns were observed: (i) Initiation, discontinuation, and type of pre-engraftment bacterial prophylaxis are highly similar in auto- and allo-HCT settings, but with some between-center variability. Initiation is date-driven in ~70% of centers (55% before and ~15% after day 0) and triggered by the onset of neutropenia in the remainder (Figure 1A–B). Discontinuation in ~95% of centers occurs at the onset of neutropenic fever or with neutrophil recovery, whichever is sooner (Figure 1C–D). A fluoroquinolone (levofloxacin in 80% and ciprofloxacin in 20%) is used for bacterial prophylaxis in ~75% of centers, and 15% use no bacterial prophylaxis (Figure 1E–F). (ii) Greater heterogeneity exists among centers in their bacterial prophylaxis in patients with GVHD. The most common approaches to bacterial prophylaxis in patients with acute GVHD are no prophylaxis (55%), followed by a fluoroquinolone (25%) (Figure 1G). Bacterial prophylaxis in acute GVHD is continued until patients discontinue the added immunosuppression in 63% of centers, while no specific algorithm is followed in 21% (Figure 1H). The most common approaches to bacterial prophylaxis in patients with chronic GVHD are penicillin VK (42%), followed by no prophylaxis (35%) and a fluoroquinolone (10%) (Figure 1I). Bacterial prophylaxis in chronic GVHD is continued until patients come off the added immunosuppression in 77% of centers, while no specific algorithm is followed in 19% (Figure 1J). (iii) Centers are relatively uniform in their antibacterial choice for empiric frontline treatment of neutropenic fever (Figure 1K). Cefepime is used in this setting by 77% of the centers. Antibiotics with strong anti-anaerobic activity (meropenem or piperacillin-tazobactam) are used in this setting by 15% of the centers. With a negative infectious disease work up, the empiric frontline antibiotic is continued until neutrophil recovery in 57% of the centers, while 23% de-escalate or discontinue it at the conclusion of microbiologic work up and resolution of fever, even without neutrophil recovery.
Figure 1:
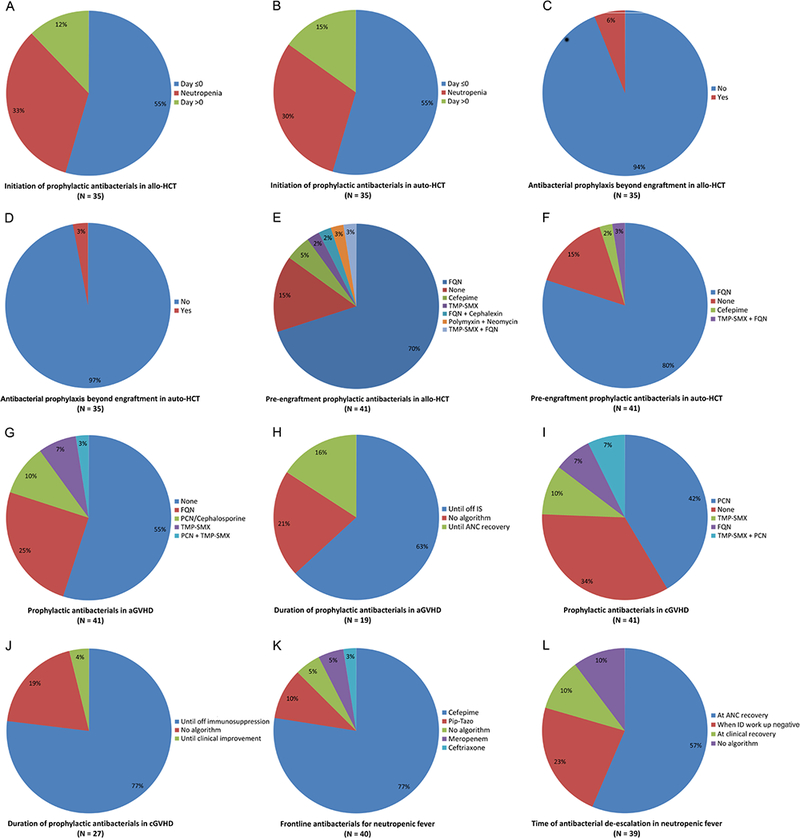
Summary of the survey results
aGVHD: Acute graft-versus-host disease; Allo: Allogeneic; ANC: Absolute neutrophil count; Auto: Autologous; cGVHD: chronic graft-versus-host disease; FQN: Fluoroquinolone; HCT: Hematopoietic cell transplantation; ID: Infectious diseases; IS: Immunosuppression; PCN: Penicillin; Pip-Tazo: Piperacillin-Tazobactam; TMP-SMX: Trimethoprim-Sulfamethoxazole
The results from this first BMT CTN survey of antibiotic practices indicate that the largest heterogeneity among centers is in their prophylactic antibacterial choice in chronic GVHD, whether they use any antibacterial prophylaxis in GVHD, and de-escalation/discontinuation strategies. This information should be considered when designing multi-center trials with endpoints that are potentially influenced by microbiota. Such endpoints include acute GVHD, bloodstream infection4, survival5, and relapse6. While efforts to reduce antibiotic practice heterogeneity may facilitate the analysis of such trials, comparative multi-center studies (e.g., altering the timing and type of antibacterial prophylaxis) can be informative.
Supplementary Material
Survey questions Three major domains were addressed: Pre-engraftment bacterial prophylaxis (Q1–2), bacterial prophylaxis in graft-versus-host disease (Q3–6), and empiric frontline antibacterial in neutropenic fever (Q7–8). GVHD: Graft-versus-host disease. Q: Question
Acknowledgements:
The authors thank the Blood and Marrow Transplantation Clinical Trials Network (BMT CTN) for reviewing the survey and providing the principal investigator emails. BMT CTN is supported by grant #U10HL069294 from the National Heart, Lung, and Blood Institute and the National Cancer Institute. Sabrina Porter is acknowledged for her assistance with graphics. The following investigators (in alphabetical order) and centers completed and returned the survey: Sunil Abhyankar (University of Kansas), Luke Akard (Indiana Blood and Marrow Transplantation), Amin Alousi (MD Anderson Cancer Center), Joseph Antin (Dana-Farber Cancer Institute), Andrew Artz (University of Chicago), Karen Ballen (University of Virginia), Natalie Callander (University of Wisconsin), Jan Cerny (University of Massachusetts), Luciano Costa (University of Alabama), Lloyd Damon (University of California in San Francisco), David Delgado (Riley Children’s Hospital), Sherif Farag (Indiana University), Steven Goldstein (Florida Hospital Cancer Institute), Betty Hamilton (Cleveland Clinic), Parameswaran Hari (Medical College of Wisconsin), Gerhard Hildebrandt (University of Kentucky), LaQuisa Hill (Baylor College of Medicine), William Hogan (Mayo Clinic, Rochester), Murali Janakiram (Montefiore Medical Center), Sonata Jodele (Children’s Hospital of Los Angeles), Mohamed Kharfan-Dabaja (Mayo Clinic, Florida), Nandita Khera (Mayo Clinic, Arizona), Jennifer Krajewski (Hackensack University Medical Center), John Levine (Mount Sinai), Robert Lowsky (Stanford University), John Maciejewski (Rush University), Richard Maziarz (Oregon Health & Science University), Ryotaro Nakamura (City of Hope National Medical Center), Miguel Perales (Memorial Sloan Kettering Cancer Center), George Selby (Oklahoma University Medical Center), Zainab Shahid (Levine Cancer Institute), Thomas Shea (University of North Carolina), Kellie Sprague (Tufts New England Medical Center), Patrick Stiff (Loyola University Medical Center), Julie Talano (Children’s Hospital of Wisconsin), Joseph Uberti (Karmanos Cancer Institute), Edmund Waller (Emory University), Mark Walters (UCSF Benioff Children’s Hospital, Oakland), Peter Westervelt (Washington University), and John Wingard (Shands Healthcare & University of Florida)
References
- 1.Staffas A, Burgos da Silva M, van den Brink MRM. The intestinal microbiota in allogeneic hematopoietic cell transplant and graft-versus-host disease. Blood. 2017;129(8):927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andermann T, Peled J, Ho C, et al. Microbiome-Host Interactions in Hematopoietic Stem Cell Transplant Recipients Biol Blood Marrow Transplant. February 2018. (in press). [Google Scholar]
- 3.Weber D, Jenq RR, Peled JU, et al. Microbiota Disruption Induced by Early Use of Broad-Spectrum Antibiotics Is an Independent Risk Factor of Outcome after Allogeneic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2017;23(5):845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taur Y, Xavier JB, Lipuma L, et al. Intestinal Domination and the Risk of Bacteremia in Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation. Clin Infect Dis. 2012;55(7):905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taur Y, Jenq RR, Perales M-A, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood. 2014;124(7):1174–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peled JU, Devlin SM, Staffas A, et al. Intestinal Microbiota and Relapse After Hematopoietic-Cell Transplantation. J Clin Oncol. 2017;35(15):1650–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Survey questions Three major domains were addressed: Pre-engraftment bacterial prophylaxis (Q1–2), bacterial prophylaxis in graft-versus-host disease (Q3–6), and empiric frontline antibacterial in neutropenic fever (Q7–8). GVHD: Graft-versus-host disease. Q: Question


