Abstract
About twenty years ago, the functional lipid raft model of the plasma membrane was published. It took into account decades of research showing that cellular membranes are not just homogenous mixtures of lipids and proteins. Lateral anisotropy leads to assembly of membrane domains with specific lipid and protein composition regulating vesicular traffic, cell polarity, and cell signaling pathways in a plethora of biological processes. However, what appeared to be a clearly defined entity of clustered raft lipids and proteins became increasingly fluid over the years, and many of the fundamental questions about biogenesis and structure of lipid rafts remained unanswered. Experimental obstacles in visualizing lipids and their interactions hampered progress in understanding just how big rafts are, where and when they are formed, and with which proteins raft lipids interact. In recent years, we have begun to answer some of these questions and sphingolipids may take center stage in re-defining the meaning and functional significance of lipid rafts. In addition to the archetypical cholesterol-sphingomyelin raft with liquid ordered (Lo) phase and the liquid-disordered (Ld) non-raft regions of cellular membranes, a third type of microdomains termed ceramide-rich platforms (CRPs) with gel-like structure has been identified. CRPs are “ceramide rafts” that may offer some fresh view on the membrane mesostructure and answer several critical questions for our understanding of lipid rafts.
1. Raft biophysics and cell biology
1.1. A brief history of lipid rafts
Public databases such as Pubmed list about 6500 studies published on “lipid rafts” till 2018, about 20% of those are reviews. This number is large compared to other areas in cell biology such as “membrane” or “cytoskeleton” with a proportion of about 10% being reviews and the remaining publications being original research articles. The large proportion of reviews indicates a looming challenge underlying research on lipid rafts: their existence and function is still controversial (Sonnino and Prinetti, 2010). For one, there is only little doubt that the distribution of lipids and proteins in cellular membranes is anisotropic: these components are not homogenously distributed, but show some degree of lateral order, mainly in form of “crowding” or “clustering”. On the other hand, it has been notoriously difficult to understand the biophysics of lipid rafts, mainly how lipid rafts are formed and what interactions between lipids and proteins determine the function of rafts. More seriously, however, is the technical difficulty to investigate lipid rafts, particularly in living cells. The following sections will discuss the basics in biophysics and analysis of lipid rafts, the history of discovery, and the progress made to determine their structure and function.
1.1.1. The pre-raft era of membrane biology: lipids, proteins, or both?
The official birth date of formalizing the lipid raft hypothesis is June 5th, 1997 when Elina Ikonen and Kai Simons published a concept paper on “functional rafts” in Nature (Simons and Ikonen, 1997), although Simons discussed the function of “glycolipid rafts” already in previous studies on the regulation of polarized vesicular trafficking originating in the Golgi of MDCK cells (Fiedler et al., 1994; Simons and van Meer, 1988). However, the first ideas of membrane anisotropy in form of “membrane subdomains” or “microdomains” appeared more than 20 years earlier, shortly after Singer and Nicolson postulated the “fluid mosaic model” of the plasma membrane with homogeneously distributed proteins and lipids (Singer and Nicolson, 1971, 1972). Most of this early work investigated the effect of different lipid compositions on anisotropy in liposomes, with several remarkable studies in 1975 by Hui and Parsons and 1980–82 by Klausner and Karnovsky visualizing lipid domains and postulating a membrane model akin to the raft model, respectively (Hui and Parsons, 1975; Karnovsky et al., 1982a; Karnovsky et al., 1982b; Klausner et al., 1980). One may even find the first ideas on a modular mesostructure of membrane lipids emerging a decade prior to the Singer-Nicolson paper, when the peculiar appearance of helical and hexagonal structures in negative electronmicroscopic stains of lipid mixtures were interpreted as lipid micelles representative of the structure of cellular membranes (Lucy, 1964). Considering what was known about membrane anisotropy at the time the fluid mosaic model was published it is surprising that the domain-driven mesostructure of the membrane was not included in their model. In fact, Singer and Nicolson described protein clusters in the membrane as merely driven by protein interaction and an experimentally induced artifact (Singer and Nicolson, 1972), a vantage point that continued to burden research on lipid rafts till now. Probably the closest to what was later classified as subtypes of lipid rafts were membrane domain structures identified as “caveolae”. The caveolae vs. lipid domain dichotomy apprehended from early on the theoretical as well as experimental challenges to cope with understanding the functional significance of protein-lipid interaction in rafts: if proteins cluster first, why do they need lipids, and if lipid domains drive protein clustering, how do they do it?
Caveolae.
Caveolae were first described by Eichi Yamada in 1955, although the original discovery by electron microscopic analysis of the plasma membrane of capillary endothelial cells dates back to George E. Palade in 1953 (Yamada, 1955). Palade also proposed that the numerous vesicles detected underneath of pits seen in the capillary membrane were generated by inward pinching off the plasma membrane. Till today, his interpretation is valid in that caveolae initiate one type of clathrin-independent and raft-dependent endocytosis. The composition of caveolae consisting of oligomerized caveolin protein embedded into a cholesterol-enriched microdomain was determined in the early 1990’s (Rothberg et al., 1992). Cholesterol enrichment in caveolae was found by using the fluorescent cholesterol binding drug fillipin for microscopy. Since then, fluorescence microscopy techniques have been instrumental in identifying formation and function of caveolae in cells, particularly when fluorescent protein-tagged caveolins and other caveolae-associated proteins became available. However, apart from interrupting caveolae by cholesterol depletion, the function of lipids in caveolae remained elusive.
Lipid microdomains.
The critical function of lipids in sorting of membrane components during intracellular trafficking was demonstrated in a series of seminal studies conducted by Kai Simons and his post-doctoral fellow at that time, Gerrit van Meer in the 1980’s (Simons and van Meer, 1988; van Meer et al., 1987). They showed that in MDCK cells, fluorescently labeled glycosphingolipids are asymmetrically transported to the apical cell membrane, suggesting that sphingolipids are critical for the membrane distribution of proteins. It was already shown in the early 1970’s that alkaline or cold detergent (Trion X-100) insoluble membranes in cell lysates are enriched with cholesterol and sphingolipids (Butters and Hughes, 1974; Gurd et al., 1972).Decades later, isolation of detergent-resistant membranes (DRMs) should become the commonly used method to biochemically characterize lipid rafts. However, there were only few attempts to conceptually combine the lipid microdomain with caveolin models until the famous “lipid raft” paper in 1997 (Simons and Ikonen, 1997). The major conceptual difference of lipid microdomains and caveolae is as put by van Meer when cited “lipids can do it by themselves” (Lai, 2003), meaning that lipid microdomains are mainly self-organized by lipids (Fig. 1A). Caveolins were found to be associated with DRMs just a few years before the raft hypothesis, and they are not present in all types of lipid rafts.
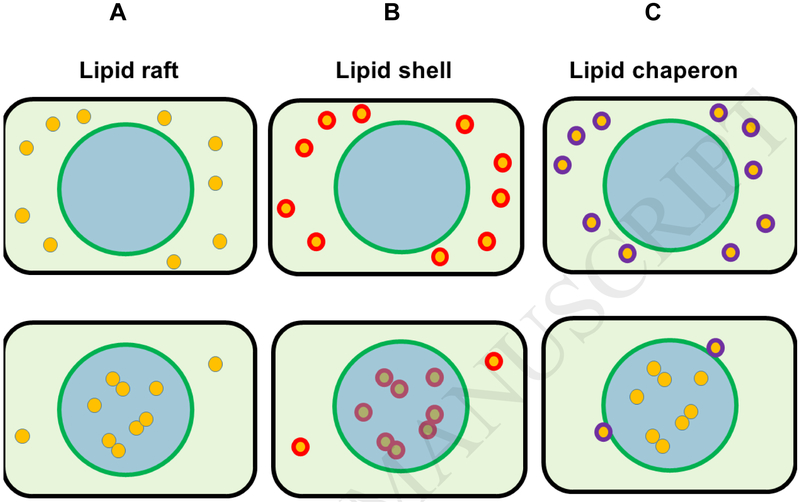
Figure 1.
Different models of lipid-protein interaction in membrane microdomains and rafts A. In the original “lipid raft” model formalized by Simons in 1997, lipids such as cholesterol and sphingomyelin form a liquid ordered phase (Lo) in the membrane, the lipid raft (blue). Raft proteins (yellow) are enriched in rafts and form complexes. One obstacle in this model is the diffusion barrier (green), which requires activation energy for transfer of raft proteins into rafts. B. In 2002, Anderson and Jacobson published the “lipid shell” model. Annular or boundary lipids surround membrane proteins in lipid shells (red) that target raft proteins to lipid rafts where they form larger lipid-protein or protein-protein complexes. However, in this model, the relationship between lipids in a raft and lipid shells surrounding raft proteins is not clear. C. The “lipid chaperon” model suggested in this review attempts to integrate and refine the raft and shell model. Protein-bound chaperon lipids interact with raft lipids to overcome the diffusion barrier between raft and non-raft areas of the membrane. Once entering the raft, chaperon lipids (purple) may be replaced by raft lipids, which is different from the shell model. Chaperon lipids act like a catalyst lowering the activation energy for overcoming the diffusion barrier to the lipid raft, but they do not need to stay with the protein complex once formed. They may actually prevent protein complex formation unless stripped off from raft proteins when entering the raft.
Lipid shells.
Probably one of the least advocated models on the mesostructure of cellular membranes integrating clustering of proteins and lipids is the annular lipid shell hypothesis. Annular shells are formed by so-called “boundary lipids” that tightly bind to proteins and surround them like a shell (Fig. 1B). They were discovered by spin labeling almost 50 years ago when detecting immobilized phospholipids forming a single layered shell separating the protein from the fluid bilayer of the membrane (Jost et al., 1973). While lipid shells explain why proteins are tightly bound into membranes through hydrophobic interaction, they do not by themselves predict clustering of proteins in lipid microdomains. It was not until recently that they were suggested to drive the formation of lipid rafts (Anderson and Jacobson, 2002). In recent years, the lipid shell hypothesis was revisited and refined in the “induced-fit raft model of raft heterogeneity” proposed by Linda Pike in 2004 (Pike, 2004), a step-wise remodeling mechanism driving lipid-bound proteins into membrane rafts.
1.2. The post-raft era: breaking down the barrier between lipids and proteins
Biophysicists are strict in their definition of different phases in a lipid membrane as introduced by Ipsen et al. in 1987: there are liquid ordered (Lo) and disordered (Ld) phases with particular lipids such as cholesterol promoting Lo, while others such as non-saturated phospholipids favoring Ld phases (Ipsen et al., 1987). In Lo phases, fatty acid chains are stretched (ordered), but lipids do not adopt hexagonal lateral arrangement (liquid-crystalline vs. gel state). Separation into phases with spatial extension comparable to rafts occurs when the mixing energy exceeds the Boltzmann energy of 0.55kT (Heberle and Feigenson, 2011). However, lipid domains, rafts, and shells are less defined in terms of thermodynamic underpinnings, particularly when interaction with proteins is implicated. In a consensus definition of lipid rafts formulated at the 2006 “Keystone Symposium on Lipid Rafts and Cell Function” it was concluded that “Membrane rafts are small (10–200 nm), heterogeneous, highly dynamic, sterol- and sphingolipid-enriched domains that compartmentalize cellular processes. Small rafts can sometimes be stabilized to form larger platforms through protein-protein and protein-lipid interactions” (Pike, 2006). The omission of lipid-lipid interactions in this definition reflects the full gravity of a lack in our understanding the function of lipids in rafts. The question at stake is relatively simple: how do lipid rafts induce clustering of proteins to be relevant in a biological process? Obviously, the answer is simple when protein clusters are stabilized by protein-protein interaction: the binding energy is equivalent to the energy required to dissociate the protein complex. If a protein complex is stabilized long enough, a biological response ensues. Likewise, pure lipid domains are stabilized by binding of lipids to each other, however, binding energies are usually lower by orders of magnitude than those of proteins. Hence, which forces drive formation of lipid rafts and segregation of proteins into rafts, and why is this segregation biologically relevant?
Diffusion-driven lipid raft formation.
The main difference between the (homogeneous) fluid mosaic and the (heterogeneous) lipid raft model of cellular membranes is that in the latter, lipids and proteins are segregated in different phases. Lipid rafts are considered Lo, they are enriched with sterols such as cholesterol and sphingolipids such as sphingomyelin and glycosphingolipids, and they are associated with specific proteins, so-called raft proteins (Fig. 1A). Raft proteins are often post-translationally modified by glycosylphosphatidylinositol (GPI) anchors or fatty acids (e.g., palmitoyl- or myristoyl-residues), although covalently bound lipid anchors are not a general requirement for lipid raft association and in most cases, they are rather critical for translocation of proteins to the plasma membrane. Non-raft domains of the membrane are considered Ld, they are typically enriched with non-saturated phospholipids and associated with non-raft proteins. Unless raft and non-raft proteins are targeted directly into the respective domains in the membrane by anterograde transport from within the cell, they have to be laterally “moved” within the membrane. While the “driving force” for this movement is lateral diffusion, it is not clear how diffusion of proteins is regulated by phase separation into raft and non-raft domains of the membrane. Diffusion or flux of a particle is governed by Fick’s law, which is dependent on the viscosity of the medium in which the particle moves. Denser packing of lipids in rafts increases their viscosity compared to non-raft domains. One could imagine that segregating a protein into the raft area is like stepping into a pile of mud, the protein gets stuck and immobilized in the raft. However, if rafts function as diffusion barriers “trapping” proteins in viscous lipid patches, then the barrier goes both ways and energy is needed to get the protein in (Fig. 1A). Hence, diffusion alone is not able to explain how lipids in rafts alter the distribution of proteins in the membrane. In addition, experimental observations using fluorescence recovery after photobleaching (FRAP) microscopy on the apical membrane of MDCK cells argue against immobility of proteins in rafts (Meder et al., 2006). On the contrary, raft proteins move freely, while non-raft proteins are segregated to isolated domains, suggesting percolation through a continuous raft-domain extending over the apical plasma membrane (Simons and Sampaio, 2011). This observation is in line with a more recent interpretation of lipid rafts as diffusion barriers invoking the cortical actin cytoskeleton underneath the plasma membrane. A mesh of actin filaments with periodic openings lining the membrane domains allows for percolation and “hop diffusion” of raft proteins (picket fence model) (Ritchie et al., 2003). However, while these models reconcile experimental data with theoretical predictions about diffusion, they do not address the central question of how rafts are formed and proteins segregated at first place.
Entropy and line tension-driven lipid raft formation.
Entropy is a powerful force for coalescence of immiscible liquids. Illustrative examples are the merging of oil drops in water or wax drops in lava lamps. The driving force for the process of coalescence is reducing the degree of order by minimizing the surface of the immiscible phases. However, since phase separation in artificial membranes increases with temperature, increasing entropy must be counterbalanced by an energy term, which is called line tension. Line tension along the borders of raft and non-raft membrane domains is mediated by van der Waals interaction between lipids, which leads to lateral separation (demixing) of lipid phases when the mixing energy exceeds 0.55kT (Ackerman and Feigenson, 2015). In artificial membranes, reducing line tension as well as increasing entropy by coalescence will lead to formation of larger rafts. Hence, the main question is how the lipid raft organization is sustained without all phases coalescing into just two large raft and non-raft membrane domains? A potential solution to this question has already been touched upon in the previous paragraph on diffusion-driven raft segregation: cortical actin “fences” underneath the membrane separate raft domains and prevent coalescence. There is direct experimental evidence for this hypothesis from state-of-the art combination of fluorescence microscopy and (secondary) imaging mass spectrometry (SIMS) methods. In a landmark study from 2013, the laboratory of Mary Kraft showed that in fibroblasts, membrane domains are enriched with sphingolipids and interrupted by cholesterol depletion and reagents depolymerizing the cortical actin cytoskeleton (Frisz et al., 2013). There is also evidence that some sphingolipids, particularly ceramide and lysosphingolipids, can induce coalescence of lipid rafts into larger platforms or domains (Gulbins and Kolesnick, 2003; Spassieva and Bieberich, 2016). Probably, coalescence is triggered by enzymatic conversion of raft lipids such as sphingomyelin into ceramide by activation of sphingomyelinases (SMases), or hydrolytic breakdown of glycosphingolipids by glycosidases; and therefore, it can be a well-regulated and tunable, but also a dysregulated and pathophysiological process. However, while line tension and entropy can explain how lipid phases separate and fuse, and how this can be regulated or disrupted by specific lipids, it is still unclear how this extends to the segregation of raft and non-raft proteins.
Affinity-driven lipid raft formation.
We discussed that lipid rafts are diffusion barriers separating non-raft and raft domains (Fig. 1A), which demands for an explanation how certain proteins are enriched in rafts, and whether this enrichment is biologically relevant. In the simplest case, we assume that no additional energy is needed and that overcoming the diffusion barrier resembles a catalytic process that lowers “activation energy” for moving a protein from a non-raft area of the membrane into a lipid raft. Once inside the raft, protein distribution stabilizes at a lower potential energy with the activation energy for transition into the raft being equal to that of the mixing energy for lipid phases, 0.55kT (Ackerman and Feigenson, 2015; Heberle and Feigenson, 2011). However, how is the activation process mediated? In enzymes, the activation energy is lowered by binding of the substrate in a transition state within the enzyme substrate (ES) complex. The equivalent of the transition state is a complex between the raft protein and lipids we will term “chaperon lipids”. To overcome the diffusion barrier, chaperon lipids bound to raft proteins engage into van der Waals interaction with raft lipids (Fig. 1C). The fundamental difference to the shell hypothesis is that chaperon lipids are only weakly bound and will be replaced by either raft lipids or other proteins forming stable complexes once translocated into the raft. One may even speculate that chaperon lipids prevent protein oligomerization in the non-raft phase, while giving way to forming stable protein complexes once moved into the lipid raft (Fig. 1C). The “lipid chaperon” model resembles the induced-fit raft model proposed by Linda Pike (Pike, 2004), however, it defines lipid-protein as a driving force for raft formation. In any case, discussing lipid-protein affinity as a critical driving force in raft formation and protein translocation demands for new experimental approaches in testing lipid-protein binding and its function for the biological relevance of lipid rafts. Unfortunately, apart from post-translational modification sites for GPI anchors and acylation, only little is known about raft targeting motifs in proteins that could serve as binding sites for raft, shell, or chaperon lipids. In recent years, focus was on the transmembrane domain (TMD) of raft proteins that have to be long and slim to accommodate for the increased thickness of lipid rafts and to reduce surface tension (Diaz-Rohrer et al., 2014b; Lorent and Levental, 2015). However, these studies did not specify the lipids the TMD interacts with and the biological significance of the TMD for raft association was not distinct from its function in the translocation of proteins to the plasma membrane. There are only very few studies that clearly show raft-specific targeting motifs with biological significance. One study identified a six-amino acid, phospholipid-specific raft targeting motif in the C-terminus of Src homology 2 containing phosphatase 1 (SHP-1) (Sankarshanan et al., 2007). Importantly, the study performed site-directed mutation demonstrating that raft association of the phosphatase was crucial for its cell signaling activity. Lipid raft localization, however, was solely based on copurification of the protein with DRMs, while imaging using microscopy was not performed. This limitation makes it difficult to decide if the targeting motif is specific for raft association or also mediates translocation of the phosphatase to the plasma membrane.
Interestingly, the phospholipids with the highest affinity to the raft-targeting 6-mer were phosphatidic acid, phosphatidylinositol-4 phosphate (PI4P) and PI4,5P. PI4KIIα, the PI4 kinase generating PI4P, is instrumental for lipid raft formation at the trans Golgi network (TGN) (Clayton et al., 2013), and PI4,5P has been implicated in the lipid regulation of caveolae (Fujita et al., 2009). It is not known if these phospholipids have a general function in chaperoning the translocation of proteins into lipid rafts. Most recently, novel methods have been developed to cross-link lipid analogs to proteins, providing the opportunity to define a lipid-protein interactome. By identifying and mutating raft-targeting sequences that bind to specific lipids, one may be able to clearly demonstrate the biological significance of lipid rafts for protein function in cell signaling and other biological processes.
1.3. Lipid-enriched compartments and road maps for vesicle traffic
Prior to proposing the raft as concrete entity in cellular membranes, lipid rafts were operationally defined as lipid-regulated sorting mechanism for vesicle flow from the Golgi and TGN to the plasma membrane and back into the endosome. Instrumental for identifying lipid-regulated polarity in vesicle flow was cultivation of polarized epithelial (MDCK) cells and radioactive tracer analysis of the lipid envelope of apically vs. basally budded viruses used as reporters for the lipid composition of the apical or basolateral domain of the plasma membrane (van Meer and Simons, 1982). Simons and van Meer showed that a similar “lipid polarity” underlies budding and sorting of exocytotic vesicles in MDCK cells, which was hypothesized to be regulated by local enrichment of specific sphingolipids and phospholipids at the TGN (van Meer and Simons, 1988). The next milestone was a study by Deborah Brown and John Rose in 1992 showing that a GPI-anchored protein is recovered from the glycosphingolipid-enriched DRM fraction of post-Golgi vesicles (Brown and Rose, 1992). The authors concluded that “protein-sphingolipid microdomains form in the Golgi apparatus and plasma membrane”, which were dubbed “rafts” one year later by Simons and colleagues (Fiedler et al., 1993). Hence, the postulate of lipid rafts in the Golgi and TGN as polarized exit points for secretory vesicles preceded the formal description of rafts in the plasma membrane by several years.
Since these original discoveries, sorting regulated by specific enrichment of lipids in the Golgi and many other compartments has been implicated in intra- and inter-organelle vesicle flow. Sphingolipids, particularly glycosphingolipids, sphingomyelin, and ceramide, and phospholipids, particularly phosphatidylinositol phosphates (PIPs) have taken center stage in understanding regulation of vesicle flow and membrane fusion and fission. We and others demonstrated that sphingolipids including ceramide are organized in sphingolipid- or ceramide-enriched compartments (sphingosomes or CECs) (Bieberich, 2008, 2011a, 2012; Burgert et al., 2017; Cremesti et al., 2001; Gulbins and Grassme, 2002; Gulbins and Kolesnick, 2003; He et al., 2012; Stancevic and Kolesnick, 2010; Zhang et al., 2009). Our group found that an apical CEC (ACEC) is important for biogenesis and function of cilia and cell polarity (Bieberich, 2011a; He et al., 2012; He et al., 2014; Kong et al., 2015a; Krishnamurthy et al., 2007; Wang et al., 2009; Wang et al., 2008). In addition, various lipids are distributed in a gradient with cholesterol and sphingomyelin enriched in the plasma membrane, while ceramide appears to be enriched in the endosomal compartment (Schulze et al., 2009; van Meer and de Kroon, 2011; van Meer et al., 2008). Based on these observations we hypothesize that the lateral anisotropy of lipids leads to formation of rafts that are integrated with a lipid gradient orthogonal to the membrane. This integration leads to lipid compartmentalization and resembles a “road map” regulating intracellular vesicle traffic and cell polarity. The vesicular identity is even preserved during mitosis when many compartments such as the Golgi apparatus and the nuclear envelope are disintegrated into a myriad of vesicles (“Golgi haze”) and yet reassemble in the daughter cells with a composition identical to the original cell (Axelsson and Warren, 2004). The following sections will discuss the functional compartmentalization of lipids in various organelles and the potential contribution of lipid rafts in vesicular traffic.
Golgi apparatus and TGN.
The secretory route from endoplasmic reticulum (ER) to Golgi/TGN and then the plasma membrane was once the role model for regulating polarity of vesicle cargo and direction by lipid rafts. Consensus was that cholesterol (or ergosterol in yeast) was indispensable and accompanied by sphingolipids such as sphingomyelin and glycosphingolipids to initiate at TGN exit points what should then become a lipid raft in the plasma membrane. However, since several pioneering studies appeared about two decades ago (Brown and Rose, 1992; Fiedler et al., 1993), there was not much progress in our understanding on how these lipids actually achieve the sorting process. While focusing on an active role of lipid rafts as organizers for sorting, it was less appreciated that a well-investigated sorting process mediated by coat protein (COPI) polymerization for intercisternal Golgi and retrograde vesicle transport to the ER does not rely on lipid rafts (Arakel and Schwappach, 2018; Dodonova et al., 2015). After coating starts with binding of a GTPase (Arf1) via myristoyl-residue insertion into the Golgi membrane, coat polymerization is confined to the non-raft (liquid disordered or Ld) domains, probably to prevent phase separation during coat assembly (Manneville et al., 2008; Pinot et al., 2010). These data suggested that raft and non-raft domains in the Golgi/TGN are equally important for vesicle transport within or from the Golgi. For sorting and transport from TGN to the plasma membrane, specific GPI-anchored, raft-associated proteins are important, while non-raft, coated vesicles are instrumental for trafficking to the basolateral plasma membrane or within the Golgi-ER compartment (Guerriero et al., 2008; Surma et al., 2012).
Golgi morphology and intra-Golgi transport is disrupted by reducing cholesterol content by just about 10% (Stuven et al., 2003), which implies a critical role for this archetypical raft lipid in intra-Golgi and other Golgi-related vesicle transport. There is undisputable evidence that a lipid gradient from ER to Golgi to plasma membrane increases the concentration of cholesterol and sphingomyelin and that lipid transport is instrumental for this enrichment of raft lipids in the plasma membrane (Brugger et al., 2000). However, it is debatable whether lipids or proteins initiate or mediate the raft formation or sorting process. Probably, in Golgi-related vesicle trafficking, proteins are the key players, but they cannot achieve sorting without the proper lipid environment. This hypothesis is based on two important observations made in recent years: 1) a portion of lipids is not transported by vesicles, but by lipid transport proteins (LTPs) such as the glycolipid transport protein (GLTP) superfamily, with four phosphate (PI4P) adaptor protein 2 (FAPP2) as the most prominent example (Brown and Mattjus, 2007; D’Angelo et al., 2007; Godi et al., 2004; Mattjus, 2016; Vieira et al., 2005; Yamaji et al., 2008), oxysterol binding proteins OSBPs (Olkkonen and Li, 2013; Ridgway, 2010), and ceramide transport protein (CERT) (Hanada et al., 2003; Kumagai et al., 2005); and 2) lipid transport proteins LTPs recognize specific lipid signals at the TGN, particularly PI4P and PI4,5P (Martin, 2001). PIPs themselves can be localized in rafts and therefore, determine transport and metabolism of other raft lipids in an autoregulatory process that is not completely understood yet (Martin, 2001).
Ceramide is another raft regulatory lipid the interaction of which with CERT may play a critical role in shaping the lipid environment of lipid rafts in the Golgi. Ceramide is de novo synthesized in the ER and then transported via vesicles and CERT to the Golgi. CERT-transported ceramide is used to generate sphingomyelin, which is transported through vesicles to the plasma membrane. Hence, the transport and catalytic activity of CERT and Golgi-resident sphingomyelin synthase 1 (SMS1), respectively, may determine the lipid raft composition at the Golgi, and therefore, lipid polarity-regulated trafficking to the plasma membrane.
Endosomes, lysosomes, autophagosomes, and extracellular vesicles.
The endosome is at the crossroads of different vesicle flows into and from the Golgi, lysosomes, and other compartments. Since any continuity of the cell size requires that endocytosis balances exocytosis it is likely that similar sorting mechanisms, including those mediated by lipid rafts are active in biogenesis and turnover of the endosome. The endocytic compartment can be roughly divided into two domains, the early and recycling endosome, and the multivesicular (MVE) and late endosome (Scott et al., 2014). The early endosome is the source for the recycling endosome which is in constant exchange with the plasma membrane. The early endosome is also the source for the MVE, which matures into the late endosome or fuses with the plasma membrane and releases exosomes. The late endosome is in exchange with the TGN and lysosomes. Separation of these compartments and lipid analysis showed that the early and recycling endosomes are rich in raft lipids such as cholesterol and sphingomyelin, while the late endosome is depleted of these lipids, probably with the aid of cholesterol binding proteins such as NPC1 that sequesters cholesterol to the recycling endosome (Diaz-Rohrer et al., 2014a; Gagescu et al., 2000; Hao et al., 2002; Lusa et al., 2001; Pipalia et al., 2007). It is possible that a two-step process consisting of protein-mediated enrichment of raft lipids in the recycling endosome, followed by lipid-induced anisotropy mediates the function of lipid rafts in endocytosis and membrane recycling. This hypothesis is supported by data showing that abrogation of raft partitioning by mutation of a raft-associated protein (linker for activation of T-cells or LAT) leads to re-localization from the recycling endosome to the late endosome (Diaz-Rohrer et al., 2014b).
Exosomes are lipid vesicles initially generated as intraluminal vesicles by inward budding and fission of the MVE membrane. The composition of lipids in exosomes is distinct from that of the plasma membrane, mainly due to several-fold enrichment of sphingomyelin, cholesterol, glcyosphingolipids, and ceramide (Record et al., 2014; Skotland et al., 2017; Tan et al., 2013). Hence, the question is how typical raft lipids are enriched in exosomes, while the later endosome compartments are depleted of them. One possible solution is that proteins assisting in exosome formation enrich raft lipids for inward budding of the MVE membrane. Proteins assisting in exosomes formation are those of the ESCRT family. It was shown that lysobisphosphatidic acid (LBPA), an MVE specific glycerophospholipid, binds to Alix1, an ESCRT and exosomal marker protein (Kufareva et al., 2014). Another study using liposomes showed that cholesterol is required for assembly of the ESCRT protein complex, while the assembled complex then binds to phospholipids such as PI3P to increase line tension (Boura et al., 2012). Line tension is relieved by forming a neck-shaped membrane curvature that initiates inward budding and fission of the endosomal membrane (Hurley et al., 2010). The authors suggest that cholesterol does not bind directly to ESCRT proteins, but ESCRT-associated phospholipids interact with cholesterol prior to neck formation, which is consistent with the lipid chaperon model. Apart from the ESCRT-driven intraluminal vesicle/exosome formation, there is an ESCRT-independent mechanism. ESCRT-independent exosome formation is likely to invoke another mode of lipid-protein interaction that critically relies on neutral sphingomyelinase 2 (nSMase2)-dependent conversion of sphingomyelin into ceramide (Marsh and van Meer, 2008; Trajkovic et al., 2008). Ceramide is sequestered into exosomes, suggesting that it plays a role in formation as well as function of exosomes (Kong et al., 2015b; Wang et al., 2012). Recently, we found that ceramide is critical for association of astrocyte-derived exosomes with amyloid peptide Aβ and may contribute to plaque formation and neurodegeneration in Alzheimer’s disease (AD) (Dinkins et al., 2014; Dinkins et al., 2016a; Dinkins et al., 2016b; Wang et al., 2012). We also suggested that exosomes may serve as extracellular signalosomes for “mobile rafts” that copy and broadcast activation of a particular raft-associated cell signaling pathway from a donor into a recipient cell (manuscript in press). Accordingly, uptake of exosomes is likely to change the lipid composition of the plasma membrane or that of the endosomal compartment of the recipient cell. However, only a few studies investigated the role of lipid rafts in endocytosis of exosomes. One study shows that caveolin 1 negatively regulates exosome uptake by non-classical, lipid-raft mediated endocytosis, suggesting that uptake of exosomes is mediated by caveolae-independent, lipid raft-mediated endocytosis (Svensson et al., 2013). In this regard, it is interesting that increase of ceramide in the plasma membrane was reported to displace cholesterol and caveolin 1 from lipid rafts, suggesting that ceramide association with other raft lipids and proteins may play a role in endocytosis of exosomes (Yu et al., 2005). One of the raft-associated proteins critically involved in endocytosis and also enriched in exosomes is flotillin (Frick et al., 2007; Phuyal et al., 2014), suggesting that flotillin may contribute to lipid raft formation and recycling of rafts through endo- and exocytotic trafficking routes.
Further downstream of the early endosome is the late endosome that feeds into lysosomes. As mentioned before, raft lipids such as cholesterol are sequestered from the late endosome by NPC1, which suggests that lipid rafts may not be important for lysosomes. In pathological settings such as NPC1 deficiency (Niemann Pick disease C), redistribution of raft lipids to lysosomes may become important, suggesting that lysosomes are devoid of rafts unless lipid metabolism is dysregulated. However, our view on the significance of lipid rafts for lysosomes may get a different twist when considering the endosomal and lysosomal compartments as a functional continuum with respect to luminal acidification. The pH value of the early to late endosome and then lysosomes drops to activate acid hydrolases, including those generating ceramide from sphingomyelin (acid SMase) or glycosylceramides (sialidases and glucosyl- and galactosylceramidases). The main proton pump achieving acidification is the vacuolar ATPase (V-ATPase), a multiunit transmembrane protein that was co-purified with DRMs from the plasma membrane, synaptic vesicles, and the endosome (Dermine et al., 2001; Lafourcade et al., 2008). In addition, re-acidification of lysosomes requires the activity of cystic fibrosis transmembrane conductance regulator (CFTR) a ceramide raft-associated chloride channel (Grassme et al., 2003b). A recent study on the effect of the lysosphingolipid psychosine (galactosylsphingosine) in Krabbe’s disease found that V-ATPase and CFTR are affected by these lipids (Folts et al., 2016). Therefore, one may speculate that lipid interaction of V-ATPase and CFTR is critical for lysosomal acidification, although involvement of lipid rafts in this interaction is unclear.
Lysosomes fuse with autophagosomes to form autolysosomes, a step instrumental for recycling a cellular material in autophagy. It is hypothesized that autophagosomes originate in mitochondria-associated membranes (MAMs), a subcompartment of the endoplasmic reticulum (ER) that forms membrane contact sites (MCS) with mitochondria (see also next paragraph in this review) (Garofalo et al., 2016). It was also shown that the raft ganglioside GD3 is critical for biogenesis of autophagosomes, suggesting that lipid rafts in MAMs are important for autophagy and autophagosome formation (Garofalo et al., 2007; Sorice et al., 2012). Since dysregulation of autophagy is associated with many pathological processes from cancer to senescence, the composition of lipid rafts during aging and disease may change and provide an explanation for many processes involving “healthy” membrane flow and vesicle trafficking in cells.
ER, MAMs, mitochondria, and MCS.
The endoplasmic reticulum (ER) is instrumental in at least four important aspects of cell biology: 1) it is the locus for translation of transmembrane proteins and initial post-translational modification or ER degradation (e.g., N-glycosylation, ERAD) as well as the translation site of intraluminal and many secreted proteins; 2) it houses enzymes for de novo lipid biosynthesis of sphingolipids (e.g., sphingosine, ceramide) and sterols; 3) it serves as storage compartment for calcium (Ca2+) and regulates its exchange with the cytosol and organelles such as mitochondria; and 4) it is the source for ER-derived membranes and subcompartments such as the perinuclear ER, mitochondria-associated membranes (MAMs), autophagosomes, and oil droplets. In all of these aspects of ER biology, lipid rafts may play important roles in the regulation of ER function.
To function properly, ER membranes engage into numerous contact sites (MCS) with other compartments such as the nucleus, mitochondria, and the plasma membrane, and they form attachment sites with the cytoskeleton. Proteins mediating attachment of membranes within MCS are well described, while the function of lipids and lipid rafts is largely unknown. The MCS between the ER and the plasma membrane mediate Ca2+ influx from outside of the cell to replenish Ca2+ that was stored for release to the cytosol or mitochondria. This so-called “store-operated Ca2+ entry” (SOCE) mainly relies on a complex between two proteins, the Ca2+ sensing stromal interaction molecule 1 (STIM1) in the ER and the calcium release-activated calcium channel (ORAI)-transient receptor potential cation channel (TRPC) in the plasma membrane (Liao et al., 2009; Pani and Singh, 2009; Vaca, 2010). It was found that gating of the pore forming ORAI-TRPC requires its association with lipid rafts that become part of the MCS between the plasma membrane and the ER (Liao et al., 2009). Since the discovery about 40 years ago that MCS between the plasma membrane and the sarcoplasmic reticulum in smooth muscle cells are localized at caveolae (Gabella, 1971), Ca2+ regulation at the plasma membrane was associated with caveolin-containing rafts. Investigating the role of lipids was limited to cholesterol depletion, which is a rather crude method for testing the physiological significance of lipid rafts as discussed in the next section.
Another type of MCS is formed between MAMs, a subcompartment of the ER, and mitochondria. MAMs themselves are compartmentalized in different subdomains with enrichment of distinct lipids, probably specific for the function of these subdomains. MCS between MAMs and the outer mitochondrial membrane (OMM) regulate Ca2+ influx from the ER to mitochondria and exchange of lipids important for the function of mitochondria (Herrera-Cruz and Simmen, 2017; Simmen and Tagaya, 2017). These MCS are mainly stabilized by complexes of proteins associated with MAMs and the outer mitochondrial membrane (OMM). The importance of lipids and lipid rafts for Ca2+-regulating MCS was shown for the complex of sigma receptor 1 (SigR1) and inositol-3-phosphate receptor (IP3R) and their glucose-related protein 78 (GRP78/BiP)-mediated interaction with voltage-dependent anion channel 1 (VDAC1) in the OMM. SigR1, a chaperon of IP3R, is a transmembrane protein that associates with cholesterol, is activated by sphingolipids such as sphingosine, and embedded into ceramide and galactosylceramide-enriched subdomains of MAMs (Bieberich, 2012; Fujimoto and Hayashi, 2011; Hayashi and Fujimoto, 2010; Hayashi et al., 2009; Hayashi and Su, 2004, 2010; Palmer et al., 2007; Ramachandran et al., 2009). Knockdown of SigR1 was shown to disrupt lipid raft distribution of raft marker proteins such as flotillin-1 and caveolin-1 (Vollrath et al., 2014). Disruption of lipid rafts by MCβD-mediated cholesterol depletion shifted distribution of SigR1 into the non-raft fraction and impaired Ca2+ influx into mitochondria. Several studies showed that in neural differentiation, association of SigR1 with galactosylceramide-enriched lipid rafts is important for oligodendrocyte differentiation (Hayashi and Su, 2004). These data suggest that lipid rafts regulate Ca2+ influx in mitochondria and are important for cell differentiation, although the specific roles of ceramide, galactosylceramide, and other raft lipids are still unknown.
Our own research showed that ceramide-enrichment in MAMs modulates another important function of MCS with mitochondria: cellular ATP regulation. Oxidative phosphorylation (OxPhos) in mitochondria is the main supply for cellular ATP unless impaired by oxygen deprivation or mitochondrial malfunction as observed with the Warburg effect when aerobic glycolysis generates the bulk of ATP. Apart from regulation of enzymes in OxPhos, transport of ADP and ATP between mitochondria and other compartments is critical for cellular ATP levels. This transport is mediated by an ADP/ATP adenine nucleotide translocator (ANT) in the inner mitochondrial membrane (IMM) and VDAC1. As discussed VDAC1 engages into a complex with IP3R-SigR1, but it is also regulated by tubulin. This form of MAM-associated tubulin blocks ADP/ATP transport by inserting its C-terminal tail into the VDAC1 pore (Maldonado and Lemasters, 2012; Maldonado et al., 2013; Noskov et al., 2013; Rostovtseva et al., 2008; Sheldon et al., 2015). It was proposed that lipids regulate that interaction, although this was confined to the microenvironment of VDAC1 in the OMM (Hoogerheide et al., 2017; Rostovtseva and Bezrukov, 2008; Rostovtseva et al., 2012; Rostovtseva et al., 2006). Our study showed that ceramide enrichment in MAMs and its interaction with tubulin promotes closure of VDAC1 and impairs ATP generation when ceramide levels increase (Kong et al., 2018). It should be noted that the function of ceramide in or at mitochondria has gained growing attention in many recent studies ranging from inducing mitophagy to forming pro-apoptotic pores (Dany and Ogretmen, 2015; Hernandez-Corbacho et al., 2017; Law et al., 2017; Novgorodov et al., 2011; Novgorodov and Gudz, 2011; Novgorodov et al., 2018; Patwardhan et al., 2016; Perera et al., 2016; Schwartz et al., 2018; Siskind et al., 2002, 2006), which may involve processes such as lipid raft speciation for membrane remodeling. Regulation of ceramide levels to modify the lipid composition and function of lipid rafts in MAMs or mitochondria is mainly achieved by ER-resident enzymes such as ceramide synthases (CerSs) or membrane-associated SMases such as nSMase2 and potentially, a recently discovered mitochondrial neutral SMase (Kong et al., 2018; Novgorodov et al., 2018; Rajagopalan et al., 2015; Wu et al., 2010).
In addition to regulating Ca2+ and ADP/ATP transport, lipid rafts in MAMs may be critical for many pathophysiological processes. It was shown that pathological protein aggregates such as prion protein, amyloid peptide, synuclein or huntingtin associate with lipid rafts and are transported to mitochondria, where they bind to proteins regulating mitochondrial function (Area-Gomez and Schon, 2017; Garofalo et al., 2015; Manczak and Reddy, 2012; Rouvinski et al., 2014; Smilansky et al., 2015). It remains to be elucidated if ceramide or other raft lipids are instrumental in protein aggregate formation, endocytosis, transport, and mitochondrial dysfunction, a pathophysiological chain reaction shared among the majority of neurodegenerative diseases, including frontotemporal dementia (FTD), amyotrophic lateral sclerosis (ALS), Parkinson’s disease (PD), Huntington’s disease (HD), and Alzheimer’s disease (AD) (Area-Gomez et al., 2018; Area-Gomez and Schon, 2017; Burte et al., 2015; Cardoso et al., 2017; Chu et al., 2014; Golpich et al., 2017; Johri and Beal, 2012; Kozlov et al., 2017; Krols et al., 2016; Paillusson et al., 2016; Rodriguez-Arribas et al., 2016; Shoshan-Barmatz et al., 2018; Wakade and Chong, 2014; Wong and Krainc, 2017). Studies in our laboratory found that amyloid peptide (Aβ42) impaired mitochondrial motility and induced fragmentation of mitochondria in astrocytes, which was not observed in ceramide-depleted cells (Kong et al., 2018). This data suggests that ceramide-enriched lipid rafts or CRPs in mitochondria and MAMs may contribute to mitochondrial dysfunction in AD and other neurodegenerative diseases.
Nucleus, chloroplasts, and peroxisomes.
These organelles contain raft lipids that are important for their function, however, it is not known if the lipids are organized in functional rafts. In the nuclear envelop, GM1, a typical raft glycosphingolipid, was shown to regulate Ca2+ influx into the nucleus and to affect epigenetic regulation of gene expression (Itokazu et al., 2017; Ledeen and Wu, 2008, 2010, 2015; Tsai et al., 2016; Tsai and Yu, 2014; Wu et al., 2009; Xie et al., 2004). Lipid rafts were also found in plants, particularly in the plasma membrane of pollen tubes, root hairs, and chloroplasts, although the function is less investigated than in animals (Bhat and Panstruga, 2005; Cacas et al., 2012; Gronnier et al., 2016; Grosjean et al., 2018; Jarsch and Ott, 2011; Malinsky et al., 2013; Mongrand et al., 2010; Shabala et al., 2015; Simon-Plas et al., 2011). Peroxisomes are indispensable for functioning of photosynthesis in plant cells, although they were first discovered in kidney and liver cells not too long ago (De Duve and Baudhuin, 1966). They are instrumental in oxidation of glycolate (plants and algae) and for biosynthesis of important lipids such as polyunsaturated fatty acids, phosphatidylserine, phosphatidylethanolamine, and ether lipids. It was shown that lipid rafts are critical for peroxisome biogenesis and ceramide-enriched membrane domains are important for peroxisome fusion (Boukh-Viner et al., 2005). It is not known which and how raft-associated proteins are involved in these processes and if dysregulation of peroxisomal lipid rafts contributes to a plethora of disorders caused by peroxisome dysfunction, including Zellweger syndrome and neonatal and X-linked adrenoleukodystrophy. In addition to endosomes, peroxisomes are interesting in understanding membrane and raft lipid remodeling since they transition between the ER and mitochondria to fulfill their metabolic function. Therefore, isolation of vesicular compartments followed by lipid raft analysis may be a promising approach to understand the function of lipid rafts.
2. Detection, preparation, and visualization of rafts and their interaction with lipids and proteins
The methods to purify and test the biological function of lipid rafts have not changed for decades. The purification relies on density gradient centrifugation of detergent or alkaline-insoluble membranes, two techniques introduced in the 1970’s. Testing of the biological function relies on cholesterol depletion using incubation of cell cultures with MβCD, which was introduced in the 1980’s. Unfortunately, these “classical” methods used for purification or cholesterol depletion have severe limitations. Detergents and to some extent, alkaline treatment may change the biophysics of cellular membranes thereby inducing the formation of lipid rafts as artifacts. MβCD will deplete cholesterol from all of the cellular membranes, thereby disturbing Golgi architecture and Golgi-derived vesicle transport. Therefore, disruption of cell signaling pathways is not surprising and may not rely on formation for rafts, but vesicular transport to the plasma membrane or other compartments or organelles. Fortunately, there are experiments instilling some degree of confidence that insoluble membranes are a reasonable equivalent to lipid rafts and MβCD is able to test the biological significance of rafts. These classical and novel methods to prepare and investigate lipid rafts and their benefits and limitations will be discussed in the next paragraphs.
Biochemical preparation and manipulation of lipid rafts.
Two decades before lipid rafts became a formal concept, two studies described the isolation of detergent-resistant and alkaline insoluble membranes as low density buoyant fraction after ultracentrifugation on density gradients. This method relies on the ability of certain non-ionic detergent such as cold Triton X-100 to exclude particular membrane vesicles from solubilization, leading to a fraction of detergent-insoluble particles of low density (due to the high lipid content) that is separated from a denser protein fraction solubilized by forming micelles. The method initially introduced in 1974 by Butters and Hughes to prepare DRMs from a cell membrane fraction using cold 0.5% Triton-X 100 and then refined by Brown and Rose 20 years later has essentially been the same till now (Brown and Rose, 1992; Butters and Hughes, 1974). Thin layer chromatography (TLC) of DRMs showed that DRMs are enriched with typical raft lipids such as sphingomyelin and cholesterol. The alkaline method of membrane extraction was introduced in 1972 by Gurd et al. who reported that pyridine-insoluble fractions of the plasma membrane contain a specific selection of glycoproteins, although lipids were not further analyzed (Gurd et al., 1972). Later, this method was refined by replacing pyridine with Na2CO3 (carbonate method). Lipid and protein components were found to be similar (but not always identical) to DRMs (Luria et al., 2002; Macdonald and Pike, 2005; Persaud-Sawin et al., 2009). However, the cold Triton X-100 extraction method was criticized for introducing artifacts due to altering the lipid distribution by exposing membranes to low temperature and detergent, or disassembling the original rafts into smaller micellar components that are mistaken for rafts (Heerklotz, 2002). This criticism led to a variety of methods using cations such as Mg2+ to stabilize DRMs or different detergents such as Brij98 to isolate rafts at physiological temperature (Chamberlain, 2004; Chen et al., 2009; Lingwood and Simons, 2007; Morris et al., 2011; Schuck et al., 2003; Shogomori and Brown, 2003; Williamson et al., 2010). A review by Linda Pike comprehensively discusses the effect of different detergents on the ability to isolate heterogeneous rafts as proposed by the induced fit, lipid shell, or lipid chaperon model (Pike, 2004). Non-detergent and detergent methods were refined in recent years by using shear force and immunoaffinity isolation of lipid vesicles from different membrane and organelle fractions to isolate rafts (Brugger et al., 2004; Pike, 2004). Physical methods of membrane disruption based on shear force (Wheaton Dounce or Potter Elvehjem homogenizer or extrusion through needles) or cavitation (high frequency ultrasound) are likely to partition the membrane into vesicles of 100–150 nm covering membrane surface areas of 200–300 nm, which is in the size range of lipid rafts (Garcia-Marcos et al., 2006). While there are several examples of GPI-anchored proteins that are not found in DRMs but detected in rafts by other methods (and vice versa), there is broad consensus that DRMs are not identical to, but they are the closest equivalent to rafts that can be isolated by biochemical methods.
In the last 20 years, studies with synthetic vesicles and immunocytochemistry using antibodies against ceramide showed that it is a main regulator of lipid rafts. SMases activated at the plasma membrane and other compartments catalyzes hydrolysis of the raft lipid sphingomyelin to generate ceramide. This leads to ceramide enrichment of rafts that can also be found in DRMs. However, at a particular level, ceramide is no longer part of a raft with Lo structures, but forms its own lipid microdomain structure. Studies with synthetic vesicles showed that there are two distinct types of ceramide microdomains: clusters of sphingomyelin-ceramide interaction with ceramide displacing cholesterol and those with ceramide-cholesterol interaction. Ceramide-enriched rafts and these two types of ceramide microdomains are now combined under the term ceramide-rich platforms (CRPs) that can be detected in fixed cells using immunocytochemistry with anti ceramide antibody. Currently, there are no thorough studies investigating the detergent insolubility of CRPs isolated from cells, however, results from synthetic vesicles show that ceramide enrichment >5 mol% in mixtures with sphingomyelin increases detergent resistance at room temperature (Sot et al., 2006). CRPs have been detected in the plasma membrane and membranes of other compartments and they are likely to form functional units in CECs such as MAMs and exosomes. Probably, it is reasonable to assume that CRPs are best isolated by immunoaffinity methods using anti-ceramide antibody as introduced by our group several years ago (Bieberich, 2008, 2011b; He et al., 2012; Wang et al., 2005).
To test the functional significance of lipid rafts, methods that reduce or abolish rafts in cells and DRMs by removing or disrupting the membrane distribution of raft lipids were introduced many years ago. Ideally, a reagent disrupting rafts would also abolish the association of raft lipids and proteins with detergent or alkaline resistant membranes and impair the biological function of rafts in cells. One of the first reagents disrupting rafts was the fungal polyene filipin. Filipin was isolated in 1955 from Streptomycesfilipinensis and is a potent anti-fungal antibiotic because of its high affinity to membrane sterols. It was initially used to visualize caveolae, although it was debated whether it truthfully shows caveolae or rather disrupts them and forms clusters detectable by fluorescence microscopy and freeze edge electron microscopy (Fields et al., 1987; Schlosser and Gottlieb, 1966; Severs and Robenek, 1983; van Deenen et al., 1975). In 1995, a study showed that filipin disrupts association of GPI-anchored CD59 with detergent-resistant high molecular weight protein complexes, although distribution to DRMs was not discussed (van den Berg et al., 1995). The association of filipin with lipid rafts or specialized microdomains termed glycolipid-enriched domains (GEMs) and “glycosphingolipid signaling domains” was reported by Hakomori’s group in 1998 and it has been used since then to disrupt rafts in biological assays testing their function in cell signaling pathways (Iwabuchi et al., 1998). Another more commonly used reagent to disrupt rafts is MβCD. In 1973, MβCD was found to form inclusion complexes with activated fatty acids, and then about 20 years later, it was shown to extract cholesterol from cells and lipid rafts (Keller and Simons, 1998; Kilsdonk et al., 1995; Machida and Bloch, 1973). Currently, MβCD is the most commonly used reagent to disrupt rafts, abolish association of raft proteins with DRMs, and disrupt raft-regulated cell signaling pathways. However, caution is advised since MβCD will not only extract cholesterol from the plasma membrane, but also from intracellular compartments thereby severely disturbing organelle structure and function, and vesicular transport.
Since formation of lipid rafts relies on generation of sphingolipids and sterol biosynthesis inhibition or silencing of enzymes in metabolism of the two lipid classes will affect raft and DRM distribution as well as cell signaling pathways regulated by raft-associated lipids and proteins. About 20 years ago, lovostatin, a specific inhibitor of hydroxymethylglutaryl CoA reductase, the first enzyme in cholesterol biosynthesis, was shown to disrupt Golgi-derived transport and raft as well as DRM association of apical, but not basolateral plasma membrane proteins in polarized enterocytes (Hansen et al., 2000). This effect was similar to that of MβCD, demonstrating the critical contribution of cholesterol to apicobasal vesical sorting and raft formation. The use of statins, however, was never as popular as that of MβCD in disrupting rafts, probably because inhibition of the entry step of isoprenoid biosynthesis will not only reduce cholesterol levels, but also that of lipid anchors for raft-associated proteins. While MβCD destabilizes rafts, studies from several laboratories found that docosahexaenoic acid (DHA), a poly-unsaturated omega 3 fatty acid (PUFA) widely available as dietary supplement in fish oil, increases the degree of disorder in non-raft domains, thereby enhancing liquid-liquid segregation and stabilizing sphingomyelin-cholesterol type lipid rafts (Levental et al., 2016; Shaikh et al., 2002; Wassall et al., 2004; Wassall and Stillwell, 2008, 2009). Treatment of mesenchymal stem cells (MSCs) with DHA at physiologically relevant concentration (20 μM) increased the amount of raft lipids in giant plasma-membrane derived vesicles (GPMVs) and led to osteogenic differentiation of MSCs into osteoblasts due to activation of the Akt cell signaling pathway (Levental et al., 2017). However, most recent studies suggest that the effect of PUFAs on membrane lipid organization is more complicated and depends on the type of fatty acid used for incubation of cells (Hellwing et al., 2018).
Inhibition of enzymes in sphingolipid metabolism is another method to interfere with raft formation and function. The biosynthesis of raft lipids such as sphingomyelin and glycosphingolipids is initiated in the ER by de novo biosynthesis of the long chain bases and aminoalcohols sphingosine and dihydrosphingosine (Gault et al., 2010; Hannun and Obeid, 2011, 2018; Pruett et al., 2008; Tidhar and Futerman, 2013). The entry reaction by condensation of serine with activated palmitic acid is catalyzed by serine palmitoyl transferase (SPT), an enzyme that can be specifically inhibited with the fungus toxin myriocin (Hanada et al., 2000). The long chain base is then attached to fatty acids, a reaction catalyzed by a variety of CerSs in the ER (Levy and Futerman, 2010; Park et al., 2014; Schiffmann et al., 2012; Stiban et al., 2010). This reaction can be specifically inhibited by the fungus toxin fumonisin B1 (FB1) (Wang et al., 1991). Most ceramides are then transported to the Golgi to be converted into glycosphingolipids and sphingomyelin. Ceramide transport protein (Cert) mediates ceramide transport for sphingomyelin biosynthesis, which can be inhibited by HPA-12 (Berkes et al., 2016; Duris et al., 2011; Hanada, 2014, 2017; Hanada et al., 2003; Kumagai et al., 2005; Santos et al., 2015). Conversion of ceramide to glucosylceramide is the first step in biosynthesis of raft-associated glycosphingolipids and it is catalyzed by glucosylceramide synthase, a Golgi resident enzyme inhibited by l-phenyl-2-decanoylamino-3-morpholino-1-propanol (PDMP) or deoxynojirimycin-type compounds (Bieberich et al., 1999; Gu et al., 2017; Mellor et al., 2004). Another Golgi-resident enzyme is sphingomyelin synthase 1 (SMS1) that catalyzes conversion of ceramide to sphingomyelin and is inhibited by D609 (Adada et al., 2016; Adibhatla et al., 2012; Chen and Cao, 2017; Holthuis and Luberto, 2010; Li et al., 2007; Luberto and Hannun, 1998; Tafesse et al., 2006; Taniguchi and Okazaki, 2014). In addition to enzymes in sphingolipid biosynthesis, there is a variety of hydrolases breaking down glycosphingolipids or sphingomyelin, which can be inhibited or silenced to manipulate raft formation or function.
Many of these inhibitors or knockdown/knockout of enzymes have been used to disrupt lipid rafts or association of lipids and proteins with DRMs (Decker and ffrench-Constant, 2004; Gelineau-van Waes et al., 2005; Kilkus et al., 2003; Li et al., 2007; Martin and Konopka, 2004; Meszaros et al., 2013; Naslavsky et al., 1999; Saghy et al., 2015; Schwan et al., 2011; Van der Luit et al., 2007). Probably, the most interesting results come from two cell lines, SPB-1 or LY-B CHO cells, in which a mutation of SPT prevents sphingolipid biosynthesis, and SMS1 and 2 double knockout mouse embryonic fibroblasts (MEFs) that are devoid of sphingomyelin. In a remarkable study from 1995, Hanada and Pagano found that SPT deficiency mimicked MβCD treatment and reduced levels of DRMs and association of GPI-anchored alkaline phosphatase with the raft fraction, which was restored by supplementation of the medium with sphingosine and cholesterol (Hanada et al., 1995; Hanada and Pagano, 1995). On the other hand, SMS1 and 2 double knockout MEFs did not show reduced levels of DRMs, which was consistent with an earlier study showing that silencing or inhibiting SMS1 with D609 reduces the sphingomyelin content in DRMs (Li et al., 2007; Ogiso et al., 2015).
In addition to SMS 1 and 2, a variety of SMases alter the level of sphingomyelin in cellular membranes, which is particularly interesting since the product of this reaction, ceramide forms its own lipid rafts or CRPs. Acid SMase was shown to generate spatially extended CRPs, which is prevented by the tricyclic antidepressant imipramine, a drug that stimulates proteolytic degradation of acid SMase (Grassme et al., 2003a; Grassme et al., 2005; Gulbins and Kolesnick, 2003). Imipramine reduces association of proteins with rafts and DRMs as shown for Gα and Toll like receptor 4 (TLR4), respectively (Cuschieri et al., 2007; Lu et al., 2012; Singh et al., 2018). Apparently, this would argue against increase of sphingomyelin levels always being correlated with more rafts, however, enrichment of ceramide in rafts may be critical for raft translocation of particular proteins. A similar result was found with nSMase2, the enzyme we have already discussed as being critical for exosome secretion. nSMase2 does not only increase the amount of ceramide in rafts, but the enzyme itself is associated with DRMs, which was not shown yet for acid SMase (Goswami et al., 2005a; Stoffel et al., 2016). Interestingly, nSMase2 was found to be associated with DRMs isolated from Golgi fractions, consistent with its ability to shuttle between the Golgi and the plasma membrane (Milhas et al., 2010; Stoffel et al., 2016). Knockout of nSMase2 led to perturbation of secretory transport suggesting that sphingomyelin and ceramide regulate vesicular traffic consistent with the proposed function of lipid rafts in the Golgi (Stoffel et al., 2016). Skin fibroblasts from the nSMase2-deficient fro/fro mouse showed decreased flotillin 1 association with DRMs, while enzyme inhibition with GW4869 reduced association of variety of proteins with DRMs (Qin et al., 2012; Tan et al., 2017). These results are consistent with biophysical studies using synthetic sphingomyelin/ceramide bilayers and membrane vesicles from immortalized Schwann cells and suggest that ceramide enrichment and probably displacement of cholesterol from lipid rafts increases detergent resistance concurrent with translocation of proteins to rafts and DRMs (Megha and London, 2004; Sot et al., 2006; Yu et al., 2005).
Another method to affect lipid rafts and DRMs is incubation of cells with drugs interfering with the cytoskeleton, particularly actin filaments. As discussed in one of the previous sections of this review, cortical actin regulates lateral partitioning of the plasma membranes into microdomains or lipid rafts (picket fence and actin aster model), which is obliterated by drugs altering actin polymerization and stability (Ma et al., 2015). Incubation with phalloidin, an actin filament stabilizer, prevented distribution of the raft protein CD44 to the basolateral membrane of polarized epithelial cells after cholesterol depletion with digitonin, while actin filament depolymerization with lacuntrulin A increased the amount of CD44 in DRMs (Oliferenko et al., 1999). Preventing F-actin filament formation by cytochalasin D had a similar effect on GPI-anchored proteins crosslinked by fixation following antibody incubation or other crosslinking methods (Holowka et al., 2000). Using cytochalasin D and immunoaffinity isolation of Mg2+-stabilized DRMs, a recent study found that two archetypical GPI-anchored raft proteins, Thy-1 and PrP, are distributed to actin-dependent and independent DRMs, respectively, suggesting that a combination of methods interfering with the cytoskeleton is useful to differentiate specific lipid rafts (Chen et al., 2009). As standard operating procedure (SOP) it is recommended to use two methods to isolate DRMs (1% Triton X-100 at 4 °C and 1% Brij98 at 37 °C) or the detergent-free carbonate method should be combined with cholesterol depletion using pre-incubation with MβCD and F-actin depolymerization (latrunculin A or cytochalasin D), followed by immunoaffinity isolation of DRMs to determine lipid and protein composition in different lipid raft fractions (Cubi et al., 2013). Raft fractions should be tested for presence raft or raft-associated proteins such as flotillin (not GPI anchored), alkaline phosphatase (GPI-anchored), caveolin (not GPI anchored, specific for caveolae-type rafts), and transferrin receptor as a non-raft protein. This SOP is compatible with other means of lipid raft detection such as immunocytochemistry or TEM and proximity assays testing co-localization with raft marker proteins, lipids, and the cortical cytoskeleton in fixed cells.
Once certain proteins were co-purified in DRMs, the actual protein (or lipid-protein) complex formation within (or outside) a lipid raft has to be tested separately. The dilemma with this approach is that the commonly used co-immunoprecipitation assays (co-IPs) are incompatible with the detergent insolubility of proteins associated with DRMs. Any detergent solubilizing protein complexes from DRMs will obliterate the effect of raft lipids on protein complex formation, unless complexes remain stable once induced by rafts. One has to be aware that co-IP of proteins from a detergent-insoluble fraction without further solubilization only means that proteins are in the same DRMs, but they are not necessarily interacting in a complex. In our laboratory, we have used Digitonin as a detergent that removes cholesterol from rafts to perform co-IPs while avoiding the problem of insoluble protein-lipid vesicles in DRMs (Kong et al., 2018). In addition to co-IPs, there are other ways of determining protein-lipid and protein-protein complexes such as proximity assays.
Synthetic and plasma membrane-derived vesicles.
Studies using synthetic lipid vesicles or liposomes as model bilayer membranes showed lateral anisotropy and lipid domain formation already at the advent of the Singer Nicolson fluid mosaic model and decades before the lipid raft concept was formalized. Initially, this lipid domain formation was found in different mixtures of phosphatidyl choline with cholesterol using TEM (Hui and Parsons, 1975). Studies correlating the lipid domain concept with biological effects in cellular membranes appeared shortly thereafter (Karnovsky et al., 1982a; Karnovsky et al., 1982b; Klausner et al., 1980). A report from Kleemann and McConnell in 1976 showed that ATPase isolated from sarcoplasmic reticulum is segregated to fluid phases while cholesterol forms solid phases in the bilayer membrane (Kleemann and McConnell, 1976). An early study from Wim van Blitterswijk in 1979 was probably the first showing that EVs from leukemia cells are made of membranes that are less fluid and enriched with cholesterol and sphingomyelin (van Blitterswijk et al., 1979). Blitterswijk correctly noted that membrane rigidity was due to these two lipids and different from the plasma membrane, implying that EVs were actively formed by lipid anisotropy of the plasma membrane and not the remnants of dead cells. The formation of a connected cholesterol and sphingomyelin network or domain was then demonstrated in 1980, the first evidence for what is now known as sphingomyelin and cholesterol-enriched lipid rafts (Freire and Snyder, 1980). In the mid 1980’s, it was well established that cholesterol and sphingomyelin form rigid lipid domains in cell membranes. The description of sphingomyelin-cholesterol clusters as liquid ordered (Lo) vs. other phospholipid-containing membrane domains as liquid disordered (Ld) phases was introduced in 1987 (Ipsen et al., 1987). Since then, studies with liposomes and other types of synthetic vesicles are the best evidence that “lipids can do it by themselves” (van Meer) when self-organizing rafts.
Currently, lipid vesicles such as synthetic giant unilamellar vesicles (GUVs) or giant plasma membrane-derived GPMVs are used to test the effect of specific lipids on the “classical” cholesterol-sphingomyelin raft structure and function. Particularly, the conversion of sphingomyelin to ceramide in rafts has become a vibrant field in biophysics showing that increased levels of ceramide lead to fundamental restructuring of the raft and non-raft domains to form CRPs. The use of anti ceramide antibodies in visualizing ceramide-enriched cellular membranes, a research field in which our group has made significant contributions, suggested that there are CRPs of a wide range of sizes from less than 100 nm to more than 1 μm of diameter (Bollinger et al., 2005; Burgert et al., 2017; Chao et al., 2010; Goldschmidt-Arzi et al., 2011; Grassme et al., 2002; Lacour et al., 2004; Scheffer et al., 2007). These are known as ceramide rafts, platforms, or caps, and can even encompass whole membrane compartments termed CECs. Three questions arise from these observations: 1) what is the CRP structure; 2) how are CRPs regulated, particularly with respect to size and location; and 3) what is the function of CRPs. When it comes to question 2 and 3, CRPs may actually be the better candidates in understanding the biology of lipid rafts than the classical sphingomyelincholesterol type. Regulation of CRPs by ceramide biosynthesis and SMases has already been discussed in previous sections of this review and is clearly dependent on the intracellular location of these enzymes.
With respect to the raft structure, there are two distinct options dictated by the biophysics of lipid interaction: the sphingomyelin-ceramide raft in which ceramide displaces cholesterol, and the ceramide-cholesterol raft in which ceramide simply replaces sphingomyelin after being generated by SMases (Boulgaropoulos et al., 2011; Boulgaropoulos et al., 2012; Castro et al., 2007; Castro et al., 2009; Chao et al., 2010; Garcia-Arribas et al., 2016; Goldschmidt-Arzi et al., 2011; Megha and London, 2004; Pinto et al., 2011; Sot et al., 2006). It was suggested that DRMs isolated from red blood cell membranes contain cholesterol-ceramide CRPs equivalent to those described from lipid vesicles (Garcia-Arribas et al., 2016). Cholesterolceramide CRPs were also detected by using an anti ceramide antibody raised against this type of microdomains (Goldschmidt-Arzi et al., 2011; Scheffer et al., 2007). Both, the gel phases formed by cholesterol-ceramide and sphingomyelin-ceramide CRPs are detergent resistant, highly ordered, and more densely packed than the original Lo phase and thus more rigid (Sot et al., 2006). Changes in the length and desaturation of the fatty acid linked to sphingosine in ceramide are critical for the biophysics of these rafts and therefore, depending on the activity of specific CerSs. Collaborative studies from Manuel Prieto’s and Tony Futerman’s laboratories clearly showed that the results from model membranes are consistent with those from transgenic mice, particularly those from ectopic CerS2 expression and knockout mice (Pinto et al., 2011; Silva et al., 2012). CerS2 catalyzes condensation of activated very-long chain (VLC) fatty acids (C22-C26) with (dihydro)sphingosine to generate VLC ceramides (Levy and Futerman, 2010; Stiban et al., 2010; Tidhar and Futerman, 2013). These ceramide species favor formation of rigid, highly ordered gel phases such as described for ceramide-sphingomyelin rafts in GUVs. When GUVs were prepared from cell membranes of cells with altered CerS2 expression, they showed biophysical properties consistent with those of synthetic vesicles: increased gel phase when CerS2 was overexpressed, while membranes were more fluid in GUVs from CerS2 knockout cells (Park et al., 2014; Pewzner-Jung et al., 2010; Silva et al., 2012). CerS2 knockout mice are particularly interesting because loss of VLC ceramide is compensated by increased levels of medium-chain ceramide such as C16 ceramide. Increased C16 ceramide levels make membranes more fluid, although this is likely to occur in liquid-ordered CRPs with elevated content of ceramide instead of gel-like ceramide-sphingomyelin CRPs. Fluidity is also increased (and the formation of gel phases reduced) by unsaturation, particularly by replacing VLC C24:0 ceramide with C24:1 ceramide. In vesicles, C24:1 ceramide promotes formation of tubular structures consistent with its critical contribution to ciliogenesis reported by our laboratory (Pinto et al., 2008; Pinto et al., 2011). On the other hand, the brain-typical C18:0 ceramide generated by CerS1, an enzyme predominantly expressed in neurons, favors vesicular structures consistent with its critical contribution to exosome formation also reported by our group (Wang et al., 2012). From these observations it is clear that ceramide is an ideal lipid to fine tune the raft composition of the membrane and function in CECs depending on the activities of enzymes such as CerSs and SMases.
Protein-lipid interaction is an important aspect of our current understanding how lipid rafts are organized. We have discussed the cortical actin cytoskeleton as “picket fence” separating raft from non-raft regions of the plasma membrane. We have also discussed the potential function of lipid shells and chaperon lipids for translocation of proteins into lipid rafts. Finally, several proteins interacting with cholesterol such as caveolin, NPC1, or flotillin were shown to be critical for the function of caveolae or other lipid rafts. Therefore, it is reasonable to ask if CRPs are also regulated by protein-lipid interaction and whether synthetic vesicles can provide model membranes to test the function of this interaction for CRPs and other lipid raft structures. It was not until recently that we began understanding the role of proteins interacting with ceramide for the function of CRPs. In a landmark study from 1986, Yusuf Hannun and Bob Bell found that sphingosine interacts with protein kinase C (PKC), which was followed by the finding that ceramide activates protein phosphatase 2 (PP2a) and PP1 (Chalfant et al., 2000; Chalfant et al., 1999; Chalfant et al., 2004; Dobrowsky and Hannun, 1992; Dobrowsky et al., 1993; Hannun and Bell, 1989; Hannun et al., 1986; Mukhopadhyay et al., 2008; Perry et al., 2012). His group found that ceramide-activated PP1 dephosphorylates and activates the ezrin, radixin, and moesin (ERM) family proteins, which anchor lipid rafts to the cytoskeleton, particularly to cortical F-actin (Adada et al., 2014; Canals et al., 2012). It is not known if CRPs function as “hot spots” for F-actin anchoring to the plasma membrane involving ERMs. We and others found that aPKC is a protein binding to ceramide (Bieberich et al., 2000; Bourbon et al., 2000; Lozano et al., 1994; Muller et al., 1995; Wang et al., 2005; Wang et al., 1999). Recently, we have identified another two proteins that interact with ceramide, GSK3 and tubulin (Bieberich, 2004, 2008; Kong et al., 2015a; Kong et al., 2018). Common to aPKC, GSK3, and tubulin is their localization in lipid rafts (at least in some cell types) and their role in regulation of the cytoskeleton (Dremina et al., 2005; Etienne-Manneville and Hall, 2003; Fox et al., 2007; Palazzo et al., 2004; Singh et al., 2018; Sui et al., 2006). Therefore, it is possible that CRPs are critical for the interdependence and interaction of lipid rafts and the cytoskeleton. While it is known that lipid vesicles made of ceramide and sphingomyelin are detergent resistant, even at higher temperature, there is only little information on the protein association with CRPs isolated in the DRM fraction. The few studies showing increase of the proportion of CRPs and analysis of proteins associated with DRMs report that concurrent with cholesterol displacement from lipid rafts, cholesterol-associated proteins such as caveolins are also depleted from DRMs (Yu et al., 2005). The most prominent example are studies showing that CD95 clustering is associated with CRPs. Visualization of CRPs using anti ceramide antibody and isolation of DRMs clearly show that acid SMase-mediated increase of ceramide labeling coincides with elevated levels of CD95 and CD40 in DRMs (Fanzo et al., 2003; Grassme et al., 2003a; Grassme et al., 2002; Gulbins and Grassme, 2002). Although imaging studies suggest association of ceramide binding proteins with CRPs, it not clear if these proteins are also enriched in DRMs.
Imaging of lipid rafts.
The first evidence of lipid microdomains emerged from contrast-stained TEM images of caveolae and liposomes more than 50 years ago (Hui and Parsons, 1975; Yamada, 1955). The first report on lipid-lipid and lipid-protein interaction in membrane microdomains using freeze etch or fracture TEM then appeared in 1975 (van Deenen et al., 1975). One of the obstacles using TEM for visualization of rafts is the use of organic solvents or SDS as the detergent for immunolabeling, which makes it difficult to image lipids using antibodies. The “unfixability” of lipids can be overcome by introducing a second fixation step after applying the lipid-specific antibody assuming that the initial fixation of membrane proteins preserves the distribution of lipids in the membrane. While cryo-EM works without these setbacks and has yielded images of stunning resolution for membrane proteins embedded in liposomes or micelles, the actual visualization of the lipid environment of these proteins, including that of lipid shells and rafts has still to be developed. Another recently developed imaging method applied to lipid rafts is Atomic Force Microscopy or AFM. AFM scans membrane surface potential at sub-nanometer resolution and has provided clear evidence that membranes actually become thicker in CRPs, and when combined with high resolution fluorescence microscopy (Correlated Fluorescence AFM), they are confined to areas visualized with fluorescently labeled sphingolipid analogs (Chiantia et al., 2006; Chiantia et al., 2008; Orsini et al., 2012; Popov et al., 2008; Shaw et al., 2006). To date, however, correlated AFM has been limited to imaging supported model membranes generated from synthetic lipid mixtures or matrix-adsorbed DRMs, which will still need to be developed into analysis of lipid rafts in actual cell membranes.
From the very beginning of lipid raft analysis, fluorescence microscopy has been the most important technique for raft imaging in cells and lipid vesicles. In principle, there are three different methods of using fluorescence labeling for raft imaging: 1) fluorescent dyes binding to raft lipids such as filipin or laurdan; 2) fluorescently labeled lipids such as BODIPY-cholesterol or fluorescently labeled gangliosides incorporating into rafts; and 3) proteins and antibodies binding to raft lipid and proteins such as lysenin (binds to sphingomyelin), cholera toxin B (binds to GM1 ganglioside), or anti lipid (e.g., gangliosides, ceramide) antibodies. As previously discussed filipin specifically binding to cholesterol is one of the first fluorescent dye used for lipid raft analysis (Fields et al., 1987; Schlosser and Gottlieb, 1966; Severs and Robenek, 1983; van Deenen et al., 1975). However, filipin disrupts the raft organization and is therefore not suitable for imaging. Laurdan was introduced to lipid domain imaging about 30 years ago and shows a large red shift from 440 nm (gel phase, non-polar) to 490 nm (liquid phase, polar) emission known as fluorescence relaxation (Barucha-Kraszewska et al., 2013; Dietrich et al., 2001; Dinic et al., 2013; Kindzelskii et al., 2004; Parasassi et al., 1990). Since the early 2000’s, laurdan and later, C-laurdan was used to visualize rafts equivalent to DRMs in model membranes and living cells (Barucha-Kraszewska et al., 2013). The drawback of these lipophilic dyes is their rapid internalization and incompatibility with fixation that have prevented them from moving into novel fields of superresolution fluorescence microscopy. A similar obstacle arises for fluorescent analogs of raft lipids. While they were originally used to distinguish apical and basolateral transport suggesting the function of lipid raft at Golgi exit sites form vesicle transport, it was later noted that membrane lipid packing and therefore, partitioning of these lipid analogs is critically affected by the conjugated fluorophore (Klymchenko and Kreder, 2014). To solve this problem, Bob Bittman and others synthesized a variety of cholesterol and sphingolipid compounds with the fluorophore attached to different positions of the analogs (Li et al., 2006). The most recent development of fluorescent lipid analogs are ultrabright ganglioside analogs (e.g., ATTO-labeled GM1) and clickable cholesterol or sphingolipids that are administered to living cells and are only minimally different from endogenous lipids (Haberkant and Holthuis, 2014; Haberkant et al., 2016; Kong et al., 2015a; Kong et al., 2018; Walter et al., 2016). The new generation of ganglioside analogs carries the fluorophore in the oligosaccharide moiety which does not interfere with incorporation of the sphingolipid portion into the membrane (Suzuki et al., 2017; Suzuki et al., 2018). They were successfully used in high-speed, superresolution or single molecule fluorescence microscopy (particularly TIRF, see further down) to track the movement and resting time of raft lipids in the plasma membrane of living cells at the millisecond range. In the new generation of clickable sphingolipid analogs developed by Per Haberkant and colleagues, the fluorophore is introduced via click reaction after incorporation of these analogs into membranes of living or fixed cells. We and our collaborators found that clickable ceramide analogs colocalize with CECs detected by immunocytochemistry using anti ceramide antibody (Kong et al., 2015a; Kong et al., 2018; Walter et al., 2016). Bifunctional and trifunctional analogs can even be used for cross-linking to lipid binding proteins and administered as caged compounds, respectively (Hoglinger et al., 2017). Ultimately, any fluorescent lipid analogs to image rafts are only reliable if combined with other methods such as AFM (Correlated AFM), immunocytochemistry, or imaging mass spectrometry (Chiantia et al., 2008; Shaw et al., 2006).
The use of lipid binding proteins and antibodies for lipid raft imaging generally requires fixation of cells and tissue. It is understood that organic solvents such as cold acetone or methanol cannot be used for initial fixation. Unfortunately, there is no ideal method for fixation of endogenous lipids. The most commonly used fixation method is a mixture of 4% pformaldehyde/0.5% glutaraldehyde in PBS at room temperature. We and others showed that this fixation method will preserve CRPs and CECs, and imaging of other lipid raft types was verified by using a second method such as mass imaging spectrometry as shown by Mary Kraft’s group (Burgert et al., 2017; Frisz et al., 2013; He et al., 2012). However, one has to keep in mind that it is still debated if any fixation method is even capable of genuinely preserving the in situ raft organization of cellular membranes. Nevertheless, the use of antibodies and specific binding proteins allows to characterize and distinguish raft subtypes based on lipid composition. For example, lysenin binds to clusters of sphingomyelin and cholesterol and should not recognize CRPs, cholera toxin B recognizes GM1 in all types of rafts, and anti ceramide antibody has been raised in our group against different ceramide species and binds to CRPs and CECs, while others have developed an antibody specifically recognizing CRPs of the ceramide-cholesterol type (Abe and Kobayashi, 2014; Hullin-Matsuda and Kobayashi, 2007; Makino et al., 2015; Skocaj et al., 2013; Yamaji et al., 1998; Yilmaz et al., 2018). Combining our antibody with lysenin labeling, we showed that in polarized MDCK cells, sphingomyelin-cholesterol clusters are gradually converted into an apical CEC (ACEC), a ceramide-enriched compartment at the apical plasma membrane that is important for the formation of primary cilia (He et al., 2012). Segregation of sphingolipids into subdomains of the apical plasma membrane of MDCK cells was also observed for the gangliosides GM1 and GM3 (Fujita et al., 2007; Janich and Corbeil, 2007). However, in this and many other studies, the resolution of confocal immunofluorescence microscopy was not sufficiently high to resolve structures of lipid rafts.
Most recently, immunocytochemistry of lipid rafts has been combined with super resolution fluorescence microscopy, mainly using stimulation emission depletion (STED), photoactivated localization microscopy (PALM), and stochastic optical reconstruction microscopy (STORM). STED coupled with fluorescence correlation spectroscopy (STED-FCS) yields images of high spatiotemporal resolution thereby catching transient resting or trapping of single molecules in raft and non-raft domains of the membrane. A recent study from Stefan Hell, a pioneer of STED microscopy and nobel laureate, found that in contrast to glycerophospholipids, sphingolipids (sphingomyelin and GM1) and GPI-anchored proteins (GPIACP) are transiently confined to very small areas of less than 20 nm for a very short period of time (10–20 ms), which is dependent on cholesterol (Eggeling, 2015; Eggeling et al., 2009; Sezgin et al., 2012). While this observation is consistent with the prediction of lipid raft composition and dependence on cholesterol, it raises serious questions about functional significance of rafts, at least when considering the interpretation from 2006, that lipid rafts are “…stabilized to form larger platforms through protein-protein and protein-lipid interactions.” While the half-life of the observed “nanodomains” is well within the association rate of receptor complex formation, dissociation rates in the second-to-minutes range for protein complexes preclude stable association with lipid rafts once protein interaction has occurred (Fallahi-Sichani and Linderman, 2009). Therefore, data from STED microscopy are rather compatible with our lipid chaperon model predicting that lipid rafts are transient catalysts, but not stabilizers for protein interaction within rafts. PALM and the related STORM allows for reconstruction of fluorophore distribution throughout the entire cell (Castro et al., 2013; Curthoys et al., 2015). PALM combined with total internal reflection fluorescence (TIRF) microscopy of quantum dot-labeled receptor proteins (type 1 interferon receptor) found that the plasma membrane is microcompartmentalized and rapidly dissociated receptor complexes re-associate within a secondary confinement domain of about 300 nm diameter depending on the cortical actin meshwork or “membrane skeleton” (You et al., 2016). This study did not test the effect of lipids, but its results are compatible with the “picket fence” model and suggests that lipid rafts are hierarchically organized ranging from very small domains (few nanometers) that are clustered (>100 nm) and segregated from non-raft domains by cortical actin. In another study, PALM images of raft-associated receptors (GPCR β2-adrenergic receptor) revealed that cluster formation was susceptible to actin filament dissociation, but not cholesterol depletion, suggesting that our previous view on the role of cholesterol in lipid raft formation needs revision (Annibale et al., 2011; Scarselli et al., 2013; Scarselli et al., 2012). Another study combining dSTORM with TIRF showed segregation of PIPs into 60–100 nm nanodomains containing either PIP2 or PIP3, but not both PIPs at the same time (Wang and Richards, 2012). Of note, membrane availability of PIP2 depends on cholesterol and PIP2 regulates cortical actin, which may mediate interdependence of lipid rafts and the cytoskeleton as suggested by the “cluster feed-back” model of rafts (Curthoys et al., 2015). Using dSTORM and anti ceramide antibody, our collaborative study showed that CRPs in the plasma membrane were about 70 nm in diameter and contained approximately 30 ceramide molecules (Burgert et al., 2017). In another study, we used N-STORM to visualize the distribution of ceramide and the bifunctional ceramide analog N-(9-(3-pent-4-ynyl-3-H-diazirin-3-yl)-nonanoyl)-D-erythro-sphingosine (pacFACer) in astrocytes (Kong et al., 2018). pacFACer can be cross-linked to ceramide binding proteins and derivatized with photoactivatable fluorophores using click chemistry (Kong et al., 2015a). We imaged CECs in mitochondria-associated membranes (MAMs) that were cross-linked to tubulin, suggesting that hierarchical or modular organization and interaction of lipid domains with the cytoskeleton is a general principle for regulation and compartmentalization of cellular membranes (Kong et al., 2018).
In the few years that superresolution microscopy was applied to imaging of lipid rafts, new compelling data demand for a fresh view on the structure and function of lipid rafts. In line with this conclusion are recent approaches to specifically map the lipid content in rafts by secondary imaging mass spectrometry (SIMS). SIMS uses an ion beam to eject and collect isotope-labeled components of surfaces such as lipids in the plasma membrane, the composition of which is analyzed by mass spectrometry. Using this technique at a lateral resolution of 50 nm and a depth of <5 nm and combined with TIRF immunofluorescence microscopy, Mary Kraft’s laboratory found 200 nm lipid microdomains in the plasma membrane that were enriched with 15N-labeled sphingolipids (Frisz et al., 2013). Surprisingly, even 30% reduction of cholesterol by treatment with MβCD did not eliminate the sphingolipid-enriched rafts, while latrunculin-induced disruption of the F-actin cytoskeleton did. This observation demonstrates that cholesterol depletion does not abolish lipid rafts, which demands for a fundamental revision of our understanding of lipid rafts. It also shows that the interaction of the cytoskeleton with rafts is critical for the formation and function of lipid rafts.
4. Function of CRPs and Conclusions
When discussing the evolving concepts on the structural organization of lipid rafts, one may have noticed that we did not further elaborate on their function in different biological settings. The reason for this limitation is several-fold: 1) there are many excellent reviews on the functional significance of the archetypical cholesterol-sphingomyelin lipid raft, and the reader is kindly referred to the following selection that is by no means complete, but certainly representative of various aspects of lipid raft function in different cell types and organisms (Lu and Fairn, 2018; Pacheco et al., 2016; Sezgin et al., 2017; Simons and Gerl, 2010; Sonnino et al., 2015; Varshney et al., 2016); 2) with the changing view on lipid rafts shifting from the central role of cholesterol toward sphingolipids, many of the experiments studying lipid raft function based on cholesterol depletion by MβCD will have to be revisited and probably re-evaluated using new approaches and re-interpreted using new concepts. Hence, it may be premature to reiterate conclusions drawn from these studies; and 3) the concept of CRPs as functionally defined rafts is too preliminary to clearly determine what their function is. It is unlikely that there is simply a structural or functional continuum between tiny raft-like CRPs and large ceramide membrane domains or even CECs. CECs may contain a large number of CRPs, but they are by no means just big CRPs. To provide the reader with some information on current directions in research on ceramide rafts we have to keep in mind that ceramide is ideally suited to bridge structure and function for several reasons.
Firstly, ceramide levels in cellular membranes are low when compared to “classical” raft lipids such as sphingomyelin and cholesterol. The typical plasma membrane contains <1 Mol% ceramide and 10 Mol% sphingomyelin (Das et al., 2014). To be of regulatory function, the regulatory factor should be low in concentration and enzymatically adjustable. Which brings us to the second benefit: the ceramide concentration in cellular membranes is tightly controlled by mainly three enzyme families: CerSs, SMSases, and SMases. Particularly, acid and neutral SMases increase the ceramide concentration in the outer and inner leaflet of the plasma membrane, respectively. Which brings us to third benefit: SMases are activated by environmental cues such as cytokines and pathogens, which makes ceramide an ideal membrane signaling lipid interfacing extracellular signals and intracellular cell signaling pathways. Along comes the fourth benefit that ceramide binds to upstream protein phosphatases and kinases, which complements activation of signaling effectors by their recruitment and clustering in spatially defined ceramide domains. Finally, and already discussed in previous sections: ceramide forms domains on its own that are more gel-like than the classical Lo-type domains. While this is still a matter of intense research, ceramide domains may undergo various mesostructural transitions of the hexagonal or porous type, which was suggested to be important for receptor clustering/activation and punching holes in membranes, respectively (Cremesti et al., 2002).
Receptor clustering was the first function of extended ceramide domains discovered in the early 2000’s, mainly based on the use of anti-ceramide antibody in flow cytometry and immunofluorescence microscopy and co-localization analyses. The groups led by Erich Gulbins and Richard Kolesnick found that ceramide domains dubbed “ceramide-rich membrane rafts” fuse and induce clustering of CD95 (capping), a process induced by the CD95 ligand TNF-α and activation of acid SMase, eventually leading to apoptosis (Cremesti et al., 2001; Grassme et al., 2003a; Grassme et al., 2001; Gulbins and Grassme, 2002). Subsequently, these groups also showed that clustering and activation of other receptors such as CD40 or pathogen infection is regulated by ceramide domains (Grassme and Becker, 2013; Grassme et al., 2002; Grassme et al., 2003b; Grassme et al., 2005; Grassme et al., 2013; Gulbins et al., 2004). However, it is not clear whether receptors or pathogens actually interact with ceramide. Atypical PKCς/λ (aPKC) is one of the first proteins found to bind to ceramide and at the same time, being recruited to lipid rafts. Mark Kester’s group showed that ceramide binding and raft recruitment leads to aPKC-dependent inactivation of protein kinase B/Akt, an activator for glucose transport and cell survival (Fox et al., 2007). Likewise, a study by Glyn Dawson’s laboratory found that ceramide recruits PTEN to rafts, which also leads to inactivation of the PI3K/Akt in the insulin cell signaling pathway (Goswami et al., 2005b). Our group showed that interaction of aPKC with ceramide-enriched membrane domains organizes cell polarity, probably by a sphingolipid-induced protein scaffold (SLIPS) involving polarity protein Par6 and the small Rho-type GTPase Cdc42 (Bieberich, 2008, 2011a, 2012; Krishnamurthy et al., 2007; Wang et al., 2008; Wang et al., 2018). Our studies also provided evidence that enrichment of ceramide in the apical plasma membrane and interaction with ceramide is instrumental for primitive ectoderm differentiation and neuronal cell polarity and cortical layer formation (lamination) of the embryonic brain. However, it still needs to be investigated whether ceramide-aPKC interaction described by Kester’s and our group involved CRPs of the sphingomyelin-ceramide type.
The function of CRPs of the cholesterol-ceramide type is also not clearly defined yet. The group led by Lia Addadi who reported development of an anti-ceramide antibody detecting this type of CRPs found that cholesterol-ceramide rafts are localized in late endosomes and trans-Golgi, but not in early endosomes, ER, or lysosomes (Goldschmidt-Arzi et al., 2011; Scheffer et al., 2007). In collaboration with Tony Futerman’s group, they discovered that in Farber disease, a deficiency of acid ceramidase, cholesterol-ceramide CRPs are also found in mitochondria and the plasma membrane, suggesting that they contribute to the pathophysiology of the disease (Ferreira et al., 2014). Another potentially pathophysiological role of ceramidecholesterol CRPs is suggested by data from Felix Goni’s group obtained with lipid vesicles. These studies found that mixtures of cholesterol and ceramide may increase permeability of cellular membranes including those of mitochondria (Goni et al., 2005). The mechanism is related but not identical to ceramide pores described by Marco Colombini’s group (Perera et al., 2016; Siskind et al., 2002). They form at a certain concentration in isolated mitochondria and may induce apoptosis, an alternative (or complementary) mechanism to death receptor clustering previously described for ceramide rafts. It is obvious that CRPs of the sphingomyelin-ceramide or cholesterol-ceramide type can be generated by activation of SMases, the activity of which is modulated by the extent and composition of lipid rafts, suggesting a self-propelled feedback activation mechanism. However, the biological function or dysfunction of this activation and emergence of CRPs is still not fully understood.
Highlights.
This review discusses the history and new developments in research on lipid rafts. Specifically, the lipid raft and lipid shell model are explained and the lipid chaperon model introduced. Additional topics are methods to detect and characterize lipid rafts in vitro and in vivo with focus on the physiological and pathophysiological function of ceramide-rich platforms.
Acknowledgments:
This work was the supported by the grants R01AG034389, R01NS095215, and NSF 1615874. The authors thank the Department of Physiology (Chair Dr. Alan Daugherty). College of Medicine, University of Kentucky, Lexington, KY for institutional support.
Abbreviations:
- ACEC
apical CEC
- aSMase
acid sphingomyelinase
- ATP
adenosine triphosphate
- CEC
ceramide-enriched comportment
- CRP
ceramide-rich platform
- ESCRT
endosomal sorting complexes required for transport
- EV
extracellular vesicle
- GPI
glycosylphosphatidylinositol
- GPCR
G-protein coupled receptor
- LC-MS/MS
liquid chromatography tandem mass spectrometry
- MVE
multivesicular endosome
- nSMase2
neutral sphingomyelinase 2
- RAP
raft-associated protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe M, Kobayashi T, 2014. Imaging local sphingomyelin-rich domains in the plasma membrane using specific probes and advanced microscopy. Biochimica et biophysica acta 1841, 720–726. [DOI] [PubMed] [Google Scholar]
- Ackerman DG, Feigenson GW, 2015. Lipid bilayers: clusters, domains and phases. Essays in biochemistry 57, 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adada M, Canals D, Hannun YA, Obeid LM, 2014. Sphingolipid regulation of ezrin, radixin, and moesin proteins family: implications for cell dynamics. Biochimica et biophysica acta 1841, 727–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adada M, Luberto C, Canals D, 2016. Inhibitors of the sphingomyelin cycle: Sphingomyelin synthases and sphingomyelinases. Chemistry and physics of lipids 197, 45–59. [DOI] [PubMed] [Google Scholar]
- Adibhatla RM, Hatcher JF, Gusain A, 2012. Tricyclodecan-9-yl-xanthogenate (D609) mechanism of actions: a mini-review of literature. Neurochemical research 37, 671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RG, Jacobson K, 2002. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science 296, 1821–1825. [DOI] [PubMed] [Google Scholar]
- Annibale P, Vanni S, Scarselli M, Rothlisberger U, Radenovic A, 2011. Quantitative photo activated localization microscopy: unraveling the effects of photoblinking. PloS one 6, e22678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakel EC, Schwappach B, 2018. Formation of COPI-coated vesicles at a glance. Journal of cell science 131. [DOI] [PubMed] [Google Scholar]
- Area-Gomez E, de Groof A, Bonilla E, Montesinos J, Tanji K, Boldogh I, Pon L, Schon EA, 2018. A key role for MAM in mediating mitochondrial dysfunction in Alzheimer disease. Cell death & disease 9, 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Area-Gomez E, Schon EA, 2017. On the Pathogenesis of Alzheimer’s Disease: The MAM Hypothesis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 31, 864–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson MA, Warren G, 2004. Rapid, endoplasmic reticulum-independent diffusion of the mitotic Golgi haze. Molecular biology of the cell 15, 1843–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barucha-Kraszewska J, Kraszewski S, Ramseyer C, 2013. Will C-Laurdan dethrone Laurdan in fluorescent solvent relaxation techniques for lipid membrane studies? Langmuir : the ACS journal of surfaces and colloids 29, 1174–1182. [DOI] [PubMed] [Google Scholar]
- Berkes D, Daich A, Santos C, Ballereau S, Genisson Y, 2016. Chemistry and Biology of HPAs: A Family of Ceramide Trafficking Inhibitors. Chemistry 22, 17514–17525. [DOI] [PubMed] [Google Scholar]
- Bhat RA, Panstruga R, 2005. Lipid rafts in plants. Planta 223, 5–19. [DOI] [PubMed] [Google Scholar]
- Bieberich E, 2004. Integration of glycosphingolipid metabolism and cell-fate decisions in cancer and stem cells: review and hypothesis. Glycoconjugate journal 21, 315–327. [DOI] [PubMed] [Google Scholar]
- Bieberich E, 2008. Ceramide signaling in cancer and stem cells. Future Lipidol 3, 273–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieberich E, 2011a. Ceramide in stem cell differentiation and embryo development: novel functions of a topological cell-signaling lipid and the concept of ceramide compartments. J Lipids 2011, 610306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieberich E, 2011b. Lipid vesicle-mediated affinity chromatography using magnetic activated cell sorting (LIMACS): a novel method to analyze protein-lipid interaction. J Vis Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieberich E, 2012. It’s a lipid’s world: bioactive lipid metabolism and signaling in neural stem cell differentiation. Neurochemical research 37, 1208–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieberich E, Freischutz B, Suzuki M, Yu RK, 1999. Differential effects of glycolipid biosynthesis inhibitors on ceramide-induced cell death in neuroblastoma cells. Journal of neurochemistry 72, 1040–1049. [DOI] [PubMed] [Google Scholar]
- Bieberich E, Kawaguchi T, Yu RK, 2000. N-acylated serinol is a novel ceramide mimic inducing apoptosis in neuroblastoma cells. The Journal of biological chemistry 275, 177–181. [DOI] [PubMed] [Google Scholar]
- Bollinger CR, Teichgraber V, Gulbins E, 2005. Ceramide-enriched membrane domains. Biochimica et biophysica acta 1746, 284–294. [DOI] [PubMed] [Google Scholar]
- Boukh-Viner T, Guo T, Alexandrian A, Cerracchio A, Gregg C, Haile S, Kyskan R, Milijevic S, Oren D, Solomon J, Wong V, Nicaud JM, Rachubinski RA, English AM, Titorenko VI, 2005. Dynamic ergosterol- and ceramide-rich domains in the peroxisomal membrane serve as an organizing platform for peroxisome fusion. The Journal of cell biology 168, 761–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulgaropoulos B, Arsov Z, Laggner P, Pabst G, 2011. Stable and unstable lipid domains in ceramide-containing membranes. Biophysical journal 100, 2160–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulgaropoulos B, Rappolt M, Sartori B, Amenitsch H, Pabst G, 2012. Lipid sorting by ceramide and the consequences for membrane proteins. Biophysical journal 102, 2031–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boura E, Ivanov V, Carlson LA, Mizuuchi K, Hurley JH, 2012. Endosomal sorting complex required for transport (ESCRT) complexes induce phase-separated microdomains in supported lipid bilayers. The Journal of biological chemistry 287, 28144–28151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbon NA, Yun J, Kester M, 2000. Ceramide directly activates protein kinase C zeta to regulate a stress-activated protein kinase signaling complex. The Journal of biological chemistry 275, 35617–35623. [DOI] [PubMed] [Google Scholar]
- Brown DA, Rose JK, 1992. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68, 533–544. [DOI] [PubMed] [Google Scholar]
- Brown RE, Mattjus P, 2007. Glycolipid transfer proteins. Biochimica et biophysica acta 1771, 746–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugger B, Graham C, Leibrecht I, Mombelli E, Jen A, Wieland F, Morris R, 2004. The membrane domains occupied by glycosylphosphatidylinositol-anchored prion protein and Thy-1 differ in lipid composition. The Journal of biological chemistry 279, 7530–7536. [DOI] [PubMed] [Google Scholar]
- Brugger B, Sandhoff R, Wegehingel S, Gorgas K, Malsam J, Helms JB, Lehmann WD, Nickel W, Wieland FT, 2000. Evidence for segregation of sphingomyelin and cholesterol during formation of COPI-coated vesicles. The Journal of cell biology 151, 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgert A, Schlegel J, Becam J, Doose S, Bieberich E, Schubert-Unkmeir A, Sauer M, 2017. Characterization of Plasma Membrane Ceramides by Super-Resolution Microscopy. Angewandte Chemie. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burte F, Carelli V, Chinnery PF, Yu-Wai-Man P, 2015. Disturbed mitochondrial dynamics and neurodegenerative disorders. Nature reviews. Neurology 11, 11–24. [DOI] [PubMed] [Google Scholar]
- Butters TD, Hughes RC, 1974. Solubilization and fractionation of glycoproteins and glycolipids of KB cell membranes. The Biochemical journal 140, 469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacas JL, Furt F, Le Guedard M, Schmitter JM, Bure C, Gerbeau-Pissot P, Moreau P, Bessoule JJ, Simon-Plas F, Mongrand S, 2012. Lipids of plant membrane rafts. Progress in lipid research 51, 272–299. [DOI] [PubMed] [Google Scholar]
- Canals D, Roddy P, Hannun YA, 2012. Protein phosphatase 1alpha mediates ceramide-induced ERM protein dephosphorylation: a novel mechanism independent of phosphatidylinositol 4, 5-biphosphate (PIP2) and myosin/ERM phosphatase. The Journal of biological chemistry 287, 10145–10155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso S, Seica RM, Moreira PI, 2017. Mitochondria as a target for neuroprotection: implications for Alzheimer s disease. Expert review of neurotherapeutics 17, 77–91. [DOI] [PubMed] [Google Scholar]
- Castro BM, de Almeida RF, Silva LC, Fedorov A, Prieto M, 2007. Formation of ceramide/sphingomyelin gel domains in the presence of an unsaturated phospholipid: a quantitative multiprobe approach. Biophysical journal 93, 1639–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro BM, Silva LC, Fedorov A, de Almeida RF, Prieto M, 2009. Cholesterol-rich fluid membranes solubilize ceramide domains: implications for the structure and dynamics of mammalian intracellular and plasma membranes. The Journal of biological chemistry 284, 22978–22987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro BM, Torreno-Pina JA, van Zanten TS, Gracia-Parajo MF, 2013. Biochemical and imaging methods to study receptor membrane organization and association with lipid rafts. Methods in cell biology 117, 105–122. [DOI] [PubMed] [Google Scholar]
- Chalfant CE, Kishikawa K, Bielawska A, Hannun YA, 2000. Analysis of ceramide-activated protein phosphatases. Methods in enzymology 312, 420–428. [DOI] [PubMed] [Google Scholar]
- Chalfant CE, Kishikawa K, Mumby MC, Kamibayashi C, Bielawska A, Hannun YA, 1999. Long chain ceramides activate protein phosphatase-1 and protein phosphatase-2A. Activation is stereospecific and regulated by phosphatidic acid. The Journal of biological chemistry 274, 20313–20317. [DOI] [PubMed] [Google Scholar]
- Chalfant CE, Szulc Z, Roddy P, Bielawska A, Hannun YA, 2004. The structural requirements for ceramide activation of serine-threonine protein phosphatases. Journal of lipid research 45, 496–506. [DOI] [PubMed] [Google Scholar]
- Chamberlain LH, 2004. Detergents as tools for the purification and classification of lipid rafts. FEBS letters 559, 1–5. [DOI] [PubMed] [Google Scholar]
- Chao L, Gast AP, Hatton TA, Jensen KF, 2010. Sphingomyelinase-induced phase transformations: causing morphology switches and multiple-time-domain ceramide generation in model raft membranes. Langmuir : the ACS journal of surfaces and colloids 26, 344–356. [DOI] [PubMed] [Google Scholar]
- Chen X, Jen A, Warley A, Lawrence MJ, Quinn PJ, Morris RJ, 2009. Isolation at physiological temperature of detergent-resistant membranes with properties expected of lipid rafts: the influence of buffer composition. The Biochemical journal 417, 525–533. [DOI] [PubMed] [Google Scholar]
- Chen Y, Cao Y, 2017. The sphingomyelin synthase family: proteins, diseases, and inhibitors. Biological chemistry 398, 1319–1325. [DOI] [PubMed] [Google Scholar]
- Chiantia S, Kahya N, Ries J, Schwille P, 2006. Effects of ceramide on liquid-ordered domains investigated by simultaneous AFM and FCS. Biophysical journal 90, 4500–4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiantia S, Ries J, Chwastek G, Carrer D, Li Z, Bittman R, Schwille P, 2008. Role of ceramide in membrane protein organization investigated by combined AFM and FCS. Biochimica et biophysica acta 1778, 1356–1364. [DOI] [PubMed] [Google Scholar]
- Chu Y, Goldman JG, Kelly L, He Y, Waliczek T, Kordower JH, 2014. Abnormal alphasynuclein reduces nigral voltage-dependent anion channel 1 in sporadic and experimental Parkinson’s disease. Neurobiology of disease 69, 1–14. [DOI] [PubMed] [Google Scholar]
- Clayton EL, Minogue S, Waugh MG, 2013. Mammalian phosphatidylinositol 4-kinases as modulators of membrane trafficking and lipid signaling networks. Progress in lipid research 52, 294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremesti A, Paris F, Grassme H, Holler N, Tschopp J, Fuks Z, Gulbins E, Kolesnick R, 2001. Ceramide enables fas to cap and kill. The Journal of biological chemistry 276, 23954–23961. [DOI] [PubMed] [Google Scholar]
- Cremesti AE, Goni FM, Kolesnick R, 2002. Role of sphingomyelinase and ceramide in modulating rafts: do biophysical properties determine biologic outcome? FEBS letters 531, 47–53. [DOI] [PubMed] [Google Scholar]
- Cubi R, Matas LA, Pou M, Aguilera J, Gil C, 2013. Differential sensitivity to detergents of actin cytoskeleton from nerve endings. Biochimica et biophysica acta 1828, 2385–2393. [DOI] [PubMed] [Google Scholar]
- Curthoys NM, Parent M, Mlodzianoski M, Nelson AJ, Lilieholm J, Butler MB, Valles M, Hess ST, 2015. Dances with Membranes: Breakthroughs from Super-resolution Imaging. Current topics in membranes 75, 59–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuschieri J, Bulger E, Billgrin J, Garcia I, Maier RV, 2007. Acid sphingomyelinase is required for lipid Raft TLR4 complex formation. Surgical infections 8, 91–106. [DOI] [PubMed] [Google Scholar]
- D’Angelo G, Polishchuk E, Di Tullio G, Santoro M, Di Campli A, Godi A, West G, Bielawski J, Chuang CC, van der Spoel AC, Platt FM, Hannun YA, Polishchuk R, Mattjus P, De Matteis MA, 2007. Glycosphingolipid synthesis requires FAPP2 transfer of glucosylceramide. Nature 449, 62–67. [DOI] [PubMed] [Google Scholar]
- Dany M, Ogretmen B, 2015. Ceramide induced mitophagy and tumor suppression. Biochimica et biophysica acta 1853, 2834–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Brown MS, Anderson DD, Goldstein JL, Radhakrishnan A, 2014. Three pools of plasma membrane cholesterol and their relation to cholesterol homeostasis. eLife 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Duve C, Baudhuin P, 1966. Peroxisomes (microbodies and related particles). Physiological reviews 46, 323–357. [DOI] [PubMed] [Google Scholar]
- Decker L, ffrench-Constant C, 2004. Lipid rafts and integrin activation regulate oligodendrocyte survival. The Journal of neuroscience : the official journal of the Society for Neuroscience 24, 3816–3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermine JF, Duclos S, Garin J, St-Louis F, Rea S, Parton RG, Desjardins M, 2001. Flotillin-1-enriched lipid raft domains accumulate on maturing phagosomes. The Journal of biological chemistry 276, 18507–18512. [DOI] [PubMed] [Google Scholar]
- Diaz-Rohrer B, Levental KR, Levental I, 2014a. Rafting through traffic: Membrane domains in cellular logistics. Biochimica et biophysica acta 1838, 3003–3013. [DOI] [PubMed] [Google Scholar]
- Diaz-Rohrer BB, Levental KR, Simons K, Levental I, 2014b. Membrane raft association is a determinant of plasma membrane localization. Proceedings of the National Academy of Sciences of the United States of America 111, 8500–8505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich C, Bagatolli LA, Volovyk ZN, Thompson NL, Levi M, Jacobson K, Gratton E, 2001. Lipid rafts reconstituted in model membranes. Biophysical journal 80, 1417–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinic J, Ashrafzadeh P, Parmryd I, 2013. Actin filaments attachment at the plasma membrane in live cells cause the formation of ordered lipid domains. Biochimica et biophysica acta 1828, 1102–1111. [DOI] [PubMed] [Google Scholar]
- Dinkins MB, Dasgupta S, Wang G, Zhu G, Bieberich E, 2014. Exosome reduction in vivo is associated with lower amyloid plaque load in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol Aging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkins MB, Enasko J, Hernandez C, Wang G, Kong J, Helwa I, Liu Y, Terry AV Jr., Bieberich E, 2016a. Neutral Sphingomyelinase-2 Deficiency Ameliorates Alzheimer’s Disease Pathology and Improves Cognition in the 5XFAD Mouse. The Journal of neuroscience : the official journal of the Society for Neuroscience 36, 8653–8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkins MB, Wang G, Bieberich E, 2016b. Sphingolipid-Enriched Extracellular Vesicles and Alzheimer’s Disease: A Decade of Research. Journal of Alzheimer’s disease : JAD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrowsky RT, Hannun YA, 1992. Ceramide stimulates a cytosolic protein phosphatase. The Journal of biological chemistry 267, 5048–5051. [PubMed] [Google Scholar]
- Dobrowsky RT, Kamibayashi C, Mumby MC, Hannun YA, 1993. Ceramide activates heterotrimeric protein phosphatase 2A. The Journal of biological chemistry 268, 15523–15530. [PubMed] [Google Scholar]
- Dodonova SO, Diestelkoetter-Bachert P, von Appen A, Hagen WJ, Beck R, Beck M, Wieland F, Briggs JA, 2015. VESICULAR TRANSPORT. A structure of the COPI coat and the role of coat proteins in membrane vesicle assembly. Science 349, 195–198. [DOI] [PubMed] [Google Scholar]
- Dremina ES, Sharov VS, Schoneich C, 2005. Protein tyrosine nitration in rat brain is associated with raft proteins, flotillin-1 and alpha-tubulin: effect of biological aging. Journal of neurochemistry 93, 1262–1271. [DOI] [PubMed] [Google Scholar]
- Duris A, Wiesenganger T, Moravcikova D, Baran P, Kozisek J, Daich A, Berkes D, 2011. Expedient and practical synthesis of CERT-dependent ceramide trafficking inhibitor HPA 12 and its analogues. Organic letters 13, 1642–1645. [DOI] [PubMed] [Google Scholar]
- Eggeling C, 2015. Super-resolution optical microscopy of lipid plasma membrane dynamics. Essays in biochemistry 57, 69–80. [DOI] [PubMed] [Google Scholar]
- Eggeling C, Ringemann C, Medda R, Schwarzmann G, Sandhoff K, Polyakova S, Belov VN, Hein B, von Middendorff C, Schonle A, Hell SW, 2009. Direct observation of the nanoscale dynamics of membrane lipids in a living cell. Nature 457, 1159–1162. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A, 2003. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature 421, 753–756. [DOI] [PubMed] [Google Scholar]
- Fallahi-Sichani M, Linderman JJ, 2009. Lipid raft-mediated regulation of G-protein coupled receptor signaling by ligands which influence receptor dimerization: a computational study. PloS one 4, e6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanzo JC, Lynch MP, Phee H, Hyer M, Cremesti A, Grassme H, Norris JS, Coggeshall KM, Rueda BR, Pernis AB, Kolesnick R, Gulbins E, 2003. CD95 rapidly clusters in cells of diverse origins. Cancer biology & therapy 2, 392–395. [DOI] [PubMed] [Google Scholar]
- Ferreira NS, Goldschmidt-Arzi M, Sabanay H, Storch J, Levade T, Ribeiro MG, Addadi L, Futerman AH, 2014. Accumulation of ordered ceramide-cholesterol domains in farber disease fibroblasts. JIMD reports 12, 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler K, Kobayashi T, Kurzchalia TV, Simons K, 1993. Glycosphingolipid-enriched, detergent-insoluble complexes in protein sorting in epithelial cells. Biochemistry 32, 6365–6373. [DOI] [PubMed] [Google Scholar]
- Fiedler K, Parton RG, Kellner R, Etzold T, Simons K, 1994. VIP36, a novel component of glycolipid rafts and exocytic carrier vesicles in epithelial cells. The EMBO journal 13, 1729–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD, Black JA, Waxman SG, 1987. Filipin-cholesterol binding in CNS axons prior to myelination: evidence for microheterogeneity in premyelinated axolemma. Brain research 404, 21–32. [DOI] [PubMed] [Google Scholar]
- Folts CJ, Scott-Hewitt N, Proschel C, Mayer-Proschel M, Noble M, 2016. Lysosomal Reacidification Prevents Lysosphingolipid-Induced Lysosomal Impairment and Cellular Toxicity. PLoS biology 14, e1002583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox TE, Houck KL, O’Neill SM, Nagarajan M, Stover TC, Pomianowski PT, Unal O, Yun JK, Naides SJ, Kester M, 2007. Ceramide recruits and activates protein kinase C zeta (PKC zeta) within structured membrane microdomains. The Journal of biological chemistry 282, 12450–12457. [DOI] [PubMed] [Google Scholar]
- Freire E, Snyder B, 1980. Monte Carlo studies of the lateral organization of molecules in two-component lipid bilayers. Biochimica et biophysica acta 600, 643–654. [DOI] [PubMed] [Google Scholar]
- Frick M, Bright NA, Riento K, Bray A, Merrified C, Nichols BJ, 2007. Coassembly of flotillins induces formation of membrane microdomains, membrane curvature, and vesicle budding. Current biology : CB 17, 1151–1156. [DOI] [PubMed] [Google Scholar]
- Frisz JF, Lou K, Klitzing HA, Hanafin WP, Lizunov V, Wilson RL, Carpenter KJ, Kim R, Hutcheon ID, Zimmerberg J, Weber PK, Kraft ML, 2013. Direct chemical evidence for sphingolipid domains in the plasma membranes of fibroblasts. Proceedings of the National Academy of Sciences of the United States of America 110, E613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M, Hayashi T, 2011. New insights into the role of mitochondria-associated endoplasmic reticulum membrane. International review of cell and molecular biology 292, 73–117. [DOI] [PubMed] [Google Scholar]
- Fujita A, Cheng J, Hirakawa M, Furukawa K, Kusunoki S, Fujimoto T, 2007. Gangliosides GM1 and GM3 in the living cell membrane form clusters susceptible to cholesterol depletion and chilling. Molecular biology of the cell 18, 2112–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita A, Cheng J, Tauchi-Sato K, Takenawa T, Fujimoto T, 2009. A distinct pool of phosphatidylinositol 4,5-bisphosphate in caveolae revealed by a nanoscale labeling technique. Proceedings of the National Academy of Sciences of the United States of America 106, 9256–9261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabella G, 1971. Caveolae intracellulares and sarcoplasmic reticulum in smooth muscle. Journal of cell science 8, 601–609. [DOI] [PubMed] [Google Scholar]
- Gagescu R, Demaurex N, Parton RG, Hunziker W, Huber LA, Gruenberg J, 2000. The recycling endosome of Madin-Darby canine kidney cells is a mildly acidic compartment rich in raft components. Molecular biology of the cell 11, 2775–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Arribas AB, Alonso A, Goni FM, 2016. Cholesterol interactions with ceramide and sphingomyelin. Chemistry and physics of lipids 199, 26–34. [DOI] [PubMed] [Google Scholar]
- Garcia-Marcos M, Pochet S, Tandel S, Fontanils U, Astigarraga E, Fernandez-Gonzalez JA, Kumps A, Marino A, Dehaye JP, 2006. Characterization and comparison of raft-like membranes isolated by two different methods from rat submandibular gland cells. Biochimica et biophysica acta 1758, 796–806. [DOI] [PubMed] [Google Scholar]
- Garofalo T, Manganelli V, Grasso M, Mattei V, Ferri A, Misasi R, Sorice M, 2015. Role of mitochondrial raft-like microdomains in the regulation of cell apoptosis. Apoptosis : an international journal on programmed cell death 20, 621–634. [DOI] [PubMed] [Google Scholar]
- Garofalo T, Matarrese P, Manganelli V, Marconi M, Tinari A, Gambardella L, Faggioni A, Misasi R, Sorice M, Malorni W, 2016. Evidence for the involvement of lipid rafts localized at the ER-mitochondria associated membranes in autophagosome formation. Autophagy 12, 917–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo T, Tinari A, Matarrese P, Giammarioli AM, Manganelli V, Ciarlo L, Misasi R, Sorice M, Malorni W, 2007. Do mitochondria act as “cargo boats” in the journey of GD3 to the nucleus during apoptosis? FEBS letters 581, 3899–3903. [DOI] [PubMed] [Google Scholar]
- Gault CR, Obeid LM, Hannun YA, 2010. An overview of sphingolipid metabolism: from synthesis to breakdown. Advances in experimental medicine and biology 688, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelineau-van Waes J, Starr L, Maddox J, Aleman F, Voss KA, Wilberding J, Riley RT, 2005. Maternal fumonisin exposure and risk for neural tube defects: mechanisms in an in vivo mouse model. Birth Defects Res A Clin Mol Teratol 73, 487–497. [DOI] [PubMed] [Google Scholar]
- Godi A, Di Campli A, Konstantakopoulos A, Di Tullio G, Alessi DR, Kular GS, Daniele T, Marra P, Lucocq JM, De Matteis MA, 2004. FAPPs control Golgi-to-cell-surface membrane traffic by binding to ARF and PtdIns(4)P. Nature cell biology 6, 393–404. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Arzi M, Shimoni E, Sabanay H, Futerman AH, Addadi L, 2011. Intracellular localization of organized lipid domains of C16-ceramide/cholesterol. J Struct Biol 175, 21–30. [DOI] [PubMed] [Google Scholar]
- Golpich M, Amini E, Mohamed Z, Azman Ali R, Mohamed Ibrahim N, Ahmadiani A, 2017. Mitochondrial Dysfunction and Biogenesis in Neurodegenerative diseases: Pathogenesis and Treatment. CNS neuroscience & therapeutics 23, 5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goni FM, Contreras FX, Montes LR, Sot J, Alonso A, 2005. Biophysics (and sociology) of ceramides. Biochem Soc Symp, 177–188. [DOI] [PubMed] [Google Scholar]
- Goswami R, Ahmed M, Kilkus J, Han T, Dawson SA, Dawson G, 2005a. Differential regulation of ceramide in lipid-rich microdomains (rafts): antagonistic role of palmitoyl:protein thioesterase and neutral sphingomyelinase 2. Journal of neuroscience research 81, 208–217. [DOI] [PubMed] [Google Scholar]
- Goswami R, Singh D, Phillips G, Kilkus J, Dawson G, 2005b. Ceramide regulation of the tumor suppressor phosphatase PTEN in rafts isolated from neurotumor cell lines. Journal of neuroscience research 81, 541–550. [DOI] [PubMed] [Google Scholar]
- Grassme H, Becker KA, 2013. Bacterial infections and ceramide. Handb Exp Pharmacol, 305–320. [DOI] [PubMed] [Google Scholar]
- Grassme H, Cremesti A, Kolesnick R, Gulbins E, 2003a. Ceramide-mediated clustering is required for CD95-DISC formation. Oncogene 22, 5457–5470. [DOI] [PubMed] [Google Scholar]
- Grassme H, Jekle A, Riehle A, Schwarz H, Berger J, Sandhoff K, Kolesnick R, Gulbins E, 2001. CD95 signaling via ceramide-rich membrane rafts. The Journal of biological chemistry 276, 20589–20596. [DOI] [PubMed] [Google Scholar]
- Grassme H, Jendrossek V, Bock J, Riehle A, Gulbins E, 2002. Ceramide-rich membrane rafts mediate CD40 clustering. Journal of immunology 168, 298–307. [DOI] [PubMed] [Google Scholar]
- Grassme H, Jendrossek V, Riehle A, von Kurthy G, Berger J, Schwarz H, Weller M, Kolesnick R, Gulbins E, 2003b. Host defense against Pseudomonas aeruginosa requires ceramide-rich membrane rafts. Nature medicine 9, 322–330. [DOI] [PubMed] [Google Scholar]
- Grassme H, Riehle A, Wilker B, Gulbins E, 2005. Rhinoviruses infect human epithelial cells via ceramide-enriched membrane platforms. The Journal of biological chemistry 280, 26256–26262. [DOI] [PubMed] [Google Scholar]
- Grassme H, Riethmuller J, Gulbins E, 2013. Ceramide in cystic fibrosis. Handb Exp Pharmacol, 265–274. [DOI] [PubMed] [Google Scholar]
- Gronnier J, Germain V, Gouguet P, Cacas JL, Mongrand S, 2016. GIPC: Glycosyl Inositol Phospho Ceramides, the major sphingolipids on earth. Plant signaling & behavior 11, e1152438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean K, Der C, Robert F, Thomas D, Mongrand S, Simon-Plas F, Gerbeau-Pissot P, 2018. Interactions between Lipids and Proteins are Critical for Plasma Membrane Ordered Domain Organization in BY-2 Cells. Journal of experimental botany. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Gupta V, Yang Y, Zhu JY, Carlson EJ, Kingsley C, Tash JS, Schonbrunn E, Hawkinson J, Georg GI, 2017. Structure-Activity Studies of N-Butyl-1-deoxynojirimycin (NB-DNJ) Analogues: Discovery of Potent and Selective Aminocyclopentitol Inhibitors of GBA1 and GBA2. ChemMedChem 12, 1977–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerriero CJ, Lai Y, Weisz OA, 2008. Differential sorting and Golgi export requirements for raft-associated and raft-independent apical proteins along the biosynthetic pathway. The Journal of biological chemistry 283, 18040–18047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbins E, Dreschers S, Wilker B, Grassme H, 2004. Ceramide, membrane rafts and infections. J Mol Med 82, 357–363. [DOI] [PubMed] [Google Scholar]
- Gulbins E, Grassme H, 2002. Ceramide and cell death receptor clustering. Biochimica et biophysica acta 1585, 139–145. [DOI] [PubMed] [Google Scholar]
- Gulbins E, Kolesnick R, 2003. Raft ceramide in molecular medicine. Oncogene 22, 7070–7077. [DOI] [PubMed] [Google Scholar]
- Gurd JW, Evans WH, Perkins HR, 1972. Chemical characterization of the proteins and glycoproteins of mouse liver plasma membranes solubilized by sequential extraction with aqueous and organic solvents. The Biochemical journal 126, 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberkant P, Holthuis JC, 2014. Fat & fabulous: bifunctional lipids in the spotlight. Biochimica et biophysica acta 1841, 1022–1030. [DOI] [PubMed] [Google Scholar]
- Haberkant P, Stein F, Hoglinger D, Gerl MJ, Brugger B, Van Veldhoven PP, Krijgsveld J, Gavin AC, Schultz C, 2016. Bifunctional Sphingosine for Cell-Based Analysis of Protein Sphingolipid Interactions. ACS chemical biology 11, 222–230. [DOI] [PubMed] [Google Scholar]
- Hanada K, 2014. Co-evolution of sphingomyelin and the ceramide transport protein CERT. Biochimica et biophysica acta 1841, 704–719. [DOI] [PubMed] [Google Scholar]
- Hanada K, 2017. Ceramide Transport from the Endoplasmic Reticulum to the Trans Golgi Region at Organelle Membrane Contact Sites. Advances in experimental medicine and biology 997, 69–81. [DOI] [PubMed] [Google Scholar]
- Hanada K, Kumagai K, Yasuda S, Miura Y, Kawano M, Fukasawa M, Nishijima M, 2003. Molecular machinery for non-vesicular trafficking of ceramide. Nature 426, 803–809. [DOI] [PubMed] [Google Scholar]
- Hanada K, Nishijima M, Akamatsu Y, Pagano RE, 1995. Both sphingolipids and cholesterol participate in the detergent insolubility of alkaline phosphatase, a glycosylphosphatidylinositol-anchored protein, in mammalian membranes. The Journal of biological chemistry 270, 6254–6260. [DOI] [PubMed] [Google Scholar]
- Hanada K, Nishijima M, Fujita T, Kobayashi S, 2000. Specificity of inhibitors of serine palmitoyltransferase (SPT), a key enzyme in sphingolipid biosynthesis, in intact cells. A novel evaluation system using an SPT-defective mammalian cell mutant. Biochemical pharmacology 59, 1211–1216. [DOI] [PubMed] [Google Scholar]
- Hanada K, Pagano RE, 1995. A Chinese hamster ovary cell mutant defective in the nonendocytic uptake of fluorescent analogs of phosphatidylserine: isolation using a cytosol acidification protocol. The Journal of cell biology 128, 793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun YA, Bell RM, 1989. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science 243, 500–507. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Loomis CR, Merrill AH Jr., Bell RM, 1986. Sphingosine inhibition of protein kinase C activity and of phorbol dibutyrate binding in vitro and in human platelets. The Journal of biological chemistry 261, 12604–12609. [PubMed] [Google Scholar]
- Hannun YA, Obeid LM, 2011. Many ceramides. The Journal of biological chemistry 286, 27855–27862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM, 2018. Sphingolipids and their metabolism in physiology and disease. Nature reviews. Molecular cell biology 19, 175–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen GH, Niels-Christiansen LL, Thorsen E, Immerdal L, Danielsen EM, 2000. Cholesterol depletion of enterocytes. Effect on the Golgi complex and apical membrane trafficking. The Journal of biological chemistry 275, 5136–5142. [DOI] [PubMed] [Google Scholar]
- Hao M, Lin SX, Karylowski OJ, Wustner D, McGraw TE, Maxfield FR, 2002. Vesicular and non-vesicular sterol transport in living cells. The endocytic recycling compartment is a major sterol storage organelle. The Journal of biological chemistry 277, 609–617. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Fujimoto M, 2010. Detergent-resistant microdomains determine the localization of sigma-1 receptors to the endoplasmic reticulum-mitochondria junction. Mol Pharmacol 77, 517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Rizzuto R, Hajnoczky G, Su TP, 2009. MAM: more than just a housekeeper. Trends in cell biology 19, 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP, 2004. Sigma-1 receptors at galactosylceramide-enriched lipid microdomains regulate oligodendrocyte differentiation. Proceedings of the National Academy of Sciences of the United States of America 101, 14949–14954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP, 2010. Cholesterol at the endoplasmic reticulum: roles of the sigma-1 receptor chaperone and implications thereof in human diseases. Subcell Biochem 51, 381–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Wang G, Dasgupta S, Dinkins M, Zhu G, Bieberich E, 2012. Characterization of an apical ceramide-enriched compartment regulating ciliogenesis. Molecular biology of the cell 23, 3156–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Wang G, Wakade S, Dasgupta S, Dinkins M, Kong JN, Spassieva SD, Bieberich E, 2014. Primary cilia in stem cells and neural progenitors are regulated by neutral sphingomyelinase 2 and ceramide. Molecular biology of the cell 25, 1715–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberle FA, Feigenson GW, 2011. Phase separation in lipid membranes. Cold Spring Harbor perspectives in biology 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerklotz H, 2002. Triton promotes domain formation in lipid raft mixtures. Biophysical journal 83, 2693–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellwing C, Tigistu-Sahle F, Fuhrmann H, Kakela R, Schumann J, 2018. Lipid composition of membrane microdomains isolated detergent-free from PUFA supplemented RAW264.7 macrophages. Journal of cellular physiology 233, 2602–2612. [DOI] [PubMed] [Google Scholar]
- Hernandez-Corbacho MJ, Salama MF, Canals D, Senkal CE, Obeid LM, 2017. Sphingolipids in mitochondria. Biochimica et biophysica acta 1862, 56–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera-Cruz MS, Simmen T, 2017. Of yeast, mice and men: MAMs come in two flavors. Biology direct 12, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoglinger D, Nadler A, Haberkant P, Kirkpatrick J, Schifferer M, Stein F, Hauke S, Porter FD, Schultz C, 2017. Trifunctional lipid probes for comprehensive studies of single lipid species in living cells. Proceedings of the National Academy of Sciences of the United States of America 114, 1566–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowka D, Sheets ED, Baird B, 2000. Interactions between Fc(epsilon)RI and lipid raft components are regulated by the actin cytoskeleton. Journal of cell science 113 (Pt 6), 1009–1019. [DOI] [PubMed] [Google Scholar]
- Holthuis JC, Luberto C, 2010. Tales and mysteries of the enigmatic sphingomyelin synthase family. Advances in experimental medicine and biology 688, 72–85. [DOI] [PubMed] [Google Scholar]
- Hoogerheide DP, Noskov SY, Jacobs D, Bergdoll L, Silin V, Worcester DL, Abramson J, Nanda H, Rostovtseva TK, Bezrukov SM, 2017. Structural features and lipid binding domain of tubulin on biomimetic mitochondrial membranes. Proceedings of the National Academy of Sciences of the United States of America 114, E3622–E3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui SW, Parsons DF, 1975. Direct observation of domains in wet lipid bilayers. Science 190, 383–384. [DOI] [PubMed] [Google Scholar]
- Hullin-Matsuda F, Kobayashi T, 2007. Monitoring the distribution and dynamics of signaling microdomains in living cells with lipid-specific probes. Cellular and molecular life sciences : CMLS 64, 2492–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley JH, Boura E, Carlson LA, Rozycki B, 2010. Membrane budding. Cell 143, 875–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipsen JH, Karlstrom G, Mouritsen OG, Wennerstrom H, Zuckermann MJ, 1987. Phase equilibria in the phosphatidylcholine-cholesterol system. Biochimica et biophysica acta 905, 162–172. [DOI] [PubMed] [Google Scholar]
- Itokazu Y, Tsai YT, Yu RK, 2017. Epigenetic regulation of ganglioside expression in neural stem cells and neuronal cells. Glycoconjugate journal 34, 749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwabuchi K, Handa K, Hakomori S, 1998. Separation of “glycosphingolipid signaling domain” from caveolin-containing membrane fraction in mouse melanoma B16 cells and its role in cell adhesion coupled with signaling. The Journal of biological chemistry 273, 33766–33773. [DOI] [PubMed] [Google Scholar]
- Janich P, Corbeil D, 2007. GM1 and GM3 gangliosides highlight distinct lipid microdomains within the apical domain of epithelial cells. FEBS letters 581, 1783–1787. [DOI] [PubMed] [Google Scholar]
- Jarsch IK, Ott T, 2011. Perspectives on remorin proteins, membrane rafts, and their role during plant-microbe interactions. Mol Plant Microbe Interact 24, 7–12. [DOI] [PubMed] [Google Scholar]
- Johri A, Beal MF, 2012. Mitochondrial dysfunction in neurodegenerative diseases. The Journal of pharmacology and experimental therapeutics 342, 619–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost PC, Griffith OH, Capaldi RA, Vanderkooi G, 1973. Evidence for boundary lipid in membranes. Proceedings of the National Academy of Sciences of the United States of America 70, 480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnovsky MJ, Kleinfeld AM, Hoover RL, Dawidowicz EA, McIntyre DE, Salzman EA, Klausner RD, 1982a. Lipid domains in membranes. Annals of the New York Academy of Sciences 401, 61–75. [DOI] [PubMed] [Google Scholar]
- Karnovsky MJ, Kleinfeld AM, Hoover RL, Klausner RD, 1982b. The concept of lipid domains in membranes. The Journal of cell biology 94, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller P, Simons K, 1998. Cholesterol is required for surface transport of influenza virus hemagglutinin. The Journal of cell biology 140, 1357–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkus J, Goswami R, Testai FD, Dawson G, 2003. Ceramide in rafts (detergent-insoluble fraction) mediates cell death in neurotumor cell lines. Journal of neuroscience research 72, 65–75. [DOI] [PubMed] [Google Scholar]
- Kilsdonk EP, Yancey PG, Stoudt GW, Bangerter FW, Johnson WJ, Phillips MC, Rothblat GH, 1995. Cellular cholesterol efflux mediated by cyclodextrins. The Journal of biological chemistry 270, 17250–17256. [DOI] [PubMed] [Google Scholar]
- Kindzelskii AL, Sitrin RG, Petty HR, 2004. Cutting edge: optical microspectrophotometry supports the existence of gel phase lipid rafts at the lamellipodium of neutrophils: apparent role in calcium signaling. Journal of immunology 172, 4681–4685. [DOI] [PubMed] [Google Scholar]
- Klausner RD, Kleinfeld AM, Hoover RL, Karnovsky MJ, 1980. Lipid domains in membranes. Evidence derived from structural perturbations induced by free fatty acids and lifetime heterogeneity analysis. The Journal of biological chemistry 255, 1286–1295. [PubMed] [Google Scholar]
- Kleemann W, McConnell HM, 1976. Interactions of proteins and cholesterol with lipids in bilayer membranes. Biochimica et biophysica acta 419, 206–222. [DOI] [PubMed] [Google Scholar]
- Klymchenko AS, Kreder R, 2014. Fluorescent probes for lipid rafts: from model membranes to living cells. Chemistry & biology 21, 97–113. [DOI] [PubMed] [Google Scholar]
- Kong JN, Hardin K, Dinkins M, Wang G, He Q, Mujadzic T, Zhu G, Bielawski J, Spassieva S, Bieberich E, 2015a. Regulation of Chlamydomonas flagella and ependymal cell motile cilia by ceramide-mediated translocation of GSK3. Molecular biology of the cell 26, 4451–4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong JN, He Q, Wang G, Dasgupta S, Dinkins MB, Zhu G, Kim A, Spassieva S, Bieberich E, 2015b. Guggulsterone and bexarotene induce secretion of exosome-associated breast cancer resistance protein and reduce doxorubicin resistance in MDA-MB-231 cells. International journal of cancer 137, 1610–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong JN, Zhu Z, Itokazu Y, Wang G, Dinkins MB, Zhong L, Lin HP, Elsherbini A, Leanhart S, Jiang X, Qin H, Zhi W, Spassieva SD, Bieberich E, 2018. Novel function of ceramide for regulation of mitochondrial ATP release in astrocytes. Journal of lipid research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov S, Afonin A, Evsyukov I, Bondarenko A, 2017. Alzheimer’s disease: as it was in the beginning. Reviews in the neurosciences 28, 825–843. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy K, Wang G, Silva J, Condie BG, Bieberich E, 2007. Ceramide Regulates Atypical PKC{zeta}/{lambda}-mediated Cell Polarity in Primitive Ectoderm Cells: A NOVEL FUNCTION OF SPHINGOLIPIDS IN MORPHOGENESIS. The Journal of biological chemistry 282, 3379–3390. [DOI] [PubMed] [Google Scholar]
- Krols M, van Isterdael G, Asselbergh B, Kremer A, Lippens S, Timmerman V, Janssens S, 2016. Mitochondria-associated membranes as hubs for neurodegeneration. Acta neuropathologica 131, 505–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufareva I, Lenoir M, Dancea F, Sridhar P, Raush E, Bissig C, Gruenberg J, Abagyan R, Overduin M, 2014. Discovery of novel membrane binding structures and functions. Biochemistry and cell biology = Biochimie et biologie cellulaire 92, 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai K, Yasuda S, Okemoto K, Nishijima M, Kobayashi S, Hanada K, 2005. CERT mediates intermembrane transfer of various molecular species of ceramides. The Journal of biological chemistry 280, 6488–6495. [DOI] [PubMed] [Google Scholar]
- Lacour S, Hammann A, Grazide S, Lagadic-Gossmann D, Athias A, Sergent O, Laurent G, Gambert P, Solary E, Dimanche-Boitrel MT, 2004. Cisplatin-induced CD95 redistribution into membrane lipid rafts of HT29 human colon cancer cells. Cancer research 64, 3593–3598. [DOI] [PubMed] [Google Scholar]
- Lafourcade C, Sobo K, Kieffer-Jaquinod S, Garin J, van der Goot FG, 2008. Regulation of the V-ATPase along the endocytic pathway occurs through reversible subunit association and membrane localization. PloS one 3, e2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai EC, 2003. Lipid rafts make for slippery platforms. The Journal of cell biology 162, 365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law BA, Liao X, Moore KS, Southard A, Roddy P, Ji R, Sculz Z, Bielawska A, Schulze PC, Cowart LA, 2017. Lipotoxic very-long-chain ceramides cause mitochondrial dysfunction, oxidative stress, and cell death in cardiomyocytes. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledeen RW, Wu G, 2008. Nuclear sphingolipids: metabolism and signaling. Journal of lipid research 49, 1176–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledeen RW, Wu G, 2010. In search of a solution to the sphinx-like riddle of GM1. Neurochemical research 35, 1867–1874. [DOI] [PubMed] [Google Scholar]
- Ledeen RW, Wu G, 2015. The multi-tasked life of GM1 ganglioside, a true factotum of nature. Trends in biochemical sciences 40, 407–418. [DOI] [PubMed] [Google Scholar]
- Levental KR, Lorent JH, Lin X, Skinkle AD, Surma MA, Stockenbojer EA, Gorfe AA, Levental I, 2016. Polyunsaturated Lipids Regulate Membrane Domain Stability by Tuning Membrane Order. Biophysical journal 110, 1800–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levental KR, Surma MA, Skinkle AD, Lorent JH, Zhou Y, Klose C, Chang JT, Hancock JF, Levental I, 2017. omega-3 polyunsaturated fatty acids direct differentiation of the membrane phenotype in mesenchymal stem cells to potentiate osteogenesis. Science advances 3, eaao1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M, Futerman AH, 2010. Mammalian ceramide synthases. IUBMB life 62, 347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Hailemariam TK, Zhou H, Li Y, Duckworth DC, Peake DA, Zhang Y, Kuo MS, Cao G, Jiang XC, 2007. Inhibition of sphingomyelin synthase (SMS) affects intracellular sphingomyelin accumulation and plasma membrane lipid organization. Biochimica et biophysica acta 1771, 1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Mintzer E, Bittman R, 2006. First synthesis of free cholesterol-BODIPY conjugates. The Journal of organic chemistry 71, 1718–1721. [DOI] [PubMed] [Google Scholar]
- Liao Y, Plummer NW, George MD, Abramowitz J, Zhu MX, Birnbaumer L, 2009. A role for Orai in TRPC-mediated Ca2+ entry suggests that a TRPC:Orai complex may mediate store and receptor operated Ca2+ entry. Proceedings of the National Academy of Sciences of the United States of America 106, 3202–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingwood D, Simons K, 2007. Detergent resistance as a tool in membrane research. Nature protocols 2, 2159–2165. [DOI] [PubMed] [Google Scholar]
- Lorent JH, Levental I, 2015. Structural determinants of protein partitioning into ordered membrane domains and lipid rafts. Chemistry and physics of lipids 192, 23–32. [DOI] [PubMed] [Google Scholar]
- Lozano J, Berra E, Municio MM, Diaz-Meco MT, Dominguez I, Sanz L, Moscat J, 1994. Protein kinase C zeta isoform is critical for kappa B-dependent promoter activation by sphingomyelinase. The Journal of biological chemistry 269, 19200–19202. [PubMed] [Google Scholar]
- Lu DY, Chen HC, Yang MS, Hsu YM, Lin HJ, Tang CH, Lee CH, Lai CK, Lin CJ, Shyu WC, Lin FY, Lai CH, 2012. Ceramide and Toll-like receptor 4 are mobilized into membrane rafts in response to Helicobacter pylori infection in gastric epithelial cells. Infection and immunity 80, 1823–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SM, Fairn GD, 2018. Mesoscale organization of domains in the plasma membrane - beyond the lipid raft. Critical reviews in biochemistry and molecular biology 53, 192–207. [DOI] [PubMed] [Google Scholar]
- Luberto C, Hannun YA, 1998. Sphingomyelin synthase, a potential regulator of intracellular levels of ceramide and diacylglycerol during SV40 transformation. Does sphingomyelin synthase account for the putative phosphatidylcholine-specific phospholipase C? The Journal of biological chemistry 273, 14550–14559. [DOI] [PubMed] [Google Scholar]
- Lucy JA, 1964. Globular Lipid Micelles and Cell Membranes. Journal of theoretical biology 7, 360–373. [DOI] [PubMed] [Google Scholar]
- Luria A, Vegelyte-Avery V, Stith B, Tsvetkova NM, Wolkers WF, Crowe JH, Tablin F, Nuccitelli R, 2002. Detergent-free domain isolated from Xenopus egg plasma membrane with properties similar to those of detergent-resistant membranes. Biochemistry 41, 13189–13197. [DOI] [PubMed] [Google Scholar]
- Lusa S, Blom TS, Eskelinen EL, Kuismanen E, Mansson JE, Simons K, Ikonen E, 2001. Depletion of rafts in late endocytic membranes is controlled by NPC1-dependent recycling of cholesterol to the plasma membrane. Journal of cell science 114, 1893–1900. [DOI] [PubMed] [Google Scholar]
- Ma Y, Hinde E, Gaus K, 2015. Nanodomains in biological membranes. Essays in biochemistry 57, 93–107. [DOI] [PubMed] [Google Scholar]
- Macdonald JL, Pike LJ, 2005. A simplified method for the preparation of detergent-free lipid rafts. Journal of lipid research 46, 1061–1067. [DOI] [PubMed] [Google Scholar]
- Machida Y, Bloch K, 1973. Complex formation between mycobacterial polysaccharides and fatty acyl-CoA derivatives. Proceedings of the National Academy of Sciences of the United States of America 70, 1146–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino A, Abe M, Murate M, Inaba T, Yilmaz N, Hullin-Matsuda F, Kishimoto T, Schieber NL, Taguchi T, Arai H, Anderluh G, Parton RG, Kobayashi T, 2015. Visualization of the heterogeneous membrane distribution of sphingomyelin associated with cytokinesis, cell polarity, and sphingolipidosis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 29, 477–493. [DOI] [PubMed] [Google Scholar]
- Maldonado EN, Lemasters JJ, 2012. Warburg revisited: regulation of mitochondrial metabolism by voltage-dependent anion channels in cancer cells. The Journal of pharmacology and experimental therapeutics 342, 637–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado EN, Sheldon KL, DeHart DN, Patnaik J, Manevich Y, Townsend DM, Bezrukov SM, Rostovtseva TK, Lemasters JJ, 2013. Voltage-dependent anion channels modulate mitochondrial metabolism in cancer cells: regulation by free tubulin and erastin. The Journal of biological chemistry 288, 11920–11929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinsky J, Opekarova M, Grossmann G, Tanner W, 2013. Membrane microdomains, rafts, and detergent-resistant membranes in plants and fungi. Annual review of plant biology 64, 501–529. [DOI] [PubMed] [Google Scholar]
- Manczak M, Reddy PH, 2012. Abnormal interaction of VDAC1 with amyloid beta and phosphorylated tau causes mitochondrial dysfunction in Alzheimer’s disease. Human molecular genetics 21, 5131–5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manneville JB, Casella JF, Ambroggio E, Gounon P, Bertherat J, Bassereau P, Cartaud J, Antonny B, Goud B, 2008. COPI coat assembly occurs on liquid-disordered domains and the associated membrane deformations are limited by membrane tension. Proceedings of the National Academy of Sciences of the United States of America 105, 16946–16951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M, van Meer G, 2008. Cell biology. No ESCRTs for exosomes. Science 319, 1191–1192. [DOI] [PubMed] [Google Scholar]
- Martin SW, Konopka JB, 2004. Lipid raft polarization contributes to hyphal growth in Candida albicans. Eukaryotic cell 3, 675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TF, 2001. PI(4,5)P(2) regulation of surface membrane traffic. Current opinion in cell biology 13, 493–499. [DOI] [PubMed] [Google Scholar]
- Mattjus P, 2016. Specificity of the mammalian glycolipid transfer proteins. Chemistry and physics of lipids 194, 72–78. [DOI] [PubMed] [Google Scholar]
- Meder D, Moreno MJ, Verkade P, Vaz WL, Simons K, 2006. Phase coexistence and connectivity in the apical membrane of polarized epithelial cells. Proceedings of the National Academy of Sciences of the United States of America 103, 329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megha, London E, 2004. Ceramide selectively displaces cholesterol from ordered lipid domains (rafts): implications for lipid raft structure and function. The Journal of biological chemistry 279, 9997–10004. [DOI] [PubMed] [Google Scholar]
- Mellor HR, Neville DC, Harvey DJ, Platt FM, Dwek RA, Butters TD, 2004. Cellular effects of deoxynojirimycin analogues: uptake, retention and inhibition of glycosphingolipid biosynthesis. The Biochemical journal 381, 861–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meszaros P, Klappe K, van Dam A, Ivanova PT, Milne SB, Myers DS, Brown HA, Permentier H, Hoekstra D, Kok JW, 2013. Long term myriocin treatment increases MRP1 transport activity. The international journal of biochemistry & cell biology 45, 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milhas D, Clarke CJ, Idkowiak-Baldys J, Canals D, Hannun YA, 2010. Anterograde and retrograde transport of neutral sphingomyelinase-2 between the Golgi and the plasma membrane. Biochimica et biophysica acta 1801, 1361–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongrand S, Stanislas T, Bayer EM, Lherminier J, Simon-Plas F, 2010. Membrane rafts in plant cells. Trends in plant science 15, 656–663. [DOI] [PubMed] [Google Scholar]
- Morris RJ, Jen A, Warley A, 2011. Isolation of nano-meso scale detergent resistant membrane that has properties expected of lipid ‘rafts’. Journal of neurochemistry 116, 671–677. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay A, Saddoughi SA, Song P, Sultan I, Ponnusamy S, Senkal CE, Snook CF, Arnold HK, Sears RC, Hannun YA, Ogretmen B, 2008. Direct interaction between the inhibitor 2 and ceramide via sphingolipid-protein binding is involved in the regulation of protein phosphatase 2A activity and signaling. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller G, Ayoub M, Storz P, Rennecke J, Fabbro D, Pfizenmaier K, 1995. PKC zeta is a molecular switch in signal transduction of TNF-alpha, bifunctionally regulated by ceramide and arachidonic acid. The EMBO journal 14, 1961–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslavsky N, Shmeeda H, Friedlander G, Yanai A, Futerman AH, Barenholz Y, Taraboulos A, 1999. Sphingolipid depletion increases formation of the scrapie prion protein in neuroblastoma cells infected with prions. The Journal of biological chemistry 274, 20763–20771. [DOI] [PubMed] [Google Scholar]
- Noskov SY, Rostovtseva TK, Bezrukov SM, 2013. ATP transport through VDAC and the VDAC-tubulin complex probed by equilibrium and nonequilibrium MD simulations. Biochemistry 52, 9246–9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novgorodov SA, Chudakova DA, Wheeler BW, Bielawski J, Kindy MS, Obeid LM, Gudz TI, 2011. Developmentally regulated ceramide synthase 6 increases mitochondrial Ca2+ loading capacity and promotes apoptosis. The Journal of biological chemistry 286, 4644–4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novgorodov SA, Gudz TI, 2011. Ceramide and mitochondria in ischemic brain injury. Int J Biochem Mol Biol 2, 347–361. [PMC free article] [PubMed] [Google Scholar]
- Novgorodov SA, Voltin JR, Gooz MA, Li L, Lemasters JJ, Gudz TI, 2018. Acid sphingomyelinase promotes mitochondrial dysfunction due to glutamate-induced regulated necrosis. Journal of lipid research 59, 312–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogiso H, Taniguchi M, Okazaki T, 2015. Analysis of lipid-composition changes in plasma membrane microdomains. Journal of lipid research 56, 1594–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliferenko S, Paiha K, Harder T, Gerke V, Schwarzler C, Schwarz H, Beug H, Gunthert U, Huber LA, 1999. Analysis of CD44-containing lipid rafts: Recruitment of annexin II and stabilization by the actin cytoskeleton. The Journal of cell biology 146, 843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olkkonen VM, Li S, 2013. Oxysterol-binding proteins: sterol and phosphoinositide sensors coordinating transport, signaling and metabolism. Progress in lipid research 52, 529–538. [DOI] [PubMed] [Google Scholar]
- Orsini F, Cremona A, Arosio P, Corsetto PA, Montorfano G, Lascialfari A, Rizzo AM, 2012. Atomic force microscopy imaging of lipid rafts of human breast cancer cells. Biochimica et biophysica acta 1818, 2943–2949. [DOI] [PubMed] [Google Scholar]
- Pacheco J, Ramirez-Jarquin JO, Vaca L, 2016. Microdomains Associated to Lipid Rafts. Advances in experimental medicine and biology 898, 353–378. [DOI] [PubMed] [Google Scholar]
- Paillusson S, Stoica R, Gomez-Suaga P, Lau DH, Mueller S, Miller T, Miller CC, 2016. There’s Something Wrong with my MAM; the ER-Mitochondria Axis and Neurodegenerative Diseases. Trends in neurosciences 39, 146–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzo AF, Eng CH, Schlaepfer DD, Marcantonio EE, Gundersen GG, 2004. Localized stabilization of microtubules by integrin- and FAK-facilitated Rho signaling. Science 303, 836–839. [DOI] [PubMed] [Google Scholar]
- Palmer CP, Mahen R, Schnell E, Djamgoz MB, Aydar E, 2007. Sigma-1 receptors bind cholesterol and remodel lipid rafts in breast cancer cell lines. Cancer research 67, 11166–11175. [DOI] [PubMed] [Google Scholar]
- Pani B, Singh BB, 2009. Lipid rafts/caveolae as microdomains of calcium signaling. Cell calcium 45, 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasassi T, De Stasio G, d’Ubaldo A, Gratton E, 1990. Phase fluctuation in phospholipid membranes revealed by Laurdan fluorescence. Biophysical journal 57, 1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JW, Park WJ, Futerman AH, 2014. Ceramide synthases as potential targets for therapeutic intervention in human diseases. Biochimica et biophysica acta 1841, 671–681. [DOI] [PubMed] [Google Scholar]
- Patwardhan GA, Beverly LJ, Siskind LJ, 2016. Sphingolipids and mitochondrial apoptosis. Journal of bioenergetics and biomembranes 48, 153–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera MN, Ganesan V, Siskind LJ, Szulc ZM, Bielawska A, Bittman R, Colombini M, 2016. Ceramide channel: Structural basis for selective membrane targeting. Chemistry and physics of lipids 194, 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry DM, Kitatani K, Roddy P, El-Osta M, Hannun YA, 2012. Identification and characterization of protein phosphatase 2C activation by ceramide. Journal of lipid research 53, 1513–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persaud-Sawin DA, Lightcap S, Harry GJ, 2009. Isolation of rafts from mouse brain tissue by a detergent-free method. Journal of lipid research 50, 759–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pewzner-Jung Y, Park H, Laviad EL, Silva LC, Lahiri S, Stiban J, Erez-Roman R, Brugger B, Sachsenheimer T, Wieland F, Prieto M, Merrill AH Jr., Futerman AH, 2010. A critical role for ceramide synthase 2 in liver homeostasis: I. alterations in lipid metabolic pathways. The Journal of biological chemistry 285, 10902–10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phuyal S, Hessvik NP, Skotland T, Sandvig K, Llorente A, 2014. Regulation of exosome release by glycosphingolipids and flotillins. The FEBS journal 281, 2214–2227. [DOI] [PubMed] [Google Scholar]
- Pike LJ, 2004. Lipid rafts: heterogeneity on the high seas. The Biochemical journal 378, 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike LJ, 2006. Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function. Journal of lipid research 47, 1597–1598. [DOI] [PubMed] [Google Scholar]
- Pinot M, Goud B, Manneville JB, 2010. Physical aspects of COPI vesicle formation. Molecular membrane biology 27, 428–442. [DOI] [PubMed] [Google Scholar]
- Pinto SN, Silva LC, de Almeida RF, Prieto M, 2008. Membrane domain formation, interdigitation, and morphological alterations induced by the very long chain asymmetric C24:1 ceramide. Biophysical journal 95, 2867–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto SN, Silva LC, Futerman AH, Prieto M, 2011. Effect of ceramide structure on membrane biophysical properties: the role of acyl chain length and unsaturation. Biochimica et biophysica acta 1808, 2753–2760. [DOI] [PubMed] [Google Scholar]
- Pipalia NH, Hao M, Mukherjee S, Maxfield FR, 2007. Sterol, protein and lipid trafficking in Chinese hamster ovary cells with Niemann-Pick type C1 defect. Traffic 8, 130–141. [DOI] [PubMed] [Google Scholar]
- Popov J, Vobornik D, Coban O, Keating E, Miller D, Francis J, Petersen NO, Johnston LJ, 2008. Chemical mapping of ceramide distribution in sphingomyelin-rich domains in monolayers. Langmuir : the ACS journal of surfaces and colloids 24, 13502–13508. [DOI] [PubMed] [Google Scholar]
- Pruett ST, Bushnev A, Hagedorn K, Adiga M, Haynes CA, Sullards MC, Liotta DC, Merrill AH Jr., 2008. Biodiversity of sphingoid bases (“sphingosines”) and related amino alcohols. Journal of lipid research 49, 1621–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Berdyshev E, Poirer C, Schwartz NB, Dawson G, 2012. Neutral sphingomyelinase 2 deficiency increases hyaluronan synthesis by up-regulation of Hyaluronan synthase 2 through decreased ceramide production and activation of Akt. The Journal of biological chemistry 287, 13620–13632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan V, Canals D, Luberto C, Snider J, Voelkel-Johnson C, Obeid LM, Hannun YA, 2015. Critical determinants of mitochondria-associated neutral sphingomyelinase (MAnSMase) for mitochondrial localization. Biochimica et biophysica acta 1850, 628–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran S, Chu UB, Mavlyutov TA, Pal A, Pyne S, Ruoho AE, 2009. The sigma1 receptor interacts with N-alkyl amines and endogenous sphingolipids. Eur J Pharmacol 609, 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Record M, Carayon K, Poirot M, Silvente-Poirot S, 2014. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochimica et biophysica acta 1841, 108–120. [DOI] [PubMed] [Google Scholar]
- Ridgway ND, 2010. Oxysterol-binding proteins. Subcell Biochem 51, 159–182. [DOI] [PubMed] [Google Scholar]
- Ritchie K, Iino R, Fujiwara T, Murase K, Kusumi A, 2003. The fence and picket structure of the plasma membrane of live cells as revealed by single molecule techniques (Review). Molecular membrane biology 20, 13–18. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Arribas M, Yakhine-Diop SM, Pedro JM, Gomez-Suaga P, Gomez-Sanchez R, Martinez-Chacon G, Fuentes JM, Gonzalez-Polo RA, Niso-Santano M, 2016. Mitochondria-Associated Membranes (MAMs): Overview and Its Role in Parkinson’s Disease. Molecular neurobiology. [DOI] [PubMed] [Google Scholar]
- Rostovtseva TK, Bezrukov SM, 2008. VDAC regulation: role of cytosolic proteins and mitochondrial lipids. Journal of bioenergetics and biomembranes 40, 163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostovtseva TK, Gurnev PA, Chen MY, Bezrukov SM, 2012. Membrane lipid composition regulates tubulin interaction with mitochondrial voltage-dependent anion channel. The Journal of biological chemistry 287, 29589–29598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostovtseva TK, Kazemi N, Weinrich M, Bezrukov SM, 2006. Voltage gating of VDAC is regulated by nonlamellar lipids of mitochondrial membranes. The Journal of biological chemistry 281, 37496–37506. [DOI] [PubMed] [Google Scholar]
- Rostovtseva TK, Sheldon KL, Hassanzadeh E, Monge C, Saks V, Bezrukov SM, Sackett DL, 2008. Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration. Proceedings of the National Academy of Sciences of the United States of America 105, 18746–18751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG, 1992. Caveolin, a protein component of caveolae membrane coats. Cell 68, 673–682. [DOI] [PubMed] [Google Scholar]
- Rouvinski A, Karniely S, Kounin M, Moussa S, Goldberg MD, Warburg G, Lyakhovetsky R, Papy-Garcia D, Kutzsche J, Korth C, Carlson GA, Godsave SF, Peters PJ, Luhr K, Kristensson K, Taraboulos A, 2014. Live imaging of prions reveals nascent PrPSc in cell-surface, raft-associated amyloid strings and webs. The Journal of cell biology 204, 423–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghy E, Szoke E, Payrits M, Helyes Z, Borzsei R, Erostyak J, Janosi TZ, Setalo G Jr., Szolcsanyi J, 2015. Evidence for the role of lipid rafts and sphingomyelin in Ca2+-gating of Transient Receptor Potential channels in trigeminal sensory neurons and peripheral nerve terminals. Pharmacological research 100, 101–116. [DOI] [PubMed] [Google Scholar]
- Sankarshanan M, Ma Z, Iype T, Lorenz U, 2007. Identification of a novel lipid raft-targeting motif in Src homology 2-containing phosphatase 1. Journal of immunology 179, 483–490. [DOI] [PubMed] [Google Scholar]
- Santos C, Fleury L, Rodriguez F, Markus J, Berkes D, Daich A, Ausseil F, Baudoin Dehoux C, Ballereau S, Genisson Y, 2015. The CERT antagonist HPA-12: first practical synthesis and individual binding evaluation of the four stereoisomers. Bioorganic & medicinal chemistry 23, 2004–2009. [DOI] [PubMed] [Google Scholar]
- Scarselli M, Annibale P, Gerace C, Radenovic A, 2013. Enlightening G-protein-coupled receptors on the plasma membrane using super-resolution photoactivated localization microscopy. Biochemical Society transactions 41, 191–196. [DOI] [PubMed] [Google Scholar]
- Scarselli M, Annibale P, Radenovic A, 2012. Cell type-specific beta2-adrenergic receptor clusters identified using photoactivated localization microscopy are not lipid raft related, but depend on actin cytoskeleton integrity. The Journal of biological chemistry 287, 16768–16780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer L, Futerman AH, Addadi L, 2007. Antibody labeling of cholesterol/ceramide ordered domains in cell membranes. Chembiochem : a European journal of chemical biology 8, 2286–2294. [DOI] [PubMed] [Google Scholar]
- Schiffmann S, Hartmann D, Fuchs S, Birod K, Ferreiros N, Schreiber Y, Zivkovic A, Geisslinger G, Grosch S, Stark H, 2012. Inhibitors of specific ceramide synthases. Biochimie 94, 558–565. [DOI] [PubMed] [Google Scholar]
- Schlosser E, Gottlieb D, 1966. Sterols and the sensitivity of Pythium species to filipin. Journal of bacteriology 91, 1080–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck S, Honsho M, Ekroos K, Shevchenko A, Simons K, 2003. Resistance of cell membranes to different detergents. Proceedings of the National Academy of Sciences of the United States of America 100, 5795–5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze H, Kolter T, Sandhoff K, 2009. Principles of lysosomal membrane degradation: Cellular topology and biochemistry of lysosomal lipid degradation. Biochimica et biophysica acta 1793, 674–683. [DOI] [PubMed] [Google Scholar]
- Schwan C, Nolke T, Kruppke AS, Schubert DM, Lang AE, Aktories K, 2011. Cholesterol- and sphingolipid-rich microdomains are essential for microtubule-based membrane protrusions induced by Clostridium difficile transferase (CDT). The Journal of biological chemistry 286, 29356–29365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz NU, Linzer RW, Truman JP, Gurevich M, Hannun YA, Senkal CE, Obeid LM, 2018. Decreased ceramide underlies mitochondrial dysfunction in Charcot-Marie-Tooth 2 F. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 32, 1716–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott CC, Vacca F, Gruenberg J, 2014. Endosome maturation, transport and functions. Seminars in cell & developmental biology 31, 2–10. [DOI] [PubMed] [Google Scholar]
- Severs NJ, Robenek H, 1983. Detection of microdomains in biomembranes. An appraisal of recent developments in freeze-fracture cytochemistry. Biochimica et biophysica acta 737, 373–408. [DOI] [PubMed] [Google Scholar]
- Sezgin E, Levental I, Grzybek M, Schwarzmann G, Mueller V, Honigmann A, Belov VN, Eggeling C, Coskun U, Simons K, Schwille P, 2012. Partitioning, diffusion, and ligand binding of raft lipid analogs in model and cellular plasma membranes. Biochimica et biophysica acta 1818, 1777–1784. [DOI] [PubMed] [Google Scholar]
- Sezgin E, Levental I, Mayor S, Eggeling C, 2017. The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nature reviews. Molecular cell biology 18, 361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala S, Wu H, Bose J, 2015. Salt stress sensing and early signalling events in plant roots: Current knowledge and hypothesis. Plant science : an international journal of experimental plant biology 241, 109–119. [DOI] [PubMed] [Google Scholar]
- Shaikh SR, Brzustowicz MR, Gustafson N, Stillwell W, Wassall SR, 2002. Monounsaturated PE does not phase-separate from the lipid raft molecules sphingomyelin and cholesterol: role for polyunsaturation? Biochemistry 41, 10593–10602. [DOI] [PubMed] [Google Scholar]
- Shaw JE, Epand RF, Epand RM, Li Z, Bittman R, Yip CM, 2006. Correlated fluorescence-atomic force microscopy of membrane domains: structure of fluorescence probes determines lipid localization. Biophysical journal 90, 2170–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon KL, Gurnev PA, Bezrukov SM, Sackett DL, 2015. Tubulin tail sequences and post-translational modifications regulate closure of mitochondrial voltage-dependent anion channel (VDAC). The Journal of biological chemistry 290, 26784–26789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shogomori H, Brown DA, 2003. Use of detergents to study membrane rafts: the good, the bad, and the ugly. Biological chemistry 384, 1259–1263. [DOI] [PubMed] [Google Scholar]
- Shoshan-Barmatz V, Nahon-Crystal E, Shteinfer-Kuzmine A, Gupta R, 2018. VDAC1, mitochondrial dysfunction, and Alzheimer’s disease. Pharmacological research 131, 87–101. [DOI] [PubMed] [Google Scholar]
- Silva LC, Ben David O, Pewzner-Jung Y, Laviad EL, Stiban J, Bandyopadhyay S, Merrill AH Jr., Prieto M, Futerman AH, 2012. Ablation of ceramide synthase 2 strongly affects biophysical properties of membranes. Journal of lipid research 53, 430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmen T, Tagaya M, 2017. Organelle Communication at Membrane Contact Sites (MCS): From Curiosity to Center Stage in Cell Biology and Biomedical Research. Advances in experimental medicine and biology 997, 1–12. [DOI] [PubMed] [Google Scholar]
- Simon-Plas F, Perraki A, Bayer E, Gerbeau-Pissot P, Mongrand S, 2011. An update on plant membrane rafts. Current opinion in plant biology 14, 642–649. [DOI] [PubMed] [Google Scholar]
- Simons K, Gerl MJ, 2010. Revitalizing membrane rafts: new tools and insights. Nature reviews. Molecular cell biology 11, 688–699. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E, 1997. Functional rafts in cell membranes. Nature 387, 569–572. [DOI] [PubMed] [Google Scholar]
- Simons K, Sampaio JL, 2011. Membrane organization and lipid rafts. Cold Spring Harbor perspectives in biology 3, a004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, van Meer G, 1988. Lipid sorting in epithelial cells. Biochemistry 27, 6197–6202. [DOI] [PubMed] [Google Scholar]
- Singer SJ, Nicolson GL, 1971. The structure and chemistry of mammalian cell membranes. The American journal of pathology 65, 427–437. [PMC free article] [PubMed] [Google Scholar]
- Singer SJ, Nicolson GL, 1972. The fluid mosaic model of the structure of cell membranes. Science 175, 720–731. [DOI] [PubMed] [Google Scholar]
- Singh H, Wray N, Schappi JM, Rasenick MM, 2018. Disruption of lipid-raft localized Galphas/tubulin complexes by antidepressants: a unique feature of HDAC6 inhibitors, SSRI and tricyclic compounds. Neuropsychopharmacology : official publication of the American College of N europsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Siskind LJ, Kolesnick RN, Colombini M, 2002. Ceramide channels increase the permeability of the mitochondrial outer membrane to small proteins. The Journal of biological chemistry 277, 26796–26803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskind LJ, Kolesnick RN, Colombini M, 2006. Ceramide forms channels in mitochondrial outer membranes at physiologically relevant concentrations. Mitochondrion 6, 118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skocaj M, Bakrac B, Krizaj I, Macek P, Anderluh G, Sepcic K, 2013. The sensing of membrane microdomains based on pore-forming toxins. Current medicinal chemistry 20, 491–501. [DOI] [PubMed] [Google Scholar]
- Skotland T, Sandvig K, Llorente A, 2017. Lipids in exosomes: Current knowledge and the way forward. Progress in lipid research 66, 30–41. [DOI] [PubMed] [Google Scholar]
- Smilansky A, Dangoor L, Nakdimon I, Ben-Hail D, Mizrachi D, Shoshan-Barmatz V, 2015. The Voltage-dependent Anion Channel 1 Mediates Amyloid beta Toxicity and Represents a Potential Target for Alzheimer Disease Therapy. The Journal of biological chemistry 290, 30670–30683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnino S, Aureli M, Mauri L, Ciampa MG, Prinetti A, 2015. Membrane lipid domains in the nervous system. Frontiers in bioscience 20, 280–302. [DOI] [PubMed] [Google Scholar]
- Sonnino S, Prinetti A, 2010. Lipids and membrane lateral organization. Frontiers in physiology 1, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorice M, Garofalo T, Misasi R, Manganelli V, Vona R, Malorni W, 2012. Ganglioside GD3 as a raft component in cell death regulation. Anti-cancer agents in medicinal chemistry 12, 376–382. [DOI] [PubMed] [Google Scholar]
- Sot J, Bagatolli LA, Goni FM, Alonso A, 2006. Detergent-resistant, ceramide-enriched domains in sphingomyelin/ceramide bilayers. Biophysical journal 90, 903–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassieva S, Bieberich E, 2016. Lysosphingolipids and sphingolipidoses: Psychosine in Krabbe’s disease. Journal of neuroscience research 94, 974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancevic B, Kolesnick R, 2010. Ceramide-rich platforms in transmembrane signaling. FEBS letters 584, 1728–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiban J, Tidhar R, Futerman AH, 2010. Ceramide synthases: roles in cell physiology and signaling. Advances in experimental medicine and biology 688, 60–71. [DOI] [PubMed] [Google Scholar]
- Stoffel W, Hammels I, Jenke B, Binczek E, Schmidt-Soltau I, Brodesser S, Schauss A, Etich J, Heilig J, Zaucke F, 2016. Neutral sphingomyelinase (SMPD3) deficiency disrupts the Golgi secretory pathway and causes growth inhibition. Cell death & disease 7, e2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuven E, Porat A, Shimron F, Fass E, Kaloyanova D, Brugger B, Wieland FT, Elazar Z, Helms JB, 2003. Intra-Golgi protein transport depends on a cholesterol balance in the lipid membrane. The Journal of biological chemistry 278, 53112–53122. [DOI] [PubMed] [Google Scholar]
- Sui Z, Kovacs AD, Maggirwar SB, 2006. Recruitment of active glycogen synthase kinase-3 into neuronal lipid rafts. Biochemical and biophysical research communications 345, 1643–1648. [DOI] [PubMed] [Google Scholar]
- Surma MA, Klose C, Simons K, 2012. Lipid-dependent protein sorting at the trans-Golgi network. Biochimica et biophysica acta 1821, 1059–1067. [DOI] [PubMed] [Google Scholar]
- Suzuki KGN, Ando H, Komura N, Fujiwara TK, Kiso M, Kusumi A, 2017. Development of new ganglioside probes and unraveling of raft domain structure by singlemolecule imaging. Biochimica et biophysica acta 1861, 2494–2506. [DOI] [PubMed] [Google Scholar]
- Suzuki KGN, Ando H, Komura N, Konishi M, Imamura A, Ishida H, Kiso M, Fujiwara TK, Kusumi A, 2018. Revealing the Raft Domain Organization in the Plasma Membrane by Single-Molecule Imaging of Fluorescent Ganglioside Analogs. Methods in enzymology 598, 267–282. [DOI] [PubMed] [Google Scholar]
- Svensson KJ, Christianson HC, Wittrup A, Bourseau-Guilmain E, Lindqvist E, Svensson LM, Morgelin M, Belting M, 2013. Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1. The Journal of biological chemistry 288, 17713–17724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafesse FG, Ternes P, Holthuis JC, 2006. The multigenic sphingomyelin synthase family. The Journal of biological chemistry 281, 29421–29425. [DOI] [PubMed] [Google Scholar]
- Tan LH, Tan AJ, Ng YY, Chua JJ, Chew WS, Muralidharan S, Torta F, Dutta B, Sze SK, Herr DR, Ong WY, 2017. Enriched Expression of Neutral Sphingomyelinase 2 in the Striatum is Essential for Regulation of Lipid Raft Content and Motor Coordination.Molecular neurobiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan SS, Yin Y, Lee T, Lai RC, Yeo RW, Zhang B, Choo A, Lim SK, 2013. Therapeutic MSC exosomes are derived from lipid raft microdomains in the plasma membrane. Journal of extracellular vesicles 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M, Okazaki T, 2014. The role of sphingomyelin and sphingomyelin synthases in cell death, proliferation and migration-from cell and animal models to human disorders. Biochimica et biophysica acta 1841, 692–703. [DOI] [PubMed] [Google Scholar]
- Tidhar R, Futerman AH, 2013. The complexity of sphingolipid biosynthesis in the endoplasmic reticulum. Biochimica et biophysica acta 1833, 2511–2518. [DOI] [PubMed] [Google Scholar]
- Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, Simons M, 2008. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319, 1244–1247. [DOI] [PubMed] [Google Scholar]
- Tsai YT, Itokazu Y, Yu RK, 2016. GM1 Ganglioside is Involved in Epigenetic Activation Loci of Neuronal Cells. Neurochemical research 41, 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai YT, Yu RK, 2014. Epigenetic activation of mouse ganglioside synthase genes: implications for neurogenesis. Journal of neurochemistry 128, 101–110. [DOI] [PubMed] [Google Scholar]
- Vaca L, 2010. SOCIC: the store-operated calcium influx complex. Cell calcium 47, 199–209. [DOI] [PubMed] [Google Scholar]
- van Blitterswijk WJ, Emmelot P, Hilkmann HA, Hilgers J, Feltkamp CA, 1979. Rigid plasma-membrane-derived vesicles, enriched in tumour-associated surface antigens (MLr), occurring in the ascites fluid of a murine leukaemia (GRSL). International journal of cancer 23, 62–70. [DOI] [PubMed] [Google Scholar]
- van Deenen LL, de Gier J, Demel RA, de Kruyff B, Blok MC, van der Neut-Kok EC, Haest CW, Ververgaert PH, Verkleij AJ, 1975. Lipid-lipid and lipid-protein interaction in model systems and membranes. Annals of the New York Academy of Sciences 264, 124–141. [DOI] [PubMed] [Google Scholar]
- van den Berg CW, Cinek T, Hallett MB, Horejsi V, Morgan BP, 1995. Exogenous glycosyl phosphatidylinositol-anchored CD59 associates with kinases in membrane clusters on U937 cells and becomes Ca(2+)-signaling competent. The Journal of cell biology 131, 669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Luit AH, Budde M, Zerp S, Caan W, Klarenbeek JB, Verheij M, Van Blitterswijk WJ, 2007. Resistance to alkyl-lysophospholipid-induced apoptosis due to downregulated sphingomyelin synthase 1 expression with consequent sphingomyelin- and cholesterol-deficiency in lipid rafts. The Biochemical journal 401, 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G, de Kroon AI, 2011. Lipid map of the mammalian cell. Journal of cell science 124, 5–8. [DOI] [PubMed] [Google Scholar]
- van Meer G, Simons K, 1982. Viruses budding from either the apical or the basolateral plasma membrane domain of MDCK cells have unique phospholipid compositions. The EMBO journal 1, 847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G, Simons K, 1988. Lipid polarity and sorting in epithelial cells. Journal of cellular biochemistry 36, 51–58. [DOI] [PubMed] [Google Scholar]
- van Meer G, Stelzer EH, Wijnaendts-van-Resandt RW, Simons K, 1987. Sorting of sphingolipids in epithelial (Madin-Darby canine kidney) cells. The Journal of cell biology 105, 1623–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G, Voelker DR, Feigenson GW, 2008. Membrane lipids: where they are and how they behave. Nature reviews. Molecular cell biology 9, 112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney P, Yadav V, Saini N, 2016. Lipid rafts in immune signalling: current progress and future perspective. Immunology 149, 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira OV, Verkade P, Manninen A, Simons K, 2005. FAPP2 is involved in the transport of apical cargo in polarized MDCK cells. The Journal of cell biology 170, 521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollrath JT, Sechi A, Dreser A, Katona I, Wiemuth D, Vervoorts J, Dohmen M, Chandrasekar A, Prause J, Brauers E, Jesse CM, Weis J, Goswami A, 2014. Loss of function of the ALS protein SigR1 leads to ER pathology associated with defective autophagy and lipid raft disturbances. Cell death & disease 5, e1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakade C, Chong R, 2014. A novel treatment target for Parkinson’s disease. J Neurol Sci 347, 34–38. [DOI] [PubMed] [Google Scholar]
- Walter T, Collenburg L, Japtok L, Kleuser B, Schneider-Schaulies S, Muller N, Becam J, Schubert-Unkmeir A, Kong JN, Bieberich E, Seibel J, 2016. Incorporation and visualization of azido-functionalized N-oleoyl serinol in Jurkat cells, mouse brain astrocytes, 3T3 fibroblasts and human brain microvascular endothelial cells. Chem Commun (Camb) 52, 8612–8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E, Norred WP, Bacon CW, Riley RT, Merrill AH Jr., 1991. Inhibition of sphingolipid biosynthesis by fumonisins. Implications for diseases associated with Fusarium moniliforme. The Journal of biological chemistry 266, 14486–14490. [PubMed] [Google Scholar]
- Wang G, Dinkins M, He Q, Zhu G, Poirier C, Campbell A, Mayer-Proschel M, Bieberich E, 2012. Astrocytes secrete exosomes enriched with proapoptotic ceramide and prostate apoptosis response 4 (PAR-4): potential mechanism of apoptosis induction in Alzheimer disease (AD). The Journal of biological chemistry 287, 21384–21395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Krishnamurthy K, Bieberich E, 2009. Regulation of primary cilia formation by ceramide. Journal of lipid research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Krishnamurthy K, Chiang YW, Dasgupta S, Bieberich E, 2008. Regulation of neural progenitor cell motility by ceramide and potential implications for mouse brain development. Journal of neurochemistry 106, 718–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Silva J, Krishnamurthy K, Tran E, Condie BG, Bieberich E, 2005. Direct binding to ceramide activates protein kinase Czeta before the formation of a pro-apoptotic complex with PAR-4 in differentiating stem cells. The Journal of biological chemistry 280, 26415–26424. [DOI] [PubMed] [Google Scholar]
- Wang G, Spassieva SD, Bieberich E, 2018. Ceramide and S1 P Signaling in Embryonic Stem Cell Differentiation. Methods in molecular biology 1697, 153–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Richards DA, 2012. Segregation of PIP2 and PIP3 into distinct nanoscale regions within the plasma membrane. Biology open 1, 857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YM, Seibenhener ML, Vandenplas ML, Wooten MW, 1999. Atypical PKC zeta is activated by ceramide, resulting in coactivation of NF-kappaB/JNK kinase and cell survival. Journal of neuroscience research 55, 293–302. [DOI] [PubMed] [Google Scholar]
- Wassall SR, Brzustowicz MR, Shaikh SR, Cherezov V, Caffrey M, Stillwell W, 2004. Order from disorder, corralling cholesterol with chaotic lipids. The role of polyunsaturated lipids in membrane raft formation. Chemistry and physics of lipids 132, 79–88. [DOI] [PubMed] [Google Scholar]
- Wassall SR, Stillwell W, 2008. Docosahexaenoic acid domains: the ultimate non-raft membrane domain. Chemistry and physics of lipids 153, 57–63. [DOI] [PubMed] [Google Scholar]
- Wassall SR, Stillwell W, 2009. Polyunsaturated fatty acid-cholesterol interactions: domain formation in membranes. Biochimica et biophysica acta 1788, 24–32. [DOI] [PubMed] [Google Scholar]
- Williamson R, Thompson AJ, Abu M, Hye A, Usardi A, Lynham S, Anderton BH, Hanger DP, 2010. Isolation of detergent resistant microdomains from cultured neurons: detergent dependent alterations in protein composition. BMC neuroscience 11, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong YC, Krainc D, 2017. alpha-synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nature medicine 23, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu BX, Rajagopalan V, Roddy PL, Clarke CJ, Hannun YA, 2010. Identification and characterization of murine mitochondria-associated neutral sphingomyelinase (MA-nSMase), the mammalian sphingomyelin phosphodiesterase 5. The Journal of biological chemistry 285, 17993–18002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Xie X, Lu ZH, Ledeen RW, 2009. Sodium-calcium exchanger complexed with GM1 ganglioside in nuclear membrane transfers calcium from nucleoplasm to endoplasmic reticulum. Proceedings of the National Academy of Sciences of the United States of America 106, 10829–10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Wu G, Lu ZH, Rohowsky-Kochan C, Ledeen RW, 2004. Presence of sodium-calcium exchanger/GM1 complex in the nuclear envelope of non-neural cells: nature of exchanger-GM1 interaction. Neurochemical research 29, 2135–2146. [DOI] [PubMed] [Google Scholar]
- Yamada E, 1955. The fine structure of the gall bladder epithelium of the mouse. The Journal of biophysical and biochemical cytology 1, 445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji A, Sekizawa Y, Emoto K, Sakuraba H, Inoue K, Kobayashi H, Umeda M, 1998. Lysenin, a novel sphingomyelin-specific binding protein. The Journal of biological chemistry 273, 5300–5306. [DOI] [PubMed] [Google Scholar]
- Yamaji T, Kumagai K, Tomishige N, Hanada K, 2008. Two sphingolipid transfer proteins, CERT and FAPP2: their roles in sphingolipid metabolism. IUBMB life 60, 511–518. [DOI] [PubMed] [Google Scholar]
- Yilmaz N, Yamaji-Hasegawa A, Hullin-Matsuda F, Kobayashi T, 2018. Molecular mechanisms of action of sphingomyelin-specific pore-forming toxin, lysenin. Seminars in cell & developmental biology 73, 188–198. [DOI] [PubMed] [Google Scholar]
- You C, Marquez-Lago TT, Richter CP, Wilmes S, Moraga I, Garcia KC, Leier A, Piehler J, 2016. Receptor dimer stabilization by hierarchical plasma membrane microcompartments regulates cytokine signaling. Science advances 2, e1600452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Alterman M, Dobrowsky RT, 2005. Ceramide displaces cholesterol from lipid rafts and decreases the association of the cholesterol binding protein caveolin-1. Journal of lipid research 46, 1678–1691. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li X, Becker KA, Gulbins E, 2009. Ceramide-enriched membrane domains--structure and function. Biochimica et biophysica acta 1788, 178–183. [DOI] [PubMed] [Google Scholar]