Abstract
PCB 11 (3,3’-dichlorobiphenyl), a contemporary congener produced as a byproduct of current pigment production processes, has recently emerged as a prevalent worldwide pollutant. We recently demonstrated that exposure to PCB 11 increases dendritic arborization in vitro, but the mechanism(s) mediating this effect remain unknown. To address this data gap, primary cortical neuron-glia co-cultures derived from neonatal Sprague Dawley rats were exposed for 48 h to either vehicle (0.1% DMSO) or PCB 11 at concentrations ranging from 1 fM to 1 nM in the absence or presence of pharmacologic antagonists of established molecular targets of higher chlorinated PCBs. Reporter cell lines were used to test activity of PCB 11 at the aryl hydrocarbon receptor (AhR) and thyroid hormone receptor (THR). PCB 11 lacked activity at the AhR and THR, and antagonism of these receptors had no effect on the dendrite promoting activity of PCB 11. Pharmacologic antagonism of various calcium channels or treatment with antioxidants also did not alter PCB 11-induced dendritic arborization. In contrast, pharmacologic blockade or shRNA knockdown of cAMP response element binding protein (CREB) significantly decreased dendritic growth in PCB 11-exposed cultures, suggesting PCB 11 promotes dendritic growth via CREB-mediated mechanisms. Since CREB signaling is crucial for normal neurodevelopment, and perturbations of CREB signaling have been associated with neurodevelopmental disorders, our findings suggest that this contemporary pollutant poses a threat to the developing brain, particularly in individuals with heritable mutations that promote CREB signaling.
Keywords: developmental neurotoxicity, in vitro model, neuronal connectivity, persistent organic pollutants
Introduction
Polychlorinated biphenyls (PCBs) are a class of persistent organic pollutants that are widely considered to be developmental neurotoxicants (Boucher et al. 2009; Schantz et al. 2003). Despite a worldwide ban on their production since the early 2000’s, PCBs are still widely detected in various environmental media (Ampleman et al. 2015; Herrick et al. 2004; Robson et al. 2010), including the human food chain (Chen et al. 2017; Cimenci et al. 2013; Llobet et al. 2003), and there has been little to no decrease in human body burdens over the past two decades (Consonni et al. 2012; Dewailly et al. 1999; Hopf et al. 2009; Koh et al. 2015). Developmental neurotoxicity remains the primary endpoint of concern for PCBs (Berghuis et al. 2015; Schantz et al. 2003), and recent epidemiologic data identifies PCBs as risk factors for neurodevelopmental disorders (NDDs) (Cheslack-Postava et al. 2013; Lyall et al. 2016; Rosenquist et al. 2017; Sagiv et al. 2010; Sealey et al. 2016; Ye et al. 2017). At the cellular level, legacy PCBs have been shown to alter neuronal connectivity by increasing dendritic growth both in vitro (Wayman et al. 2012a; Yang et al. 2014) and in vivo (Lein et al. 2007; Wayman et al. 2012b; Yang et al. 2009). These effects on dendritic growth are postulated to contribute to the behavioral deficits associated with developmental exposure to PCBs because altered dendritic morphology is a commonly shared pathology of many neurodevelopmental disorders (NDDs), including autism spectrum disorder (ASD) (Copf 2016).
PCB 11 is a contemporary pollutant of concern produced as a byproduct of current pigment production processes (Guo et al. 2014; Hu and Hornbuckle 2010). Although PCB 11 has been detected in our environment for the past century (Hu et al. 2011), it has gained recent attention following reports of its presence in environmental media around the world (Du et al. 2008; Heo et al. 2014; Hu et al. 2008; King et al. 2002), in milk produced for human consumption in northern California (Chen et al. 2017), and in the serum of mothers and their children in various regions of the United States (Koh et al. 2015; Sethi et al. 2017). We previously demonstrated that PCB 11 promotes dendritic arborization in primary rat cortical and hippocampal neurons at concentrations comparable to levels found in the serum of pregnant women at risk for having a child diagnosed with a NDD (Sethi et al. 2017). However, the mechanism(s) underlying this effect remain unknown. To address this data gap, we investigated a role for known molecular targets of higher chlorinated PCBs in PCB 11-induced dendritic growth. The aryl hydrocarbon receptor (AhR) is activated by a number of PCB congeners (Glazer et al. 2016; Vondracek et al. 2005), and has been implicated in dendritic development both in vitro (Dever et al. 2016) and in vivo (Kimura et al. 2015). Thyroid hormone (TH) promotes dendritic growth in Purkinje cells (Hatsukano et al. 2017; Ibhazehiebo and Koibuchi 2012), and TH receptor (THR) function can be positively modulated by PCBs (Zoeller 2007; Zoeller et al. 2000). Reactive oxygen species (ROS) have also been identified as signaling molecules involved in dendritic outgrowth (Chandrasekaran et al. 2015; Olguin-Albuerne and Moran 2017), and PCBs have been shown to increase ROS both in vitro (Howard et al. 2003) and in vivo (Lee et al. 2012). Multiple receptors involved in Ca2+ signaling were also investigated because of the essential role Ca2+ plays in activity-dependent dendritic growth (Brini et al. 2014; Wayman et al. 2006). Moreover, a number of non-dioxin-like (NDL) PCBs are potent sensitizers of the ryanodine receptor (RyR), and this molecular interaction has been causally linked to dendritic growth via activation of Ca2+-dependent signaling pathways that activate cAMP response element binding protein (CREB) (Wayman et al. 2012a; Wayman et al. 2012b; Yang et al. 2014).
Here, we report that PCB 11 has no activity at the AhR or THR in reporter cell lines, and pharmacological antagonism of these receptors or of various calcium ion channels has no significant effect on PCB 11-induced dendritic growth in primary cortical neurons. Antioxidant treatment also did not alter dendritic arborization in PCB 11-exposed neurons. However, the dendritic promoting activity of PCB 11 was blocked by pharmacologic inhibition of CREB signaling or by genetic knockdown of CREB. These results indicate that PCB 11 increases dendritic arborization via CREB-dependent mechanism(s) independent of interactions with established molecular targets of legacy PCBs.
Materials and Methods
Materials
PCB 11 (3,3’-dichlorobiphenyl, CAS # 2050-67-1) was synthesized by Dr. Hans-Joachim Lehmler (The University of Iowa, Iowa City, IA) and confirmed to be > 99% pure as determined by 1H NMR, 13C NMR, and GC-MS (Sethi et al. 2017). Map2B-pCAG-fusion protein red (FusRed) and short hairpin cAMP response element binding (shCREB) cDNA constructs were generous gifts from Dr. Gary Wayman (University of Washington, Pullman, WA) and have been previously characterized (Wayman et al. 2006). Scrambled shRNA control was purchased from Santa Cruz Biotechnology (Santa Cruz Biotechnology Inc., Dallas, TX). FLA 365 (Chen et al. 2016) and NH3 (Singh et al. 2016) were synthesized by Dr. Heike Wulff (University of California Davis, Davis, CA). CH 223-191, 666-15, and xestospongin C were purchased from Tocris (Bio-Techne, Minneapolis, MN). Verapamil and triiodo-L-thryonine were purchased from Sigma-Aldrich (St. Louis, MO). TCDD was provided by Dr. Stephen Safe (Texas A&M University, College Station, TX). The GH3.TRE-Luc cell line was generously provided by Dr. Dave Furlow (University of California Davis, Davis, CA). All stock solutions were made in dry sterile dimethylsulfoxide (DMSO, Sigma-Aldrich).
Luciferase Assays
GH3.TRE-Luc cells (Freitas et al. 2011) were plated at a density of 150,000 cells/well in 24-well cell culture plates (Thermo Scientific) in DMEM-F12 (Thermo Scientific) supplemented with 10% fetal bovine serum (Thermo Scientific). After 24 h, cultures were rinsed with phosphate-buffered saline (PBS) and 0.5 ml PCM, a serum-free medium (Sirbasku et al. 1991), was added. After a 24 h incubation in PCM, media were changed to PCM with PCB 11 or vehicle (0.1% DMSO). After a 24 h exposure, cultures were washed with PBS, lysed with 100 μl of Reporter Lysis Buffer (Promega, Madison, WI), and immediately frozen at −80°C. For analysis, 5 μl of thawed lysate was combined with 20 μl of Luciferase assay reagent (Promega). Luciferase activity was measured in a Synergy H1 hybrid microplate reader (BioTek Instruments, Winooski, VT), and normalized to protein concentrations measured using a BCA assay kit (Thermo Scientific). Each assay was performed in duplicate and repeated using 5-6 independent cell preparations. All assays were performed using cells within passage number 4-10.
Recombinant mouse (H1L6.1c3), rat (H4L1.1c4) and human HG2L6.1c1) hepatoma cells, which contain the stably transfected enhanced AhR-responsive reporter gene plasmid pGudLuc6.1 (Brennan et al. 2015; Han et al. 2004) were plated at a density of 750,000 cells/well into white, clear-bottomed 96-well plates and incubated at 37°C for 24 h prior to chemical treatment. Cells were then incubated for 24 h with vehicle (1% DMSO), TCDD (1 nM), or PCB 11 (1 or 10 μM). After incubation, cultures were visually inspection for signs of toxicity, then washed with PBS and lysed in 50 μl of Promega passive lysis buffer for 20 min at room temperature with shaking. Luciferase activity in each well was measured following automatic injection of Promega stabilized luciferase reagent in a Berthold microplate luminometer (Berthold Technologies, Bad Wildbad, Germany). Luciferase activity in each well was expressed relative to that maximally induced by TCDD. Luciferase activity was corrected for background luciferase activity present in DMSO-treated cells and values expressed as relative light units (RLU). Each assay was repeated 3-4 times independently. All assays were performed using cells below passage number 20.
Primary Rat Cortical Cell Culture
All procedures involving animals were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals following protocols approved by the University of California, Davis Institutional Animal Care and Use Committee. Timed-pregnant Sprague Dawley rats were purchased from Charles River Laboratory (Hollister, CA). All animals were housed in clear plastic shoebox cages containing corn cob bedding under constant temperature (22 ± 2 °C) and a 12 h light-dark cycle. Food and water were provided ad libitum.
Primary cortical neuron-glia cocultures were prepared from postnatal day 0 rat pups as previously described (Sethi et al. 2017; Wayman et al. 2012a). Neocortices from all pups in the litter were pooled, dissociated and plated at 83,000 cells/cm2 on glass coverslips (BellCo, Vineland, NJ) precoated with 0.5 mg/ml poly-L-lysine (Sigma-Aldrich) and maintained in a 5% CO2 humidified incubator at 37°C in NeuralQ Basal Medium supplemented with 2% GS21 (MTI-GlobalStem, Gaithersburg, MD) and GlutaMAX (ThermoScientific, Waltham, MA). At 4 days in vitro (DIV), cultures were treated with cytosine β-D-arabinofuranoside (Sigma-Aldrich) at 2.5 μM to limit glial growth. At 7 DIV, cultures were exposed for 48 h to vehicle (DMSO; 1:1,000 dilution), 1 femtomolar (fM), 1 picomolar (pM) or 1 nanomolar (nM) of PCB 11 in the absence or presence of various pharmacologic antagonists. Pharmacological antagonists were added 30 min prior to addition of PCB 11. All chemicals were diluted from 1,000x stocks directly into culture medium. At the end of the exposure period, cultures were fixed with 4% paraformaldehyde (Sigma-Aldrich) in PBS.
Morphometric Analyses of Dendritic Arborization
To visualize dendrites, cortical cultures were transfected with a Map2B-pCAG-FusRed plasmid at 6 DIV using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. Previous studies have demonstrated that expression of this construct does not alter dendritic growth of primary rat neurons (Wayman et al. 2006). A subset of cultures were co-transfected with a shCREB construct or a scrambled shRNA control. Images of FusRed positive neurons were acquired using unbiased automated image acquisition software (Metaxpress Version 5.3.0.5, Molecular Devices, Sunnyvale, CA) interfaced to a ImageExpressXL high content imaging system (Molecular Devices). Neurons were chosen for analysis using previously described criteria (Keil et al. 2017). Dendritic arborization was quantified manually by counting the number of dendritic tips and primary dendrites per neuron. All morphometric analyses of dendritic arborization were performed by an experienced individual blinded to experimental group.
Statistical Analyses
Data were assessed for normality using the Shapiro-Wilks test using GraphPad Prism v 6.07 (San Diego, CA). Normal data was analyzed using a parametric one-way analysis of variance (ANOVA) with significance set at p ≤ 0.05. Differences between groups were identified by post-hoc Holm-Sidak’s multiple comparisons test.
Results
Neither AhR nor THR signaling are necessary for PCB 11-induced dendritic growth
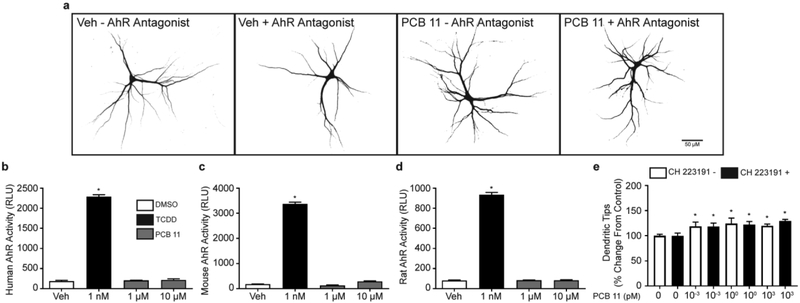
The AhR is a primary molecular target of dioxin-like PCBs (Vondracek et al. 2005), but there are no data regarding PCB 11 activity at this receptor. As determined using AhR reporter hepatoma cell lines, PCB 11 had no activity at the human, rat, or mouse AhR (Figure 1b, c, d). To ensure that AhR activity was not mediating the dendrite promoting effects of PCB 11, neurons were exposed to PCB 11 in the absence or presence of the AhR antagonist, CH223191 at 10 μM (Kim et al. 2006). Consistent with previous work (Sethi et al. 2017), PCB 11 had no effect on the number of primary dendrites (data not shown) but did significantly enhance dendritic arborization at 1 fM, 1 pM, and 1 nM, as evidenced by an increased number of dendritic tips per neuron in cultures exposed to PCB 11 relative to sister cultures exposed to vehicle alone (Figure 1a, e). CH223191 did not block this dendritic promoting activity of PCB 11 (Figure 1a, e).
Fig. 1. PCB 11 does not activate the aryl hydrocarbon receptor (AhR) and antagonism of the AhR does not attenuate the dendritic promoting activity of PCB 11.
(a) Representative photomicrographs of DIV 9 cortical neurons exposed to the AhR antagonist, CH223191 (10 μM) or PCB 11 (1 pM) singly or combined. (b-d) PCB 11 does not agonize the human, mouse or rat AhR. (e) Quantification of the number of dendritic tips per neuron in cortical neurons exposed to vehicle (Veh, 0.1% DMSO) or varying concentrations of PCB 11 in the absence or presence of CH223191. Data presented as the mean ± SD (n = 6 wells from 2 independent dissections). *Significantly different from vehicle control at p < 0.05 as determined using a nonparametric one-way ANOVA (p < 0.05) followed by Holm-Sidak’s multiple comparisons test
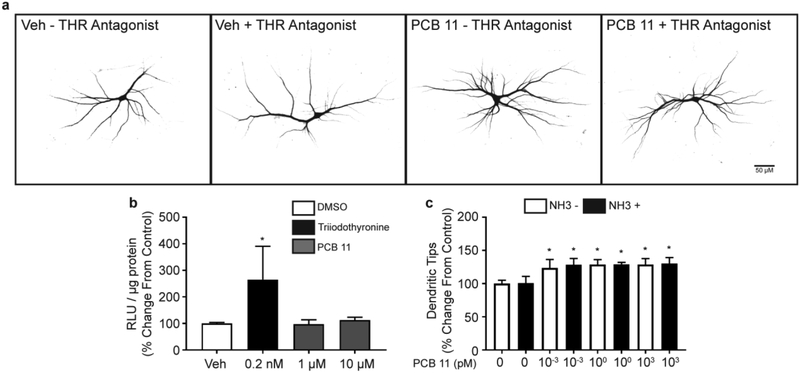
Altered THR signaling is widely posited to mediate the neurotoxic effects of various PCBs (Zoeller 2007), so a luciferase-based THR reporter cell line (Freitas et al. 2011) was used to assess the THR activity of PCB 11. PCB 11 was not active at the THR in the GH3.TRE cell line (Figure 2b). To confirm that THR signaling was not involved in PCB 11-induced dendritic arborization, cortical cultures were exposed to PCB 11 in the absence or presence of the THR antagonist, NH3 (Singh et al. 2016). At 100 pM, a concentration identified to inhibit upregulation of TH-responsive genes in cortical cultures exposed to a physiologically relevant concentration of T3 (Supplemental Figure S1), NH3 had no effect on PCB 11-induced dendritic growth (Figure 2a, c). Neither PCB 11 nor NH3 changed the number of primary dendrites when applied singly or in combination (data not shown).
Fig. 2. PCB 11 has no agonistic activity at the thyroid hormone receptor (THR) and THR antagonism does not block the dendritic effects of PCB 11.
(a) Representative photomicrographs of DIV 9 cortical neurons exposed to the THR antagonist, NH3 (100 pM), or PCB 11 (1 pM), singly or in combination. (b) PCB 11 does not agonize the THR in a rat pituitary THR reporter cell line. (c) Quantification of the number of dendritic tips per neuron in cortical neurons exposed to vehicle (Veh, 0.1% DMSO) or varying concentrations of PCB 11 in the absence or presence of NH3. Data presented as the mean ± SD (n = 6 wells from 2 independent dissections). *Significantly different from vehicle control at p < 0.05 as determined using a nonparametric one-way ANOVA (p < 0.05) followed by Holm-Sidak’s multiple comparisons test
The dendrite promoting activity of PCB 11 is not mediated by Ca2+ or ROS-dependent mechanisms
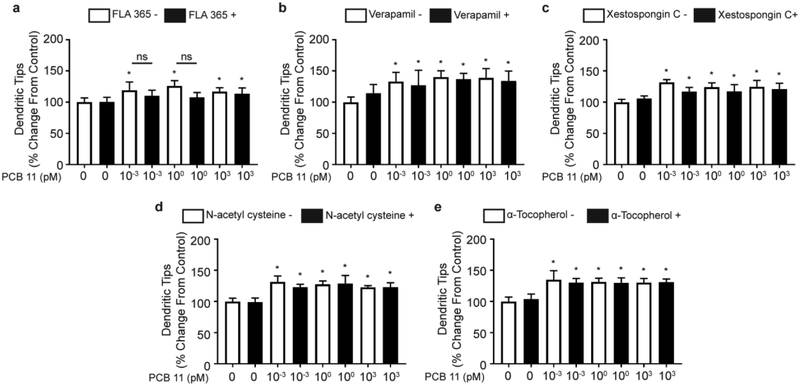
Calcium is a critical signaling molecule during neurodevelopment, and NDL PCBs have been shown to alter dendritic arborization via Ca2+-dependent mechanisms (Wayman et al. 2012a). To test whether PCB 11 effects on dendritic growth were due to increased levels of intracellular Ca2+, we blocked Ca2+ ion channels using pharmacologic antagonists of the L-type calcium channel, IP3 receptor (IP3R) and ryanodine receptor (RyR). At a concentration previously shown to block PCB 95-induced dendritic growth in primary rat neurons (Wayman et al. 2012b), the RyR antagonist, FLA365 (10 μM), had minimal effect on PCB 11-induced dendritic growth (Figure 3a). Antagonism of L-type calcium channels by verapamil (30 μM) or the IP3R by xestospongin C (1 μM) at concentrations previously shown to block these channels (Howard et al. 2003) did not inhibit the dendrite promoting activity of PCB 11 (Figure 3b, c). None of these experimental treatments altered the number of primary dendrites relative to vehicle controls (data not shown).
Fig. 3. Pharmacological antagonism of the ryanodine receptor (RyR), L-type calcium channels or the IP3 receptor or antioxidant treatment does not attenuate PCB 11-induced dendritic arborization.
The number of dendritic tips per neurons, presented as the % change from vehicle control, was quantified in cortical neurons exposed to vehicle (0.1% DMSO) or varying concentrations of PCB 11 in the absence or presence of (a) the RyR blocker, FLA 365 (10 μM); (b) the L-type calcium channel blocker, verapamil (30 μM); (c) the IP3 receptor antagonist, xestospongin C (1 μM); (d) the antioxidant, N-acetyl-cysteine (NAC, 100 μM); or (e) the antioxidant α-tocopherol (100 μM). Data presented as the mean ± SD (n = 6 wells from 2 independent dissections). *Significantly different from vehicle control at p < 0.05 as determined using a nonparametric one-way ANOVA (p < 0.05) followed by Holm-Sidak’s multiple comparisons test
Recent studies have shown that physiological levels of ROS function as critical signaling molecules in dendritic growth (Chandrasekaran et al. 2015; Olguin-Albuerne and Moran 2017). To investigate whether PCB 11-induced dendritic arborization is mediated by increased levels of intracellular ROS, neurons were exposed to PCB 11 in the absence or presence of one of two mechanistically and structurally distinct antioxidants, N-acetyl-cysteine (NAC) or α-tocopherol. Neither NAC (100 μM) nor α-tocopherol (100 μM) significantly altered the number of dendritic tips (Figure 3d, e) or the number of primary dendrites (data not shown) in cortical neurons exposed to PCB 11.
PCB 11-induced dendritic growth requires CREB activity
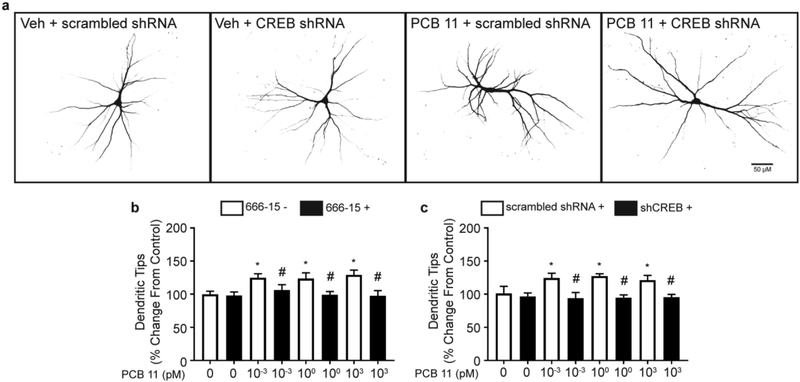
CREB is a signaling molecule that is required for activity-dependent dendritic growth in central neurons (Wayman et al. 2006), and has been shown to be a downstream target of higher chlorinated, RyR active, PCBs (Wayman et al. 2012a). To determine if PCB 11 promotes dendritic growth via CREB-dependent mechanisms, we tested whether PCB 11-induced dendritic growth was blocked by compound 666-15, a pharmacologic inhibitor of CREB signaling (Xie et al. 2015). At 500 nM, compound 666-15 reduced the number of dendritic tips in PCB 11-exposed cultures to control levels (Figure 4b), but had no effect on the number of primary dendrites (data not shown). To confirm that the effects of 666-15 were specific to CREB, neurons were transfected with a shCREB construct or scrambled shRNA control one day prior to PCB 11 exposure. The dendrite promoting effects of PCB 11 were blocked in neurons transfected with shCREB but not altered in neurons transfected with the scrambled shRNA control (Figure 4a, c). Transfection with either shRNA construct had no effect on the number of primary dendrites in vehicle control or PCB 11-exposed neurons (data not shown).
Fig. 4. Pharmacologic blockade and shRNA knockdown of CREB abolishes the dendritic promoting effects of PCB 11.
(a) Representative photomicrographs of DIV 9 cortical neurons transfected with shCREB or a scrambled shRNA and then exposed to PCB 11 (1 pM) for 48 h. (b, c) The number of dendritic tips per neuron, presented as the % change from vehicle control, was quantified in cortical neurons exposed to vehicle (0.1% DMSO) or varying concentrations of PCB 11 (b) in the absence or presence of the CREB blocker, compound 666–15 (500 nM) or (c) following transfection with shCREB or a scrambled (control) shRNA. Data presented as the mean ± SD (n = 6 wells from 2 independent dissections). *Significantly different from vehicle control at p < 0.05, #Significantly different from corresponding PCB 11 concentration at p < 0.05 as determined using a nonparametric one-way ANOVA (p < 0.05) followed by Holm-Sidak’s multiple comparisons test
Discussion
This study extends our previous work characterizing the effects of PCB 11 on neuronal morphology in vitro (Sethi et al. 2017) to address the mechanism(s) by which PCB 11 enhances dendritic arborization. More highly chlorinated legacy PCBs have been shown to mediate their biological effects via interaction with the AhR (White and Birnbaum 2009), RyR (Pessah et al. 2010) or THR (Zoeller 2007). The major findings from this study suggest that the lightly chlorinated contemporary congener, PCB 11, phenocopies the effect of higher chlorinated legacy PCBs on dendritic arborization independent of interactions with the canonical receptor targets of the legacy PCBs. It has previously been reported that PCB 11 has no activity at the RyR (Holland et al. 2016), and here we report that PCB 11 also has no activity at the AhR or THR. Consistent with these observations, pharmacologic antagonism of these three receptors did not significantly attenuate the increase in dendritic arborization triggered by PCB 11. In contrast, pharmacologic blockade or genetic knockdown of CREB abolished the effects of PCB 11 on dendritic arborization in primary cortical neurons, indicating that PCB 11 promotes dendritic growth through a CREB-mediated mechanism. We previously demonstrated that the NDL legacy congener, PCB 95, also promotes dendritic arborization via activation of CREB (Wayman et al. 2012a), suggesting that CREB may be a point of convergence for PCB-induced dendritic growth. However, the upstream targets of PCB 95 vs. PCB 11 diverge, with PCB 95 effects on CREB mediated by RyR sensitization (Wayman et al. 2012b), and the PCB 11 activation of CREB mediated by upstream target(s) other than RyR.
The proximal molecular target(s) mediating PCB 11’s effects on dendritic growth have yet to be identified. Results of a recent screening study confirm that PCB 11 lacks activity at the AhR and the THR, and further demonstrate that PCB 11 also lacks activity at the estrogen, androgen and glucocorticoid receptors at concentrations ≤ 1 μM (Takeuchi et al. 2017). An independent study replicated these findings, but also reported slight activity of PCB 11 at the constitutive androstane receptor (CAR), as indicated by increased CYP2B6 expression (Pencikova et al. 2018). However, the EC25 for this effect was 26 μM, which raises serious doubts regarding its relevance to PCB 11 effects on dendritic arborization. Another possible mechanism by which PCB 11 may promote dendritic growth is by increasing intracellular Ca2+ concentrations. Our data shows that PCB 11 is not acting through the major Ca2+ ion channels implicated in dendritic growth, including the RyR, L-type Ca2+ channels, or the IP3R. However, another lightly chlorinated congener, PCB 19, which has three chlorine substitutions, has been shown to briefly increase intracellular Ca2+ levels prior to eventually inhibiting store operated Ca2+entry (Choi et al. 2016). Similar to our findings, these effects of PCB 19 were independent of the IP3R. Other voltage gated calcium channels, such as the N, P/Q, R and T channels, are also expressed in dendrites (Catterall 2011), but were not investigated in this study, therefore, we cannot rule out the possibility that Ca2+-dependent mechanisms mediate PCB 11 activation of CREB. However, CREB activity is regulated by multiple mechanisms other than increased intracellular Ca2+ (Sakamoto et al. 2011). Briefly, transducers of CREB regulatory activity (TORCs) assist in CREB-mediated transcription, multiple miRNAs translationally control CREB levels, and several posttranslational modifications, such as acetylation, glycosylation, ubiquitination, and phosphorylation modulate CREB activity (Sakamoto et al. 2011). Teasing out potential effects of PCB 11 on these diverse mechanisms for regulating CREB activity will provide mechanistic insight(s) of how PCB 11 induces dendritic growth.
Altered CREB signaling has been implicated in various NDDs (Bu et al. 2017; D'Andrea et al. 2015; Ngounou Wetie et al. 2015; Todd and Mack 2001). Consistent with these clinical observations, PI3Kγ knockout mice exhibit increased CREB signaling coincident with an attention deficit hyperactivity disorder phenotype (D'Andrea et al. 2015), and transgenic mice expressing human mutations in CREB binding protein exhibit increased repetitive behaviors, social deficits, and deficits in learning and memory (Zheng et al. 2016). These observations, together with clinical data identifying increased dendritic complexity and hyperconnectivity as a shared pathology of diverse NDDs (Alaerts et al. 2016; Copf 2016; Supekar et al. 2013), heighten the concern that PCB 11 poses a threat to the developing human brain, particularly in individuals with heritable mutations that increase CREB signaling.
Supplementary Material
Acknowledgements:
This research was supported by the National Institutes of Health (R01 ES014901, P30 ES023513, P01 ES011269, T32 ES007059 [predoctoral fellowship to SS] and F32 HD088016 [postdoctoral fellowship to KPK]) and by the United States Environmental Protection Agency (RD 83543201). This project used core facilities supported by the MIND Institute Intellectual and Developmental Disabilities Research Center (grant U54 HD079125). The synthesis of PCB 11 provided by Dr. Xueshu Li (The University of Iowa, Iowa City, IA) was supported by the Superfund Research Center at The University of Iowa (P42 ES013661). We gratefully acknowledge Dr. Michael Denison (University of California-Davis, Davis, CA), who performed the AhR luciferase assays, and Dr. J. David Furlow (University of California-Davis, Davis, CA) who provided the TRE-Luc cell line. The contents of this work do not necessarily represent the official views of the funding agencies, and the funding agencies do not endorse the purchase of any commercial products or services mentioned in the publication.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical approval: All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The manuscript does not contain clinical studies or patient data.
References
- Alaerts K, Swinnen S, Wenderoth N (2016) Sex differences in Autism: A resting-state fMRI investigation of functional brain connectivity in males and females. Soc Cogn Affect Neurosci doi: 10.1093/scan/nsw027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ampleman MD, Martinez A, DeWall J, Rawn DF, Hombuckle KC, Thorne PS (2015) Inhalation and dietary exposure to PCBs in urban and rural cohorts via congener-specific measurements. Environ Sci Technol 49(2):1156–64 doi: 10.1021/es5048039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghuis SA, Bos AF, Sauer PJ, Roze E (2015) Developmental neurotoxicity of persistent organic pollutants: an update on childhood outcome. Arch Toxicol 89(5):687–709 doi: 10.1007/s00204-015-1463-3 [DOI] [PubMed] [Google Scholar]
- Boucher O, Muckle G, Bastien CH (2009) Prenatal exposure to polychlorinated biphenyls: a neuropsychologic analysis. Environ Health Perspect 117(1):7–16 doi: 10.1289/ehp.11294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan JC, He G, Tsutsumi T, et al. (2015) Development of Species-Specific Ah Receptor-Responsive Third Generation CALUX Cell Lines with Enhanced Responsiveness and Improved Detection Limits. Environ Sci Technol 49(19):11903–12 doi: 10.1021/acs.est.5b02906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brini M, Cali T, Ottolini D, Carafoli E (2014) Neuronal calcium signaling: function and dysfunction. Cell Mol Life Sci 71(15):2787–814 doi: 10.1007/s00018-013-1550-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu Q, Wang A, Hamzah H, et al. (2017) CREB Signaling Is Involved in Rett Syndrome Pathogenesis. J Neurosci 37(13):3671–3685 doi: 10.1523/JNEUROSCI.3735-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA (2011) Voltage-gated calcium channels. Cold Spring Harb Perspect Biol 3(8):a003947 doi: 10.1101/cshperspect.a003947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran V, Lea C, Sosa JC, Higgins D, Lein PJ (2015) Reactive oxygen species are involved in BMP-induced dendritic growth in cultured rat sympathetic neurons. Mol Cell Neurosci 67:116–25 doi: 10.1016/j.mcn.2015.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Streifel KM, Singh V, et al. (2016) BDE-47 and BDE-49 Inhibit Axonal Growth in Primary Rat Hippocampal Neuron-Glia Co-Cultures via Ryanodine Receptor-Dependent Mechanisms. Toxicol Sci doi: 10.1093/toxsci/kfw259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Lin Y, Dang K, Puschner B (2017) Quantification of Polychlorinated Biphenyls and Polybrominated Diphenyl Ethers in Commercial Cows' Milk from California by Gas Chromatography-Triple Quadruple Mass Spectrometry. PLoS One 12(1):e0170129 doi: 10.1371/journal.pone.0170129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheslack-Postava K, Rantakokko PV, Hinkka-Yli-Salomaki S, et al. (2013) Maternal serum persistent organic pollutants in the Finnish Prenatal Study of Autism: A pilot study. Neurotoxicol Teratol 38:1–5 doi: 10.1016/j.ntt.2013.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SY, Lee K, Park Y, et al. (2016) Non-Dioxin-Like Polychlorinated Biphenyls Inhibit G-Protein Coupled Receptor-Mediated Ca2+ Signaling by Blocking Store-Operated Ca2+ Entry. PLoS One 11(3):e0150921 doi: 10.1371/journal.pone.0150921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimenci O, Vandevijvere S, Goscinny S, et al. (2013) Dietary exposure of the Belgian adult population to non-dioxin-like PCBs. Food Chem Toxicol 59:670–9 doi: 10.1016/j.fct.2013.06.020 [DOI] [PubMed] [Google Scholar]
- Consonni D, Sindaco R, Bertazzi PA (2012) Blood levels of dioxins, furans, dioxin-like PCBs, and TEQs in general populations: a review, 1989-2010. Environ Int 44:151–62 doi: 10.1016/j.envint.2012.01.004 [DOI] [PubMed] [Google Scholar]
- Copf T (2016) Impairments in dendrite morphogenesis as etiology for neurodevelopmental disorders and implications for therapeutic treatments. Neurosci Biobehav Rev doi: 10.1016/j.neubiorev.2016.04.008 [DOI] [PubMed] [Google Scholar]
- D'Andrea I, Fardella V, Fardella S, et al. (2015) Lack of kinase-independent activity of PI3Kgamma in locus coeruleus induces ADHD symptoms through increased CREB signaling. EMBO Mol Med 7(7):904–17 doi: 10.15252/emmm.201404697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dever DP, Adham ZO, Thompson B, et al. (2016) Aryl hydrocarbon receptor deletion in cerebellar granule neuron precursors impairs neurogenesis. Dev Neurobiol 76(5):533–50 doi: 10.1002/dneu.22330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewailly E, Mulvad G, Pedersen HS, et al. (1999) Concentration of organochlorines in human brain, liver, and adipose tissue autopsy samples from Greenland. Environ Health Perspect 107(10):823–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du S, Belton TJ, Rodenburg LA (2008) Source apportionment of polychlorinated biphenyls in the tidal Delaware River. Environ Sci Technol 42(11):4044–51 [DOI] [PubMed] [Google Scholar]
- Freitas J, Cano P, Craig-Veit C, Goodson ML, Furlow JD, Murk AJ (2011) Detection of thyroid hormone receptor disruptors by a novel stable in vitro reporter gene assay. Toxicol In Vitro 25(1):257–66 doi: 10.1016/j.tiv.2010.08.013 [DOI] [PubMed] [Google Scholar]
- Glazer L, Hahn ME, Aluru N (2016) Delayed effects of developmental exposure to low levels of the aryl hydrocarbon receptor agonist 3,3',4,4',5-pentachlorobiphenyl (PCB126) on adult zebrafish behavior. Neurotoxicology 52:134–43 doi: 10.1016/j.neuro.2015.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Capozzi SL, Kraeutler TM, Rodenburg LA (2014) Global distribution and local impacts of inadvertently generated polychlorinated biphenyls in pigments. Environ Sci Technol 48(15):8573–80 doi: 10.1021/es502291b [DOI] [PubMed] [Google Scholar]
- Han D, Nagy SR, Denison MS (2004) Comparison of recombinant cell bioassays for the detection of Ah receptor agonists. Biofactors 20(1):11–22 [DOI] [PubMed] [Google Scholar]
- Hatsukano T, Kurisu J, Fukumitsu K, Fujishima K, Kengaku M (2017) Thyroid Hormone Induces PGC-1alpha during Dendritic Outgrowth in Mouse Cerebellar Purkinje Cells. Front Cell Neurosci 11:133 doi: 10.3389/fncel.2017.00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo J, Kim D, Lee G (2014) Congener profiles and source-wise phase partitioning analysis of PCDDs/Fs and PCBs in Gyeonggi-do ambient air, South Korea. Int J Environ Res Public Health 11(11):11065–80 doi: 10.3390/ijerph111111065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick RF, McClean MD, Meeker JD, Baxter LK, Weymouth GA (2004) An unrecognized source of PCB contamination in schools and other buildings. Environ Health Perspect 112(10):1051–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland EB, Feng W, Zheng J, et al. (2016) An extended structure-activity relationship of non-dioxin-like PCBs evaluates and supports modeling predictions and identifies picomolar potency of PCB 202 towards ryanodine receptors. Toxicol Sci doi: 10.1093/toxsci/kfw189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf NB, Ruder AM, Succop P (2009) Background levels of polychlorinated biphenyls in the U.S. population. Sci Total Environ 407(24):6109–19 doi: 10.1016/j.scitotenv.2009.08.035 [DOI] [PubMed] [Google Scholar]
- Howard AS, Fitzpatrick R, Pessah I, Kostyniak P, Lein PJ (2003) Polychlorinated biphenyls induce caspase-dependent cell death in cultured embryonic rat hippocampal but not cortical neurons via activation of the ryanodine receptor. Toxicol Appl Pharmacol 190(1):72–86 [DOI] [PubMed] [Google Scholar]
- Hu D, Hornbuckle KC (2010) Inadvertent polychlorinated biphenyls in commercial paint pigments. Environ Sci Technol 44(8):2822–7 doi: 10.1021/es902413k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Martinez A, Hornbuckle KC (2008) Discovery of non-aroclor PCB (3,3'-dichlorobiphenyl) in Chicago air. Environ Sci Technol 42(21):7873–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Martinez A, Hornbuckle KC (2011) Sedimentary Records of Non-Aroclor and Aroclor PCB mixtures in the Great Lakes. J Great Lakes Res 37(2):359–364 doi: 10.1016/j.jglr.2011.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibhazehiebo K, Koibuchi N (2012) Temporal effects of thyroid hormone (TH) and decabrominated diphenyl ether (BDE209) on Purkinje cell dendrite arborization. Niger J Physiol Sci 27(1):11–7 [PubMed] [Google Scholar]
- Keil KP, Sethi S, Wilson MD, Chen H, Lein PJ (2017) In vivo and in vitro sex differences in the dendritic morphology of developing murine hippocampal and cortical neurons. Sci Rep 7(1):8486 doi: 10.1038/s41598-017-08459-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Henry EC, Kim DK, et al. (2006) Novel compound 2-methyl-2H-pyrazole-3-carboxylic acid (2-methyl-4-o-tolylazo-phenyl)-amide (CH-223191) prevents 2,3,7,8-TCDD-induced toxicity by antagonizing the aryl hydrocarbon receptor. Mol Pharmacol 69(6):1871–8 doi: 10.1124/mol.105.021832 [DOI] [PubMed] [Google Scholar]
- Kimura E, Kubo K, Matsuyoshi C, et al. (2015) Developmental origin of abnormal dendritic growth in the mouse brain induced by in utero disruption of aryl hydrocarbon receptor signaling. Neurotoxicol Teratol 52(Pt A):42–50 doi: 10.1016/j.ntt.2015.10.005 [DOI] [PubMed] [Google Scholar]
- King TL, Yeats P, Hellou J, Niven S (2002) Tracing the source of 3,3'-dichlorobiphenyl found in samples collected in and around Halifax Harbour. Mar Pollut Bull 44(7):590–6 [DOI] [PubMed] [Google Scholar]
- Koh WX, Hornbuckle KC, Thorne PS (2015) Human Serum from Urban and Rural Adolescents and Their Mothers Shows Exposure to Polychlorinated Biphenyls Not Found in Commercial Mixtures. Environ Sci Technol 49(13):8105–12 doi: 10.1021/acs.est.5b01854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DW, Notter SA, Thiruchelvam M, et al. (2012) Subchronic polychlorinated biphenyl (Aroclor 1254) exposure produces oxidative damage and neuronal death of ventral midbrain dopaminergic systems. Toxicol Sci 125(2):496–508 doi: 10.1093/toxsci/kfr313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein PJ, Yang D, Bachstetter AD, et al. (2007) Ontogenetic alterations in molecular and structural correlates of dendritic growth after developmental exposure to polychlorinated biphenyls. Environ Health Perspect 115(4):556–63 doi: 10.1289/ehp.9773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llobet JM, Bocio A, Domingo JL, Teixido A, Casas C, Muller L (2003) Levels of polychlorinated biphenyls in foods from Catalonia, Spain: estimated dietary intake. J Food Prot 66(3):479–84 [DOI] [PubMed] [Google Scholar]
- Lyall K, Croen LA, Sjodin A, et al. (2016) Polychlorinated Biphenyl and Organochlorine Pesticide Concentrations in Maternal Mid-Pregnancy Serum Samples: Association with Autism Spectrum Disorder and Intellectual Disability. Environ Health Perspect doi: 10.1289/EHP277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AG Ngounou Wetie, KL Wormwood, L Charette, JP Ryan, AG Woods, CC Darie (2015) Comparative two-dimensional polyacrylamide gel electrophoresis of the salivary proteome of children with autism spectrum disorder. J Cell Mol Med 19(11):2664–78 doi: 10.1111/jcmm.12658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olguin-Albuerne M, Moran J (2017) Redox signaling mechanisms in nervous system development. Antioxid Redox Signal doi: 10.1089/ars.2017.7284 [DOI] [PubMed] [Google Scholar]
- Pencikova K, Svrzkova L, Strapacova S, et al. (2018) In vitro profiling of toxic effects of prominent environmental lower-chlorinated PCB congeners linked with endocrine disruption and tumor promotion. Environ Pollut 237:473–486 doi: 10.1016/j.envpol.2018.02.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah IN, Cherednichenko G, Lein PJ (2010) Minding the calcium store: Ryanodine receptor activation as a convergent mechanism of PCB toxicity. Pharmacol Ther 125(2):260–85 doi: 10.1016/j.pharmthera.2009.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson M, Melymuk L, Csiszar SA, Giang A, Diamond ML, Helm PA (2010) Continuing sources of PCBs: the significance of building sealants. Environ Int 36(6):506–13 doi: 10.1016/j.envint.2010.03.009 [DOI] [PubMed] [Google Scholar]
- Rosenquist AH, Hoyer BB, Julvez J, et al. (2017) Prenatal and Postnatal PCB-153 and p,p'-DDE Exposures and Behavior Scores at 5-9 Years of Age among Children in Greenland and Ukraine. Environ Health Perspect 125(10):107002 doi: 10.1289/EHP553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv SK, Thurston SW, Bellinger DC, Tolbert PE, Altshul LM, Korrick SA (2010) Prenatal organochlorine exposure and behaviors associated with attention deficit hyperactivity disorder in school-aged children. Am J Epidemiol 171(5):593–601 doi: 10.1093/aje/kwp427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Karelina K, Obrietan K (2011) CREB: a multifaceted regulator of neuronal plasticity and protection. J Neurochem 116(1):1–9 doi: 10.1111/j.1471-4159.2010.07080.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantz SL, Widholm JJ, Rice DC (2003) Effects of PCB exposure on neuropsychological function in children. Environmental health perspectives 111(3):357–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealey LA, Hughes BW, Sriskanda AN, et al. (2016) Environmental factors in the development of autism spectrum disorders. Environ Int 88:288–298 doi: 10.1016/j.envint.2015.12.021 [DOI] [PubMed] [Google Scholar]
- Sethi S, Keil KP, Chen H, et al. (2017) Detection of 3,3'-Dichlorobiphenyl in Human Maternal Plasma and Its Effects on Axonal and Dendritic Growth in Primary Rat Neurons. Toxicol Sci doi: 10.1093/toxsci/kfx100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh L, Pressly B, Mengeling BJ, et al. (2016) Chasing the Elusive Benzofuran Impurity of the THR Antagonist NH-3: Synthesis, Isotope Labeling, and Biological Activity. J Org Chem 81(5):1870–6 doi: 10.1021/acs.joc.5b02665 [DOI] [PubMed] [Google Scholar]
- Sirbasku DA, Pakala R, Sato H, Eby JE (1991) Thyroid hormone dependent pituitary tumor cell growth in serum-free chemically defined culture. A new regulatory role for apotransferrin. Biochemistry 30(30):7466–77 [DOI] [PubMed] [Google Scholar]
- Supekar K, Uddin LQ, Khouzam A, et al. (2013) Brain hyperconnectivity in children with autism and its links to social deficits. Cell Rep 5(3):738–47 doi: 10.1016/j.celrep.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi S, Anezaki K, Kojima H (2017) Effects of unintentional PCBs in pigments and chemical products on transcriptional activity via aryl hydrocarbon and nuclear hormone receptors. Environ Pollut 227:306–313 doi: 10.1016/j.envpol.2017.04.059 [DOI] [PubMed] [Google Scholar]
- Todd PK, Mack KJ (2001) Phosphorylation, CREB, and Mental Retardation. Pediatr Res 50(6):672 doi: 10.1203/00006450-200112000-00002 [DOI] [PubMed] [Google Scholar]
- Vondracek J, Machala M, Bryja V, et al. (2005) Aryl hydrocarbon receptor-activating polychlorinated biphenyls and their hydroxylated metabolites induce cell proliferation in contact-inhibited rat liver epithelial cells. Toxicol Sci 83(1):53–63 doi: 10.1093/toxsci/kfi009 [DOI] [PubMed] [Google Scholar]
- Wayman GA, Bose DD, Yang D, et al. (2012a) PCB-95 modulates the calcium-dependent signaling pathway responsible for activity-dependent dendritic growth. Environ Health Perspect 120(7):1003–9 doi: 10.1289/ehp.1104833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman GA, Impey S, Marks D, et al. (2006) Activity-dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron 50(6):897–909 doi: 10.1016/j.neuron.2006.05.008 [DOI] [PubMed] [Google Scholar]
- Wayman GA, Yang D, Bose DD, et al. (2012b) PCB-95 promotes dendritic growth via ryanodine receptor-dependent mechanisms. Environ Health Perspect 120(7):997–1002 doi: 10.1289/ehp.1104832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SS, Birnbaum LS (2009) An overview of the effects of dioxins and dioxin-like compounds on vertebrates, as documented in human and ecological epidemiology. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 27(4):197–211 doi: 10.1080/10590500903310047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie F, Li BX, Kassenbrock A, et al. (2015) Identification of a Potent Inhibitor of CREB-Mediated Gene Transcription with Efficacious in Vivo Anticancer Activity. J Med Chem 58(12):5075–87 doi: 10.1021/acs.jmedchem.5b00468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Kania-Korwel I, Ghogha A, et al. (2014) PCB 136 atropselectively alters morphometric and functional parameters of neuronal connectivity in cultured rat hippocampal neurons via ryanodine receptor-dependent mechanisms. Toxicol Sci 138(2):379–92 doi: 10.1093/toxsci/kft334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Kim KH, Phimister A, et al. (2009) Developmental exposure to polychlorinated biphenyls interferes with experience-dependent dendritic plasticity and ryanodine receptor expression in weanling rats. Environ Health Perspect 117(3):426–35 doi: 10.1289/ehp.11771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye BS, Leung AOW, Wong MH (2017) The association of environmental toxicants and autism spectrum disorders in children. Environ Pollut 227:234–242 doi: 10.1016/j.envpol.2017.04.039 [DOI] [PubMed] [Google Scholar]
- Zheng F, Kasper LH, Bedford DC, Lerach S, Teubner BJ, Brindle PK (2016) Mutation of the CH1 Domain in the Histone Acetyltransferase CREBBP Results in Autism-Relevant Behaviors in Mice. PLoS One ll(l):e0146366 doi: 10.1371/journal.pone.0146366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller RT (2007) Environmental chemicals impacting the thyroid: targets and consequences. Thyroid 17(9):811–7 doi: 10.1089/thy.2007.0107 [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Dowling AL, Vas AA (2000) Developmental exposure to polychlorinated biphenyls exerts thyroid hormone-like effects on the expression of RC3/neurogranin and myelin basic protein messenger ribonucleic acids in the developing rat brain. Endocrinology 141(1):181–9 doi: 10.1210/endo.141.1.7273 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.