Abstract
There is now compelling evidence for membrane-associated estrogen receptors in hypothalamic neurons that are critical for the hypothalamic control of homeostatic functions. It has been known for some time that estradiol (E2) can rapidly alter hypothalamic neuronal activity within seconds, indicating that some cellular effects can occur via membrane initiated events. However, our understanding of how E2 signals via membrane-associated receptors and how these signals impact physiological functions is only just emerging. Thus, E2 can affect second messenger systems including calcium mobilization and a plethora of kinases to alter cell excitability and even gene transcription in hypothalamic neurons. One population of hypothalamic neurons, the anorexigenic proopiomelanocortin (POMC) neurons, has long been considered to be a target of E2’s actions based on gene (Pomc) expression studies. However, we now know that E2 can rapidly alter POMC neuronal activity within seconds and activate several intracellular signaling cascades that ultimately affect gene expression, actions which are critical for maintaining sensitivity to insulin in metabolically stressed states. E2 also affects the orexigenic Neuropeptide Y/Agouti-related Peptide (NPY/AgRP) neurons in similarly rapid but antagonistic manner. Therefore, this review will summarize our current state of knowledge of how E2 signals via rapid membrane-initiated and intracellular signaling cascades in POMC and NPY/AgRP neurons to regulate energy homeostasis.
Keywords: β-endorphin, ERα, ERβ, GABAB receptor, Gαq-mER, GIRK channels, NPY/AgRP neurons, PKA, PKC, POMC neurons
Estrogen Neurobiology—Classical Signaling
17β-estradiol (E2) modulates hypothalamic neuronal excitability that ultimately regulates reproduction, energy balance, temperature, circadian rhythms, and stress. In addition, E2 is involved in neuronal synaptic plasticity in the hippocampus, striatum and cerebellum (Grove-Strawser et al., 2010; Hedges et al., 2012; Woolley, 2007). E2 signaling in the hypothalamus is the quintessential function that controls reproduction (Kelly and Ronnekleiv, 2008; Kelly and Rønnekleiv, 2015; Kelly et al., 2013; Micevych and Kelly, 2012; Moenter et al., 2003; Sinchak and Wagner, 2012). In females, E2 signaling in the hypothalamus is the basis of positive and negative feedback within the hypothalamic-pituitary-ovarian axis. The endocrine status of gonads is communicated to the brain by circulating E2 that activates hypothalamic circuits that regulate ovulation. E2 both inhibits and stimulates the release of gonadotropin-releasing hormone (GnRH) and luteinizing hormone (LH), as well as follicle stimulating hormone (FSH) and stimulates sexual behavior. E2 binds to and activates the classical estrogen receptors ERα and ERβ, but also G protein-coupled metabotropic receptors. We now know that many of these actions of E2 are mediated via its presynaptic effects on Kisspeptin (Kiss1) neurons in the anteroventral periventricular/periventricular nuclei (AVPV/PeN) (Clarkson and Herbison, 2009; Smith et al., 2005; Zhang et al., 2015).
Classically, ERs were defined by their ability to bind estrogens and elicit a specific response (Jensen and DeSombre, 1973). They were initially considered cytosolic receptors that upon E2 binding underwent a conformational change and translocation to the nucleus where they interacted with DNA to regulate the expression of targeted genes. Now it is thought that they are found either in the nucleus or associated with the plasma membrane (Levin, 2009). ERα (ESR1) and ERβ (ESR2) were cloned in the 1980’s and 1990’s, respectively (Kuiper et al., 1996; Walter et al., 1985). Although they are the product of different genes, ERα and ERβ share a similar modular structure that binds E2 and have significant sequence homology, especially in their DNA and ligand binding domains. Also, ERα and ERβ interact with other transcription factors, such as Fos and Jun, which bind DNA at the activator protein-1 (AP-1) site, to regulate transcription independent of the unique DNA sequences known as estrogen response elements (EREs) (Kushner et al., 2000; Paech et al., 1997).
Early studies utilizing 3H-17β-estradiol identified binding sites in the brain and revealed that estradiol-concentrating neurons were localized in hypothalamic regions including the preoptic (POA), periventricular (PV) and arcuate nuclei (Pfaff and Keiner, 1973; Sar, 1984; Sar and Stumpf, 1975; Tardy and Pasqualini, 1983; Warembourg, 1977). Once ERα and ERβ were cloned, their distribution was thoroughly elucidated using in situ hybridization and/or immunocytochemistry (DonCarlos et al., 1991; Gréco et al., 2001; Gundlah et al., 2000; Kruijver et al., 2002, 2003; Laflamme et al., 1998; Osterlund et al., 2000; Sar and Parikh, 1986; Shughrue et al., 1997; Shughrue and Merchenthaler, 2001; Simerly et al., 1990). ERα is robustly expressed in regions such as the preoptic area (POA), bed nucleus stria terminalis (BNST), amygdala, periventricular nucleus (PeN), ventrolateral part of the ventromedial nucleus of the hypothalamus (VMH) and the arcuate nucleus. ERβ is found in many of the same regions, but is more highly expressed in the BNST, POA, paraventricular nucleus of the hypothalamus (PVH) and supraoptic nuclei (SON), with some notable species differences (Kruijver et al., 2003; Laflamme et al., 1998; Mitra et al., 2003; Shughrue et al., 1997; Warembourg and Leroy, 2004). ERα and ERβ are also found in other brain regions including the cortex, hippocampus, midbrain, striatum (Merchenthaler et al., 2004; Shughrue et al., 1997) and in dorsal root ganglion neurons (Chaban and Micevych, 2007). Co-localization studies have identified ERα in hypothalamic neurons containing GABA, neurotensin, somatostatin, galanin, dopamine, norepinephrine, neuropeptide Y (NPY), proopiomelanocortin (POMC) and kisspeptin (Flugge et al., 1986; Herbison, 1994; Herbison and Theodosis, 1992; Horvath et al., 1995; Hu et al., 2006; Laflamme et al., 1998; Lehman and Karsch, 1993; Roepke et al., 2007; Skinner and Herbison, 1997). ERβ is expressed in different populations of hypothalamic neurons: GnRH, vasopressin, oxytocin, and nociceptin/orphanin FQ, as well as in midbrain serotonin neurons (Cardona-Gomez et al., 2000; Gundlah et al., 2001; Herbison et al., 2001; Hrabovszky et al., 1998; Hrabovszky et al., 2004; Hrabovszky et al., 2000; Hrabovszky et al., 2001; Isgor et al., 2003; Kallo et al., 2001; Skynner et al., 1999). ERα and ERβ are co-localized in neurons expressing corticotropin releasing hormone and insulin-like growth factor I (IGF-I), as well as in subpopulations of unidentified hypothalamic neurons (Bao et al., 2005; Cardona-Gomez et al., 2000; Gréco et al., 2001; Shughrue et al., 1998).
The nuclear-initiated signaling of estradiol via ERα and ERβ exerts diverse effects in a number of tissues that involve gene stimulation as well as gene repression (Couse and Korach, 1999; Etgen et al., 2001; Herbison, 1998; Kininis et al., 2007; Nilsson et al., 2001; Stossi et al., 2006). In general, the “classical” signaling pathway of E2 involves steroid-dependent formation of nuclear estrogen receptor homo- or heterodimers and the subsequent binding of this complex to an ERE, in E2-responsive gene promoters and enhancers (Gruber et al., 2004; Muramatsu and Inoue, 2000; O’Malley and Tsai, 1992).
However, there are many genes in the brain that are estrogen-responsive that do not appear to contain ERE sequences (Gruber et al., 2004; Malyala et al., 2004). There is compelling evidence that ERα and ERβ can regulate transcription of some of these “estrogen-responsive” genes by interacting with other DNA-bound transcription factors, such as specificity protein-1 (SP-1) and activator protein 1 (AP-1), rather than binding directly to DNA (Gruber et al., 2004; Jacobson et al., 2003; Paech et al., 1997). In contrast to ERα, the ligand-induced responses with ERβ at an AP-1 site illustrate the negative transcriptional regulation by estrogens and strong positive regulation by ER antagonists like ICI 164,384 (Paech et al., 1997). In addition, Kiss1 mRNA is differentially regulated by E2 in the AVPV/PeN and arcuate nucleus. Although the positive E2 regulation of Kiss1 mRNA expression in the AVPV is dependent on an ERE-binding site, the down regulation of Kiss1 mRNA in the arcuate nucleus is via an ERE-independent mechanism (Gottsch et al., 2009). Therefore, there are potentially multiple mechanisms for differential regulation of gene expression by E2 via nuclear-initiated signaling.
Another parallel line of research developed in the 1970’s that implicated E2 in rapid, non-genomic actions in numerous neuronal and non-neuronal cells: E2 membrane signaling rapidly increased levels of cAMP in the uterus (Szego and Davis, 1967), altered firing of hypothalamic neurons within seconds (Kelly et al., 1976) and the release of neuropeptides (Sarkar and Fink, 1980). However, the concept of “rapid” non-genomic effects for estrogen signaling was foreign to neuroendocrinologists. Although E2 elicited effects on hypothalamic and striatal neurons at subnanomolar concentrations, there did not appear to be identifiable steroid receptors associated with the plasma membrane for mediating these rapid actions (Lagrange et al., 1997; Mermelstein et al., 1996). This changed in the 1990’s when membrane localization of ERα was documented in pituitary cells and primary cultures of hippocampal CA1 neurons (Clarke et al., 2000; Pappas et al., 1994). Moreover, Razandi et al., (Razandi et al., 1999) discovered that nuclear and membrane receptors were encoded by the same estrogen receptor genes, and ERα and ERβ were shown to complex with G protein signaling cascades. In addition, several groups identified membrane estrogen receptors (mERs) that were not derived from ERα or ERβ transcripts (Qiu et al., 2003; Qiu et al., 2006; Toran-Allerand et al., 2002) including a bona fide G protein-coupled receptor, GPR30/GPER1 (Filardo et al., 2000; Revankar et al., 2005). It was evident from the investigation of “non-genomic” signaling that while some of these signaling cascades initiated at the membrane were tied to rapid membrane effects on ion channel activity, others led to the regulation of gene transcription - similar to the membrane-to-nucleus signaling described for many neurotransmitters (Wu et al., 2001). With this caveat in mind, it has been more accurate to differentiate between membrane-initiated signaling and nuclear-initiated signaling when discussing hormone actions in neurons and non-neural cells (Hammes and Levin, 2007). Therefore, this review will focus on the role of mERs in hypothalamic functions with an emphasis on energy homeostasis, keeping in mind that similar membrane-initialed actions of E2 have been documented in other brain structures such as the hippocampus, striatum and cerebellum, CNS structures involved in cognition and motor functions, respectively (Grove-Strawser et al., 2010; Hedges et al., 2012; Woolley, 2007).
Estrogen Neurobiology—Non-classical signaling
Selective membrane binding sites for E2 were first identified on endometrial cells (Pietras and Szego, 1977; Pietras and Szego, 1979), and later studies revealed relatively high affinity, specific binding of [3H]-17β-estradiol to synaptosomal membranes prepared from the adult rat brain (Towle and Sze, 1983). The binding in the central nervous system (CNS) was later corroborated using the membrane impermeant 17β-estradiol-6-[125I]-conjugated to bovine serum albumin (BSA) (Zheng and Ramirez, 1997). Furthermore, competition-binding assays of synaptosomal membranes showed that the hypothalamus exhibited a relatively high affinity (3 nM) binding site for E2 and somewhat lower affinity binding sites in the olfactory bulb and cerebellum (Ramirez and Zheng, 1996; Ramirez et al., 1996). The stereospecificity of the binding was demonstrated by displacement of the radiolabeled E2 with cold E2 or E2-BSA, but not by 17α-estradiol or 17α-estradiol-BSA even at micromolar concentrations (Ramirez et al., 1996).
In parallel electrophysiological studies E2 was shown to have acute, rapid membrane-initiated signaling actions in many CNS structures including the hypothalamus (Kelly et al., 1976; Kelly et al., 1977a, 1978a, b; Kelly et al., 1977b; Kelly and Rønnekleiv, 2002; Kelly et al., 1984; Micevych and Dominguez, 2009; Qiu et al., 2003; Qiu et al., 2006; Ronnekleiv and Kelly, 2005; Smith et al., 2013). Three decades ago the nature and physiological significance of these actions were a matter of debate, but it is now generally accepted that some of the actions of E2 are much too fast to be attributed to the classical nuclear-initiated steroid signaling of ERα or ERβ. However, ERα and ERβ can associate with signaling complexes in the plasma membrane—e.g., caveolins (Bondar et al., 2009; Boulware et al., 2005; Dewing et al., 2007; Pedram et al., 2006; Razandi et al., 1999; Szegõ et al., 2006). Caveolin-dependent clustering allows ERα to activate an associated metabotropic glutamate receptor (mGluR) (Boulware et al., 2007), altering the phosphorylation of CREB, with protein kinase C acting as an intermediary (Dewing et al., 2008). In addition, mERs can trigger mitogen-activated protein kinase (MAPK) via mGluR1a and phospholipase C (PLC) or inhibit L-type Ca2+ channels through mGluR2/3 and decreased cAMP production (Boulware et al., 2005) (see Kelly and Rønnekleiv, 2008 for review). Finally, many of the rapid effects of E2 can be induced by selective ERα or ERβ ligands, antagonized by the ER antagonist ICI 182,780 and abrogated in animals bearing mutations in ERα and/or ERβ genes (Abraham et al., 2003; Boulware et al., 2007; Boulware et al., 2005; Couse and Korach, 1999; Dubal et al., 2001; Singer et al., 1999; Wade et al., 2001).
It is also evident that E2 can activate bona fide G protein-coupled receptors (GPCRs), the most notable being GPR30 and a putative Gαq-coupled membrane ER (Gu et al., 1999; Kenealy et al., 2011; Noel et al., 2009; Qiu et al., 2003; Qiu et al., 2006; Toran-Allerand, 2004; Toran-Allerand, 2005). Over the years, evidence has been generated in the support of a novel Gαq-coupled membrane ER (Gαq-mER). Intracellular sharp electrode and whole cell patch recording from guinea pig and mouse hypothalamic slices were used to characterize this Gαq-mER (Lagrange et al., 1997; Qiu et al., 2003; Qiu et al., 2006; Smith et al., 2013). These two independent electrophysiological methods established that E2 acts rapidly and stereospecifically within physiologically-relevant concentrations to significantly reduce the potency of μ-opioid and GABAB agonists (i.e., heterologous desensitization) to activate G protein-coupled inwardly rectifying K+ (GIRK) channels (Lagrange et al., 1997; Qiu et al., 2003). Estrogenic desensitization of μ-opioid and GABAB receptors was mimicked by stimulation of adenylyl cyclase with forskolin or by direct protein kinase A (PKA) activation with the non-hydrolyzable cAMP analog Sp-cAMP, in a concentration-dependent manner (Lagrange et al., 1997; Qiu et al., 2003). Furthermore, the selective PKA antagonists KT5720 and Rp-cAMP blocked the effects of E2. As predicted from the literature on desensitization of GPCRs (Gainetdinov et al., 2004), PKA is downstream in a signaling cascade that is initiated by a Gαq-coupled mER that is linked to activation of phospholipase C (PLC)-protein kinase C (PKC)-protein kinase A (PKA) (Qiu et al., 2003; Qiu et al., 2006). It should be emphasized that E2 does not alter the affinity of the μ-opioid and GABAB ligands for their respective receptors (Cunningham et al., 1998). In addition, E2 uncouples opioid receptor-like 1 (Mela et al., 2016) and cannabinoid receptor one (Conde et al., 2016) from their respective effector systems in POMC neurons, more specifically A-type K+ channels and Ca2+-activated K+ channels. Presynaptic to POMC neurons, E2 rapidly attenuates the ability of cannabinoid signaling to reduce glutamate release (Jeffery et al., 2011; Washburn et al., 2013). The actions of E2 on metabotropic receptors is not restricted to negative modulation as the activity of 5HT2C (Gq-coupled) receptor agonists are augmented by E2 in POMC neurons thereby augmenting the anorexigenic activity of serotonin drugs (Qiu et al., 2007). Therefore, E2 attenuates Gi,o-coupled receptor signaling but augments Gq-coupled receptor signaling in POMC neurons. Furthermore, the ER antagonists ICI 164,384 and ICI 182,780 block the actions of E2 with subnanomolar affinity (K i = 0.5 nM), which is similar to K i for antagonism of ERα (Lagrange et al., 1997; Weatherill et al., 1988). These pharmacological findings clearly argued for a G protein-coupled membrane receptor with high selectivity for E2.
The results from these early physiological and pharmacological experiments led to the design of STX, which is structurally similar to 4-OH tamoxifen, for selectively targeting the Gαq-mER signaling pathway (Qiu et al., 2003). As predicted, STX has greater affinity (~20-fold) for the Gαq-mER than E2, and most importantly, does not bind to ERα or ERβ (Qiu et al., 2006; Tobias et al., 2006). Furthermore, both STX and E2 activate the Gαq signaling pathway in POMC neurons in mice lacking both ERα and ERβ and in GPR30-knockout mice (Qiu et al., 2006; Qiu et al., 2008). More recent studies indicate that STX can rapidly increase neurotransmitter release from POMC neurons onto NPY/AgRP neurons, which is indicative of its presynaptic actions to inhibit the inhibitory GABAB receptor in POMC nerve terminals (Figure 1) (Stincic et al., 2017). These actions would further enhance the anorexigenic actions of E2 in the POMC-NPY/AgRP circuitry. The ability of STX to robustly mimic the rapid effects of E2 on POMC neuronal activity led to the hypothesis that the putative Gαq-mER has a role in the control of energy homeostasis. Indeed, peripheral administration of STX is found to mimic the effects of E2 in controlling energy homeostasis (Qiu et al., 2006; Roepke et al., 2010; Roepke et al., 2008). Both E2 and STX reduce food intake, body weight gain and abdominal fat accumulation following ovariectomy.
Figure 1. Presynaptic actions of 17β-estradiol (E2) in POMC neurons.
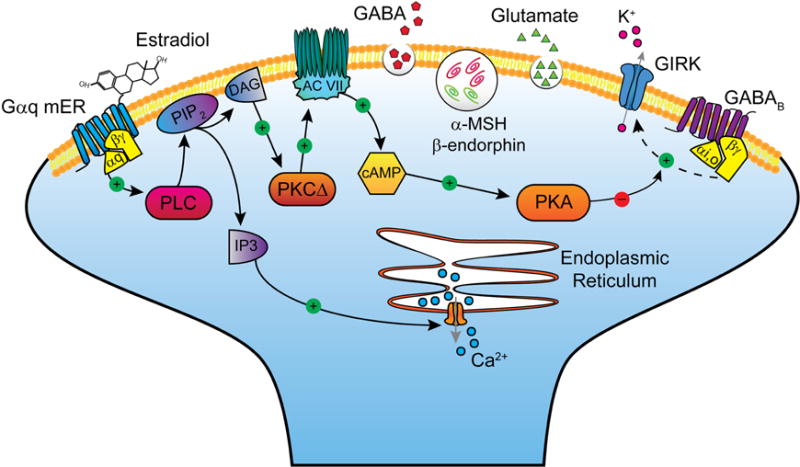
Schematic overview of the E2-mediated modulation of Gαi,o-coupled GABAB receptors via a membrane-associated receptor (mER) in hypothalamic POMC nerve terminals. E2 binds to a mER that is Gαq-coupled to activate phospholipase C and catalyzes the hydrolysis of membrane-bound phosphatidylinositol 4,5-biphosphate (PIP2) to inositol 1,4,5 triphosphate (IP3) and diacylglycerol (DAG). Calcium is released from intracellular stores (endoplasmic reticulum) by IP3, and DAG activates protein kinase C Δ (PKCΔ). Through phosphorylation, adenylyl cyclase VII (AC VII) activity is upregulated by PKC. The generation of cAMP activates PKA, which can uncouple (dashed line) GABAB receptors from their signaling pathway through phosphorylation of a downstream effector molecule (e.g., G protein-coupled, inwardly rectifying K+, GIRK, channels). Together, elevated intracellular Ca2+ and attenuation of GABAB-mediated inhibition will facilitate the release of multiple neurotransmitters—GABA, glutamate, β-endorphin and α-melanocyte stimulating hormone (α-MSH)—from POMC neurons.
Estradiol Signaling in POMC and NPY/AgRP neurons
Energy Homeostasis
Hypothalamic POMC and neuropeptide Y/agouti-related peptide (NPY/AgRP) neurons reside within the arcuate nucleus and compose a critical circuit for regulating energy homeostasis (Gao and Horvath, 2007). Selective optogenetic stimulation of NPY/AgRP neurons evokes intense feeding (Aponte et al., 2011), and ablation of these neurons in adults causes starvation (Arroyo et al., 2006; Luquet et al., 2005; Wu et al., 2009). Similar genetic approaches have revealed that POMC cells have the exact opposite actions in the control of energy homeostasis (Aponte et al., 2011; Gao et al., 2007; Kavalali et al., 2011; Qiu et al., 2006; Shi et al., 2010; Xu et al., 2011). Moreover, these two populations of neurons regulate feeding through sensing circulating levels of metabolic hormones, thereby altering their firing frequency and the release of peptide and/or amino acid neurotransmitters onto target neurons in the paraventricular nucleus and other hypothalamic nuclei (Gao and Horvath, 2007).
POMC and NPY/AgRP neurons are also sensitive to circulating estrogens. Experiments dating back 30-40 years determined that the anorectic effects of E2 in rodents are mediated through CNS sites of action since direct injections of E2 into the arcuate/ventromedial nucleus were effective to reduce food intake, body weight and increase wheel running activity in females (Ahdieh and Wade, 1982; Butera and Czaja, 1984; Colvin and Sawyer, 1969). Moreover, E2 increases the expression of the peptide β-endorphin in POMC neurons in ovariectomized female guinea pigs (Bethea et al., 1995; Thornton et al., 1994), and in postmortem studies there is a decrease in hypothalamic β-endorphin expression associated with weight gain in postmenopausal women who abstained from hormone replacement therapy (Leal et al., 1998). More recently it has been shown that E2 signaling via ERα is a critical component in the regulation of energy homeostasis (Geary et al., 2001). In rodents, hypo-estrogenic states are clearly associated with decreased activity and an increase in body weight (Asarian and Geary, 2002; Butera and Czaja, 1984; Clegg et al., 2006; Clegg et al., 2007; Czaja, 1984; Czaja and Goy, 1975; Jones et al., 2000; McCaffrey and Czaja, 1989; Qiu et al., 2006). In humans a loss-of-function mutation in ERα, reported in a case history from one male individual, resulted in a clear metabolic phenotype with expression of type 2 diabetes, hyperinsulinemia and obesity (Smith et al., 1994). However, global reinstatement of an ERα that is lacking the ERE targeting domain is sufficient for “rescuing” the metabolic deficits in mice (Park et al., 2011). These findings suggest an important role for non-ERE mediated E2 signaling, albeit this could be via other transcriptional activity (Hewitt et al., 2014) or membrane-initiated signaling of ERα as in NPY/AgRP neurons (see discussion below). Indeed, a point mutation in ERα (C451A), which precludes palmitoylation and membrane trafficking of ERα globally, creates an obese phenotype with excessive visceral fat deposition in mice fed a normal chow diet (Pedram et al., 2014). Moreover, brain-specific knockout of ERα causes hyperphagia and hypometabolism (Musatov et al., 2007; Xu et al., 2011), and selective knockdown of ERα in POMC neurons recapitulates the hyperphagic phenotype in female mice (Xu et al., 2011). However, one must be cautious in interpreting global and conditional ERα gene deletion experiments since ERα is a transcription factor affecting the expression of hundreds of genes important for cell signaling, and many of these genes are essential for membrane initiated actions of E2 that contribute to POMC excitability and hence control of energy homeostasis (Malyala et al., 2004). With this caveat in mind, it appears that the arcuate nucleus and specifically POMC neurons are major targets for the anorectic actions of estrogens via ERα signaling, which underscores their importance in the control of energy homeostasis. In addition, POMC neurons are also involved in the rewarding aspects of food ingestion (Appleyard et al., 2003; Hayward and Low, 2007; Hayward et al., 2002).
There is also a complementary Gαq-mER-mediated anorexigenic pathway in NPY/AgRP neurons. E2 and the Gαq-mER ligand STX rapidly enhance (sensitize) the GABAB receptor agonist baclofen activation of GIRK channels, and this effect is blocked by the estrogen receptor antagonist ICI 182,780 (Smith et al., 2013). On the other hand, an ERα-mediated signaling pathway exists that opposes the Gαq-mER signaling cascade: activating ERα with the selective agonist propyl pyrazole triol (PPT) rapidly suppresses GABAB mediated activation of GIRK channels in NPY/AgRP neurons (Smith et al., 2013). In gonadectomized mice, the “non-selective” ligand E2 can either enhance or suppress GABAB-mediated currents (Smith et al., 2013). However, co-administering phosphatidylinositol 3 Kinase (PI3K) inhibitors, specifically a selective inhibitor of p110β, results in E2 enhancing the GABAB receptor-mediated response similar to effects of STX. Thus, ERα via PI3K attenuates (desensitizes) the GABAB receptor–mediated response in NPY/AgRP neurons. Physiologically, the effects of E2 could depend on the relative expression of ERα versus Gαq-mER in these orexigenic cells. NPY/AgRP neurons may serve a metabolic function when Gαq-mER expression predominates, whereas they may serve a reproductive function when more ERα is expressed (Figure 2) (see (Acosta-Martinez et al., 2006) for review).
Figure 2. Effects of E2 and STX on hypothalamic POMC and NPY/AgRP neurons.
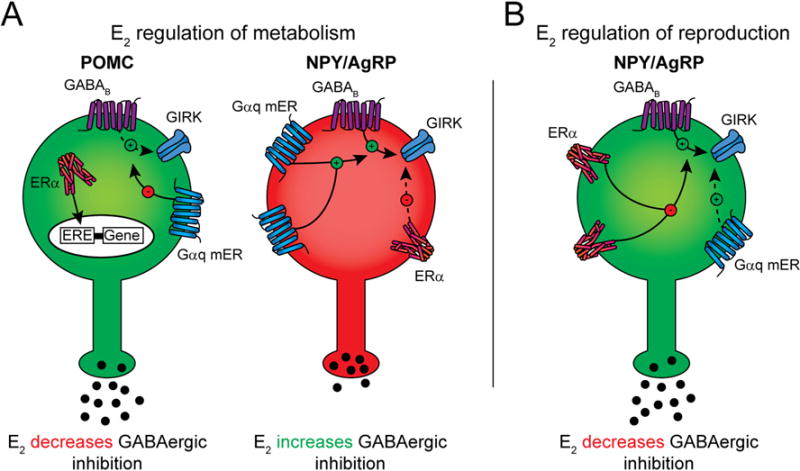
Summary of how E2 excites POMC neurons and inhibits NPYAgRP neurons via a Gαq-mER and ERα mediated signaling pathways to regulate metabolism (A). Activation of the Gαq pathway attenuates the coupling of the GABAB receptor to GIRK channel activation in POMC neurons, thereby increasing the excitability, but enhances the GABAB receptor activation of GIRK channels in NPY/AgRP neurons, thus decreasing their excitability. In addition, upon binding E2, ERα inside the cell can activate estrogen response elements (ERE) to initiate changes in gene transcription. However, in NPY/AgRP neurons, ERα is also associated with the membrane, and its activation leads to attenuation of the GABAB receptor coupling to GIRK channels (B), which is hypothesized to be involved in the control of reproduction. Abbreviations: Gαq-mER, Gαq-coupled membrane estrogen receptor; GIRK, G protein-coupled inwardly rectifying potassium channel; ERα, estrogen receptor α; NPY/AgRP, neuropeptide Y/Agouti-related peptide; POMC, proopiomelanocortin.
Systemic treatment with STX, similar to E2, regulates gene transcription in the arcuate nucleus, and many of the genes are involved in the control of neuronal excitability (e.g., the T-type calcium channel transcript Cav3.1) and intracellular signaling cascades in arcuate neurons (Roepke et al., 2008). For example, the PI3K regulatory subunits are regulated by E2 and STX: PI3K p55γ mRNA is increased by E2 treatment (Malyala et al., 2008) and PI3K p85α mRNA is upregulated by STX (Roepke et al., 2008). Therefore, the putative Gαq-mER may also function in the estrogenic control of energy homeostasis through direct excitation of POMC and direct inhibition of NPY/AgRP neurons through a Gαq signaling cascade to alter gene transcription in these anorexigenic and orexigenic neurons, respectively (Figure 2). Indeed, the electrophysiological effects of STX in NPY/AgRP neurons are consistent with the finding that STX down-regulates arcuate NPY mRNA expression in ovariectomized female guinea pigs (Roepke et al., 2008).
Circulating levels of E2 are in the low to high pM range, and these actions of E2 to rapidly alter POMC and NPY/AgRP neuronal activity are in the high picomolar range (Ki of ICI 164,384 = 0.5 nM (Lagrange et al., 1997)). Also, there is evidence that E2 is locally synthesized and released (>1 μg/ml) from the mediobasal hypothalamus of rhesus macaques (Kenealy et al., 2013; Kenealy et al., 2017). Kennealy et al. (Kenealy et al., 2017) suggest that there is an obligatory role for “neuroestradiol” in the estrogen-induced LH surge in the rhesus macaque. Neuroestrogen production has also been measured in the hippocampus, cerebellum and brainstem (see (Terasawa and Kenealy, 2012) for review). Therefore, not only ovarian estrogens but locally produced E2 could activate the Gαq-mER signaling cascade to provide continued excitation of POMC neurons and inhibition of NPY/AgRP neurons. However, food intake is depressed during the preovulatory phase of the menstrual cycle in humans, monkeys and guinea pigs that correlates with peak levels of circulating E2 (see (Dye and Blundell, 1997) for review); and ovariectomy (or menopause) often leads to increased food intake and weight gain, which argues for a substantial role of ovarian E2 feedback in the hypothalamic control of energy homeostasis.
POMC neurons and insulin resistance
The pleiotropic effects of insulin (and leptin) in POMC neurons are vital for both the short term (excitability) and long term (transcriptional) modulation of POMC neuronal activity and the control of food intake and energy homeostasis. POMC and NPY/AgRP neurons are major CNS targets of insulin and leptin actions (Belgardt and Bruning, 2010; Morton et al., 2006; Qiu et al., 2014; Schwartz et al., 2000). Insulin delivered directly into the third ventricle decreases food intake in guinea pigs (Qiu et al., 2014), mice (Benoit et al., 2002; Brown et al., 2006) and rats (Clegg et al., 2011). Insulin depolarizes POMC neurons in both males and females via activation of canonical transient receptor potential (TRPC5) channels, and hyperpolarizes NPY/AgRP neurons via activation of KATP channels (Qiu et al., 2014), activity that is congruent with the anorexigenic effects of insulin. The increase in POMC cell excitability induced by insulin translates into heightened transcriptional activity—i.e., an increase in Fos expression in the arcuate nucleus and specifically in POMC neurons following icv insulin (Qiu et al., 2014).
In POMC neurons the insulin receptor (InsR) couples to PI3K p110β activation (Al-Qassab et al., 2009; Xu et al., 2005), and the InsR-mediated excitation of POMC neurons is abrogated by inhibition of PI3K activity (Al-Qassab et al., 2009; Hill et al., 2008; Qiu et al., 2010; Qiu et al., 2014). Activation of PI3K generates phosphatidylinositol-3,4,5-triphosphate (PIP3), which stimulates phospholipase C (PLC) and protein kinase B (Akt) (Bae et al., 1998; Falasca et al., 1998; Qiu et al., 2014; Rameh et al., 1998). PLC also hydrolyzes PIP2, which modulates TRPC5 channel activity (Figure 3) (Qiu et al., 2014; Rodríguez-Menchaca et al., 2012; Zhang et al., 2013). In addition, PI3K increases the insertion of TRPC5 channels into the plasma membrane from intracellular vesicular pools to further boost depolarization and Ca2+ entry into POMC neurons (Bezzerides et al., 2004). Collectively, all of these PI3K-mediated effects are involved in the actions of insulin in POMC neurons.
Figure 3. A cellular model of insulin signaling via TRPC5 channel activation in POMC neurons.
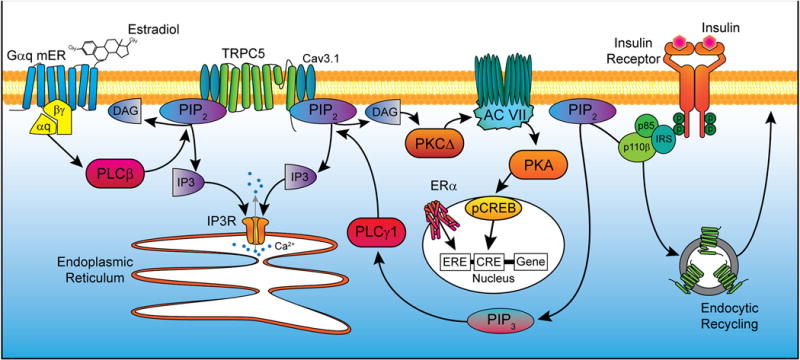
Activation of a Gq-mER by E2 stimulates phospholipase C β (PLCβ) to catalyze the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) into diacylglycerol and inositol 1,4,5-trisphosphate (IP3). IP3 activates the IP3 receptor, causing release of calcium (Ca2+) from the endoplasmic reticulum. DAG drives protein kinase CΔ to increase the activity of cAMP production by adenylyl cyclase VII which in turn drives protein kinase A activity (PKA). cAMP response element-binding protein (CREB) in the nucleus is phosphorylated, allowing for interactions with certain DNA sequences to alter gene transcription. Alternatively, ERα can dimerize after binding E2 and act on estrogen response elements (ERE) in the nucleus to similarly affect expression of target genes. Insulin signals via insulin receptor substrate-phosphoinositide 3 kinase (IRS-PI3K) to activate TRPC5 channels in POMC neurons, which generates a robust inward cationic current to depolarize POMC neurons and increase their excitability. PI3K (p85/p110) will also accelerate the rapid insertion of TPRC5 channels into the plasma membrane (Bezzerides et al., 2004). E2 facilitates TRPC channel activity through upregulation expression of Cav3.1 (T-type calcium) channels and PLC catabolism of PIP2 to facilitate TRPC5 channel opening via ERα and Gαq-mER, respectively.
We recently investigated the neuroprotective effects of E2 against the development of central insulin resistance with diet-induced obesity (Qiu et al., 2018). Although obesity produced dramatic alterations in metabolic phenotype in both males and females, E2 was able to protect females from the development of CNS (hypothalamic) insulin resistance. Insulin was fully efficacious to activate TRPC5 channels and depolarize POMC neurons in diet-induce obese (DIO), proestrous and E2-treated, ovariectomized females but not in ovariectomized female or male DIO mice. Treating ovariectomized females with an estradiol regime that mimics proestrous serum levels of E2 rescued the insulin response in POMC neurons. The neuroprotective effects of E2 were mediated, in part, by upregulation of Cav3.1 mRNA expression and T-type calcium channel currents and downregulation of Stim1 (Stromal interaction Molecule-1) mRNA. STIM1 is localized to the endoplasmic membrane and its N-terminal domain contains an EF-hand that protrudes into the lumen of the endoplasmic reticulum to sense changes in endoplasmic reticulum Ca2+ concentrations (Salido et al., 2011). Upon depletion of endoplasmic reticulum Ca2+, STIM1 undergoes a conformational change, oligomerizes and then interacts with plasma membrane TRPC channels (Salido et al., 2011; Yuan et al., 2007). Phosphorylation of STIM1 is required for oligomerization, and E2 is known to inhibit the phosphorylation of STIM1 and consequently its interaction with plasma membrane and hence store-operated Ca2+ entry (Sheridan et al., 2013). Therefore, in the absence of E2, TRPC channels are more likely to associate with STIM1 and function as store-operated Ca2+ channels (Salido et al., 2011; Yuan et al., 2007). Indeed, the insulin-induced TRPC5 current in POMC neurons in ovariectomized females was enhanced in the presence of a store-operated Ca2+ channel inhibitor (Qiu et al., 2018), and long-term treatment with E2 or STX down-regulated Stim1 mRNA expression in the arcuate nucleus of female guinea pigs (Rønnekleiv, unpublished findings). In addition, E2 prevented the increase in Socs3 expression with diet-induced obesity, which is known to inhibit the coupling of the insulin receptor with its downstream signaling cascade. Therefore, E2 protects the coupling of insulin receptors to TRPC5 channels through multiple Gq-mER and ERα signaling mechanisms.
The significance of this cellular protection of insulin signaling in POMC neurons was highlighted by the fact that icv insulin was fully efficacious in female, but not male, guinea pigs fed a high-fat diet to reduce food intake and increase energy metabolism (Qiu et al., 2018). Therefore, the insulin receptor signaling cascade in POMC neurons appears to be augmented by E2 through membrane (Gq-mER)- and nuclear-initiated (ERα)-signaling to help protect females against insulin resistance (Figure 3), and may help explain why premenopausal women are protected against development of insulin resistance in type II diabetes (Janssen et al., 2008; Margolis et al., 2004).
Thermoregulation
Concomitantly with regulation of food intake and energy metabolism, the hypothalamus is critical for the control of thermogenesis (i.e, the production of heat energy) and the maintenance of core body temperature (Tc) (Morrison et al., 2014), and POMC neurons are involved in generating heat production by brown adipocytes (Dodd et al., 2015). It is also known that circulating estrogens are critical for the maintenance of Tc (Rance et al., 2013); and approximately eighty percent of perimenopausal/postmenopausal (hypo-estrogenic) women experience hot flashes (Moline et al., 2003), which are characterized by periods of sweating and peripheral vasodilation, often associated with increased environmental temperature (Rapkin, 2007). The majority of hot flashes are preceded by elevation in Tc independent of peripheral vasoconstriction or elevated metabolic rate (Freedman, 1998; Freedman, 2005; Freedman et al., 1995). Therefore, elevated Tc may serve as one trigger of menopausal hot flashes (Freedman and Blacker, 2002; Mittelman-Smith et al., 2012; Rapkin, 2007; Roepke et al., 2010). Recent studies have implicated arcuate Kisspeptin neurons, which co-express express Neurokinin B (NKB) and Dynorphin (aka, KNDy neurons) and project to the preoptic temperature sensitive neurons (Mittelman-Smith et al., 2012; Rance et al., 2013). The KNDy neurons also project directly to and activate POMC neurons (Nestor et al., 2016). POMC neurons project directly to the preoptic area, where μ-receptors are highly expressed, and warm-sensitive neurons in this brain area are directly responsive to μ-opioid agonists (Petersen and LaFlamme, 1997; Yakimova et al., 1996; Zhou and Hammer, 1995). Clearly, there is a reduction in the incidence of hot flashes in hypo-estrogenic females with E2 treatment (Brooks et al., 1997; Freedman and Blacker, 2002; Tankersley et al., 1992); and E2 lowers Tc and reduces hot flashes by raising the Tc “sweating threshold” (Brooks et al., 1997; Freedman and Blacker, 2002; Roepke et al., 2010; Tankersley et al., 1992). Furthermore, activation of the medial preoptic GABAergic thermosensitive neurons is responsible for evoking vasomotor responses in rodents (Nakamura and Morrison, 2010), which is thought to correspond to heat dissipation responses (i.e., vasodilatation, sweating) in women experiencing hot flashes. At the cellular level E2 reduces NKB expression in the arcuate Kisspeptin neurons (Rance et al., 2013). Based on the hypothesis that the expression of vasomotor symptoms in menopausal women is due to an elevation in Tc and a reduced thermo-neutral zone in core body temperature (Freedman, 1998; Freedman, 2005; Freedman et al., 1995), we established a guinea pig “hot flash” model and found that both E2 and STX significantly reduce Tc in ovariectomized female guinea pigs compared to animals receiving vehicle injections (Roepke et al., 2010). Similar effects of E2 were reported in rats (Mittelman-Smith et al., 2012), and this was thought to be due to the down-regulation of NKB expression in arcuate Kisspeptin neurons (Ogawa et al., 2003). A complementary increase in POMC neuronal activity (Roepke et al., 2010) would also result in the inhibition of warm sensitive neurons (Petersen and LaFlamme, 1997; Yakimova et al., 1996; Zhou and Hammer, 1995). Therefore, there appears to be a convergence of membrane-initiated signaling by E2 (excitation of POMC) and E2-driven alterations in gene expression (down-regulation of NKB expression in arcuate kisspeptin neurons) to maintain Tc.
Conclusions
Based on decades of research it is now clear that E2 can signal via metabotropic (G protein-coupled) receptors to alter neuronal excitability and autonomic functions controlled by the hypothalamus. Signals initiated by E2 at the plasma membrane can trigger multiple intracellular signaling cascades (e.g., MAPK, PI3K, and PKC) that result in the phosphorylation of hundreds of proteins that ultimately affect not only cell excitability but also gene transcription. The activation of the Gαq-mER in POMC neurons leads to a rapid increase in excitability and the activation of intracellular signaling cascades that ultimately affects gene transcription. In contrast, engagement of the Gαq-mER in NPY/AgRP neurons generates the opposite effects—i.e., an increase in K+ channel activation by the GABAB receptor and downregulation of NPY mRNA expression–which is congruent with the anorexigenic effects of E2. Thus, E2 can act on hypothalamic neurons in a cell-specific manner to generate the appropriate physiological responses by eliciting a combination of rapid changes in membrane excitability accompanied by slower alterations in gene expression. A future challenge will be to identify the putative Gαq-mER, its interaction with other metabotropic receptors and its physiological/behavioral functions not only in the hypothalamus but throughout the CNS.
Highlights.
E2 rapidly alters POMC neuronal activity that ultimately affects gene expression
E2 rapidly affects the orexigenic NPY/AgRP in an antagonistic manner
E2’s actions are critical for the control of food intake and energy homeostasis
E2 maintains the response to insulin in POMC neurons in metabolically stressed states
Acknowledgments
The authors thank previous and current members of their laboratories who contributed to the work described herein, especially Ms. Martha A Bosch and Drs. Anna Malyala, Jian Qiu, Troy A. Roepke and Chunguang Zhang. Research reported in this publication was supported by National Institutes of Health R01 grants NS 038809, NS 043330 and DK 068098.
Funding: Research reported in this publication was supported by National Institutes of Health R01 grants NS 038809, NS 043330 and DK 068098. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham IM, Han SK, Todman MG, Korach KS, Herbison AE. Estrogen receptor beta mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. The Journal of Neuroscience. 2003;23:5771–5777. doi: 10.1523/JNEUROSCI.23-13-05771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta-Martinez M, Horton T, Levine JE. Estrogen receptors in neuropeptide Y neurons: at the crossroads of feeding and reproduction. Trends Endocrinol Metab. 2006;18:48–50. doi: 10.1016/j.tem.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Ahdieh HB, Wade GN. Effects of hysterectomy on sexual receptivity, food intake, running wheel activity, and hypothalamic estrogen and progestin receptors in rats. J Comp Physiol Psychol. 1982;96:886–892. [PubMed] [Google Scholar]
- Al-Qassab H, Smith MA, Irvine EE, Guillermet-Guibert J, Claret M, Choudhury AI, Selman C, Piipari K, Clements M, Lingard S, Chandarana K, Bell JD, Barsh GS, Smith AJH, Batterham RL, Ashford MLJ, Vanhaesebroeck B, Withers DJ. Dominant role of the p110β isoform of PI3K over p110α in energy homeostasis regulation by POMC and AgRP neurons. Cell Metab. 2009;10:343–354. doi: 10.1016/j.cmet.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nature Neuroscience. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleyard SM, Hayward M, Young JI, Butler AA, Cone RD, Rubinstein M, Low MJ. A role for the endogenous opioid beta-endorphin in energy homeostasis. Endocrinology. 2003;144:1753–1760. doi: 10.1210/en.2002-221096. [DOI] [PubMed] [Google Scholar]
- Arroyo A, Kim B, Rasmusson RL, Yeh J. Hyperpolarization-activated cation channels are expressed in rat hypothalamin gonadotropin-releasing hormone (GnRH) neurons and immortalized GnRH neurons. Journal of the Society of Gynecologic Investigation. 2006;13:442–450. doi: 10.1016/j.jsgi.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav. 2002;42:461–471. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- Bae YS, Cantley LG, Chen CS, Kim SR, Kwon KS, Rhee SG. Activation of phospholipase C-γ by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:4465–4469. doi: 10.1074/jbc.273.8.4465. [DOI] [PubMed] [Google Scholar]
- Bao AM, Hestiantoro A, Van Someren EJW, Swaab DF, Zhou JN. Colocalization of corticotropin-releasing hormone and oestrogen receptor-a in the paraventricular nucleus of the hypothalamus in mood disorders. Brain. 2005;128:1301–1313. doi: 10.1093/brain/awh448. [DOI] [PubMed] [Google Scholar]
- Belgardt BF, Bruning JC. CNS leptin and insulin action in the control of energy homeostasis. Annals of the New York Academy of Sciences. 2010;1212:97–113. doi: 10.1111/j.1749-6632.2010.05799.x. [DOI] [PubMed] [Google Scholar]
- Benoit SC, Air EL, Coolen LM, Strauss R, Jackman A, Clegg DJ, Seeley RJ, Woods SC. The catabolic action of insulin in the brain is mediated by melanocortins. The Journal of Neuroscience. 2002;22:9048–9052. doi: 10.1523/JNEUROSCI.22-20-09048.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea CL, Hess DL, Widmann AA, Henningfeld JM. Effects of progesterone on prolactin, hypothalamic beta-endorphin, hypothalamic substance P, and midbrain serotonin in guinea pigs. Neuroendocrinology. 1995;61:695–703. doi: 10.1159/000126897. [DOI] [PubMed] [Google Scholar]
- Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE. Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol. 2004;6:709–720. doi: 10.1038/ncb1150. [DOI] [PubMed] [Google Scholar]
- Bondar G, Kuo J, Hamid N, Micevych P. Estradiol-induced estrogen receptor-a trafficking. The Journal of Neuroscience. 2009;29:15323–15330. doi: 10.1523/JNEUROSCI.2107-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Kordasiewicz H, Mermelstein PG. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. The Journal of Neuroscience. 2007;27:9941–9950. doi: 10.1523/JNEUROSCI.1647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. The Journal of Neuroscience. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks EM, Morgan AL, Pierzga JM, Wladkowski SL, O’Gorman JT, Derr JA, Kenney WL. Chronic hormone replacement therapy alters thermoregulatory and vasomotor fuction in postmenopausal women. Journal of Applied Physiology. 1997;97:477–484. doi: 10.1152/jappl.1997.83.2.477. [DOI] [PubMed] [Google Scholar]
- Brown LM, Clegg DJ, Benoit SC, Woods SC. Intraventricular insulin and leptin reduce food intake and body weight in C57BL/6J mice. Physiol Behav. 2006;89:687–691. doi: 10.1016/j.physbeh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Butera PC, Czaja JA. Intracranial estradiol in ovariectomized guinea pigs: effects on ingestive behaviors and body weight. Brain Res. 1984;322:41–48. doi: 10.1016/0006-8993(84)91178-8. [DOI] [PubMed] [Google Scholar]
- Cardona-Gomez GP, DonCarlos L, Garcia-Segura LM. Insulin-like growth factor I receptors and estrogen recetpros colocalize in female rat brain. Neuroscience. 2000;99:751–760. doi: 10.1016/s0306-4522(00)00228-1. [DOI] [PubMed] [Google Scholar]
- Chaban VV, Micevych PE. Estrogen receptor-α mediates estrodiol attenuation of ATP-induced Ca2+ signaling in mouse dorsal root ganglion neurons. Journal of Neuroscience Research. 2007;81:31–37. doi: 10.1002/jnr.20524. [DOI] [PubMed] [Google Scholar]
- Clarke CH, Norfleet AM, Clarke MS, Watson CS, Cunningham KA, Thomas ML. Perimembrane localization of the estrogen receptor alpha protein in neuronal processes of cultured hippocampal neurons. Neuroendocrinology. 2000;71:34–42. doi: 10.1159/000054518. [DOI] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE. Oestrogen, kisspeptin, GPR54 and the pre-ovulatory luteinizing surge. Journal of Neuroendocrinology. 2009;21:305–311. doi: 10.1111/j.1365-2826.2009.01835.x. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55:978–987. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Brown LM, Zigman JM, Kemp CJ, Strader AD, Benoit SC, Woods SC, Mangiaracina M, Geary N. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes. 2007;56:1051–1058. doi: 10.2337/db06-0015. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Gotoh K, Kemp C, Wortman MD, Benoit SC, Brown LM, D’Alessio D, Tso P, Seeley RJ, Woods SC. Consumption of a high-fat diet induces central insulin resistance independent of adiposity. Physiol Behav. 2011;103:10–16. doi: 10.1016/j.physbeh.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin GB, Sawyer CH. Induction of running activity by intracerebral implants of estrogen in overiectomized rats. Neuroendocrinology. 1969;4:309–320. doi: 10.1159/000121762. [DOI] [PubMed] [Google Scholar]
- Conde K, Meza C, Kelly MJ, Sinchak K, Wagner EJ. Estradiol Rapidly Attenuates ORL-1 Receptor-Mediated Inhibition of Proopiomelanocortin Neurons via Gq-Coupled, Membrane-Initiated Signalling. Neuroendocrinology. 2016;103:787–805. doi: 10.1159/000443765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- Cunningham MJ, Fang Y, Selley DE, Kelly MJ. μ-opioid agonist-stimulated [35S]GTPgammaS binding in guinea pig hypothalamus: Effects of estrogen. Brain Res. 1998;791:341–346. doi: 10.1016/s0006-8993(98)00201-7. [DOI] [PubMed] [Google Scholar]
- Czaja JA. Sex differences in the activational effects of gonadal hormones on food intake and body weight. Physiol Behav. 1984;33:553–558. doi: 10.1016/0031-9384(84)90370-6. [DOI] [PubMed] [Google Scholar]
- Czaja JA, Goy RW. Ovarian hormones and food intake in female guinea pigs and rhesus monkeys. Horm Behav. 1975;6:329–349. doi: 10.1016/0018-506x(75)90003-3. [DOI] [PubMed] [Google Scholar]
- Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych PE. Membrane estrogen receptor-a interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. The Journal of Neuroscience. 2007;27:9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewing P, Christensen A, Bondar G, Micevych P. Protein kinase C signaling in the hypothalamic arcuate nucleus regulates sexual receptivity in female rats. Endocrinology. 2008;149:5934–5942. doi: 10.1210/en.2008-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd GT, Decherf S, Loh K, Simonds SE, Wiede F, Balland E, Merry TL, Munzberg H, Zhang ZY, Kahn BB, Neel BG, Bence KK, Andrews ZB, Cowley MA, Tiganis T. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell. 2015;160:88–104. doi: 10.1016/j.cell.2014.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DonCarlos LL, Monroy E, Morrell JI. Distribution of estrogen receptor-immunoreactive cells in the forebrain of the female guinea pig. J Comp Neurol. 1991;305:591–612. doi: 10.1002/cne.903050406. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci U S A. 2001;98:1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye L, Blundell JE. Menstrual cycle and appetite control: implications for weight regulation. Hum Reprod. 1997;12:1142–1151. doi: 10.1093/humrep/12.6.1142. [DOI] [PubMed] [Google Scholar]
- Etgen AM, Ansonoff MA, Quesada A. Mechanisms of ovarian steroid regulation of norepinephrine receptor-mediated signal transduction in the hypothalamus: implications for female reproductive physiology. Horm Behav. 2001;40:169–177. doi: 10.1006/hbeh.2001.1676. [DOI] [PubMed] [Google Scholar]
- Falasca M, Logan SK, Lehto VP, Baccante G, Lemmon MA, Schlessinger J. Activation of phospholipase Cg by PI 3-kinase-induced PH domain-mediated membrane targeting. EMBO J. 1998;17:414–422. doi: 10.1093/emboj/17.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- Flugge G, Oertel WH, Wuttke W. Evidence for estrogen-receptive GABAergic neurons in the preoptic/anterior hypothalamic area of the rat brain. Neuroendocrinology. 1986;43:1–5. doi: 10.1159/000124500. [DOI] [PubMed] [Google Scholar]
- Freedman RR. Biochemical, metabolic, and vascular mechanisms in menopausal hot flashes. Fertil Steril. 1998;70:332–337. doi: 10.1016/s0015-0282(98)00137-x. [DOI] [PubMed] [Google Scholar]
- Freedman RR. Hot flashes: behavorial treatments, mechanisms, and relation to sleep. American Journal of Medicine. 2005;118:1245–1305. doi: 10.1016/j.amjmed.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Freedman RR, Blacker CM. Estrogen raises the sweating threshold in postmenopausal women with hot flashes. Fertil Steril. 2002;77:487–490. doi: 10.1016/s0015-0282(01)03009-6. [DOI] [PubMed] [Google Scholar]
- Freedman RR, Norton D, Woodward S, Cornelissen G. Core body temperature and circadian rhythm of hot flashes in meopausal women. Journal of Clinical Endocrinology and Metabolism. 1995;80:2354–2358. doi: 10.1210/jcem.80.8.7629229. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci. 2004;27:107–144. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- Gao Q, Horvath TL. Neurobiology of feeding and energy expenditure. Annu Rev Neurosci. 2007;30:367–398. doi: 10.1146/annurev.neuro.30.051606.094324. [DOI] [PubMed] [Google Scholar]
- Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, Leranth C, Toran-Allerand D, Priest CA, Roberts JL, Gao XB, Mobbs C, Shulman GI, Diano S, Horvath TL. Anorectic estrogen mimics leptin’s effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med. 2007;13:89–94. doi: 10.1038/nm1525. [DOI] [PubMed] [Google Scholar]
- Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa S. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-alpha null mice. Endocrinology. 2001;142:4751–4757. doi: 10.1210/endo.142.11.8504. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Navarro VM, Zhao Z, Glidewell-Kenney C, Weiss J, Jameson JL, Clifton DK, Levine JE, Steiner RA. Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. The Journal of Neuroscience. 2009;29:9390–9395. doi: 10.1523/JNEUROSCI.0763-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gréco B, Allegretto EA, Telel MJ, Blaustein JD. Coexpression of ERβ and progestin receptor proteins in the female rat forebrain: effects of estradiol treatment. Endocrinology. 2001;142:5172–5181. doi: 10.1210/endo.142.12.8560. [DOI] [PubMed] [Google Scholar]
- Grove-Strawser D, Boulware MI, Mermelstein PG. Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience. 2010;170:1045–1055. doi: 10.1016/j.neuroscience.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber CJ, Gruber DM, Gruber IM, Wieser F, Huber JC. Anatomy of the estrogen response element. Trends Endocrinol Metab. 2004;15:73–78. doi: 10.1016/j.tem.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Gu Q, Korach KS, Moss RL. Rapid action of 17beta-estradiol on kainate-induced currents in hippocampal neurons lacking intracellular estrogen receptors. Endocrinology. 1999;140:660–666. doi: 10.1210/endo.140.2.6500. [DOI] [PubMed] [Google Scholar]
- Gundlah C, Kohama SG, Mirkes SJ, Garyfallou VT, Urbanski HF, Bethea CL. Distribution of estrogen receptor beta (ERbeta) mRNA in hypothalamus, midbrain and temporal lobe of spayed macaque: continued expression with hormone replacement. Brain Res Mol Brain Res. 2000;76:191–204. doi: 10.1016/s0006-8993(99)02475-0. [DOI] [PubMed] [Google Scholar]
- Gundlah C, Lu NZ, Mirkes SJ, Bethea CL. Estrogen receptor beta (ERbeta) mRNA and protein in serotonin neurons of macaques. Brain Res Mol Brain Res. 2001;91:14–22. doi: 10.1016/s0169-328x(01)00108-5. [DOI] [PubMed] [Google Scholar]
- Hammes SR, Levin ER. Extra-nuclear steroid receptors: nature and actions. Endocrine Reviews. 2007;28:726–741. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
- Hayward MD, Low MJ. The contribution of endogenous opioids to food reward is dependent on sex and background strain. Neuroscience. 2007;144:17–25. doi: 10.1016/j.neuroscience.2006.08.067. [DOI] [PubMed] [Google Scholar]
- Hayward MD, Pintar JE, Low MJ. Selective reward deficit in mice lacking b-endorphin and enkephalin. Journal of Neuroscience. 2002;22:8251–8258. doi: 10.1523/JNEUROSCI.22-18-08251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges VL, Ebner TJ, Meisel RL, Mermelstein PG. The cerebellum as a target for estrogen action. Front Neuroendocrinol. 2012;33:403–411. doi: 10.1016/j.yfrne.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE. Somatostatin-immunoreactive neurones in the hypothalamic ventromedial nucleus possess oestrogen receptors in the male and female rat. J Neuroendocrinol. 1994;6:323–328. doi: 10.1111/j.1365-2826.1994.tb00589.x. [DOI] [PubMed] [Google Scholar]
- Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocrine Reviews. 1998;19:302–330. doi: 10.1210/edrv.19.3.0332. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Skynner MJ, Sim JA. Lack of detection of estrogen receptor-α transcripts in mouse gonadotropin-releasing hormone neurons. Endocrinology. 2001;142:492–493. doi: 10.1210/endo.140.11.7146. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Theodosis DT. Localization of oestrogen receptors in preoptic neurons containing neurotensin but not tyrosine hydroxylase, cholecystokinin or luteinizing hormone-releasing hormone in the male and female rat. Neuroscience. 1992;50:283–298. doi: 10.1016/0306-4522(92)90423-y. [DOI] [PubMed] [Google Scholar]
- Hewitt SC, Li L, Grimm SA, Winuthayanon W, Hamilton KJ, Pockette B, Rubel CA, Pedersen LC, Fargo D, Lanz RB, DeMayo FJ, Schutz G, Korach KS. Novel DNA motif binding activity observed in vivo with an estrogen receptor alpha mutant mouse. Molecular Endocrinology. 2014;28:899–911. doi: 10.1210/me.2014-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK. Acute effects of leptin require PI3K signalng in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest. 2008;118:1796–1805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath TL, Leranth C, Kalra SP, Naftolin F. Galanin neurons exhibit estrogen receptor immunoreactivity in the female rat mediobasal hypothalamus. Brain Res. 1995;675:321–324. doi: 10.1016/0006-8993(94)01374-q. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Kallo I, Hajszan T, Shughrue PJ, Merchenthaler I, Liposits Z. Expression of estrogen receptor-beta messenger ribonucleic acid in oxytocin and vasopressin neurons of the rat supraoptic and paraventricular nuclei. Endocrinology. 1998;139:2600–2604. doi: 10.1210/endo.139.5.6024. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Kallo I, Steinhauser A, Merchenthaler I, Coen CW, Petersen SL, Liposits Z. Estrogen receptor-beta in oxytocin and vasopressin neurons of the rat and human hypothalamus: Immunocytochemical and in situ hybridization studies. J Comp Neurol. 2004;473:315–333. doi: 10.1002/cne.20127. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Shughrue PJ, Merchenthaler I, Hajszan T, Carpenter CD, Liposits Z, Petersen SL. Detection of estrogen receptor-b messenger ribonucleic acid and 125I-estrogen binding sites in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2000;141:3506–3509. doi: 10.1210/endo.141.9.7788. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Steinhauser A, Barabas K, Shughrue PJ, Petersen SL, Merchenthaler I, Liposits Z. Estrogen receptor-beta immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2001;142:3261–3264. doi: 10.1210/endo.142.7.8176. [DOI] [PubMed] [Google Scholar]
- Hu L, Wada K, Mores N, Krsmanovic LZ, Catt KJ. Essential role of G protein-gated inwardly rectifying potassium channels in gonadotropin-induced regulation of GnRH neuronal firing and pulsatile neurosecretion. J Biol Chem. 2006;281:25231–25240. doi: 10.1074/jbc.M603768200. [DOI] [PubMed] [Google Scholar]
- Isgor C, Cecchi M, Kabbaj M, Akil H, Watson SJ. Estrogen receptor beta in the paraventricular nucleus of hypothalamus regulates the neuroendocrine response to stress and is regulated by corticosterone. Neuroscience. 2003;121:837–845. doi: 10.1016/s0306-4522(03)00561-x. [DOI] [PubMed] [Google Scholar]
- Jacobson D, Pribnow D, Herson PS, Maylie J, Adelman JP. Determinants contributing to estrogen-regulated expression of SK3. Biochem Biophys Res Commun. 2003;303:660–668. doi: 10.1016/s0006-291x(03)00408-x. [DOI] [PubMed] [Google Scholar]
- Janssen I, Powell LH, Crawford S, Lasley B, Sutton-Tyrrell K. Menopause and the metabolic syndrome: the Study of Women’s Health Across the Nation. Arch Intern Med. 2008;168:1568–1575. doi: 10.1001/archinte.168.14.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery GS, Peng KC, Wagner EJ. The role of phosphatidylinositol-3-kinase and AMP-activated kinase in the rapid estrogenic attenuation of cannabinoid-induced changes in energy homeostasis. Pharmaceuticals. 2011;4:630–651. [Google Scholar]
- Jensen EV, DeSombre ER. Estrogen-receptor interaction. Science. 1973;182:126–134. doi: 10.1126/science.182.4108.126. [DOI] [PubMed] [Google Scholar]
- Jones MEE, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, Oz OK, Leury BJ, Robertson KM, Yao SG, Simpson ER. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci. 2000;97:12735–12740. doi: 10.1073/pnas.97.23.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallo I, Butler JA, Barkovics-Kallo M, Goubillon ML, Coen CW. Oestrogen receptor beta-immunoreactivity in gonadotropin releasing hormone-expressing neurones: regulation by oestrogen. J Neuroendocrinol. 2001;13:741–748. doi: 10.1046/j.1365-2826.2001.00708.x. [DOI] [PubMed] [Google Scholar]
- Kavalali ET, Chung CH, Khvotchev M, Leitz J, Nosyreva E, Raingo J, Ramirez DMO. Spontaneous neurotrasmission: an independent pathway form neuronal signaling? Physiology. 2011;26:45–53. doi: 10.1152/physiol.00040.2010. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Moss RL, Dudley CA. Differential sensitivity of preoptic-septal neurons to microelectrophoresed estrogen during the estrous cycle. Brain Res. 1976;114:152–157. doi: 10.1016/0006-8993(76)91017-9. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Moss RL, Dudley CA. The effects of microelecrophoretically applied estrogen, cortisol, and acetylcholine on medial preoptic-septal unit activity throughout the estrous cycle of the female rat. Exp Brain Res. 1977a;30:53–64. doi: 10.1007/BF00237858. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Moss RL, Dudley CA. The effects of ovarectomy on preoptic-septic area neurons to microelectrophoresed estrogen. Neuroendocriology. 1978a;25:204–211. doi: 10.1159/000122742. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Moss RL, Dudley CA. The stereospecific changes in the unit activity of preoptic-septal neurons to microelectrophoresed estrogen. In: Ryall RW, Kelly JS, editors. Iontophoresis and Transmitter Mechanisms in the Mammalian Central Nervous System, 1 ed. Elsevier/North-Holland Biomedical Press; New York: 1978b. pp. 113–116. [Google Scholar]
- Kelly MJ, Moss RL, Dudley CA, Fawcett CP. The specificity of the response of preoptic-septal area neurons to estrogen: 17α-estradiol versus 17β-estradiol and the response of extrahypothalamic neurons. Exp Brain Res. 1977b;30:43–52. doi: 10.1007/BF00237857. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Ronnekleiv OK. Membrane-initiated estrogen signaling in hypothalamic neurons. Mol Cell Endocrinol. 2008;290:14–23. doi: 10.1016/j.mce.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MJ, Rønnekleiv OK. Rapid membrane effects of estrogen in the central nervous system. In: Pfaff DW, editor. Hormones, Brain and behavior. 3. Academic Press; San Diego: 2002. pp. 361–380. [Google Scholar]
- Kelly MJ, Rønnekleiv OK. Mini-review: neural signaling of estradiol in the hypothalamus. Molecular Endocrinology. 2015;29:645–657. doi: 10.1210/me.2014-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MJ, Ronnekleiv OK, Eskay RL. Identification of estrogen-responsive LHRH neurons in the guinea pig hypothalamus. Brain Res Bull. 1984;12:399–407. doi: 10.1016/0361-9230(84)90112-6. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Zhang C, Qiu J, Ronnekleiv OK. Pacemaking kisspeptin neurons. Exp Physiol. 2013;98:1535–1543. doi: 10.1113/expphysiol.2013.074559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenealy BP, Kapoor A, Guerriero KA, Keen KL, Garcia JP, Kurian JR, Ziegler TE, Terasawa E. Neuroestradiol in the hypothalamus contributes to the regulation of gonadotropin releasing hormone release. The Journal of Neuroscience. 2013;33:19051–19059. doi: 10.1523/JNEUROSCI.3878-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenealy BP, Keen KL, Garcia JP, Kohlenberg LK, Terasawa E. Obligatory role of hypothalamic neuroestradiol during the estrogen-induced LH surge in female ovariectomized rhesus monkeys. Proc Natl Acad Sci U S A. 2017;114:13804–13809. doi: 10.1073/pnas.1716097115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenealy BP, Keen KL, Terasawa E. Rapid action of estradiol in primate GnRH neurons: the role of estrogen receptor alpha and estrogen receptor beta. Steroids. 2011;76:861–866. doi: 10.1016/j.steroids.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kininis M, Chen BS, Diehl AG, Isaacs GD, Zhang T, Siepel AC, Clark AG, Kraus WL. Genomic analyses of transcription factor binding, histone acetylation, and gene expression reveal mechanistically distinct classes of estrogen-regulated promoters. Mol Cell Biol. 2007;27:5090–5104. doi: 10.1128/MCB.00083-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijver FP, Balesar R, Espila AM, Unmehopa UA, Swaab DF. Estrogen receptor-alpha distribution in the human hypothalamus in relation to sex and endocrine status. J Comp Neurol. 2002;454:115–139. doi: 10.1002/cne.10416. [DOI] [PubMed] [Google Scholar]
- Kruijver FP, Balesar R, Espila AM, Unmehopa UA, Swaab DF. Estrogen-receptor-beta distribution in the human hypothalamus: similarities and differences with ER alpha distribution. J Comp Neurol. 2003;466:251–277. doi: 10.1002/cne.10899. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JÅ. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner PJ, Agard DA, Greene GL, Scanlan TS, Shiau AK, Uht RM, Webb P. Estrogen receptor pathways to AP-1. J Steroid Biochem Mol Biol. 2000;74:311–317. doi: 10.1016/s0960-0760(00)00108-4. [DOI] [PubMed] [Google Scholar]
- Laflamme N, Nappi RE, Drolet G, Labrie C, Rivest S. Expression and neuropeptidergic characterization of estrogen receptors (ERα and ERβ) throughout the rat brain: Anatomical evidence of distinct roles of each subtype. Journal of Neurobiology. 1998;36:357–378. doi: 10.1002/(sici)1097-4695(19980905)36:3<357::aid-neu5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Lagrange AH, Rønnekleiv OK, Kelly MJ. Modulation of G protein-coupled receptors by an estrogen receptor that activates protein kinase A. Mol Pharmacol. 1997;51:605–612. doi: 10.1124/mol.51.4.605. [DOI] [PubMed] [Google Scholar]
- Leal S, Andrade JP, Paula-Barbosa MM, Madeira MD. Arcuate nucleus of the hypothalamus: effects of age and sex. J Comp Neurol. 1998;401:65–88. doi: 10.1002/(sici)1096-9861(19981109)401:1<65::aid-cne5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Karsch FJ. Do gonadotropin-releasing hormone, tyrosine hydroxylase-, and β-endorphin-immunoreactive neurons contain estrogen receptors? A double-label immunocytochemical study in the Suffolk ewe. Endo. 1993;133:887–895. doi: 10.1210/endo.133.2.8102098. [DOI] [PubMed] [Google Scholar]
- Levin ER. Plasma membrane estrogen receptors. Trends Endocrinol Metab. 2009;20:477–482. doi: 10.1016/j.tem.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- Malyala A, Pattee P, Nagalla SR, Kelly MJ, Ronnekleiv OK. Suppression subtractive hybridization and microarray identification of estrogen-regulated hypothalamic genes. Neurochem Res. 2004;29:1189–1200. doi: 10.1023/b:nere.0000023606.13670.1d. [DOI] [PubMed] [Google Scholar]
- Malyala A, Zhang C, Bryant DN, Kelly MJ, Ronnekleiv OK. PI3K signaling effects in hypothalamic neurons mediated by estrogen. J Comp Neurol. 2008;506:895–911. doi: 10.1002/cne.21584. [DOI] [PubMed] [Google Scholar]
- Margolis KL, Bonds DE, Rodabough RJ, Tinker L, Phillips LS, Allen C, Bassford T, Burke G, Torrens J, Howard BV, Women’s Health Initiative I Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia. 2004;47:1175–1187. doi: 10.1007/s00125-004-1448-x. [DOI] [PubMed] [Google Scholar]
- McCaffrey TA, Czaja JA. Diverse effects of estradiol-17 beta: concurrent suppression of appetite, blood pressure and vascular reactivity in conscious, unrestrained animals. Physiol Behav. 1989;45:649–657. doi: 10.1016/0031-9384(89)90086-3. [DOI] [PubMed] [Google Scholar]
- Mela V, Vargas A, Meza C, Kachani M, Wagner EJ. Modulatory influences of estradiol and other anorexigenic hormones on metabotropic, Gi/o-coupled receptor function in the hypothalamic control of energy homeostasis. J Steroid Biochem Mol Biol. 2016;160:15–26. doi: 10.1016/j.jsbmb.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchenthaler I, Lane MV, Numan S, Dellovade TL. Distribution of estrogen receptor alpha and beta in the mouse central nervous system: in vivo autoradiographic and immunocytochemical analyses. J Comp Neurol. 2004;473:270–291. doi: 10.1002/cne.20128. [DOI] [PubMed] [Google Scholar]
- Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. The Journal of Neuroscience. 1996;16:595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych P, Dominguez R. Membrane estradiol signaling in the brain. Front Neuroendocrinol. 2009;30:315–327. doi: 10.1016/j.yfrne.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych PE, Kelly MJ. Membrane estrogen receptor regulation of hypothalamic function. Neuroendocrinology. 2012;96:103–110. doi: 10.1159/000338400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, McMullen NT, Rance NE. Role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons in cutaneous vasodilatation and the estrogen modulation of body temperature. Proc Natl Acad Sci U S A. 2012;109:19846–19851. doi: 10.1073/pnas.1211517109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moenter SM, DeFazio AR, Pitts GR, Nunemaker CS. Mechanisms underlying episodic gonadotropin-releasing hormone secretion. Front Neuroendocrinol. 2003;24:79–93. doi: 10.1016/s0091-3022(03)00013-x. [DOI] [PubMed] [Google Scholar]
- Moline ML, Broch L, Zak R, Gross V. Sleep in women across the life cycle from adulthood through menopause. Sleep Med Rev. 2003;7:155–177. doi: 10.1053/smrv.2001.0228. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Madden CJ, Tupone D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab. 2014;19:741–756. doi: 10.1016/j.cmet.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- Muramatsu M, Inoue S. Estrogen receptors: how do they control reproductive and nonreproductive functions? Biochem Biophys Res Commun. 2000;270:1–10. doi: 10.1006/bbrc.2000.2214. [DOI] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ, Kaplitt MG, Ogawa S. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci U S A. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. A thermosensory pathway mediating heat-defense responses. Proc Natl Acad Sci U S A. 2010;107:8848–8853. doi: 10.1073/pnas.0913358107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor CC, Qiu J, Padilla SL, Zhang C, Bosch MA, Fan W, Aicher SA, Palmiter RD, Ronnekleiv OK, Kelly MJ. Optogenetic Stimulation of Arcuate Nucleus Kiss1 Neurons Reveals a Steroid-Dependent Glutamatergic Input to POMC and AgRP Neurons in Male Mice. Molecular Endocrinology. 2016;30:630–644. doi: 10.1210/me.2016-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA. Mechanisms of estrogen action. Physiol Rev. 2001;81:1535–1565. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E. Involvement of G protein-coupled receptor 30 (GPR30) in rapid action of estrogen in primate LHRH neurons. Molecular Endocrinology. 2009;23:349–359. doi: 10.1210/me.2008-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley BW, Tsai MJ. Molecular pathways of steroid receptor action. Biol Reprod. 1992;46:163–167. doi: 10.1095/biolreprod46.2.163. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Chan J, Gustafsson J-Å, Korach KS, Pfaff DW. Estrogen increases locomotor activity in mice through estrogen receptor alpha: specificity for the type of activity. Endocrinology. 2003;144:230–239. doi: 10.1210/en.2002-220519. [DOI] [PubMed] [Google Scholar]
- Osterlund MK, Gustafsson JÅ, Keller E, Hurd YL. Estrogen receptor b (ERb) messenger ribonucleic acid (mRNA) expression within the human forebrain: distinct distribution pattern to ERa mRNA. J Clin Endocrinol Metab. 2000;85:3840–3846. doi: 10.1210/jcem.85.10.6913. [DOI] [PubMed] [Google Scholar]
- Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson JÅ, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ERα and ERβ at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- Pappas TC, Gametchu B, Yannariello-Brown J, Collins TJ, Watson CS. Membrane estrogen receptors in GH3/B6 cells are associated with rapid estrogen-induced release of prolactin. Endocrine. 1994;2:813–822. [Google Scholar]
- Park CJ, Zhao Z, Glidewell-Kenney C, Lazic M, Chambon P, Krust A, Weiss J, Clegg DJ, Dunaif A, Jameson JL, Levine JE. Genetic rescue of nonclassical ERa signaling normalizes energy balance in obese Era-null mutant mice. Journal of Clinical Investigation. 2011;121:604–612. doi: 10.1172/JCI41702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Molecular Endocrinology. 2006;20:1996–2009. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Lewis M, Hammes S, Levin ER. Membrane-localized estrogen receptor a is required for normal organ development and function. Dev Cell. 2014;29:482–490. doi: 10.1016/j.devcel.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SL, LaFlamme KD. Progesterone increases levels of μ-opioid receptor mRNA in the preoptic area and arcuate nucleus of ovariectomized, estradiol-treated female rats. Molecular Brain Research. 1997;52:32–37. doi: 10.1016/s0169-328x(97)00194-0. [DOI] [PubMed] [Google Scholar]
- Pfaff D, Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J Comp Neurol. 1973;151:121–158. doi: 10.1002/cne.901510204. [DOI] [PubMed] [Google Scholar]
- Pietras RJ, Szego CM. Specific binding sites for oestrogen at the outer surfaces of isolated endometrial cells. Nature. 1977;265:69–72. doi: 10.1038/265069a0. [DOI] [PubMed] [Google Scholar]
- Pietras RJ, Szego CM. Estrogen receptors in uterine plasma membrane. J Steroid Biochem. 1979;11:1471–1483. doi: 10.1016/0022-4731(79)90124-9. [DOI] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Meza C, Navarro UV, Nestor CC, Wagner EJ, Ronnekleiv OK, Kelly MJ. Estradiol Protects Proopiomelanocortin Neurons Against Insulin Resistance. Endocrinology. 2018;159:647–664. doi: 10.1210/en.2017-00793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Rønnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. The Journal of Neuroscience. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Krust A, Graham S, Murphy S, Korach KS, Chambon P, Scanlan TS, Rønnekleiv OK, Kelly MJ. A G-protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. Journal of Neuroscience. 2006;26:5649–5655. doi: 10.1523/JNEUROSCI.0327-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Fang Y, Ronnekleiv OK, Kelly MJ. Leptin excites proopiomelanocortin neurons via activation of TRPC channels. The Journal of Neuroscience. 2010;30:1560–1565. doi: 10.1523/JNEUROSCI.4816-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Rønnekleiv OK, Kelly MJ. Modulation of hypothalamic neuronal activity through a novel G-protein coupled estrogen membrane receptor. Steroids. 2008;73:985–991. doi: 10.1016/j.steroids.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Xue C, Bosch MA, Murphy JG, Fan W, Rønnekleiv OK, Kelly MJ. Serotonin 5HT2c receptor signaling in hypothalamic POMC neurons: role in energy homeostasis in females. Molecular Pharmacology. 2007;72:885–896. doi: 10.1124/mol.107.038083. [DOI] [PubMed] [Google Scholar]
- Qiu J, Zhang C, Borgquist A, Nestor CC, Smith AW, Bosch MA, Ku S, Wagner EJ, Ronnekleiv OK, Kelly MJ. Insulin excites anorexigenic proopiomelanocortin neurons via activation of canonical transient receptor potential channels. Cell Metab. 2014;19:682–693. doi: 10.1016/j.cmet.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameh LE, Rhee SG, Spokes K, Kazlauskas A, Cantley LC, Cantley LG. Phosphoinositide 3-Kinase Regulates Phospholipase Cγ-mediated Calcium Signaling. J Biol Chem. 1998;273:23750–23757. doi: 10.1074/jbc.273.37.23750. [DOI] [PubMed] [Google Scholar]
- Ramirez VD, Zheng J. Membrane sex-steroid receptors in the brain. Front Neuroendocrinol. 1996;17:402–439. doi: 10.1006/frne.1996.0011. [DOI] [PubMed] [Google Scholar]
- Ramirez VD, Zheng J, Siddique KM. Membrane receptors for estrogen, progesterone, and testosterone in the rat brain: fantasy or reality. Cell Mol Neurobiol. 1996;16:175–198. doi: 10.1007/BF02088175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rance NE, Dacks PA, Mittelman-Smith MA, Romanovsky AA, Krajewski-Hall SJ. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34:211–227. doi: 10.1016/j.yfrne.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapkin AJ. Vasomotor symptoms in menopause: physiologic condition and central nervous system approaches to treatment. American Journal of Obstetrics & Gynecology. 2007;196:97–106. doi: 10.1016/j.ajog.2006.05.056. [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: Studies of ERα and ERβ expressed in Chinese hamster ovary cells. Molecular Endocrinology. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Menchaca AA, Adney SK, Zhou L, Logothetis DE. Dual regulation of voltage-sensitive ion channels by PIP2. Front Pharmacol. 2012;3:170–170. doi: 10.3389/fphar.2012.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke TA, Bosch MA, Rick EA, Lee B, Wagner EJ, Seidlova-Wuttke D, Wuttke W, Scanlan TS, Ronnekleiv OK, Kelly MJ. Contribution of a membrane estrogen receptor to the estrogenic regulation of body temperature and energy homeostasis. Endocrinology. 2010;151:4926–4937. doi: 10.1210/en.2010-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke TA, Malyala A, Bosch MA, Kelly MJ, Ronnekleiv OK. Estrogen regulation of genes important for K+ channel signaling in the arcuate nucleus. Endocrinology. 2007;148:4937–4951. doi: 10.1210/en.2007-0605. [DOI] [PubMed] [Google Scholar]
- Roepke TA, Xue C, Bosch MA, Scanlan TS, Kelly MJ, Ronnekleiv OK. Genes associated with membrane-initiated signaling of estrogen and energy homeostasis. Endocrinology. 2008;149:6113–6124. doi: 10.1210/en.2008-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnekleiv OK, Kelly MJ. Diversity of ovarian steroid signaling in the hypothalamus. Front Neuroendocrinol. 2005;26:65–84. doi: 10.1016/j.yfrne.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Salido GM, Jardin I, Rosado JA. The TRPC ion channels: association with Orai1 and STIM1 proteins and participation in capacitative and non-capacitative calcium entry. Adv Exp Med Biol. 2011;704:413–433. doi: 10.1007/978-94-007-0265-3_23. [DOI] [PubMed] [Google Scholar]
- Sar M. Estradiol is concentrated in tyrosine hydroxylase-containing neurons of the hypothalamus. Science. 1984;223:938–940. doi: 10.1126/science.6141639. [DOI] [PubMed] [Google Scholar]
- Sar M, Parikh I. Immunohistochemical localization of estrogen receptor in rat brain, pituitary and uterus with monoclonal antibodies. J Steroid Biochem. 1986;24:497–503. doi: 10.1016/0022-4731(86)90111-1. [DOI] [PubMed] [Google Scholar]
- Sar M, Stumpf WE. Cellular locatization of progestin and estrogen in guinea pig hypothalamus by autoradiography. In: Stumpf WE, Grant LD, editors. Anatomical Neuroendocrinology. Karger; Basal: 1975. pp. 142–152. [Google Scholar]
- Sarkar DK, Fink G. Luteinizing hormone releasing factor in pituitary stalk plasma from long-term ovariectomized rats: effects of steroids. J Endocrinol. 1980;86:511–524. doi: 10.1677/joe.0.0860511. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Sheridan JT, Gilmore RC, Watson MJ, Archer CB, Tarran R. 17ß-Estradiol inhibits phosphorylation of stromal interaction molecule 1 (STIM1) protein: implication for store-operated calcium entry and chronic lung diseases. J Biol Chem. 2013;288:33509–33518. doi: 10.1074/jbc.M113.486662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Sorrell JE, Clegg DJ, Woods SC, Seeley RJ. The roles of leptin receptors on POMC neurons in the regulation of sex-specific energy homeostasis. Physiol Behav. 2010;100:165–172. doi: 10.1016/j.physbeh.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I. Distribution of estrogen receptor beta immunoreactivity in the rat central nervous system. J Comp Neurol. 2001;436:64–81. [PubMed] [Google Scholar]
- Shughrue PJ, Scrimo PJ, Merchenthaler I. Evidence for the colocalization of estrogen receptor-beta mRNA and estrogen receptor-alpha immunoreactivity in neurons of the rat forebrain. Endocrinology. 1998;139:5267–5270. doi: 10.1210/endo.139.12.6525. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Sinchak K, Wagner EJ. Estradiol signaling in the regulation of reproduction and energy balance. Front Neuroendocrinol. 2012;33:342–363. doi: 10.1016/j.yfrne.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer CA, Figueroa-Masot XA, Batchelor RH, Dorsa DM. The mitogen-activated protein kinase pathway mediates estrogen neuroprotection after glutamate toxicity in primary cortical neurons. The Journal of Neuroscience. 1999;19:2455–2463. doi: 10.1523/JNEUROSCI.19-07-02455.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner DC, Herbison AE. Effects of photoperiod on estrogen receptor, tyrosine hydroxylase, neuropeptide Y and b-endorphin immunoreactivity in the ewe hypothalamus. Endocrinology. 1997;138:2585–2595. doi: 10.1210/endo.138.6.5208. [DOI] [PubMed] [Google Scholar]
- Skynner MJ, Sim JA, Herbison AE. Detection of estrogen receptor α and β messenger ribonucleic acids in adult gonadortropin-releasing hormone neurons. Endocrinology. 1999;140:5195–5201. doi: 10.1210/endo.140.11.7146. [DOI] [PubMed] [Google Scholar]
- Smith AW, Bosch MA, Wagner EJ, Rønnekleiv OK, Kelly MJ. The membrane estrogen receptor ligand STX rapidly enhances GABAergic signaling in NPY/AgRP neurons: Role in mediating the anorexigenic effects of 17ß-estradiol. American Journal of Physiology-Endocrinology and Metabolism. 2013;305:E632–E640. doi: 10.1152/ajpendo.00281.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331:1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- Stincic TL, Grachev P, Rønnekleiv OK, Kelly MJ. Estradiol modulates hypothalamic POMC neurotransmission. Society for Neuroscience Abstract 2017 [Google Scholar]
- Stossi F, Likhite VS, Katzenellenbogen JA, Katzenellenbogen BS. Estrogen-occupied estrogen receptor represses cyclin G2 gene expression and recruits a repressor complex at the cylcin G2 promoter. J Biol Chem. 2006;281:16272–16278. doi: 10.1074/jbc.M513405200. [DOI] [PubMed] [Google Scholar]
- Szego CM, Davis JS. Adenosine 3′,5′-monophosphate in rat uterus: acute elevation by estrogen. Proc Natl Acad Sci U S A. 1967;58:1711–1718. doi: 10.1073/pnas.58.4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szegõ ÉM, Barabás K, Balog J, Szilágyi N, Korach KS, Juhász G, Abrahám IM. Estrogen induces estrogen receptor a-dependent cAMP response element-binding protein phosphorylation via mitogen activated protein kinase pathway in basal forebrain cholinergic neurons In Vivo. Journal of Neuroscience. 2006;26:4104–4110. doi: 10.1523/JNEUROSCI.0222-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tankersley CG, Nicholas WC, Deaver DR, Mikita D, Kenney WL. Estrogen replacement in middle-aged women: thermoregulatory responses to exercise in the heat. J Appl Physiol (1985) 1992;73:1238–1245. doi: 10.1152/jappl.1992.73.4.1238. [DOI] [PubMed] [Google Scholar]
- Tardy J, Pasqualini JR. Localization of [3H]-estradiol and gonadotropin-releasing hormone (GnRH) in the hypothalamus of the fetal guinea-pig. Exp Brain Res. 1983;49:77–83. doi: 10.1007/BF00235543. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Kenealy BP. Neuroestrogen, rapid action of estradiol, and GnRH neurons. Front Neuroendocrinol. 2012;33:364–375. doi: 10.1016/j.yfrne.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton JE, Loose MD, Kelly MJ, Rønnekleiv OK. Effects of estrogen on the number of neurons expressing β-endorphin in the medial basal hypothalamus of the female guinea pig. J Comp Neurol. 1994;341:68–77. doi: 10.1002/cne.903410107. [DOI] [PubMed] [Google Scholar]
- Tobias SC, Qiu J, Kelly MJ, Scanlan TS. Synthesis and biological evaluation of SERMs with potent nongenomic estrogenic activity. ChemMedChem. 2006;1:565–571. doi: 10.1002/cmdc.200500098. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD. Minireview: A plethora of estrogen receptors in the brain: where will it end? Endocrinology. 2004;145:1069–1074. doi: 10.1210/en.2003-1462. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD. Estrogen and the brain: beyond ER-a, ER-b and 17b-estradiol. Annals of the New York Academy of Sciences. 2005;1052:136–144. doi: 10.1196/annals.1347.009. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES, Jr, Nethrapalli IS, Tinnikov AA. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. The Journal of Neuroscience. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towle AC, Sze PY. Steroid binding to synaptic plasma membrane: differential binding of glucocorticoids and gonadal steroids. J Steroid Biochem. 1983;18:135–143. doi: 10.1016/0022-4731(83)90079-1. [DOI] [PubMed] [Google Scholar]
- Wade CB, Robinson S, Shapiro RA, Dorsa DM. Estrogen receptor (ER)alpha and ERbeta exhibit unique pharmacologic properties when coupled to activation of the mitogen-activated protein kinase pathway. Endocrinology. 2001;142:2336–2342. doi: 10.1210/endo.142.6.8071. [DOI] [PubMed] [Google Scholar]
- Walter P, Green S, Greene G, Krust A, Bornert JM, Jeltsch JM, Staub A, Jensen E, Scrace G, Waterfield M, et al. Cloning of the human estrogen receptor cDNA. Proc Natl Acad Sci U S A. 1985;82:7889–7893. doi: 10.1073/pnas.82.23.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warembourg M. Radioautographic localization of estrogen-concentrating cells in the brain and pituitary of the guinea pig. Brain Res. 1977;123:357–362. doi: 10.1016/0006-8993(77)90486-3. [DOI] [PubMed] [Google Scholar]
- Warembourg M, Leroy D. Comparative distribution of estrogen receptor alpha and beta immunoreactivities in the forebrain and the midbrain of the female guinea pig. Brain Res. 2004;1002:55–66. doi: 10.1016/j.brainres.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Washburn N, Borgquist A, Wang K, Jeffery GS, Kelly MJ, Wagner EJ. Receptor subtypes and signal transduction mechanisms contributing to the estrogenic attenuation of cannabinoid-induced changes in energy homeostasis. Neuroendocrinology. 2013;97:160–175. doi: 10.1159/000338669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherill PJ, Wilson AP, Nicholson RI, Davies P, Wakeling AE. Interaction of the antioestrogen ICI 164,384 with the oestrogen receptor. J Steroid Biochem. 1988;30:263–266. doi: 10.1016/0022-4731(88)90103-3. [DOI] [PubMed] [Google Scholar]
- Woolley CS. Acute effects of estrogen on neuronal physiology. Annual Review of Pharmacology and Toxicology. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- Wu GY, Deisseroth K, Tsien RW. Activity-dependent CREB phosphorylation: convergence of a fast, sensitive calmondulin kinase pathway and a slow, less sensitive mitogen-activated protein kinase pathway. PNAS. 2001;98:2808–2813. doi: 10.1073/pnas.051634198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137:1225–1234. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu AW, Kaelin CB, Takeda K, Akira S, Schwartz MW, Barsh GS. PI3K integrates the action of insulin and leptin on hypothalamic neurons. J Clin Invest. 2005;115:951–958. doi: 10.1172/JCI24301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, Zhang X, Zou F, Gent LM, Hahner LD, Khan SA, Elias CF, Elmquist JK, Clegg DJ. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14:453–465. doi: 10.1016/j.cmet.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakimova KS, Sann H, Pierau FK. Neuronal basis for the hyperthermic effect of mu-opioid agonists in rats: decrease in temperature sensitivity of warm-sensitive hypothalamic neurons. Neurosci Lett. 1996;218:115–118. doi: 10.1016/s0304-3940(96)13133-5. [DOI] [PubMed] [Google Scholar]
- Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol. 2007;9:636–645. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Bosch MA, Ronnekleiv OK, Kelly MJ. Kisspeptin activation of TRPC4 channels in female GnRH neurons requires PIP2 depletion and cSrc kinase activation. Endocrinology. 2013;154:2772–2783. doi: 10.1210/en.2013-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Bosch MA, Rønnekleiv OK, Kelly MJ. 17ß-estradiol increases persistent Na+ current and excitability of AVPV/PeN Kiss1 neurons in female mice. Molecular Endocrinology. 2015;29:518–527. doi: 10.1210/me.2014-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Ramirez VD. Demonstration of membrane estrogen binding proteins in rat brain by ligand blotting using a 17β-estradiol-[125I]bovine serum albumin conjugate. Journal of Steroid Biochemistry and Molecular Biology. 1997;62:327–336. doi: 10.1016/s0960-0760(97)00037-x. [DOI] [PubMed] [Google Scholar]
- Zhou L, Hammer RP., Jr Gonadal steroid hormones upregulate medial preoptic μ-opioid receptors in the rat. Eur J Pharmacol. 1995;278:271–274. doi: 10.1016/0014-2999(95)00175-k. [DOI] [PubMed] [Google Scholar]


