Abstract
Spontaneous chronic subdural hematoma (SDH) is a rare condition that could develop in association with hematologic disease. A 66-year-old male developed a chronic SDH as an initial manifestation of chronic myelomonocytic leukemia (CMML). He experienced recurrent chronic subdural hemorrhage and newly developed intracerebral hemorrhage. Considering the scheduled long-term chemotherapy, bilateral middle meningeal artery (MMA) embolization was performed to prevent recurrence of subdural hemorrhage. Although pancytopenia occurred during the 7 months' follow-up period, residual chronic subdural hemorrhage was absorbed without recurrence. To our best knowledge, this is the first report of CMML with spontaneous chronic SDH. MMA embolization is potentially a useful and safe treatment option in the challenging clinical situations with underlying pathologies.
Keywords: Leukemia, Myelomonocytic, Subdural hematoma, Embolization
INTRODUCTION
Chronic subdural hematoma (SDH) is commonly caused by trivial trauma followed by tearing of the cortical bridge veins or fragile vessels in the neomembrane, and repeated microhemorrhage.12) Spontaneous chronic SDH is a very rare condition which can be developed in association with rupture of a cortical vessel, tumor, metastasis, or blood dyscrasias.14),20) We report a 66-year-old man who developed spontaneous chronic SDH that presented as the initial manifestation of chronic myelomonocytic leukemia (CMML). In addition, we reviewed the literature on chronic SDH associated with leukemic neoplasm and middle meningeal artery (MMA) embolization to prevent recurrence of chronic SDH in high-risk patients.
CASE REPORT
Admission for chronic subdural hemorrhage
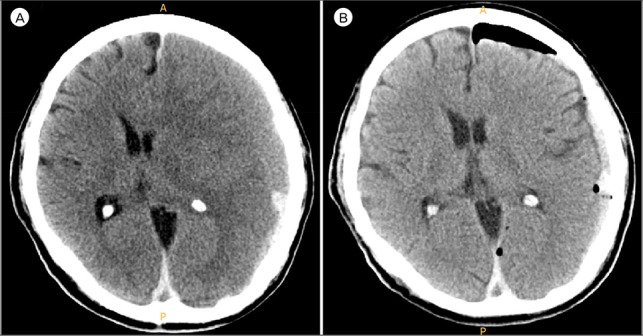
A 66-year-old right-handed male with a past medical history of hypertension and diabetes mellitus was admitted to our hospital for nausea, vomiting, and orbital pain for one day prior. He was on amolodipine and linagliptin. On the day of admission, the patient experienced a sudden severe right-sided weakness, without history of head injury or trauma. Head computed tomography (CT) scan conducted in the emergency room showed left-sided chronic SDH with significant mass effect (Fig. 1A). The patient was admitted to the neurosurgery department and underwent burr-hole drainage. Postoperative CT scan revealed decreased amount of SDH, and the symptoms of the patient were disappeared (Fig. 1B).
Fig. 1. (A) Unenhanced computed tomography (CT) scan at the time of admission showed left-sided isodensity chronic subdural hematoma. (B) CT scan performed after burr-hole drainage revealed decreased amount of subdural hematoma.
Detection of chronic myelomonocytic leukemia
At the time of evaluation, his mental status was alert and neurological examinations showed no focal deficits with full strength and intact sensation in bilateral extremities. Apart from an elevated blood pressure of 144/85 mmHg, his vital signs were stable. The patient was admitted to the neurosurgery department for further work-up including burr-hole drainage. Primary laboratory work-up showed elevated white blood cell (WBC) count of 59,300 cell/µL, with a significant left shift. Platelets count was 207,000/µL, with a hemoglobin level of 10.5 g/dL and a hematocrit of 43.8%. International normal ratio was 1.42, with prothrombin time and partial thromboplastin time values of 14.8 and 33.8 seconds, respectively. A repeat WBC count revealed an elevated level of 45,800 cell/µL. Peripheral blood morphology revealed marked neutrophilia and monocytosis, and leucoerythroblastic feature (1/100 WBCs).
Based on these results, we suspected a hematological malignancy and referred the patient to the Hematology/Oncology department. Subsequently, the patient underwent a bone marrow biopsy with cytogenetics analysis including fluorescence in situ hybridization. The results revealed hypercellular marrow (almost 100% cellularity) and diffuse interstitial infiltration of immature cells suggesting leukemic involvement. BCR/ABL rearrangement was within normal range, and the Philadelphia chromosome was not detected. Findings of the peripheral blood cell counts and bone marrow studies were suggestive of CMML.
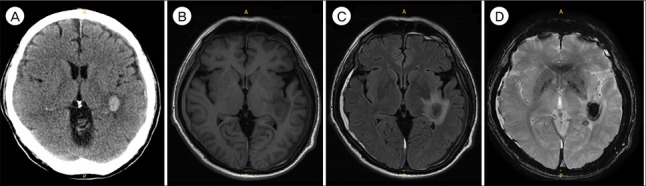
The patient was scheduled for treatment with 5 cycles of decitabin per every 4 weeks. In the first cycle, decitabin was administered at 20 mg/m2 over 1 hour daily for 5 days. A day later, the WBC count was dramatically decreased to 12,200 cell/µL. However, the patient complained of general weakness, and following brain CT showed newly developed intracerebral hemorrhage in left insular-temporal lobe. Magnetic resonance imaging to evaluate suspicious bilateral SDH, and revealed newly developed intracerebral hemorrhage in left insular-temporal lobe subcortical white matter with surrounding edema. Also, scanty amount of SDH was noted in both fronto-temporo-parietal lobes (Fig. 2). The signal intensity of left-sided hematoma was consistent with early late acute stage, and that of right-sided hematoma was consistent with subacute stage.
Fig. 2. (A) Unenhanced computed tomography scan and (B–D) magnetic resonance imaging performed 2 weeks later. Besides newly developed intracerebral hemorrhage in left insular-temporal lobe subcortical white matter with surrounding edema, scanty amount of subdural hematoma was noted in both fronto-temporo-parietal lobes. The signal intensity of left-sided hematoma was consistent with early late acute stage, and that of right-sided hematoma was consistent with subacute stage.
Middle meningeal artery (MMA) embolization
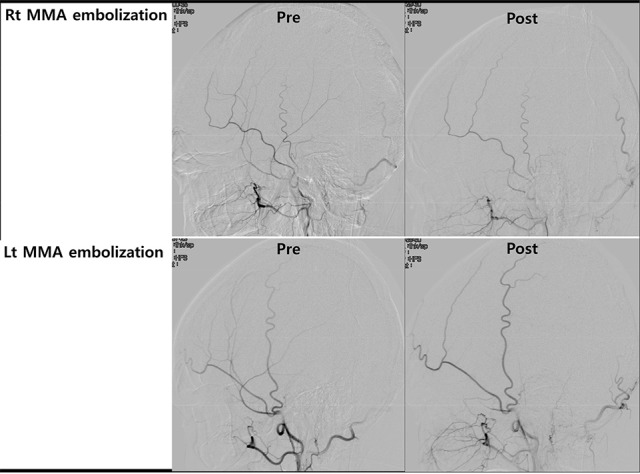
Due to expected long-term chemotherapy and possibility of repeated intracranial hemorrhage, further management was required. For prevention of recurrent SDH, bilateral MMA embolization was conducted under local anesthesia (Fig. 3). Digital subtraction angiography showed no vascular abnormality in internal carotid artery and external carotid artery angiogram. Excelsior SL 10 microcatheter (Boston Scientific, Fremont, CA, USA) was placed in the proximal portion of the MMA. After confirming absence of collateral flow between ophthalmic artery and MMA, MMA embolization was performed with polyvinyl alcohol particles (150–250 µm) mixed with contrast agent. Postoperatively, the patient complained of no headache or neurologic complications.
Fig. 3. Angiography imaging at pre- and post-embolization of bilateral middle meningeal artery. Rt = right; MMA = middle meningeal artery; Pre = pre-embolization; Post = post-embolization; Lt = left.
Clinical course
Over the 20 days following chemotherapy, WBC count was normalized to 5,930 cells/µL, and platelet count was maintained at > 80,000/µL after transient thrombocytopenia. As shown in Fig. 4, Brain CT at 2 months after bilateral MMA embolization showed slightly increased SDH in left side, but the patient had no headache or neurological complaints that were correlated with imaging. Under neurosurgical observation, he received 5 cycles of decitabin. He was admitted for neutropenic fever with pancytopenia after 2nd cycle of decitabin, but there was no further complication related with chemotherapy (Table 1). Brain CT performed 5 months later revealed complete resolution of subdural hemorrhage (Fig. 4).
Fig. 4. Brain computed tomography (CT) performed at (A) 2 weeks, (B) 1 month, (C) 2 months, and (D) 5 months after bilateral middle meningeal artery embolization. Slightly increased subdural hematoma in left side was observed in the 2 months' follow-up CT, but the patient had no headache or neurological complaints correlated with imaging. Pancytopenia with neutropenic fever after the 2nd cycle of decitabin was the only complication. Brain CT performed 5 months later revealed complete resolution of subdural hemorrhage.
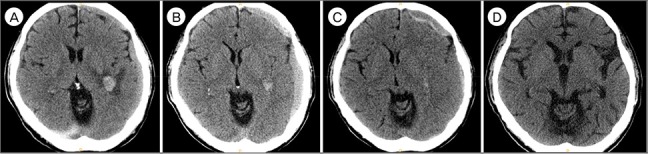
Table 1. Complete blood counts during the follow-up period.
| Events & managements | CBC counts | |||
|---|---|---|---|---|
| WBC | Hb | Platelet | ||
| At admission | Detection of left chronic subdural hematoma | 59,300 | 10.5 | 207,000 |
| After 1 week | Decitabine #1 cycle | 36,700 | 7.9 | 165,000 |
| After 5 weeks | Decitabine #2 cycle | 2,100 | 11.5 | 265,000 |
| After 2 months | Neutropenic fever | 600 | 7.9 | 7,000 |
| After 3 months | Decitabine #3 cycle | 5,800 | 10.8 | 81,000 |
| After 4 months | No clinical symptom | 2,700 | 9.8 | 32,000 |
| After 5 months | Decitabine #4 cycle | 4,700 | 11 | 71,000 |
| After 6 months | No clinical symptom | 6,400 | 11 | 18,000 |
| After 7 months | Decitabine #5 cycle | 7,100 | 11.7 | 73,000 |
CBC = complete blood count; WBC = white blood cell; Hb = hemoglobin.
DISCUSSION
CMML is a rare myeloid neoplasm defined as a clonal hematopoietic stem cell disorder which is characterized by absolute monocytosis (> 1 × 109/L) in the peripheral blood, and the presence of myelodysplastic syndrome and myeloproliferative neoplasm features in the bone marrow by 2008 World Health Organization.25) It has an inherent risk for transformation to acute myeloid leukemia. The annual incidence of CMML is estimated at 4 per 100,000.19),26) The median age at diagnosis of disease ranges from 71 to 74 years, with a male predominance (1.5–3:1).2),22) Chemotherapy such as etoposide, cytarabine, all-trans retinoic acid, topotecan, 9-nitro-campothecin (topoisomerase inhibitor), and lonafarnib (farnesyltransferase inhibitor) has been the mainstay of treatment for chronic myeloid leukemia (CML) since the 1990s.5),7) However, the outcome has been disappointing due to the low response rates and high rates of toxicities. The overall response rates of these hypomethylating agents are estimated at 30–40%, with complete remission rates of 15%.2),3)
This case is the second case report of non-traumatic SDH associated with CMML, to our best knowledge. In 1912, Ichimura et al.8) reported a 74-year-old man who was previously diagnosed as CMML and treated for non-traumatic SDH compromised platelet function due to ineffective erythropoiesis was the probable cause of SDH. There have also been a few reports on non-traumatic subdural hematomas in association with other leukemic malignancies during the disease progression or chemotherapy of such malignancies.6),21) Imatinib mesylate (IM; GleevecTM, Novartis, Basel, Switzerland), a potent and selective tyrosine kinase inhibitor that acts on Bcr/Abl product of the Philadelphia chromosome, has been used as the standard initial treatment for Philadelphia chromosome-positive CML.16) Song et al.21) reported occurrence of non-traumatic subdural hematomas in 6% (7/121 patients) of patients treated with IM for advanced CML. Subdural hematoma due to loss of complete molecular response associated with IM treatment of CML has also been reported.9),10)
Spontaneous acute SDH as the primary initial presenting manifestation of a CML is previously reported.1) A single case report described recurrent subdural hematoma as the sole manifestation of chronic lymphocytic leukemia. Other authors described a patient presenting with recurrent subdural hematomas as the primary manifestation of chronic lymphocytic leukemia.4) Besides SDH, intracerebral hematoma was also presented as initial manifestation of CML.17) This is the first case of SDH as initial manifestation of CMML. The presence of SDH in the absence of head trauma should be evaluated by basic blood work up, as well as careful medical history taking. Prompt diagnosis can be followed by prompt treatment for the causative disease. The limitation of this case report is the absence of cytological examination of the subdural fluid or dura mater. The surgical treatment was performed before hematologic evaluation. The presence of myeloid cells in the subdural fluid collection should be evaluated to rule out the malignant subdural effusion.18)
Our case had the risk of re-accumulation from the leukemia as well as the treatment. Apart from the disease progression, treatments with hypomethylating chemotherapy agents also induce the coagulopathy, which results in the rise of recurrence rates of SDH. In these situations, repeated burr-hole surgery or removal of the outer membrane with craniotomy has risks of bleeding or infection.11),15)
Since first introduced in 2000 for patient with liver cirrhosis, transarterial MMA embolization has emerged as the treatment option for frequently recurring chronic SDH.13),23) The therapeutic rationale follows from pathologic identification. Tanaka et al.24) identified that arteries originating from branches of the MMA that entered the outer membrane through histological study of dura mater and outer membrane. MMA contributes to the development of chronic SDH, by providing feeding vessels to the outer membrane that is connected with the dura matter. Angiographic findings of chronic SDH including diffuse MMA dilation and the abnormal vascular networks on outer membrane support this hypothesis.23),24) In our case, embolization of the MMA was highly effective in the patient at high risk of recurrent chronic SDH. The patient required continuous chemotherapy, and transient thrombocytopenia occurred after each chemotherapy session. Nevertheless, remnant hematoma showed spontaneous resolution with no further recurrence.
CONCLUSION
This case is the first report of CMML diagnosed from spontaneous chronic SDH, with successful MMA embolization and no recurrence. MMA embolization is potentially a useful and safe treatment option in challenging clinical situations with underlying pathologies.
Footnotes
Disclosure: The authors have no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Abdulhamid MM, Li YM, Hall WA. Spontaneous acute subdural hematoma as the initial manifestation of chronic myeloid leukemia. J Neurooncol. 2011 Feb;101(3):513–516. doi: 10.1007/s11060-010-0278-6. [DOI] [PubMed] [Google Scholar]
- 2.Adès L, Sekeres MA, Wolfromm A, Teichman ML, Tiu RV, Itzykson R, et al. Predictive factors of response and survival among chronic myelomonocytic leukemia patients treated with azacitidine. Leuk Res. 2013 Jun;37(6):609–613. doi: 10.1016/j.leukres.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Braun T, Itzykson R, Renneville A, de Renzis B, Dreyfus F, Laribi K, et al. Molecular predictors of response to decitabine in advanced chronic myelomonocytic leukemia: a phase 2 trial. Blood. 2011 Oct 06;118(14):3824–3831. doi: 10.1182/blood-2011-05-352039. [DOI] [PubMed] [Google Scholar]
- 4.Bromberg JE, Vandertop WP, Jansen GH. Recurrent subdural haematoma as the primary and sole manifestation of chronic lymphocytic leukaemia. Br J Neurosurg. 1998 Aug;12(4):373–376. doi: 10.1080/02688699844934. [DOI] [PubMed] [Google Scholar]
- 5.Cambier N, Wattel E, Menot M, Guerci A, Chomienne C, Fenaux P. All-trans retinoic acid in adult chronic myelomonocytic leukemia: results of a pilot study. Leukemia. 1996 Jul;10(7):1164–1167. [PubMed] [Google Scholar]
- 6.Comănescu A, Roşca E, Bota M, Ninulescu G. Chronic subdural hematoma in a patient with acute myeloid leukemia and dural metastatic infiltration. Rom J Morphol Embryol. 2008 Oct;49(2):259–262. [PubMed] [Google Scholar]
- 7.Feldman EJ, Cortes J, DeAngelo D, Holyoake T, Simonsson B, O'Brien SG, et al. On the use of lonafarnib in myelodysplastic syndrome and chronic myelomonocytic leukemia. Leukemia. 2008 Sep;22(9):1707–1711. doi: 10.1038/leu.2008.156. [DOI] [PubMed] [Google Scholar]
- 8.Ichimura S, Horiguchi T, Inoue S, Yoshida K. Nontraumatic acute subdural hematoma associated with the myelodysplastic/myeloproliferative neoplasms. J Neurosci Rural Pract. 2012 Jan;3(1):98–99. doi: 10.4103/0976-3147.91978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khaladkar SM, Thakkar DK, Jantre MN, Kulkarni VM, Singh A. Chronic subdural hematoma-unsual cause of headache in a patient with chronic myeloid leukemia treated with high-dose imatinib mesylate: a rare case report with review of literature. Med J DY Patil Univ. 2015 May-Jun;8(3):411–413. [Google Scholar]
- 10.Kim MS, Lee DH, Lee YR, Kim DK, Bae SH, Hwang JY, et al. A case of subdural hematoma in patient with chronic myeloid leukemia treated with high-dose imatinib mesylate. Korean J Hematol. 2010 Mar;45(1):73–75. doi: 10.5045/kjh.2010.45.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laumer R. Implantation of a reservoir for refractory chronic subdural hematoma. Neurosurgery. 2002 Mar;50(3):672. doi: 10.1097/00006123-200203000-00051. [DOI] [PubMed] [Google Scholar]
- 12.Lee KS. History of chronic subdural hematoma. Korean J Neurotrauma. 2015 Oct;11(2):27–34. doi: 10.13004/kjnt.2015.11.2.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandai S, Sakurai M, Matsumoto Y. Middle meningeal artery embolization for refractory chronic subdural hematoma. Case report. J Neurosurg. 2000 Oct;93(4):686–688. doi: 10.3171/jns.2000.93.4.0686. [DOI] [PubMed] [Google Scholar]
- 14.McDermott M, Fleming RJ, Vanderlinden GR, Tucker WS. Spontaneous arterial subdural hematoma. Neurosurgery. 1984 Jan;14(1):13–18. doi: 10.1227/00006123-198401000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Mino M, Nishimura S, Hori E, Kohama M, Yonezawa S, Midorikawa H, et al. Efficacy of middle meningeal artery embolization in the treatment of refractory chronic subdural hematoma. Surg Neurol Int. 2010 Dec 13;1:78. doi: 10.4103/2152-7806.73801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003 Mar 13;348(11):994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 17.Olfa CW, Imen R, Leila K, Hichem K, Adnane A, Mourad C, et al. Diagnosis of chronic myeloid leukemia from acute intracerebral hemorrhage: a case report. J Acute Dis. 2015 Aug;4(3):252–254. [Google Scholar]
- 18.Vasudev Rao T, Deshpande D. Malignant subdural effusion. Acta Neurochir (Wien) 1980 Mar;52(1-2):61–65. doi: 10.1007/BF01400948. [DOI] [PubMed] [Google Scholar]
- 19.Rollison DE, Howlader N, Smith MT, Strom SS, Merritt WD, Ries LA, et al. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001–2004, using data from the NAACCR and SEER programs. Blood. 2008 Jul 01;112(1):45–52. doi: 10.1182/blood-2008-01-134858. [DOI] [PubMed] [Google Scholar]
- 20.Sato M, Saito T, Yamaguchi K, Sakuma H. A case of acute subdural hematoma due to dural metastasis from malignant pleural mesothelioma. No Shinkei Geka. 1994 Mar;22(3):247–251. [PubMed] [Google Scholar]
- 21.Song KW, Rifkind J, Al-Beirouti B, Yee K, McCrae J, Messner HA, et al. Subdural hematomas during CML therapy with imatinib mesylate. Leuk Lymphoma. 2004 Aug;45(8):1633–1636. doi: 10.1080/10428190310001615666. [DOI] [PubMed] [Google Scholar]
- 22.Such E, Germing U, Malcovati L, Cervera J, Kuendgen A, Della Porta MG, et al. Development and validation of a prognostic scoring system for patients with chronic myelomonocytic leukemia. Blood. 2013 Apr 11;121(15):3005–3015. doi: 10.1182/blood-2012-08-452938. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi K, Muraoka K, Sugiura T, Maeda Y, Mandai S, Gohda Y, et al. Middle meningeal artery embolization for refractory chronic subdural hematoma: 3 case reports. No Shinkei Geka. 2002 May;30(5):535–539. [PubMed] [Google Scholar]
- 24.Tanaka T, Fujimoto S, Saitoh K, Satoh S, Nagamatsu K, Midorikawa H. Superselective angiographic findings of ipsilateral middle meningeal artery of chronic subdural hematoma in adults. No Shinkei Geka. 1998 Apr;26(4):339–347. [PubMed] [Google Scholar]
- 25.Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009 Jul 30;114(5):937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 26.Williamson PJ, Kruger AR, Reynolds PJ, Hamblin TJ, Oscier DG. Establishing the incidence of myelodysplastic syndrome. Br J Haematol. 1994 Aug;87(4):743–745. doi: 10.1111/j.1365-2141.1994.tb06733.x. [DOI] [PubMed] [Google Scholar]


