Abstract
Background and Objectives
The prognostic impact of left axis deviation (LAD) on clinical outcomes in acute heart failure syndrome (AHFS) with left bundle branch block (LBBB) is unknown. The aim of this study was to determine the prognostic significance of axis deviation in acute heart failure patients with LBBB.
Methods
Between March 2011 and February 2014, 292 consecutive AHFS patients with LBBB were recruited from 10 tertiary university hospitals. They were divided into groups with no LAD (n=189) or with LAD (n=103) groups according to QRS axis <−30 degree. The primary outcome was all-cause mortality.
Results
The median follow-up duration was 24 months. On multivariate analysis, the rate of all-cause death did not significantly differ between the normal axis and LAD groups (39.7% vs. 46.6%, adjusted hazard ratio, 1.01; 95% confidence interval, 0.66, 1.53; p=0.97). However, on the multiple linear regression analysis to evaluate the predictors of the left ventricular ejection fraction (LVEF), presence of LAD significantly predicted a worse LVEF (adjusted beta, −3.25; 95% confidence interval, −5.82, −0.67; p=0.01). Right ventricle (RV) dilatation was defined as at least 2 of 3 electrocardiographic criteria (late R in lead aVR, low voltages in limb leads, and R/S ratio <1 in lead V5) and was more frequent in the LAD group than in the normal axis group (p<0.001).
Conclusions
Among the AHFS with LBBB patients, LAD did not predict mortality, but it could be used as a significant predictor of worse LVEF and RV dilatation (Trial registry at KorAHF registry, ClinicalTrial.gov, NCT01389843).
Keywords: Bundle-branch block, Heart failure, Electrocardiography
INTRODUCTION
Left bundle branch block (LBBB) is a marker of poor prognosis in heart failure (HF) patients, and interest in LBBB is increasing due to the development of cardiac-resynchronization therapy (CRT).1),2),3),4),5) Among the randomized population included in the Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy (MADIT-CRT) trial, LBBB patients without left axis deviation (LAD) appeared to receive a greater benefit from CRT than did those with LAD.6) The most common cause of LAD is left anterior fascicular block (LAFB), but LAFB cannot explain the LAD in patients with complete LBBB.7),8),9) The proposed mechanisms of LAD in patients with LBBB include attitudinal changes in the anatomy such as a cardiomegaly and changes in myocyte mass.10) A previous study showed that patients with LBBB with LAD had greater incidence of cardiomegaly, HF, advanced conduction disease, cardiovascular mortality, and major adverse cardiac event than those with a normal axis.11),12) Another study indicated that LBBB with LAD does not confer a significant mortality risk, but those with a normal axis who developed LAD during the study period had a significantly higher mortality.13) However, limited data is available regarding the prognostic significance of LAD in acute heart failure syndrome (AHFS) patients with LBBB. Therefore, the aim of the present study is to determine the association between LAD and the clinical outcomes of AHFS with LBBB.
METHODS
Study population
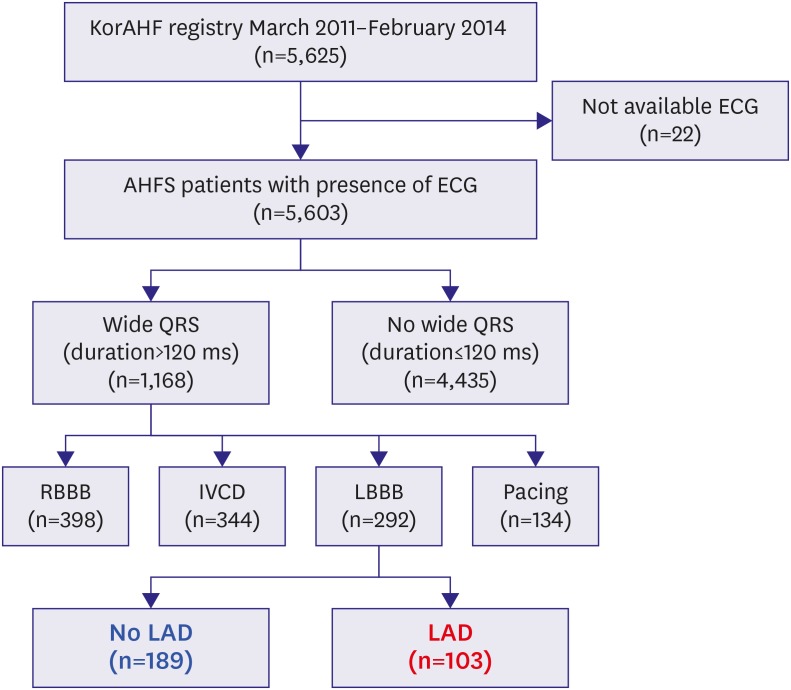
The Korean Acute Heart Failure (KorAHF) registry is a prospective multicenter cohort. Between January 2011 and February 2014, a total of 5,625 patients, who were hospitalized due to AHFS were consecutively enrolled from 10 university hospitals. The patients are planned for follow-up until 2018. Patients who had signs or symptoms of HF and one of the following criteria were eligible for the study: 1) lung congestion or 2) objective findings of LV systolic dysfunction or structural heart disease. Lung congestion was defined as ‘congestion’ on a chest X-ray or as rales on physical examination. Electrocardiograms (ECGs) were recorded at a standard paper speed of 25 mm/s and calibration of 10 mm/mV. They were read by trained physicians who were blinded to clinical data, site interpretation, and patient outcomes. LBBB was defined by the following criteria: 1) QRS duration >120 ms, 2) broad notched or slurred R waves in leads I, aVL, V5, and V6, and an occasional RS pattern in V5 and V6 attributed to a displaced transition of the QRS complex, 3) absent Q waves in leads I, V5, and V6 or a narrow Q wave in lead aVL, in the absence of a myocardial pathology, and 4) a peak R time greater than 60 ms in leads V5 and V6 but normal in leads V1, V2, and V3 when small initial r waves could be discerned in the above leads.14) Among the registered study population, 1,168 (20.8%) patients had QRS prolongation (>120 ms). Among these population, 292 patients presented with LBBB, 344 patients with intra-ventricular conduction delay (IVCD), 398 patients with RBBB, and 134 with pacing rhythm (Figure 1). We included patients with LBBB for analysis in the present study. The Institutional Review Board of each hospital approved the study protocol.
Figure 1. Study flow.
AHFS = acute heart failure syndrome; ECG = electrocardiogram; IVCD = intra-ventricular conduction delay; KorAHF = Korean Acute Heart Failure; LAD = left axis deviation; LBBB = left bundle branch block; RBBB = right bundle branch block.
Data collection
Detailed information on the study design and results from interim analysis are described in our previous paper.15),16) In brief, the investigators completed a web-based case report form at enrollment and at each visit in the Clinical Data Management System (iCReaT) from the Korea National Institute of Health. The latest information on patient clinical manifestation, laboratory results, and medications was collected at admission, at discharge, and during follow-up (30 days, 3 months, 6 months, and 1 to 5 years annually). In-hospital mortality and the cause of death were adjudicated by an independent event committee. The mortality data for patients who were lost to follow-up was collected from National Insurance data or National Death Records.
Definitions and outcomes
LAD was defined as a QRS axis <−30 degree in accordance with published criteria.14) Echocardiography was available for 96.6% (282/292) of the study population and left ventricular ejection fraction (LVEF) was assessed by the biplane Simpson technique, M-mode, or visual estimation.17) To evaluate the right ventricle (RV) dilatation on the surface ECG, any combination of at least 2 of 3 electrocardiographic criteria (late R in lead aVR, low voltages in the limb leads, and R/S ratio <1 in lead V5) was used.18)
The AHFS patients with LBBB were divided into 2 groups according to QRS axis (<−30 or ≥−30 degree). The primary end point was death from any cause. The secondary end points were reduced LVEF and RV dilatation.
Statistical analysis
Continuous variables were examined using Student's t-test or Wilcoxon rank-sum test when applicable. Categorical data were compared using the χ2 test or Fisher's exact test, as appropriate. The event-free survival was assessed by Kaplan-Meier analyses and the significance level was evaluated with the log-rank test. In multivariate Cox regression models for the survivors, covariates that were suggested to be relevant on univariate analysis with p value <0.2 or clinically relevant were considered as candidate variables. Adjusted hazard ratios (HRs) were compared by Cox regression based on age, sex, ischemic etiology, hypertension, diabetes mellitus, LVEF <40%, body mass index ≥25 kg/m2, QRS duration ≥150 ms, and creatinine ≥2 mg/dL. To assess the association between the QRS axis and echocardiographic parameters, including LVEF, E/A ratio, E/e′, deceleration time, left atrial size, and right ventricular systolic pressure, a locally weighted scatterplot smoothing (LOESS) method was utilized. To determine the predictors of LVEF, a multiple linear regression analysis was used. The variables for analysis were selected by the same criteria depicted in the Cox regression model, except LVEF <40%. Statistical analyses were performed using R Statistical Software (version 3.1.3; R Foundation for Statistical Computing, Vienna, Austria) with a p<0.05 considered statistically significant.
RESULTS
Baseline characteristics of the study population
The median (interquartile range) age of the 292 patients was 75 (68–80) years, and 46.6% were males. Patients with de novo HF accounted for 40.8% of the total, and 37.7% of the study population were classified as having ischemic cardiomyopathy. Among the study population, 80.8% of the patients were in sinus rhythm and 18.5% were in atrial fibrillation or flutter. The mean values of QRS duration and LVEF were 155.7 ms and 28.4%, respectively.
Among the study population, 189 (64.7%) patients had a normal axis, and 103 (35.3%) had LAD. The baseline clinical characteristics, treatment strategy, laboratory data, and outcomes on admission according to the presence of LAD are described in Table 1. Compared to the normal axis group, the LAD group had a higher proportion of males and a lower proportion of patients with diabetes mellitus. Also, the patients with LAD had a significantly higher creatinine level, lower body mass index, and lower LVEF values. The other variables did not significantly differ between the 2 groups.
Table 1. Baseline clinical characteristics according to the presence of LAD.
| No LAD (n=189) | LAD (n=103) | p value | |||
|---|---|---|---|---|---|
| Age (years) | 72.6±10.8 | 74.0±11.7 | 0.29 | ||
| Male | 79 (41.8) | 57 (55.3) | 0.04 | ||
| Alcohol | 63 (33.3) | 39 (37.9) | 0.52 | ||
| Current smoker | 24 (12.7) | 14 (13.6) | 0.97 | ||
| Body mass index (kg/m2) | 23.5±3.5 | 22.0±3.5 | 0.001 | ||
| Hypertension | 105 (55.6) | 68 (66.0) | 0.11 | ||
| Diabetes mellitus | 89 (47.1) | 34 (33.0) | 0.03 | ||
| Chronic kidney disease | 26 (13.8) | 18 (17.5) | 0.50 | ||
| Cerebrovascular accident | 20 (10.6) | 17 (16.5) | 0.20 | ||
| Pulmonary disease | 20 (10.6) | 18 (17.5) | 0.14 | ||
| De-novo heart failure | 83 (43.9) | 36 (35.0) | 0.17 | ||
| Ischemic etiology | 79 (41.8) | 31 (30.1) | 0.07 | ||
| NYHA classification | 0.24 | ||||
| 2 | 19 (10.1) | 17 (16.5) | |||
| 3 | 71 (37.6) | 39 (37.9) | |||
| 4 | 99 (52.4) | 47 (45.6) | |||
| Systolic blood pressure (mmHg) | 127.4±25.5 | 128.3±27.9 | 0.80 | ||
| Heart rate (beats/min) | 93.6±21.9 | 90.4±24.5 | 0.25 | ||
| Treatment | |||||
| Beta blocker at discharge | 103 (54.5) | 55 (53.4) | 0.95 | ||
| RAS blockade at discharge | 138 (73.0) | 78 (75.7) | 0.72 | ||
| AA at discharge | 105 (55.6) | 50 (48.5) | 0.31 | ||
| Mechanical ventilation | 30 (15.9) | 20 (19.4) | 0.55 | ||
| Laboratory data | |||||
| QRS axis | 21.3±34.8 | −49.8±12.3 | <0.001 | ||
| Rhythm | 0.50 | ||||
| Sinus rhythm | 152 (80.4) | 84 (81.6) | |||
| Atrial fibrillation | 31 (16.4) | 18 (17.5) | |||
| Miscellaneous | 6 (3.2) | 1 (1.0) | |||
| PR interval (ms) | 169.6±38.7 | 175.5±39.1 | 0.26 | ||
| QRS duration (ms) | 154.1±17.6 | 158.3±21.2 | 0.09 | ||
| LVEF (%) | 29.6±10.0 | 26.1±9.8 | 0.006 | ||
| Na (mg/dL) | 137.5±4.7 | 137.4±4.6 | 0.77 | ||
| Creatinine (mg/dL) | 1.3±0.8 | 1.7±1.5 | 0.01 | ||
| Outcomes on admission | |||||
| In-hospital mortality | 9 (4.8) | 3 (2.9) | 0.65 | ||
| ICU admission | 95 (50.3) | 52 (50.5) | >0.99 | ||
| Hospital days | 9 (6–15) | 9 (6–15) | 0.89 | ||
Data are presented as the mean±standard deviation, number (%) or median (interquartile range).
AA = aldosterone antagonist; ICU = intensive care unit; LAD = left axis deviation; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association; PCI = percutaneous coronary intervention; RAS = renin angiotensin system.
Clinical outcome
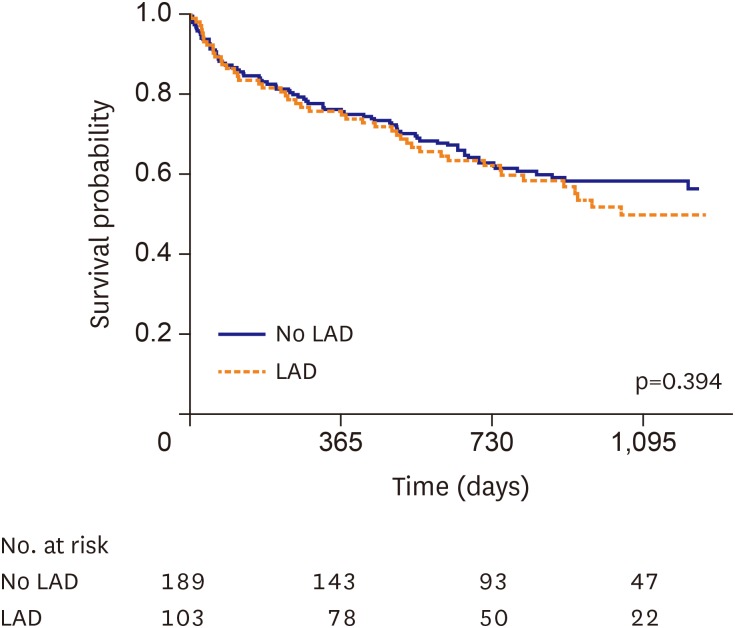
The median follow-up duration was 24 months (interquartile range, 12–35). There was a total of 123 (42.1%) all-cause deaths during the follow-up period. On univariate analysis, the patients with LAD did not have a significantly higher all-cause mortality compared to those with no LAD (LAD group vs. no LAD group, 46.6% vs. 39.7%, unadjusted HR, 1.17; 95% confidence interval [CI], 0.81, 1.68; p=0.40, Table 2 and Figure 2). Even after adjusting for the clinically relevant and statistically significant variables, multivariate analysis did not show any significant difference between the 2 groups (adjusted HR, 1.01; 95% CI, 0.66, 1.53; p=0.97, Table 2).
Table 2. Multivariate Cox regression analysis for predictors of all-cause mortality.
| Variables | Unadjusted HR (95% CI) | p value | Adjusted HR* (95% CI) | p value |
|---|---|---|---|---|
| LAD | 1.17 (0.81, 1.68) | 0.40 | 1.01 (0.66, 1.53) | 0.97 |
| Age | 1.04 (1.02, 1.06) | <0.001 | 1.05 (1.02, 1.07) | <0.001 |
| Male | 1.39 (0.97, 1.98) | 0.07 | 1.36 (0.92, 2.02) | 0.12 |
| Ischemic etiology | 1.90 (1.33, 2.70) | <0.001 | 1.65 (1.10, 2.48) | 0.02 |
| Hypertension | 1.30 (0.90, 1.88) | 0.16 | 0.98 (0.65, 1.49) | 0.94 |
| Diabetes mellitus | 1.10 (0.77, 1.58) | 0.59 | 0.80 (0.53, 1.18) | 0.26 |
| LVEF <40% | 1.01 (0.60, 1.69) | 0.97 | 1.30 (0.76, 2.22) | 0.33 |
| Body mass index ≥25 kg/m2 | 1.09 (0.72, 1.66) | 0.67 | 1.06 (0.67, 1.67) | 0.82 |
| QRS duration ≥150 ms | 0.94 (0.65, 1.35) | 0.73 | 0.82 (0.55, 1.22) | 0.33 |
| Creatinine ≥2 mg/dL | 3.05 (2.05, 4.55) | <0.001 | 3.12 (1.98, 4.93) | <0.001 |
CI = confidence interval; HR = hazard ratio; LAD = left axis deviation; LVEF = left ventricular ejection fraction.
*C-index of the Cox regression model was 0.687 (95% CI, 0.630, 0.744).
Figure 2. Kaplan-Meier curves of AHFS patients with LBBB according to the presence of LAD. Kaplan-Meier curve of all-cause death for AHFS patients with LBBB in the no LAD group (blue line) versus the LAD group (red line).
AHFS = acute heart failure syndrome; LAD = left axis deviation; LBBB = left bundle branch block.
Predictors of the echocardiographic parameters
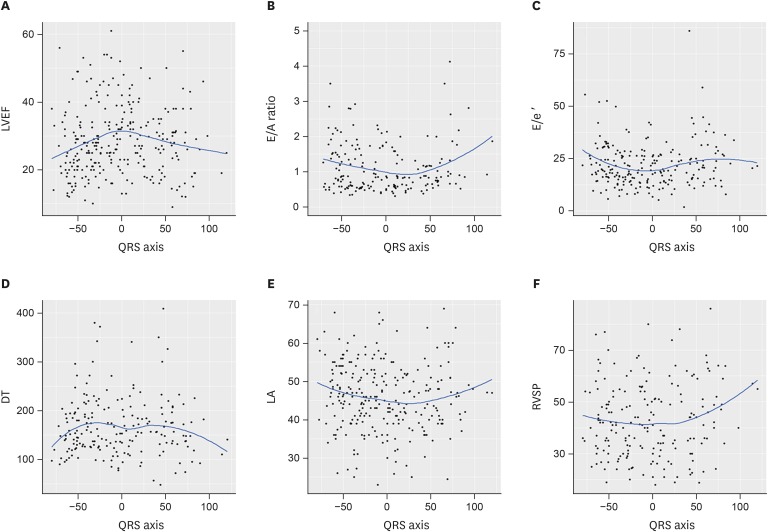
To determine the impact of the QRS axis on the echocardiographic parameters, an LOESS method was performed. The LOESS model results of the echocardiographic parameters are depicted in Figure 3. In Figure 3A, the LVEF peaked at 0 degrees of the QRS axis and trended downward when the axis was increased or decreased. After dividing the patients into 3 groups (QRS axis <−30, −30 to 90, and >90 degree), simple linear regression showed that the LAD was a significant predictor of a worse LVEF (adjusted coefficient, −3.03, 95% CI, −5.53, −0.52, p=0.02) (Table 3). The significant association between LAD and a worse LVEF was maintained on multiple linear regression analysis (adjusted coefficient, −3.25; 95% CI, −5.82, −0.67; p=0.01) (Table 3). Although the E/A ratio, LA size, and right ventricular systolic pressure appeared to vary as the QRS axis changed, as shown in Figure 3B, 3E, and 3F, there was no significant difference among the 3 groups.
Figure 3. LOESS curves of the echocardiographic parameters according to the QRS axis. LOESS curves of the LVEF (A), E/A ratio (B), E/e′ (C), DT (D), LA size (E), and RVSP (F) according to the QRS axis.
DT = deceleration time; LA = left atrium; LOESS = locally weighted scatterplot smoothing; LVEF = left ventricular ejection fraction; RVSP = right ventricular systolic pressure.
Table 3. Multiple linear regression analysis for the predictors of lower LVEF.
| Variables | Unadjusted slope (95% CI) | p value | Adjusted slope (95% CI) | p value | |
|---|---|---|---|---|---|
| Normal QRS axis (reference) | |||||
| LAD | −3.03 (−5.53, −0.52) | 0.02 | −3.25 (−5.82, −0.67) | 0.01 | |
| RAD | 0.45 (−9.47, 10.36) | 0.93 | −0.20 (−10.08, 9.67) | 0.97 | |
| Age | 0.18 (0.07, 0.28) | 0.001 | 0.16 (0.04, 0.27) | 0.007 | |
| Male | −1.31 (−3.69, 1.07) | 0.28 | −0.56 (−3.00, 1.87) | 0.65 | |
| Ischemic etiology | 2.00 (−0.44, 4.44) | 0.11 | 0.69 (−1.88, 3.26) | 0.60 | |
| Hypertension | 2.30 (0.90, 1.88) | 0.16 | 0.98 (0.65, 1.49) | 0.94 | |
| Diabetes mellitus | 2.64 (0.23, 5.06) | 0.03 | 1.57 (−1.08, 4.22) | 0.24 | |
| Body mass index ≥25 kg/m2 | 0.98 (−1.83, 3.78) | 0.49 | 0.89 (−1.97, 3.75) | 0.54 | |
| QRS duration ≥150 ms | −0.26 (−2.73, 2.22) | 0.84 | −0.26 (−2.73, 2.22) | 0.83 | |
| Creatinine ≥2 mg/dL | 1.67 (−1.58, 4.91) | 0.31 | 1.29 (−1.98, 4.57) | 0.44 | |
CI = confidence interval; LAD = left axis deviation; LVEF = left ventricular ejection fraction; RAD = right axis deviation.
RV dilatation
There was a significantly higher proportion of RV dilatation criteria in the LAD group compared to the no LAD group (LAD vs. no LAD, 40.8% vs. 12.7%, p<0.001). In particular, a late R in aVR was the strongest contributing factor (51.5% vs. 6.3%, p<0.001).
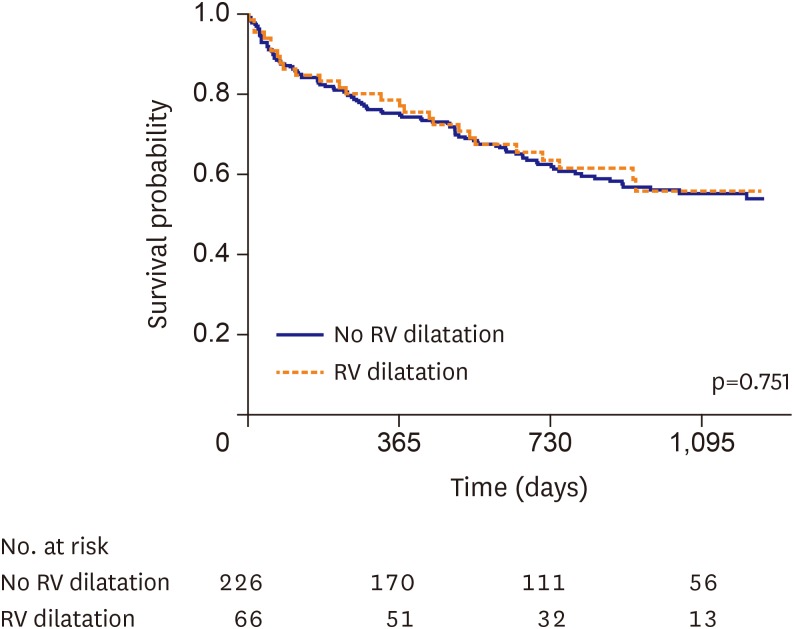
To evaluate the impact of RV dilatation criteria of the surface ECG on all-cause mortality, a Kaplan-Meier method with log-rank test was performed. In Figure 4, the Kaplan-Meier curve for all-cause death based on RV dilatation criteria showed that there was no significant difference in all-cause mortality between the 2 groups (log-rank p=0.751).
Figure 4. Kaplan-Meier curve of all-cause death in AHFS patients with LBBB according to RV dilatation criteria. Kaplan-Meier curve of all-cause death in AHFS patients with LBBB in the no RV dilatation group (blue line) versus RV dilatation group (red line).
AHFS = acute heart failure syndrome; LBBB = left bundle branch block; RV = right ventricle.
DISCUSSION
In this study, the prognostic significance of LAD in patients who had AHFS with LBBB was investigated using a prospective multicenter cohort registry. There were several main findings of our study. 1) Among the AHFS patients with LBBB, the presence of LAD did not predict all-cause mortality. 2) The LVEF was significantly lower in AHFS patients with LBBB and LAD than in those without LAD. 3) LAD was a significant predictor of RV dilatation, which was evaluated by surface ECG criteria in patients with AHFS and LBBB.
The prognostic significance of LAD in patients with LBBB has been controversial in various study populations. In chronic LBBB patients, the presence of LAD has been shown to confer greater incidence of cardiomegaly, myocardial dysfunction, advanced conduction disease, and cardiovascular mortality.11),19) Also, in patients with HF and LBBB who undergo successful implantations of CRT devices, the presence of LAD suggests a poor clinical outcome, including all-cause mortality and HF re-hospitalizations or events.6),20) Park et al.12) recently reported that LAD is associated with myocardial scarring and major adverse cardiac event in patients with LBBB. On the other hand, in patients with HF and LBBB who undergo ICD implantations, LAD does not predict all-cause mortality or HF events.6) In addition, for LBBB patients who undergo an ECG for any reason, LAD does not confer a significant mortality risk.13) Our study showed that LAD was not a significant predictor of all-cause mortality in an AHFS cohort. To the best of our knowledge, this is the first study to confirm the prognostic significance of LAD on clinical outcomes in AHFS patients with LBBB. It was estimated that the cause of the discrepancy between our data and the data of HF patients who underwent CRT implantations was due to the differences in the study populations and differences in the benefit of CRT based on the presence of LAD. Also, we speculated that the prognostic impact of LAD had little significance in a high-risk study population, such as those with AHFS and LBBB, because they already exhibit multiple poor prognostic factors and mortality risks.
A previous study showed that the presence of LAD in LBBB patients does not signify a further decrease in LVEF.21) However, the present study demonstrated that LVEF is significantly lower in AHFS patients who have LBBB with LAD than in those without LAD. Also, patients with LAD in the present study had a significantly higher proportion of RV dilatation than did those without LAD. These results support that the main cause of LAD in patients with LBBB is anatomical change, such as cardiomegaly or myocardial scarring resulting from previous study.10),22),23) Moreover, lower LVEF seen in the LAD group may be the cause or be a result of LAD. Further physiological or pathological studies are needed to evaluate the mechanism of LAD in LBBB patients.
Impaired RV function is an established marker of poor prognosis in patients with moderate to severe HF.24),25) However, our data showed that the all-cause mortality rate did not significantly differ between those who did or did not satisfy the RV dilatation criteria. Although numerous studies have been performed to evaluate the impact of RV dysfunction on clinical outcomes and CRT benefit, this issue remains controversial.26),27),28) An additional larger prospective cohort study is needed to evaluate the association between RV dysfunction and CRT benefit or mortality.
This study had several limitations. First, the current hypothesis was not defined prior to conducting the registry, and a relatively small study population was included in the final analysis, as the present study population consisted of a subgroup of the KorAHF registry. Second, it was an observational study, which may have affected the results because of confounding factors. Third, although the portion of missing data was small, 3.4% of the study population did not have echocardiographic data including that of the LVEF. Finally, due to the limitations of our data-base, we did not have any information on the RV parameters from the echocardiographic data or new onset LAD from the follow-up ECG.
Among AHFS patients with LBBB, the presence of LAD did not predict all-cause mortality, but it can be used as a significant predictor of worse LVEF and RV dilatation.
Footnotes
Funding: This work was supported by the Research of Korea Centers for Disease Control and Prevention (2010-E63003-00, 2011-E63002-00, 2012-E63005-00, 2013-E63003-00, 2013-18 E63003-01, 2013-E63003-02, and 2016-ER6303-00).
Conflict of Interest: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors have no financial conflicts of interest.
- Conceptualization: Jeon ES, Choi KH, Han S, Lee GY, Choi JO.
- Data curation: Choi KH, Han S, Lee GY, Lee HY, Lee SE, Kim JJ, Chae SC, Kang SM, Choi DJ.
- Formal analysis: Choi KH, Lee GY, Lee SE, Chae SC, Baek SH, Kang SM, Choi DJ.
- Funding acquisition: Lee HY, Cho MC, Park HY, Oh BH.
- Investigation: Choi KH, Han S, Lee GY, Choi JO, Lee SE, Kim JJ, Chae SC, Baek SH, Kang SM, Choi DJ, Yoo BS, Kim KH, Cho MC, Oh BH.
- Methodology: Choi KH.
- Resources: Lee HY, Kim JJ, Baek SH.
- Supervision: Jeon ES, Han S, Choi JO.
- Visualization: Choi KH.
- Writing - original draft: Choi KH.
- Writing - review & editing: Jeon ES, Han S.
References
- 1.Baldasseroni S, Opasich C, Gorini M, et al. Left bundle-branch block is associated with increased 1-year sudden and total mortality rate in 5517 outpatients with congestive heart failure: a report from the Italian network on congestive heart failure. Am Heart J. 2002;143:398–405. doi: 10.1067/mhj.2002.121264. [DOI] [PubMed] [Google Scholar]
- 2.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 3.Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 4.Zusterzeel R, Selzman KA, Sanders WE, et al. Cardiac resynchronization therapy in women: US Food and Drug Administration meta-analysis of patient-level data. JAMA Intern Med. 2014;174:1340–1348. doi: 10.1001/jamainternmed.2014.2717. [DOI] [PubMed] [Google Scholar]
- 5.Zareba W, Klein H, Cygankiewicz I, et al. Effectiveness of cardiac resynchronization therapy by QRS morphology in the Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy (MADIT-CRT) Circulation. 2011;123:1061–1072. doi: 10.1161/CIRCULATIONAHA.110.960898. [DOI] [PubMed] [Google Scholar]
- 6.Brenyo A, Rao M, Barsheshet A, et al. QRS axis and the benefit of cardiac resynchronization therapy in patients with mildly symptomatic heart failure enrolled in MADIT-CRT. J Cardiovasc Electrophysiol. 2013;24:442–448. doi: 10.1111/jce.12057. [DOI] [PubMed] [Google Scholar]
- 7.Rabkin SW, Mathewson FA, Tate RB. Natural history of marked left axis deviation (left anterior hemiblock) Am J Cardiol. 1979;43:605–611. doi: 10.1016/0002-9149(79)90020-1. [DOI] [PubMed] [Google Scholar]
- 8.Yano K, Peskoe SM, Rhoads GG, Moore JO, Kagan A. Left axis deviation and left anterior hemiblock among 8,000 Japanese-American men. Am J Cardiol. 1975;35:809–815. doi: 10.1016/0002-9149(75)90116-2. [DOI] [PubMed] [Google Scholar]
- 9.Swiryn S, Abben R, Denes P, Rosen KM. Electrocardiographic determinants of axis during left bundle branch block: study in patients with intermittent left bundle branch block. Am J Cardiol. 1980;46:53–58. doi: 10.1016/0002-9149(80)90605-0. [DOI] [PubMed] [Google Scholar]
- 10.Flowers NC. Left bundle branch block: a continuously evolving concept. J Am Coll Cardiol. 1987;9:684–697. doi: 10.1016/s0735-1097(87)80065-7. [DOI] [PubMed] [Google Scholar]
- 11.Dhingra RC, Amat-Y-Leon F, Wyndham C, et al. Significance of left axis deviation in patients with chronic left bundle branch block. Am J Cardiol. 1978;42:551–556. doi: 10.1016/0002-9149(78)90622-7. [DOI] [PubMed] [Google Scholar]
- 12.Park CS, Cha MJ, Choi EK, Oh S. Prognostic implication of the QRS axis and its association with myocardial scarring in patients with left bundle branch block. Korean Circ J. 2017;47:263–269. doi: 10.4070/kcj.2016.0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel PJ, Verdino RJ. Usefulness of QRS axis change to predict mortality in patients with left bundle branch block. Am J Cardiol. 2013;112:390–394. doi: 10.1016/j.amjcard.2013.03.045. [DOI] [PubMed] [Google Scholar]
- 14.Surawicz B, Childers R, Deal BJ, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III: intraventricular conduction disturbances: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. J Am Coll Cardiol. 2009;53:976–981. doi: 10.1016/j.jacc.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Lee SE, Cho HJ, Lee HY, et al. A multicentre cohort study of acute heart failure syndromes in Korea: rationale, design, and interim observations of the Korean Acute Heart Failure (KorAHF) registry. Eur J Heart Fail. 2014;16:700–708. doi: 10.1002/ejhf.91. [DOI] [PubMed] [Google Scholar]
- 16.Lee SE, Lee HY, Cho HJ, et al. Clinical characteristics and outcome of acute heart failure in Korea: results from the Korean Acute Heart Failure Registry (KorAHF) Korean Circ J. 2017;47:341–353. doi: 10.4070/kcj.2016.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 18.Van Bommel RJ, Marsan NA, Delgado V, et al. Value of the surface electrocardiogram in detecting right ventricular dilatation in the presence of left bundle branch block. Am J Cardiol. 2011;107:736–740. doi: 10.1016/j.amjcard.2010.10.051. [DOI] [PubMed] [Google Scholar]
- 19.Lichstein E, Mahapatra R, Gupta PK, Chadda KD. Significance of complete left bundle branch block with left axis deviation. Am J Cardiol. 1979;44:239–242. doi: 10.1016/0002-9149(79)90311-4. [DOI] [PubMed] [Google Scholar]
- 20.Perrotta L, Kandala JD, Biase L, et al. Prognostic Impact of QRS axis deviation in patients treated with cardiac resynchronization therapy. J Cardiovasc Electrophysiol. 2016;27:315–320. doi: 10.1111/jce.12887. [DOI] [PubMed] [Google Scholar]
- 21.Das MK, Cheriparambil K, Bedi A, et al. Prolonged QRS duration (QRS >/=170 ms) and left axis deviation in the presence of left bundle branch block: A marker of poor left ventricular systolic function? Am Heart J. 2001;142:756–759. doi: 10.1067/mhj.2001.118735. [DOI] [PubMed] [Google Scholar]
- 22.Havelda CJ, Sohi GS, Flowers NC, Horan LG. The pathologic correlates of the electrocardiogram: complete left bundle branch block. Circulation. 1982;65:445–451. doi: 10.1161/01.cir.65.3.445. [DOI] [PubMed] [Google Scholar]
- 23.Haft JI, Herman MV, Gorlin R. Left bundle branch block: etiologic, hemodynamic, and ventriculographic considerations. Circulation. 1971;43:279–287. doi: 10.1161/01.cir.43.2.279. [DOI] [PubMed] [Google Scholar]
- 24.de Groote P, Millaire A, Foucher-Hossein C, et al. Right ventricular ejection fraction is an independent predictor of survival in patients with moderate heart failure. J Am Coll Cardiol. 1998;32:948–954. doi: 10.1016/s0735-1097(98)00337-4. [DOI] [PubMed] [Google Scholar]
- 25.Ghio S, Gavazzi A, Campana C, et al. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37:183–188. doi: 10.1016/s0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 26.Damy T, Ghio S, Rigby AS, et al. Interplay between right ventricular function and cardiac resynchronization therapy: an analysis of the CARE-HF trial (Cardiac Resynchronization-Heart Failure) J Am Coll Cardiol. 2013;61:2153–2160. doi: 10.1016/j.jacc.2013.02.049. [DOI] [PubMed] [Google Scholar]
- 27.Sharma A, Bax JJ, Vallakati A, et al. Meta-analysis of the relation of baseline right ventricular function to response to cardiac resynchronization therapy. Am J Cardiol. 2016;117:1315–1321. doi: 10.1016/j.amjcard.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 28.Rapacciuolo A, Maffè S, Palmisano P, et al. Prognostic role of right ventricular function in patients with heart failure undergoing cardiac resynchronization therapy. Clin Cardiol. 2016;39:640–645. doi: 10.1002/clc.22574. [DOI] [PMC free article] [PubMed] [Google Scholar]