Abstract
Background
Correlations between volume doubling time (VDT) of primary lung cancer (PLC), histology, and ground glass opacity (GGO) components remain unclear. The purpose of this study was to evaluate and compare VDT of PLC in terms of histology and presence or absence of GGO components.
Methods
A total of 371 surgically resected PLCs from 2003 to 2015 in our institute were retrospectively reviewed. The VDT was calculated both from the diameters of the entire tumor and of consolidation by using the approximation formula of Schwartz.
Results
The median VDTs of adenocarcinoma, squamous cell carcinoma, and others (large cell neuroendocrine carcinomas, small cell lung carcinomas, pulmonary pleomorphic carcinomas, and large cell carcinomas combined) were 261, 70, and 70 days, respectively; these differ significantly (P<0.001). All PLCs with GGO were adenocarcinomas. The VDT of adenocarcinomas with GGO was significantly longer than that of those without GGO (median VDT: 725 and 177 days, respectively), squamous cell carcinomas, and others. When the VDT calculated from the maximum diameter of consolidation component was compared, adenocarcinomas with GGO also showed significantly slower growth than those without GGO (median VDT: 248 versus 177 days, respectively, P=0.040).
Conclusions
The VDT of PLCs is longest for adenocarcinomas. VDT was significantly longer in adenocarcinomas with GGO components than in those without such components, irrespective of VDT calculated on the basis of either the entire tumor diameter or consolidation diameter.
Keywords: Volume doubling time (VDT), ground glass opacity (GGO), primary lung cancer (PLC), adenocarcinoma
Introduction
The growth speed of primary lung cancer (PLC) reflects its malignant nature and is closely associated with prognosis (1-6). The time taken for a tumor to double in volume, volume doubling time (VDT), is a classical indicator of PLC proliferation and is also associated with histology and prognosis (2-8). A short VDT means rapid growth of the tumor and represents its malignant potential. A VDT less than 400 days has been suggested as a cutoff value to distinguish a benign from a malignant lesion (8,9). VDT is one of the key parameters to distinguish aggressive tumors from indolent ones.
Solid components of PLCs on high resolution computed tomography (HRCT) images represent their invasive potential and are associated with rapid growth (5-8,10). In contrast, ground glass opacity (GGO) components suggest a longer VDT and good outcome, such as in adenocarcinoma in situ and minimally invasive adenocarcinoma (10,11). It is considered that radiologically solid PLCs show rapid growth compared with GGO lesion, however, it is unclear if the growth speed of the solid component of part-solid nodules differs from that of pure solid nodules. Also, differences in VDT of radiologically solid PLCs according to histology have not yet been sufficiently explored. The main objective of this study was to compare difference in VDTs according to PLC histology, and the secondary objective was to compare difference in VDTs of PLCs with and without GGO components.
Methods
Patients
In this retrospective study, data of patients with PLC who had undergone surgical resection from 2003 through 2015 in our institute were investigated. Only patients who had undergone at least two preoperative CT scans at intervals of more than 21 days were included, leaving 371 eligible patients. All tumors were pathologically confirmed as PLCs. Histological classification was determined according to the fourth edition of the World Health Organization classification of lung tumors (12). The tumor, node and metastases staging system of the seventh edition of the American Joint Committee for Cancer Staging System was used (13). Clinical information, including age, sex, smoking history, tumor histology, pathological stage, tumor diameter, and VDT were collected from patient records for analysis. This study was approved in January 2017 by the Institutional Review Board for clinical trials of Gunma University (IRB approval number 1575). This study was conducted in accordance with the Declaration of Helsinki Principles.
CT examinations and measurement of lesions
All CT scans were performed on a 64-channel multi-detector row computed tomography (SOMATOM Definition Flash; Siemens Healthcare, Berlin, Germany). CT scans were performed on each tumor at least twice before surgery. CT images were reconstructed with a window level of 500 to 700 HU and a window width of 1,000 to 2,000 HU as lung window settings. Thin-section CT images were obtained at 1.0–2.0 mm thicknesses using a high spatial frequency reconstruction algorithm. The maximum diameter of the entire tumor, which included both GGO and consolidation components, and the maximum diameter of the consolidation component alone were measured separately on an axial CT slice that showed the longest diameter. Diameter values were measured manually using a computer workstation. GGO was defined as an area of slight increase in CT density that did not obscure lung structures such as pulmonary vessels and bronchi. Consolidation was defined as an area of homogenous increase in CT density that obscured these lung structures (14). A consolidation/tumor ratio (CTR) was calculated for each tumor by dividing the maximum diameter of consolidation by that of the entire tumor.
Tumor diameters were measured separately by two thoracic surgeons; in cases of discrepancies between measured sizes, the arithmetic mean of these diameters was used. VDTs were calculated both for the entire tumor diameter and the diameter of consolidation alone, using the approximation formula of Schwartz (15) (Figure S1).
Figure S1.
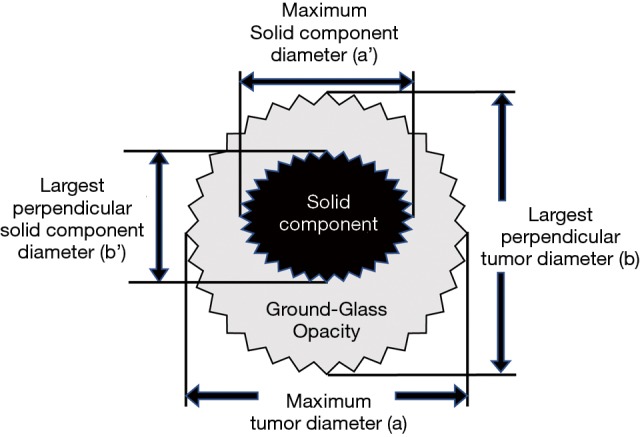
Modified Schwartz equation for calculating tumor volume. V = π/(6× ab2); VDT = (t × log2)/[log (Vt/V0)]; consolidation/tumor ratio = a’/a. a, maximum tumor diameter; b, largest perpendicular tumor diameter; a’, maximum solid component diameter; b’, largest perpendicular solid component diameter; t, interval between two CT scans; V0, tumor volume on initial CT scan; Vt, tumor volume on CT scan before surgery.
Statistical analysis of correlations between VDT and clinicopathological factors
Statistically significant correlations were identified using χ2 tests and t-tests for dichotomized factors. For factors with three or more groups, correlations were analyzed using one-way analysis of variance. Statistical analysis was performed using IBM SPSS Statistics software (version 24, Chicago, IL, USA). Probability values less than 0.05 were considered to indicate significant differences.
Results
There were 397 PLC patients who had undergone surgical resection during the described period, amongst which 371 had undergone the two preoperative CT scans. The clinicopathological characteristics of these 371 patients with PLCs are shown in Table 1.
Table 1. Comparison of histology and clinicopathological features in patients with primary lung cancer (n=371)a.
| Parameter | Adenocarcinoma, n=284 (76.5%) | Squamous cell carcinoma, n=59 (15.9%) | Others, n=28 (7.5%) | P valueb |
|---|---|---|---|---|
| Age (years) | 0.007 | |||
| ≤65 | 91 (32.0) | 7 (11.9) | 7 (25.0) | |
| >65 | 193 (68.0) | 52 (88.1) | 21 (75.0) | |
| Sex | ||||
| Male | 139 (48.9) | 54 (91.5) | 26 (92.9) | <0.001 |
| Female | 145 (51.1) | 5 (8.5) | 2 (7.1) | |
| Smoking status | <0.001 | |||
| Non-smoker | 136 (47.9) | 0 (0) | 0 (0) | |
| Current or ex-smoker | 148 (52.1) | 59 [100] | 28 [100] | |
| Pathological stage | 0.002 | |||
| I | 217 (76.4) | 37 (62.7) | 14 (50.0) | |
| II–IV | 67 (23.6) | 22 (37.3) | 14 (50.0) | |
| Lymphatic invasion | <0.001 | |||
| Not detected | 196 (69.0) | 25 (42.4) | 9 (32.1) | |
| Detected | 88 (31.0) | 34 (57.6) | 19 (67.9) | |
| Vascular invasion | <0.001 | |||
| Not detected | 201 (70.8) | 27 (45.8) | 11 (39.3) | |
| Detected | 83 (29.2) | 32 (54.2) | 17 (60.7) | |
| Maximum tumor diameter on initial CT (mm) | ||||
| Median [range] | 20 [3–87] | 21 [4–75] | 18 [5–74] | |
| 25–75% interquartile range | 14–29 | 14–31 | 14–40 | |
| Mean ± SD | 22.5±12.1 | 25.2±15.9 | 27.1±21.0 | |
| Maximum tumor diameter on preoperative CT (mm) | ||||
| Median [range] | 22 [5–102] | 26 [11–80] | 23 [12–93] | |
| 25–75% interquartile range | 17–32 | 19–42 | 18–44 | |
| Mean ± SD | 25.2±12.9 | 31.0±17.0 | 33.6±22.3 | |
| GGO component | <0.001 | |||
| Absent | 181 (63.7) | 59 [100] | 28 [100] | |
| Present | 103 (36.3) | 0 (0) | 0 (0) | |
| C/T ratio | <0.001 | |||
| Median (range) | 1 (0–1) | 1 | 1 | |
| 25–75% interquartile range | 0.6-1 | NA | NA | |
| Mean ± SD | 0.8±0.2 | NA | NA | |
| Volume doubling time (days) | <0.001 | |||
| Median [range] | 261 [24–9,150] | 70 [19–706] | 70 [21–309] | |
| 25–75% interquartile range | 131–643 | 52–138 | 42–105 | |
| Mean ± SD | 579±936 | 119±123 | 80±56 | |
a, categorical data are shown as n (%) and continuous data as median, range, 25–75% interquartile range and mean ± standard deviation (SD); b, probability value by χ2 test or one-way analysis of valiance. CT, computed tomography; GGO, ground glass opacity; C/T ratio, consolidation-tumor ratio; NA, not available.
The clinicopathological characteristics of the 371 patients with PLCs are shown in Table 1. There were 284 adenocarcinomas (76.5%), 59 squamous cell carcinomas (15.9%), 11 large cell neuroendocrine carcinomas (3.0%), 8 small cell lung carcinomas (2.2%), 5 large cell carcinomas (1.3%), and 4 pulmonary pleomorphic carcinomas (1.1%). All histologies other than adenocarcinoma and squamous cell carcinoma were grouped together and analyzed as others (n=28, 7.5%). The time between the initial CT and CT immediately before surgery ranged from 21 to 2,143 days (median, 57 days). The VDTs of the 371 tumors ranged from 19 to 9,150 days (median 189 days).
Table 1 also shows patient characteristics according to tumor histology. Patients with AC were significantly younger (P=0.007), and included more women (P<0.001) and more non-smokers (P<0.001) than patients with squamous cell carcinoma and other histologies. On pathological examination, significantly more early stage disease (P<0.001) and less lymphatic/vascular invasion was observed in the adenocarcinoma group than in the other two histology groups. The initial tumor sizes on CT were mostly the same among the three histology groups; however, squamous cell carcinomas and others showed significantly greater increases in size on the CTs taken immediately preoperatively (P<0.001).
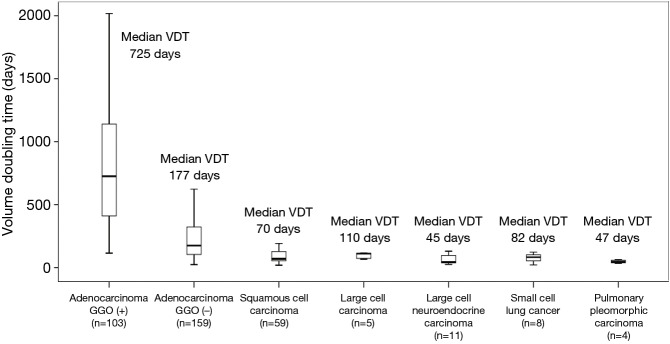
Typical CT scan images of adenocarcinoma with GGO, adenocarcinoma without GGO, Squamous cell carcinoma, and small cell lung cancer, and their tumor growth patterns are shown in Figure S2. The median VDTs of adenocarcinoma, squamous cell carcinoma and others were 261, 70, and 70 days (large cell carcinoma, 110 days; large cell neuroendocrine carcinoma, 45 days; small cell lung cancer, 82 days; pulmonary pleomorphic carcinoma, 47 days), respectively, which differ significantly (Figure 1; P<0.001). Squamous cell carcinoma and other histologies showed similar growth speeds (median VDT: 70, 70 days, respectively, P=0.106), these speeds being higher than those of adenocarcinomas without GGO (Figures 1 and 2). All PLCs with GGO were adenocarcinomas. Adenocarcinomas with GGO showed significantly slower growth than adenocarcinomas without GGO (Figure 3A; median VDT: 725 versus 177 days, respectively, P<0.001). VDTs of solid components in adenocarcinomas with GGO were significantly longer than those of adenocarcinomas without GGO (Figure 3B; median VDT: 248 versus 177 days, P=0.040).
Figure S2.
Typical CT scan images of adenocarcinoma with GGO, adenocarcinoma without GGO, squamous cell carcinoma, and small cell lung cancer. Adenocarcinoma showing part-solid GGN pattern at initial CT (A) and at preoperative CT (A’), observation interval 1,524 days, volume doubling time of whole tumor 1,136 days, volume doubling time of solid component 710 days. Adenocarcinoma showing pure-solid pattern at initial CT (B) and at preoperative CT (B’), observation interval 1,259 days, volume doubling time 521 days. Squamous cell carcinoma at initial CT (C) and at preoperative CT (C’), observation interval 107 days, volume doubling time 80 days. Small cell lung cancer at initial CT (D) and at preoperative CT (D’), observation interval 55 days, volume doubling time 77 days.
Figure 1.
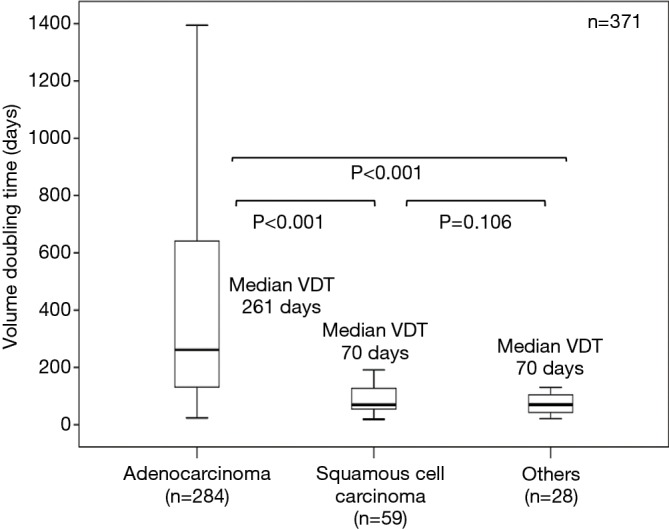
Box plots of VDT calculated from entire tumor size according to histological type. VDT, volume doubling time.
Figure 2.
Box plots of VDT of all lung cancers according to histological details. VDT, volume doubling time.
Figure 3.
Box plots of VDT calculated from entire tumor (A) and solid component diameters in adenocarcinomas according to GGO component (B). VDT, volume doubling time; GGO, ground glass opacity.
Discussion
The VDT of PLC was longest in adenocarcinomas, and VDT was significantly longer in adenocarcinomas with GGO component than those without GGO component, irrespective of VDT calculation based either on entire tumor diameter or on consolidation diameter. Previous studies have been reported that VDT is longer for adenocarcinomas than squamous cell carcinomas. Geddes used chest radiographs to review the VDTs of PLCs and reported that the mean VDT of 60 adenocarcinomas was 161 days whereas that of 111 squamous cell carcinomas was 88 days (1). Other studies have also shown that squamous cell carcinomas grow faster than adenocarcinomas (3,16,17). Our results are consistent with these findings.
Because of the rarity of these tumors, VDT has been studied only in small cohorts of histological types other than adenocarcinoma and squamous cell carcinoma. Usuda et al. reported the mean VDT of seven small cell lung cancers was 81 days and that of 12 large cell carcinomas 79 days (3). In our study, the VDTs of other histological types (large cell carcinoma, large cell neuroendocrine carcinoma, small cell lung cancer and primary pleomorphic carcinoma) were shorter than for adenocarcinomas, but were similar to those for squamous cell carcinomas. Yang et al. reported that a longer interval between diagnosis of squamous cell carcinoma and surgery was associated with worse survival (18). The survival difference is reportedly most prominent when the intervals between diagnosis and surgery were dichotomized at 80 days, which is 10 days longer than the VDT of squamous cell carcinoma and others in our study. VDT may indicate how soon surgical resection should be performed; the interval between diagnosis and surgery should likely be shorter than the VDT.
Many previous studies on VDTs of pure and part solid ground glass nodules have evaluated the entire tumor size, including the GGO component. However, in the current (eighth) edition of the TNM classification for lung cancer, the T factor has been revised to be determined only on the size of the solid component (19). In this study, we therefore calculated VDT using both the maximum entire tumor diameter and that of the solid component diameter alone. To the best of our knowledge, this is the first study to investigate VDT using both the entire tumor size and that of the solid component size only. Both these methods showed the VDTs of adenocarcinomas with GGO components were significantly longer than those of adenocarcinomas without GGO. Given that the radiologic GGO component in adenocarcinoma correlates well with a pathological lepidic growth pattern and the prognosis of lepidic-predominant adenocarcinoma is well known to be favorable, it is unsurprising that adenocarcinomas with GGO components have longer VDTs. Correlations between radiologic and pathologic findings remain controversial. The Japan Clinical Oncology Group 0201 study concluded that the radiological definition of noninvasive lung adenocarcinoma AC would be CTR ≤0.25 in c-T1a and c-T1b tumors in the eighth edition of the TNM classification (11). Hattori et al. reported that the presence of a GGO component itself rather than its ratio is a predictor of favorable outcome and that neither the maximum tumor size nor solid component size has prognostic value (20). In our study, we found that both calculations using entire tumor size and solid component size showed longer VDTs in adenocarcinomas with GGO than in those without GGO.
In the present study, VDTs of pure solid tumors varied depending on histological type. adenocarcinomas showed longer VDTs than squamous cell carcinomas and other tumors. Usuda et al. reported that the VDT of PLC was longer in women and nonsmokers and surmised that one possible explanation is that there are a greater proportion of adenocarcinomas in women and nonsmokers (3). Difference in driver mutation may also explain differences in VDT between adenocarcinomas and other tumors. In a review of 102 NSCLCs, Nakamura et al. reported that PLCs with EGFR mutations have significantly longer VDTs (median: 676 days) than those without EGFR mutations (median VDT: 139 days) (21). Takamochi et al. reported that tumors with EGFR mutations have lower glucose metabolism than tumors with wild type EGFR or KRAS mutations, suggesting that the cell cycle may be down-regulated in tumors with EGFR mutations (22). A relatively high frequency of EGFR mutations in adenocarcinomas may be responsible for the difference in VDT between adenocarcinomas and other histological types.
Several limitations of our study should be mentioned. First, there may have been a selection bias because this is a retrospective single-institution study, enrolling only patients who had undergone surgical resection. Second, the median interval between initial CT and preoperative CT and median duration of follow-up were relatively short, which may have allowed measurement errors for slow-growing tumors, especially GGO adenocarcinomas, because of their indolent nature. Third, volume measurements were derived from the results of manual segmentation and an approximation formula; thus, calculated volumes could have differed from the actual volumes.
In summary, this is the first study to examine the VDTs of adenocarcinomas calculated both from the entire tumor size and solid component size. Both calculations resulted in significantly longer VDTs in adenocarcinomas with GGO components than in those without GGO components. In pure solid tumors, the VDTs of adenocarcinomas were significantly longer those of other histologic types.
Acknowledgements
We thank Kaori Takeshita for data collection, Dr. Junji Yoshida of the National Cancer Center Hospital East for critical manuscript revision, and Dr. Trish Reynolds, MBBS, FRACP, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Funding: This work was supported by the Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (grant numbers 22590516 and 19390359).
Ethical Statement: This study was approved in January 2017 by the Institutional Review Board for clinical trials of Gunma University (IRB approval number 1575). This study was conducted in accordance with the Declaration of Helsinki Principles.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Geddes DM. The natural history of lung cancer: a review based on rates of tumour growth. Br J Dis Chest 1979;73:1-17. 10.1016/0007-0971(79)90002-0 [DOI] [PubMed] [Google Scholar]
- 2.Mizuno T, Masaoka A, Ichimura H, et al. Comparison of actual survivorship after treatment with survivorship predicted by actual tumor-volume doubling time from tumor diameter at first observation. Cancer 1984;53:2716-20. [DOI] [PubMed] [Google Scholar]
- 3.Usuda K, Sato Y, Sagawa M, et al. Tumor doubling time and prognostic assessment of patients with primary lung cancer. Cancer 1994;74:2239-44. [DOI] [PubMed] [Google Scholar]
- 4.Mackintosh JA, Marshall HM, Yang IA, et al. A retrospective study of volume doubling time in surgically resected non-small cell lung cancer. Respirology 2014;19:755-62. 10.1111/resp.12311 [DOI] [PubMed] [Google Scholar]
- 5.Aoki T, Nakata H, Watanabe H, et al. Evolution of Peripheral Lung Adenocarcinomas: CT Findings Correlated with Histology and Tumor Doubling Time. AJR Am J Roentgenol 2000;174:763-8. 10.2214/ajr.174.3.1740763 [DOI] [PubMed] [Google Scholar]
- 6.Kanashiki M, Tomizawa T, Yamaguchi I, et al. Volume doubling time of lung cancers detected in a chest radiograph mass screening program: Comparison with CT screening. Oncol Lett 2012;4:513-6. 10.3892/ol.2012.780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heuvelmans MA, Oudkerk M, de Bock GH, et al. Optimisation of volume-doubling time cutoff for fast-growing lung nodules in CT lung cancer screening reduces false-positive referrals. Eur Radiol 2013;23:1836-45. 10.1007/s00330-013-2799-9 [DOI] [PubMed] [Google Scholar]
- 8.Veronesi G, Maisonneuve P, Bellomi M, et al. Estimating overdiagnosis in low-dose computed tomography screening for lung cancer: a cohort study. Ann Intern Med 2012;157:776-84. 10.7326/0003-4819-157-11-201212040-00005 [DOI] [PubMed] [Google Scholar]
- 9.Xu DM, Gietema H, de Koning H, et al. Nodule management protocol of the NELSON randomised lung cancer screening trial. Lung Cancer 2006;54:177-84. 10.1016/j.lungcan.2006.08.006 [DOI] [PubMed] [Google Scholar]
- 10.Suzuki K, Kusumoto M, Watanabe S, et al. Radiologic classification of small adenocarcinoma of the lung: radiologic-pathologic correlation and its prognostic impact. Ann Thorac Surg 2006;81:413-9. 10.1016/j.athoracsur.2005.07.058 [DOI] [PubMed] [Google Scholar]
- 11.Asamura H, Hishida T, Suzuki K, et al. Radiographically determined noninvasive adenocarcinoma of the lung: survival outcomes of Japan Clinical Oncology Group 0201. J Thorac Cardiovasc Surg 2013;146:24-30. 10.1016/j.jtcvs.2012.12.047 [DOI] [PubMed] [Google Scholar]
- 12.Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol 2005;40:90-7. 10.1053/j.ro.2005.01.001 [DOI] [PubMed] [Google Scholar]
- 13.Edge SB, Byrd DR, Compton CC. Lung AJCC Cancer Staging Manual, 7th Ed. New York, NY: Springer, 2010:253-70. [Google Scholar]
- 14.Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: Glossary of Terms for Thoracic Imaging. Radiology 2008;246:697-722. 10.1148/radiol.2462070712 [DOI] [PubMed] [Google Scholar]
- 15.Schwartz M. A biomathematical approach to clinical tumor growth. Cancer 1961;14:1272-94. [DOI] [PubMed] [Google Scholar]
- 16.Hasegawa M, Sone S, Takashima S, et al. Growth rate of small lung cancers detected on mass CT screening. Br J Radiol 2000;73:1252-9. 10.1259/bjr.73.876.11205667 [DOI] [PubMed] [Google Scholar]
- 17.Honda O, Johkoh T, Sekiguchi J, et al. Doubling time of lung cancer determined using three-dimensional volumetric software: comparison of squamous cell carcinoma and adenocarcinoma. Lung Cancer 2009;66:211-7. 10.1016/j.lungcan.2009.01.018 [DOI] [PubMed] [Google Scholar]
- 18.Yang CJ, Wang H, Kumar A, et al. Impact of Timing of Lobectomy on Survival for Clinical Stage IA Lung Squamous Cell Carcinoma. Chest 2017;152:1239-50. 10.1016/j.chest.2017.07.032 [DOI] [PubMed] [Google Scholar]
- 19.Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203. [DOI] [PubMed] [Google Scholar]
- 20.Hattori A, Matsunaga T, Takamochi K, et al. Neither Maximum Tumor Size nor Solid Component Size Is Prognostic in Part-Solid Lung Cancer: Impact of Tumor Size Should Be Applied Exclusively to Solid Lung Cancer. Ann Thorac Surg 2016;102:407-15. 10.1016/j.athoracsur.2016.02.074 [DOI] [PubMed] [Google Scholar]
- 21.Nakamura R, Inage Y, Tobita R, et al. Epidermal Growth Factor Receptor Mutations Effect on Volume Doubling Time of Non–Small-Cell Lung Cancer Patient. J Thorac Oncol 2014;9:1340-4. 10.1097/JTO.0000000000000022 [DOI] [PubMed] [Google Scholar]
- 22.Takamochi K, Mogushi K, Kawaji H, et al. Correlation of EGFR or KRAS mutation status with 18F-FDG uptake on PET-CT scan in lung adenocarcinoma. PLoS One 2017;12:e0175622. 10.1371/journal.pone.0175622 [DOI] [PMC free article] [PubMed] [Google Scholar]