Abstract
Background
A pathologically complete response (pCR) or near pCR to neoadjuvant chemoradiotherapy (NCRT) might imply a better prognosis in patients with esophageal cancer. The aim of the study is to identify clinical factors associated with a pCR or near pCR.
Methods
We retrospectively analyzed 40 patients with radical esophagectomy after NCRT for esophageal squamous cell carcinoma (ESCC) from January 2001 to December 2006 in Sun Yat-sen University Cancer Center. Clinical factors included age, gender, weight loss, dysphagia, drinking status, smoking status, tumor location, tumor length, tumor grade, cT status, cN status, the regimen of chemotherapy and the interval between NCRT and surgery as potential predictors for a pCR or near pCR. Logistic regression was used to estimate the independent factors for a pCR or near pCR.
Results
After surgical resection, 22.5% of the patients obtained the pCR. Patients with pCR had a better prognosis than those with non-pCR. However, there was no statistically significantly difference between the two groups (P=0.124). We separated the patients into pCR or near pCR (good responders, GRs) and poor responders (PR) based on the histology. GR showed better overall survival (OS) than PR (P=0.014). Univariate analysis indicated that short tumor length, good tumor grade and never drinking were associated with GR to NCRT. Using logistic regression analysis, good tumor grade was the only independent factor for the GR to NCRT (P=0.021). Cox regression revealed that weight loss, drinking status and GR were independent factors in ESCC patients with radical esophagectomy after NCRT.
Conclusions
Our study indicated that good tumor grade were an independent significant factor for the GR to NCRT. Weight loss, drinking status and GR were independent factors in patients with radical esophagectomy after NCRT. GR may improve OS of ESCC patients receiving NCRT.
Keywords: Esophageal squamous cell carcinoma (ESCC), pathologically response, neoadjuvant chemoradiotherapy (NCRT)
Introduction
Evidence demonstrated that neoadjuvant chemoradiotherapy (NCRT) might be more appropriate for patients with locally advanced esophageal cancer (1). However, malignant cells may express different clinicopathological features and biological behavior. Pathological complete response (pCR) occurs in approximately 15% to 30% of all individuals who received NCRT (1-3). It is reported that the prognosis of patients with pCR or near pCR may be better than those who resist or do not respond to NCRT (1,3). In the meanwhile, those cases who resist or do not respond to NCRT may experience the toxicity of NCRT, or even lose the opportunity to have potentially curative resection. Thus, identifying simple and convenient indicators of patients with pCR or near pCR (4) (good responder, GR) is needed.
In the current study, we aim to investigate the factors associated with GR and analysis its prognostic impact in esophageal squamous cell carcinoma (ESCC) patients with NCRT.
Methods
The ESCC patients with radical esophagectomy after NCRT at the Sun Yat-sen University Cancer Center between January 2001 to December 2006 were included. The study was a phase II study to assess the safety and efficiency of NCRT in ESCC during the period. The study was approved by the Ethics Committee of Sun Yat-sen University Cancer Center. Written informed consents were signed by the patients. Preoperative evaluation contained esophageal barium swallow, cervical ultrasonography, CT scans of the chest and upper abdomen, bronchoscope (when tumor located at the upper PR the middle thirds of the esophagus) and an upper endoscope or endoscopic ultrasonography with biopsy. Patients diagnosed locally advanced (cT2–4N0–1M0) were recommended NCRT. The chemotherapy consisted of intravenous infusion of vinorelbine (25 mg/m2 per day) on days 1, 8, 22, and 29 or 5-fluorouracil (2.4 g/m2) on days 1 to 3 and days 22 to 24, and cisplatin (75 mg/m2) on days 1 and 22. Radiotherapy was delivered 5 days a week for 4 weeks with the total dose of 40 Gy divided into a daily fraction of 2.0 Gy. Clinical restaging was performed after the completion of chemoradiotherapy. Esophagectomy and systematic lymphadenectomy were performed in 4 to 8 weeks after the NCRT. All patients received radical esophagectomy and two-field or three-field lymphadenectomy. Three-field lymphadenectomy was performed when lymph nodes in the neck were thought to be potentially positive according to the preoperative evaluation. The surgical specimens were identified by the surgeon and submitted for pathological examination. The definition of pCR is that no tumor cells were seen in the resection specimens including the primary tumor and lymph nodes resected. Near pCR defined as residual cancer less than 10 percent of the primary tumor area without lymph nodes involvement (4). Two experienced pathologists carefully reviewed all of the removed specimens. We classified the pCR and the near pCR patients as GR because of the limit patients of the study.
Follow up
We suggested patients could follow up every 3 months during the first 2 years, every 6 months for the next 3 years and once a year afterwards. Medical history and physical examination, esophageal barium swallow, chest radiography, or CT from the neck to the upper abdomen were performed in the follow up. An upper gastrointestinal endoscopy or PET-CT were recommended if recurrence or metastasis were indicated. January 2017 was the last follow up time.
Statistical analysis
Statistical analysis was performed by SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). The cut points of the variables were assessed by receiver operator characteristic (ROC) analysis. Univariate factors of GR were estimated by the Chi-square test. Logistic regression analysis was used to identify the significant multivariate factors of GR. The overall survival (OS) was estimated by the Kaplan-Meier method. We used the log-rank test to distinguish the difference in survival between groups. multivariate Cox regression analysis was used to assess the independent prognostic factors for O). A P value less than 0.05 was defined as statistically significant.
Results
Forty ESCC patients with NCRT were reviewed in our study, consisting of 39 males and 1 female, with a median age of 58 years (range, 40–72 years). The clinicopathological characteristics of patients are summarized in Table 1. After surgical resection, 9 patients (22.5%) obtained pCR while 31 cases were non-pCR. Patients with pCR had a better prognosis than those with non-pCR. However, there was no statistically significantly difference between the two groups (P=0.124). Thus, we separated the patients into pCR or near pCR (GR) and poor responders (PR) based on the histology. GR showed better 5-year OS than PR (40.4% vs. 17.6%, P=0.014) (Figure 1). Univariate analysis indicated that short tumor length (P=0.027), good tumor grade (P=0.001) and never drinking (P=0.027) were associated with GR to NCRT. Using logistic regression analysis, good tumor grades were the only significant factor for the GR to NCRT (P=0.021, Table 2). Univariate analysis indicated that weight loss (P=0.002), drinking status (P=0.016), clinical N status (P=0.042) and GR (P=0.014) were prognostic factors. Multivariate Cox regression analysis revealed that weight loss (P=0.005), drinking status (P=0.044) and GR (P=0.043) were independent factors in ESCC patients with radical esophagectomy after NCRT (Table 3).
Table 1. The clinicopathological characteristics of patients in the cohort.
| Factors | GR | PR | P |
|---|---|---|---|
| Gender | 0.311 | ||
| Male | 20 | 19 | |
| Female | 0 | 1 | |
| Age, years | 1.000 | ||
| >60 | 10 | 10 | |
| ≤60 | 10 | 10 | |
| Weight loss | 0.736 | ||
| Yes | 13 | 14 | |
| No | 7 | 6 | |
| Dysphagia, month | 1.000 | ||
| ≤1 | 10 | 10 | |
| >1 | 10 | 10 | |
| Smoking | 0.633 | ||
| Ever | 17 | 18 | |
| Never | 3 | 2 | |
| Drinking | 0.027 | ||
| Ever | 6 | 13 | |
| Never | 14 | 7 | |
| Tumor location | 1.000 | ||
| Upper/middle | 17 | 17 | |
| Lower | 3 | 3 | |
| Tumor length, cm | 0.027 | ||
| <8 | 14 | 7 | |
| ≥8 | 6 | 13 | |
| Tumor grade | 0.001 | ||
| G1/Gx | 16 | 6 | |
| G2/G3 | 4 | 14 | |
| cT status | 0.256 | ||
| T2/3 | 17 | 14 | |
| T4 | 3 | 6 | |
| cN status | 1.000 | ||
| N− | 7 | 7 | |
| N+ | 13 | 13 | |
| Chemotherapy regimen | 0.197 | ||
| PF | 10 | 14 | |
| NP | 10 | 6 | |
| Interval between NCRT and surgery, weeks | 0.327 | ||
| >4 | 14 | 11 | |
| ≤4 | 6 | 9 |
GR, good responder; PR, poor responder; PF, 5-fluorouracil + cisplatin; NP, vinorelbine + cisplatin.
Figure 1.
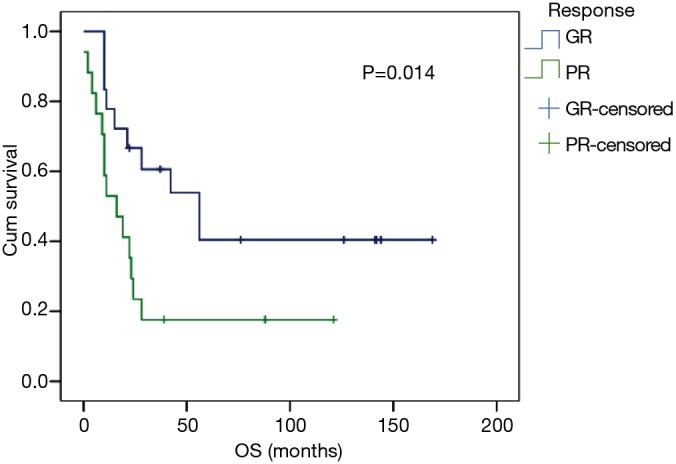
Survival in GR patients compared with PR patients (P=0.014). GR, good responder; PR, poor responder.
Table 2. Logistic regression analysis of factors for GR.
| Factors | OR | 95% CI | P |
|---|---|---|---|
| Tumor length | 6.534 | 0.768–55.608 | 0.086 |
| Drinking | 6.534 | 0.768–55.608 | 0.086 |
| Tumor grade | 11.549 | 1.454–91.742 | 0.021 |
GR, good responder.
Table 3. Univariate and multivariate analysis of prognostic factors for survival in the cohort.
| Factors | Univariate analysis (P value) | Multivariate analysis | ||
|---|---|---|---|---|
| OR | 95% CI | P | ||
| Gender | 0.798 | – | – | – |
| Age | 0.680 | – | – | – |
| Weight loss | 0.002 | 17.536 | 2.349–130.887 | 0.005 |
| Dysphagia | 0.522 | – | – | – |
| Smoking | 0.429 | – | – | – |
| Drinking | 0.016 | 4.384 | 1.040–18.173 | 0.044 |
| Tumor location | 0.513 | – | – | – |
| Tumor length | 0.887 | – | – | – |
| Tumor grade | 0.374 | – | – | – |
| cT status | 0.584 | – | – | – |
| cN status | 0.042 | 0.844 | 0.085–8.382 | 0.885 |
| Chemotherapy regimen | 0.640 | – | – | – |
| Interval between NCRT and surgery | 0.384 | – | – | – |
| Response to the NCRT (GR vs. PR) | 0.014 | 6.428 | 1.056–39.123 | 0.043 |
NCRT, neoadjuvant chemoradiotherapy; GR, good responder; PR, poor responder.
Discussion
The prognosis of locally advanced esophageal cancer with surgery alone was still unsatisfied despite of the development of the preoperative diagnose and surgical technique. Therefore, the combined treatment particular to the NCRT had attract the attention of the clinician. Previous reports indicated that NCRT might downstage the primary tumor and regional lymphatic drainage to enhance radical resection rate and reduce micrometastasis, improving local control and prolonging the survival of patients (1-3). About 30% of patients receiving NCRT followed by surgery achieve pCR (1,3). It is reported that pCR following NCRT favored long term survival, whereas patients resistant to NCRT were not benefit from the multimodal therapy (3,5,6). Therefore, non-responders may obtain the side effects of NCRT, and even miss the chance to have potentially radical resection. Thus, identifying factors associated with pCR seemed to be particularly important.
Our study revealed that patients with pCR had a better prognosis than those with non-pCR. However, there was no statistically significantly difference between the two groups perhaps due to the small sample size. Thus, the study divided the patients into GRs and poor responders according to the histology. The GR patients showed a better prognosis compared with PR patients, reaching a marked difference between the two groups. In our study, we identified tumor grade were associated with GR. Drinking status, weight loss and GR are independent factors in ESCC patients receiving NCRT.
To reduce the potential biases and the confounding factors in the NCRT cases, logistic regression analysis was used to determine the significant multivariate indicators of GR among these patients. As expected, the present study indicated that tumor grade was an independent factor that affected the efficacy in the NCRT cases. Although the mechanism between the tumor grade and the GR rate was unclear, a possible explanation could be that good tumor grade of the primary tumor may indicate lower tumor burden to be a higher response rate after NCRT compared with those with poor tumor grade. Interestingly, several reports indicated that prolonging the interval between NCRT and surgery might achieve higher pCR (7,8). However, we did not find the interval between NCRT and esophagectomy was associated with GR, similar to the result of Kim and his colleagues (9). It is possible that the sample size of our study was not enough and the cutoff of the interval varied among different institutions.
In addition, our study revealed that drinking status, weight loss and GR were associated with survival in ESCC patients with NCRT. Molecular biological research indicated that alcohol and its metabolites initiated a series of epigenetic and genetic alterations, resulting in more aggressive biological features and malignance progression (10). For example, two alcohol metabolism genes, ADH1B and ALDH2, play an important role on ESCC diagnosis and tumor spread (11). Thus, there was an urgent need to develop a better understanding of the mechanisms between the alcohol use and the esophageal cancer. In addition, worse prognosis was also found among patients who lost weight before anticancer treatment, which was consistent with the finding of van der Schaaf and his colleagues (12). Although the mechanism underlying this is unclear, reduced immune function in malnutritional patients might be associated with a reduced chance of survival. Furthermore, GR, more sensitive to the NCRT, reduced an aggressive behavior and had an improved OS compared with those who had poor response to the NCRT. Moreover, the findings above need to be further confirmed in a large cohort.
Assessment of tumor response to NCRT can help to estimate the prognosis of the patients and provide information on selecting the patients who will benefit from NCRT. EUS is a useful tool for diagnosing staging; however, it is difficult to distinguish the residual cancer or the inflammatory tissue and fibrosis after NCRT (13). Novel technologies are needed to identify those with a pCR. But imaging methods such as PET-CT following NCRT for esophageal cancer was also not accurate enough to identify patients with pathological response for clinical application (13). Recently, concerns about molecular markers related to the treatment response have been raised by the clinicians and researchers (14,15). However, the clinical application of the markers is not widely available and their clinical relevance needs to be further validated.
However, our study was a retrospective study and the sample size was small. Although more than 1,000 patients underwent esophagectomy in our department during the period of the study, few cases received NCRT due to the lack of uniform clinical standards in China. Furthermore, our results were from a single center and whether the findings were applicable to other institutions needed to be confirmed. Therefore, larger sample prospective studies are needed for verification.
Conclusions
In conclusion, our study indicated that good tumor grades were the only significant factor for the GR to NCRT. Weight loss, drinking status and GR were independent factors in patients with radical esophagectomy after NCRT. GR may improve OS of ESCC patients receiving NCRT.
Acknowledgements
We thank the members of Guangdong Esophageal Cancer Institute for their cooperation and assistance.
Funding: This work was supported by National Natural Science Foundation of China (No. 81402003), Guangzhou Science and Technology Program (2014Y2-00143), Sun Yat-sen University medical young teacher training program (15ykpy34), and Sun Yat-sen University Clinical Research 5010 Program (No. 2007048).
Ethical Statement: The study was approved by the ethics committee of Sun Yat-sen University Cancer Center (YP2007031) and written informed consent was obtained from all patients.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. 10.1056/NEJMoa1112088 [DOI] [PubMed] [Google Scholar]
- 2.Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol 2014;32:2416-22. 10.1200/JCO.2013.53.6532 [DOI] [PubMed] [Google Scholar]
- 3.Donahue JM, Nichols FC, Li Z, et al. Complete pathologic response after neoadjuvant chemoradiotherapy for esophageal cancer is associated with enhanced survival. Ann Thorac Surg 2009;87:392-8; discussion 398-9. 10.1016/j.athoracsur.2008.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chao YK, Chang Y, Yeh CJ, et al. Characterization of residual tumours at the primary site in patients with a near pathological complete response after neoadjuvant chemoradiotherapy for oesophageal cancer. Br J Surg 2016;103:1874-9. 10.1002/bjs.10293 [DOI] [PubMed] [Google Scholar]
- 5.Meredith KL, Weber JM, Turaga KK, et al. Pathologic response after neoadjuvant therapy is the major determinant of survival in patients with esophageal cancer. Ann Surg Oncol 2010;17:1159-67. 10.1245/s10434-009-0862-1 [DOI] [PubMed] [Google Scholar]
- 6.Dittrick GW, Weber JM, Shridhar R, et al. Pathologic nonresponders after neoadjuvant chemoradiation for esophageal cancer demonstrate no survival benefit compared with patients treated with primary esophagectomy. Ann Surg Oncol 2012;19:1678-84. 10.1245/s10434-011-2078-4 [DOI] [PubMed] [Google Scholar]
- 7.Shaikh T, Ruth K, Scott WJ, et al. Increased time from neoadjuvant chemoradiation to surgery is associated with higher pathologic complete response rates in esophageal cancer. Ann Thorac Surg 2015;99:270-6. 10.1016/j.athoracsur.2014.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee A, Wong AT, Schwartz D, et al. Is There a Benefit to Prolonging the Interval Between Neoadjuvant Chemoradiation and Esophagectomy in Esophageal Cancer? Ann Thorac Surg 2016;102:433-8. 10.1016/j.athoracsur.2016.02.058 [DOI] [PubMed] [Google Scholar]
- 9.Kim JY, Correa AM, Vaporciyan AA, et al. Does the timing of esophagectomy after chemoradiation affect outcome? Ann Thorac Surg 2012;93:207-12; discussion 212-3. 10.1016/j.athoracsur.2011.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Engeland M, Weijenberg MP, Roemen GM, et al. Effects of dietary folate and alcohol intake on promoter methylation in sporadic colorectal cancer: the Netherlands cohort study on diet and cancer. Cancer Res 2003;63:3133-7. [PubMed] [Google Scholar]
- 11.Lee CH, Wu DC, Wu IC, et al. Genetic modulation of ADH1B and ALDH2 polymorphisms with regard to alcohol and tobacco consumption for younger aged esophageal squamous cell carcinoma diagnosis. Int J Cancer 2009;125:1134-42. 10.1002/ijc.24357 [DOI] [PubMed] [Google Scholar]
- 12.van der Schaaf MK, Tilanus HW, van Lanschot JJ, et al. The influence of preoperative weight loss on the postoperative course after esophageal cancer resection. J Thorac Cardiovasc Surg 2014;147:490-5. 10.1016/j.jtcvs.2013.07.072 [DOI] [PubMed] [Google Scholar]
- 13.Heneghan HM, Donohoe C, Elliot J, et al. Can CT-PET and Endoscopic Assessment Post-Neoadjuvant Chemoradiotherapy Predict Residual Disease in Esophageal Cancer? Ann Surg 2016;264:831-8. 10.1097/SLA.0000000000001902 [DOI] [PubMed] [Google Scholar]
- 14.Lim SH, Hong M, Ahn S, et al. Changes in tumour expression of programmed death-ligand 1 after neoadjuvant concurrent chemoradiotherapy in patients with squamous oesophageal cancer. Eur J Cancer 2016;52:1-9. 10.1016/j.ejca.2015.09.019 [DOI] [PubMed] [Google Scholar]
- 15.Bronson NW, Diggs BS, Bakis G, et al. Molecular Marker Expression Is Highly Heterogeneous in Esophageal Adenocarcinoma and Does Not Predict a Response to Neoadjuvant Therapy. J Gastrointest Surg 2015;19:2105-10. 10.1007/s11605-015-2944-7 [DOI] [PubMed] [Google Scholar]


