Abstract
Introduction:
Infectious agents were recently implicated in Alzheimer’s disease (AD) and etiology of other dementias, notably Helicobacter pylori.
Methods:
We tested associations of H. pylori seropositivity with incident all-cause and AD dementia and with AD-related mortality among US adults in a retrospective cohort study. Data from the National Health and Nutrition Surveys III, phase 1 (1988–1991) and 1999–2000 linked with Medicare and National Death Index registries, were used (baseline age ≥45 y, follow-up to 2013, Npooled = 5927).
Results:
A positive association between H. pylori seropositivity and AD mortality was found in men (hazard ratioadj, pooled = 4.33, 95% confidence interval: 1.51–12.41, P =.006), which was replicated for incident AD and all-cause dementia, with hazard ratioadj, pooled = 1.45 (95% confidence interval: 1.03–2.04, P = .035) and hazard ratioadj, III = 1.44 (95% confidence interval: 1.05–1.98, P = .022), respectively. These associations were also positive among higher socioeconomic status groups.
Discussion:
In sum, H. pylori seropositivity’s direct association with AD mortality, all-cause dementia, and AD dementia was restricted to men and to higher socioeconomic status groups. Published by Elsevier Inc. on behalf of the Alzheimer’s Association.
Keywords: Helicobacter pylori, Dementia, Alzheimer’s disease, Mortality, Aging
1. Background
The prevalence of all-cause dementia among older adults aged ≥60 y is estimated at 4.7% [1], with 4.6–7.7 million new annual cases worldwide (3.5–10.5 per 1000 in various world regions) [1–3]. Around 60%–80% of dementia is caused by Alzheimer’s disease (AD) [1], a progressive neurodegenerative disorder with multifactorial etiology. AD manifests itself with a progressive episodic memory deterioration followed by impairment in other cognitive domains [4]. Biologically speaking, AD is thought to be caused by age-dependent and progressive amyloid β deposition in the brain—”the amyloid cascade hypothesis” [5]. Neurofibrillary tangles arising from hyperphosphorylated tau constitute the second pathological hallmark of AD [6]. AD is the leading cause of disability in old age [7] and health-care burden in developed countries [8]. It is also the sixth leading cause of death in the United States [9]. Currently, around 5.4 million Americans have AD, a number expected to reach 13.8 million by 2050 [9]. In 2016, long-term and hospice care for all-cause dementia cost the United States around $236 billion [9]. Awaiting an effective treatment, research has uncovered important genetic risk factors for late-onset AD (e.g., apolipoprotein E [ApoE] ε4). Recent reviews indicated that education, smoking, physical inactivity, depression, midlife obesity, hypertension, and type 2 diabetes collectively account for ~54% of AD risk [10], but much variation remains unexplained. Therefore, identifying novel midlife risk factors is essential for planning cost-effective interventions. Infectious agents have recently been implicated in AD etiology [8], most notably Helicobacter pylori [11–22].
H. pylori is a heterogeneous bacterial species causing numerous upper digestive diseases (e.g., peptic ulcer) [23], with similar degenerative features with AD. In fact, peptic ulcer’s etiology is multifactorial with contributions from infection, stress, chemical irritants, and genetic susceptibility [24]. Found in gastric mucosa of ≥50% of humans worldwide, H. pylori can infect children, becoming chronic during adulthood if untreated. Its seroprevalence increases with age and poorer socioeconomic conditions and was observed to be higher among minority groups [19,25,26]. Recently, a link between H. pylori and extra-digestive disorders was found. Some of those disorders, which include atherosclerosis [27], hypertension, and stroke [28], were also related to increased risk of AD through impairment of the blood-brain barrier [29–31]. Specifically, with respect to atherosclerosis, a causal relationship was suggested given the simultaneous drop in duodenal ulcer and coronary heart disease occurrence in the United States over the past 40 y, coupled with H. pylori DNA detection in atherosclerotic plaques. Underlying mediators may include inflammation, dyslipidemia, hyperglycemia, arterial stiffness, and hypertension. Other research, however, suggested that both H. pylori infection and atherosclerosis have common causes, such as smoking, low socioeconomic status (SES), and high salt intake [32].
Importantly, several hypothesized mechanisms have been identified recently for the potential causal association between H. pylori and AD: (1) Folate and vitamin B-12 malabsorption triggering increased serum homocysteine concentrations and neurotoxicity [33]; (2) apoptosis by T cell-mediated immune response, overexpression of nitric oxide, or molecular mimicry of host structures [33]; (3) increased cytokines, platelet activation, acute phase proteins, and eicosanoids [33] and; (4) H. pylori infection potentially crossing the blood-brain barrier and contributing to amyloid deposition [34]. Though slowly mounting, evidence from epidemiological studies remains limited [11–19], and most studies are case-control or cross-sectional investigations. Furthermore, given race- and sex-specific differences in H. pylori seroprevalence [19,26], there is a need to test longitudinal associations between H. pylori status and cognitive outcomes across those sociodemographic factors. Finally, there are effective ways to eradicate H. pylori, and research is under way to develop vaccines, which strengthen the rationale to study this modifiable factor [35].
Consequently, our present study examined associations of H. pylori seropositivity with incident all-cause and AD dementia, and with AD-related mortality, among US middle-aged and older adults (45 + y at baseline), (objective A). We further explored whether those associations were specific to certain sociodemographic groups, including sex, race/ethnicity, age, income, and education (objective B).
2. Methods
2.1. Database: National Health and Nutrition Surveys-Centers for Medicare and Medicaid Services
The National Health and Nutrition Examination Surveys (NHANES) provide nationally representative cross-sectional data on U.S. civilian populations health and nutritional status. Initiated in the 1970s by the National Center for Health Statistics at the Centers for Disease Control and Prevention, NHANES was noncontinuous waves before 1999, becoming a continuous survey afterward. Following a stratified, multistage probability cluster samplingdesign, the surveys included in-home basic health and demographic interviews followed by in-depth health examinations in a mobile examination center (MEC) completed by physicians, medical/health technicians, and dietary and health interviewers [36].
NHANES followed established guidelines of the Declaration of Helsinki, and the National Center for Health Statistics Institutional Review Board approved all procedures involving human subjects. Informed written and verbal consent were obtained from all participants, with verbal consent witnessed and formally recorded [36]. Moreover, our study was approved by the institutional review board of the National Institute on Aging, Intramural Research Program.
Centers for Medicare and Medicaid Services (CMS)-Medicare data were linked to NHANES III and 1999–2013 wave participants (Supplementary Appendix I). Finalized CMS-Medicare claim files are available ~9 months into the following calendar year. The annual files are available throughout the follow-up period for part A (inpatient, outpatient, Skilled Nursing Facility, hospice, or Home Health Agency) and for part B (Carrier, Durable Medical Equipment). All restricted CMS data analyses were conducted at the Research Data Center in the National Center for Health Statistics, in Rockville, MD.
2.2. Study sample
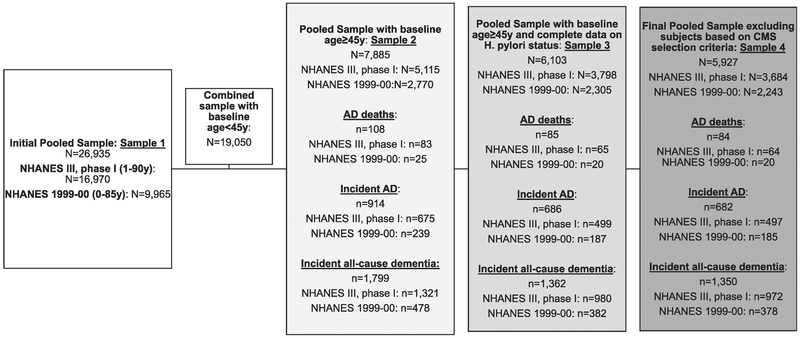
Among 16,970 participants (aged 1–90 y) interviewed in phase 1 NHANES III (1988–1991, 3 yrs) with complete sociodemographics (e.g., age, sex), 5115 were aged 45 y or older, of whom H. pylori exposure is available for n = 3798 (MEC examination). Similarly, 1999–2000 NHANES (2 yrs) consisted of 9965 participants aged 0–85 y at examination, of whom we selected those who are aged ≥45 y (N = 2770), with N = 2305 having complete data on H. pylori. Participants without matched CMS-Medicare data were assumed without follow-up event until December 31st, 2011, or censored on death. Finally, we excluded participants with Health Maintenance Organization–related CMS data to reduce bias from irregular follow-up. Therefore, the total unweighted selected sample consisted of N = 5927 adult participants, in which 84 AD-related deaths occurred up to December 31st, 2011, whereas incident all-cause dementia and AD dementia were 1350 and 682, respectively (Fig. 1).
Fig. 1.
Participant flowchart. Sample 4, mean ± SD of follow-up time (months): AD deaths: NHANES III, phase I: 174.0 ± 86.1 NHANES 1999–00: 120.0 ± 38.1. Incident AD: NHANES III, phase I: 170.3 ± 86.5 NHANES 1999–00: 117.4 ± 40.7. Incident all-cause dementia: NHANES III, phase I: 165.3 ± 87.6 NHANES 1999–00: 114.7 ± 43.6. Additional missing data (n = 41 observations for pooled analysis, n = 38 for NHANES III, phase I, and n = 3 for NHANES 1999–00) is found for each type of analysis, given that some sample weights were not valid and/or observations end before enter (i.e., prevalent cases of AD and all-cause dementia). Abbreviations: AD, Alzheimer’ disease; H. pylori, Helicobacter pylori; NHANES, National Health and Nutrition Examination Surveys; SD, standard deviation.
2.3. Dementia and AD onset
Using CMS Chronic Condition Data Warehouse Categories summary file of 21 chronic conditions, AD was diagnosed using the International Classification of Diseases and related health problems, ninth edition, code 331.0 (any diagnosis) from several claim sources including inpatient and carrier during a 3-year period, whereas all-cause dementia included the following diagnostic codes: 331.0, 331.1, 331.11, 331.19, 331.2, 331.7, 290.0, 290.10, 290.11, 290.12, 290.13, 290.20, 290.21, 290.3, 290.40, 290.41, 290.42, 290.43, 294.0, 294.1, 294.10, 294.11, 294.8, and 797. The earliest date of AD and all-cause dementia occur-rence was used to compute time-to-event starting from MEC examination date. While the 1999–2013 summary file was readily available, all-cause and AD dementia were created using the same algorithm from 1991 to 1998 raw CMS data to cover follow-up time of the NHANES III, phase 1 selected participants. Chronic Condition Data Warehouse’s integrity in estimating chronic condition prevalence was tested in NHANES I follow-up data. The study concluded that conditions that required less frequency health-care utilization, such as arthritis, were underestimated. However, all-cause dementia was among adequately estimated conditions particularly whenever ≥3 y of follow-up was made available [37].
2.4. Mortality from AD
Mortality data (National Death Index) was available through 2011. Additional AD cases were added to incident AD (obtained from CMS-Medicare claims or chronic conditions summary file) whenever earliest diagnosis date was missing but an AD-related death was assigned. All-cause death was considered as the ultimate competing event for AD incidence. Thus, follow-up was censored at time-of-death or if alive till the end of National Death Index mortality follow-up (i.e., end of 2011). Importantly, AD-related death was the primary outcome in part of our analyses. Cox proportional hazards models were conducted, from which predicted smoothed hazards were presented by H. pylori seropositivity status. Non-AD death was considered as the main competing event, and competing risk regression was carried as a sensitivity analysis.
2.5. H. pylori antibody measurement
H. pylori antibodies were measured on both NHANES III phase 1 and NHANES 1999–2000 adult participants (≥20 y) using H. pylori IgG ELISA (Wampole Laboratories, Cran-bury, NJ) [38]. An immune status ratio was determined by dividing optical density of collected specimens by mean optical density of cutoff controls. H. pylori–individuals’ immune status ratio ranged from 0 to 0.90; “equivocal”: 0.91–1.09, whereas for H. pylori+, it was ≥1.10.(36). Intended for H. pylori immunoglobulin G (IgG) detection and qualitative determination in human serum, this test has comparable sensitivity, specificity, and reproducibility to other serological tests (e.g., immunofluorescence, complement fixation, hemagglutination, and radio-immunoassays) [39].
2.6. Covariates
Key covariates considered as potential confounders in hypothesized associations were as follows: wave (NHANES III, phase 1 vs. 1999–00), age (y), sex, race/ethnicity (non-Hispanic whites, non-Hispanic blacks, Mexican-Americans), other ethnicities (other); or non-Hispanic whites versus other races/ethnicities; educational level (<high school, high school, and .>high school); poverty income ratio (≤100%,>100– and ≤200%, and >200%); current smoking status (“yes” vs. “no”); measured body mass index (weight [kg]/height-squared [m2], underweight:,<18.5, normal weight: 18.5–24.9 [referent category], overweight: 25–29.9 and obese: ≥30). Measured hypertension, hyperglycemia, high-density lipoprotein-cholesterol and triacylglycerol were categorized using the National Cholesterol Education Program Adult Treatment Panel III criteria [40,41].
2.7. Statistical analysis
Using Stata 14.0 (StataCorp, College Station, TX) [42], analyses accounted for survey design complexity, by incorporating 5-yr (pooled sample) and 3-yr (NHANES III, phase 1) sampling weights, primary sampling units, and strata and estimating standard errors using Taylor series linearization (i.e., svy: commands) [42]. We first compared sample characteristics across H. pylori seropositivity status (H. pylori IgG+vs. H. pylori IgG–) for both the pooled and the NHANES III, phase 1 samples, using design-based F-test. The latter test was also used to compare characteristic distributions across waves.
Defining time to event from age ≥45 y since baseline visit for NHANES III, phase 1 and NHANES 1999–2000 (i.e., delayed entry) until outcome occurrence or censoring, we conducted Cox PH model for incident AD/all-cause dementia and for AD-related mortality. The follow-up time was expressed in months, and models were weighted (5-yr for pooled sample and 3-yr for NHANES III, phase 1). Model 1 was adjusted for wave (1 = NHANES 1999–2000 vs. 0 = III [phase 1]), age, sex, and race/ethnicity, whereas model 2 was further adjusted for educational level and poverty income ratio. Finally, model 3 was further adjusted for smoking status, weight status, measured hypertension, hyperglycemia, dyslipidemia-high-density lipoprotein, and dyslipidemia-triacylglycerol. Subsequent models explored effect modifications by sociodemographic factors mainly sex, race, age group, poverty income ratio, and education, using two-way interaction terms with the main exposure (i.e., H. pylori seropositivity) along with stratified analyses.
From the full models, smoothed instantaneous hazards were predicted and plotted against H. pylori status for all outcomes of interest, examining sociodemographicspecific effects where applicable. A type 1 error of 0.05 was considered for statistical significance in all analyses, except for interaction terms where α was set at 0.10 [43].
3. Results
Table 1 includes baseline study characteristics among the pooled sample of middle-aged and older adults by H. pylori seropositivity status. Overall, 41.5% of the pooled sample was H. pylori IgG+(45.5% in the NHANES III, phase 1 sample). Most notably, H. pylori IgG+ individuals were older, less likely non-Hispanic whites, less likely to have .>high school education, or >200% poverty income ratio, and more likely to be hypertensive and have high-density lipoprotein-cholesterol type of dyslipidemia compared with H. pylori IgG–subjects. Similarly, and excluding missing data (data not shown), H. pylori IgG+individuals had higher hyperglycemia (20% vs. 14%, P = .005), and dyslipidemiatriacylglycerol (41.7% vs. 38.2%, P = .041) prevalence, than H. pylori IgG–participants. Similar patterns were noted among the NHANES III, phase 1 wave. The incidence proportions of AD and all-cause dementia were significantly higher among H. pylori IgG+compared with IgG– participants, as were proportions of non-AD and total deaths. Many characteristics also differed between waves, particularly metabolic and health-related covariates for which percent missingness varied between waves. Moreover, due to a longer follow-up period (Fig. 1 footnote), AD/all-cause dementia incidence proportions were higher in the NHANES III, phase 1 wave when compared with NHANES 1999–00.
Table 1.
Baseline study characteristics among selected sample (≥45 y, NHANES III, phase 1 [1988–1991] alone and combined with NHANES 1999–2000: [pooled sample]) by H. pylori seropositivity status
| Pooled sample | NHANES III, phase 1 | |||||||
|---|---|---|---|---|---|---|---|---|
| Total sample (N = 5927) |
H. pylori IgG− (N = 2707)* |
H. pylori IgG+ (N = 3220)* |
Total sample (N = 3684) | H. pylori IgG (N = 1579) |
H. pylori IgG+ (N = 2105) | |||
| Unweighted N | Mean/% (SE) | Mean/% (SE) | Mean/% (SE) | P† | Mean/% (SE) | Mean/% (SE) | Mean/% (SE) | P† |
| Weighted % | 58.4 (1.0) | 41.5 (1.0) | 54.5 (1.7) | 45.5 (1.7) | ||||
| Study characteristic, weighted means/% | ||||||||
| Sex, % | .59 | 1.00 | ||||||
| Men | 45.8 (0.7) | 46.2 (1.0) | 45.3 (1.2) | 45.7 (1.2) | 45.7 (1.4) | 45.7 (1.8) | ||
| Women | 54.2 (0.7) | 53.7 (1.0) | 54.7 (1.2) | 54.3 (1.2) | 54.3 (1.4) | 54.3 (1.8) | ||
| Age, y, mean | 60.8 (0.3) | 59.3 (0.2) | 62.8 (0.4) | <.001 | 61.2 (0.4) | 59.4 (0.4) | 63.5 (0.6) | <.001 |
| Race | <.001 | <.001 | ||||||
| NH white | 80.9* (1.6) | 88.5 (1.1) | 70.2 (2.6) | 84.1 (2.0) | 90.0 (1.1) | 77.1 (3.1) | ||
| NH black | 8.6 (1.0) | 5.6 (0.7) | 12.9 (1.6) | 8.4 (1.1) | 5.9 (0.8) | 11.5 (1.6) | ||
| MA | 3.4 (0.5) | 1.7 (0.3) | 5.9 (0.9) | 2.9 (0.4) | 15.2 (0.3) | 4.5 (0.6) | ||
| Other | 7.0 (1.4) | 4.2 (1.0) | 11.0 (2.0) | 4.5 (1.0) | 2.6 (0.6) | 6.9 (1.8) | ||
| Education | <.001 | <.001 | ||||||
| <HS | 18.5* (1.0) | 11.7 (1.1) | 28.1 (1.2) | 11.1 (1.1) | 5.8 (0.9) | 17.4 (1.5) | ||
| HS | 42.5 (1.6) | 40.0 (2.0) | 46.1 (1.4) | 55.8 (2.0) | 52.7 (2.6) | 59.5 (1.5) | ||
| >HS | 38.5 (1.7) | 47.9 (2.3) | 25.3 (1.0) | 32.5 (2.2) | 40.8 (2.8) | 22.6 (1.5) | ||
| Missing | 0.4 (0.1) | 0.4 (0.1) | 0.5 (0.1) | 0.6 (0.2) | 0.6 (0.3) | 0.5 (0.2) | ||
| Poverty income ratio, % | <.001 | |||||||
| <100% | 10.5 (0.8) | 7.4 (0.7) | 14.8 (1.1) | 9.2 (0.9) | 6.4 (0.9) | 12.6 (1.2) | <.001 | |
| >100%-<200% | 18.6 (1.5) | 16.1 (1.5) | 22.2 (1.6) | 19.0 (1.4) | 15.5 (1.6) | 23.1 (1.7) | ||
| >200% | 60.4 (1.5) | 67.0 (1.7) | 51.0 (1.5) | 62.6 (1.8) | 70.4 (2.0) | 53.3 (2.2) | ||
| Missing | 10.6 (0.8) | 9.5 (0.8) | 12.0 (1.1) | 9.2 (0.7) | 7.7 (0.6) | 10.9 (1.1) | ||
| Current smoking status, % | .032 | .25 | ||||||
| No | 58.3* (1.3) | 57.9 (1.7) | 58.7 (1.6) | 76.4 (1.2) | 77.6 (1.6) | 74.9 (1.7) | ||
| Yes | 21.4 (1.1) | 20.0 (1.4) | 23.6 (1.3) | 23.3 (1.2) | 22.2 (1.6) | 24.7 (1.7) | ||
| Missing | 20.3 (1.0) | 22.2 (1.5) | 17.7 (1.4) | 0.3 (0.1) | 0.2 (0.1) | 0.5 (0.2) | ||
| Weight status, BMI (kg.m−2), % | .51 | .031 | ||||||
| <18.5 | 1.6* (0.2) | 1.7 (0.3) | 1.5 (0.2) | 2.0 (0.2) | 2.0 (0.4) | 1.9 (0.4) | ||
| 18.5–24.9 | 33.9 (1.2) | 34.0 (1.7) | 33.9 (1.1) | 37.3 (1.3) | 38.3 (2.0) | 36.0 (1.1) | ||
| 25–29.9 | 35.7 (1.0) | 36.5 (1.0) | 34.5 (1.4) | 36.6 (1.3) | 38.3 (2.4) | 34.6 (1.4) | ||
| >30 | 28.1 (1.2) | 27.1 (1.5) | 29.6 (1.4) | 23.9 (1.0) | 21.1 (1.1) | 27.3 (1.5) | ||
| Missing | 0.6 (0.1) | 0.7 (0.2) | 0.6 (0.2) | 0.3 (0.1) | 0.4 (0.2) | 0.2 (0.1) | ||
| Hypertension, % | <.001 | <.001 | ||||||
| No | 46.1* (1.1) | 50.6 (1.4) | 39.5 (1.2) | 46.4 (1.8) | 51.6 (2.5) | 40.1 (2.0) | ||
| Yes | 52.9 (1.0) | 48.2 (1.4) | 59.5 (1.0) | 53.6 (1.8) | 48.3 (2.5) | 59.9 (2.0) | ||
| Missing | 1.0 (0.2) | 1.0 (0.2) | 1.0 (0.4) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | ||
| Hyperglycemia, % | .007 | <.001 | ||||||
| No | 63.3* (1.3) | 63.9 (1.4) | 62.5 (2.0) | 84.5 (1.1) | 87.3 (1.2) | 81.1 (1.5) | ||
| Yes | 13.0 (0.6) | 11.1 (0.8) | 15.6 (1.0) | 14.5 (1.0) | 11.9 (1.2) | 17.6 (1.3) | ||
| Missing | 23.7 (1.4) | 25.0 (1.3) | 21.9 (2.1) | 1.0 (0.5) | 0.8 (0.4) | 1.3 (0.7) | ||
| Dyslipidemia-HDL, % | .002 | .07 | ||||||
| No | 63.0 (1.1) | 65.2 (1.4) | 59.7 (1.0) | 63.3 (1.2) | 65.6 (1.7) | 60.5 (1.3) | ||
| Yes | 36.4 (1.1) | 34.0 (1.4) | 39.7 (1.1) | 36.1 (1.3) | 33.9 (1.6) | 38.8 (1.5) | ||
| Missing | 0.6 (0.2) | 0.7 (0.2) | 0.6 (0.2) | 0.6 (0.2) | 0.5 (0.3) | 0.7 (2.8) | ||
| Dyslipidemia-TA | .033 | .027 | ||||||
| No | 46.2* (1.2) | 46.4 (1.2) | 45.9 (1.7) | 60.7 (1.1) | 62.9 (1.3) | 58.1 (1.3) | ||
| Yes | 30.5 (1.0) | 28.7 (1.1) | 33.0 (1.4) | 38.9 (1.1) | 36.7 (1.3) | 41.5 (1.3) | ||
| Missing | 23.3 (1.4) | 24.9 (1.3) | 21.2 (2.0) | 0.4 (0.2) | 0.4 (0.3) | 3.6 (0.2) | ||
| Incident AD, % | 9.3* (0.5) | 7.9 (0.4) | 11.3 (1.1) | .003 | 11.4 (0.8) | 9.6 (0.7) | 13.6 (1.2) | <.001 |
| Incident all-cause dementia, % | 18.1* (0.7) | 15.8 (0.6) | 21.3 (1.3) | <.001 | 21.4 (1.1) | 18.2 (1.1) | 25.2 (1.4) | <.001 |
| AD deaths, % | 1.1 (0.2) | 0.8 (0.2) | 1.7 (0.4) | .05 | 1.5 (0.3) | 1.0 (0.4) | 2.1 (0.6) | .12 |
| Non-AD deaths, % | 38.9* (1.0) | 33.9 (1.0) | 46.0 (1.7) | <.001 | 51.0 (1.5) | 45.2 (2.0) | 58.0 (1.6) | <.001 |
| Deaths, % | 40.1* (1.0) | 34.6 (1.1) | 47.7 (1.9) | <.001 | 52.5 (1.5) | 46.2 (1.9) | 60.1 (2.1) | <.001 |
Abbreviations: AD, Alzheimer’ disease; BMI, body mass index; H. pylori, Helicobacter pylori; HS, high school; N, unweighted sample size; NHANES, National Health and Nutrition Examination Surveys; MA, Mexican-American; HDL, high-density lipoprotein; TA, triacylglycerol; IgG, Immunoglobulin G.
NOTE. Bold text indicates P<.05. Bold and italic text indicates P<.10.
P<.05 for null hypothesis of no difference in means or proportions by wave of NHANES data (NHANES III, phase 1 vs. NHANES 1999–00).
P values are two-sided and associated with design-based F-test. All percentages and SE were obtained using svy commands in Stata, to account for survey design complexity, including strata, primary sampling units, and sampling weights (combined for both in the case of the pooled sample or specific weight for NHANES III, phase 1).
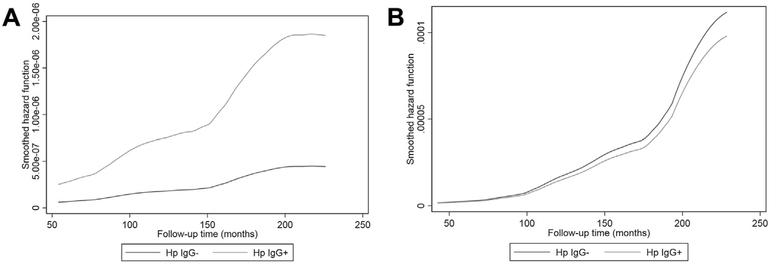
Tables 2 and 3 present the key findings from a series of Cox proportional hazards models with main outcomes being incident AD and all-cause dementia (Table 2) and AD mortality (Table 3). A direct association between H. pylori seropositivity and AD mortality was observed among men (multivariate-adjusted model hazard ratio [HR] = 4.33, 95% CI: 1.51–12.41, P =.006, pooled sample), with no association detected among women. When accounting for competing risk of non-AD deaths (i.e., competing risk regression), results did not differ markedly (data not shown). Importantly, this positive association among men was replicated with outcomes of incident AD and all-cause dementia, with a multivariable-adjusted HR = 1.45 (95% CI: 1.03–2.04, P =.035, pooled sample) and HR = 1.44 (95% CI: 1.05–1.98, P = .022, NHANES III, phase I wave), respectively. Similarly, in the NHANES III subcohort, the association of H. pylori with incident AD/all-cause dementia was restricted to individuals with more than high school educational attainment. Some differentials by poverty income ratio whereby the direct association was restricted to the upper income group were also notable, including outcomes of AD incidence and mortality (pooled sample). Using smoothed hazard rates, Figs. 2A and 2B compare mortality rate trajectories from AD by H. pylori seropositivity and sex, mirroring the significant rise in risk over time among men who are H. pylori IgG+compared with a flat trajectory among men who were H. pylori IgG–. A similar, albeit weaker pattern in men was observed for AD incidence, pooled sample (Supplementary Fig. 1A and 1B) and all-cause dementia incidence, and NHANES III, phase 1 subcohort (Supplementary Fig. 2A and 2B).
Table 2.
H. pylori seropositivity’s association with incident Alzheimer’s disease and all-cause dementia in multiple Cox proportional hazards regression models, overall and stratified by sex, race, age, income, and education groups: NHANES III, phase 1 (1988–1991) alone and combined with NHANES 1999–2000 (pooled sample)
|
H. pylori seropositivity vs. incident outcomes |
Model 1* | Model 2† | Model 3‡ | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Pooled sample | ||||||
| Alzheimer’ disease | ||||||
| Overall (N = 5876) | 1.19 (0.96–1.49) | .11 | 1.13 (0.90–1.42) | .30 | 1.10 (0.87–1.38) | .42 |
| Men (N = 2937) | 1.56 (1.12–2.18) | .008§ | 1.46 (1.03–2.05) | .031§ | 1.45 (1.03–2.04) | .035§ |
| Women (N = 2939) | 1.03 (0.78–1.37) | .82 | 0.98 (0.73–1.31) | .87 | 0.96 (0.71–1.37) | .71 |
| NH white (N = 3160) | 1.25 (0.98–1.59) | .08 | 1.14 (0.89–1.48) | .30 | 1.11 (0.86–1.45) | .40 |
| NH black (N = 1166) | 1.04 (0.67–1.61) | .87 | 0.96 (0.62–1.50) | .87 | 1.03 (0.65–1.63) | .90 |
| MA (N = 1283) | 1.17 (0.67–2.04) | .58 | 1.11 (0.64–1.9) | .71 | 1.06 (0.59–1.92) | .84 |
| ≤65 y (N = 3216) | 1.19 (0.77–1.85) | .44 | 0.94 (0.60–1.44) | .78 | 0.93 (0.59–1.45) | .74 |
| >65 y (N = 2660) | 1.17 (0.92–1.50) | .20 | 1.16 (0.90–1.50) | .26 | 1.15 (0.88–1.49) | .30 |
| PIR < 100% (N = 932) | 0.64 (0.39–1.05) | .074 | 0.72 (0.44–1.18) | .19 | 0.67 (0.41–1.11) | .12 |
| PIR: 100–200% (N = 1451) | 0.97 (0.65–1.45) | .87 | 0.97 (0.65–1.45) | .87 | 0.94 (0.61–1.45) | .79 |
| PIR: >200% (N = 2712) | 1.43 (1.03–1.99) | .032‖ | 1.42 (1.01–1.98) | .044‖ | 1.40 (0.99–1.97) | .06‖ |
| <HS (N = 1858) | 0.96 (0.63–1.47) | .85 | 0.95 (0.62–1.46) | .81 | 0.90 (0.58–1.40) | .65 |
| HS (N = 2388) | 1.06 (0.76–1.46) | .75 | 1.05 (0.75–1.45) | .79 | 1.06 (0.77–1.48) | .71 |
| >HS (N = 1598) | 1.31 (0.85–2.01) | .23 | 1.32 (0.86–2.02) | .21 | 1.32 (0.85–2.05) | .21 |
| All-cause dementia | ||||||
| Overall (N = 5865) | 1.09 (0.93–1.27) | .30 | 1.03 (0.88–1.21) | .71 | 1.01 (0.86–1.18) | .89 |
| Men (N = 2932) | 1.32 (1.05–1.65) | .018§ | 1.24 (0.98–1.56) | .07§ | 1.23 (0.97–1.56) | .09§ |
| Women (N = 2933) | 0.97 (0.78–1.19) | .74 | 0.92 (0.75–1.14) | .45 | 0.91 (0.73–1.12) | .36 |
| NH white (N = 3157) | 1.08 (0.90–1.29) | .41 | 1.00 (0.83–1.20) | .98 | 0.98 (0.82–1.18) | .86 |
| NH black (N = 1164) | 1.20 (0.88–1.64) | .24 | 1.17 (0.86–1.60) | .32 | 1.21 (0.88–1.65) | .24 |
| MA (N = 1279) | 0.93 (0.63–1.36) | .70 | 0.87 (0.60–1.27) | .47 | 0.83 (0.56–1.22) | .34 |
| ≤65 y (N = 3213) | 1.13 (0.84–1.52) | .41 | 0.97 (0.72–1.31) | .86 | 1.00 (0.74–1.35) | .99 |
| >65 y (N = 2652) | 1.03 (0.87–1.23) | .72 | 1.02 (0.85–1.21) | .86 | 1.00 (0.83–1.20) | 1.00 |
| PIR < 100% (N = 925) | 0.72 (0.50–1.04) | .08 | 0.77 (0.53–1.12) | .18 | 0.71 (0.50–1.01) | .06 |
| PIR: 100–200% (N = 1449) | 0.89 (0.66–1.20) | .45 | 0.89 (0.67–1.21) | .48 | 0.89 (0.66–1.21) | .47 |
| PIR: >200% (N = 2712) | 1.20 (0.96–1.51) | .11‖ | 1.19 (0.94–1.50) | .14‖ | 1.18 (0.93–1.49) | .18‖ |
| <HS (N = 1849) | 0.89 (0.65–1.22) | .46 | 0.84 (0.61–1.16) | .30 | 0.85 (0.61–1.18) | .33 |
| HS (N = 2388) | 1.01 (0.81–1.27) | .90 | 1.00 (0.80–1.26) | .99 | 1.01 (0.80–1.26) | .95 |
| >HS (N = 1596) | 1.18 (0.87–1.61) | .28 | 1.18 (0.88–1.60) | .28¶ | 1.18 (0.87–1.60) | .30¶ |
| NHANES III, phase 1 | ||||||
| Alzheimer’s disease | ||||||
| Overall (N = 3646) | 1.30 (1.00–1.69) | .048 | 1.23 (0.94–1.62) | .13 | 1.21 (0.92–1.60) | .17 |
| Men (N= 1856) | 2.03 (1.32–3.11) | .001§ | 1.95 (1.23–3.08) | .004§ | 1.99 (1.24–3.17) | .004§ |
| Women (N = 1790) | 1.05 (0.76–1.46) | .76 | 0.98 (0.70–1.37) | .89 | 0.96 (0.68–1.35) | .81 |
| NH white (N = 2073) | 1.30 (0.97–1.74) | .08 | 1.21 (0.89–1.64) | .22 | 1.19 (0.87–1.62) | .28 |
| NH black (N = 767) | 1.08 (0.67–1.75) | .75 | 1.03 (0.64–1.65) | .90 | 1.16 (0.71–1.89) | .56 |
| MA (N = 710) | 1.17 (0.58–2.34) | .66 | 1.07 (0.53–2.16) | .86 | 1.06 (0.50–2.27) | .88 |
| ≤65 y (N = 1949) | 1.23 (0.77–1.96) | .39 | 0.96 (0.60–1.52) | .85 | 0.97 (0.61–1.55) | .90 |
| >65 y (N = 1697) | 1.27 (0.94–1.73) | .12 | 1.28 (0.93–1.76) | .13 | 1.24 (0.89–1.73) | .20 |
| PIR < 100% (N = 570) | 0.84 (0.46–1.54) | .58 | 0.80 (0.43–1.50) | .49 | 0.69 (0.37–1.27) | .23 |
| PIR: 100–200% (N = 906) | 1.13 (0.68–1.89) | .64 | 1.12 (0.67–1.89) | .66 | 1.19 (0.68–2.08) | .54 |
| PIR: >200% (N = 1729) | 1.34 (0.93–1.95) | .11 | 1.35 (0.91–1.98) | .13 | 1.30 (0.88–1.93) | .19 |
| <HS (N = 852) | 0.76 (0.44–1.32) | .34 | 0.71 (0.41–1.23) | .22 | 0.66 (0.38–1.15) | .14 |
| HS (N = 1924) | 1.12 (0.80–1.59) | .50¶ | 1.09 (0.77–1.54) | .64¶ | 1.09 (0.77–1.55) | .62¶ |
| >HS (N = 846) | 1.75 (1.02–3.00) | .044¶ | 1.75 (1.01–3.02) | .046¶ | 1.84 (1.05–3.22) | .032¶ |
| All-cause dementia | ||||||
| Overall (N = 3646) | 1.18 (0.98–1.42) | .07 | 1.13 (0.93–1.36) | .21 | 1.11 (0.92–1.34) | .28 |
| Men (N = 1856) | 1.51 (1.13–2.01) | .005§ | 1.44 (1.06–1.95) | .020§ | 1.44 (1.05–1.98) | .022§ |
| Women (N = 1790) | 1.04 (0.82–1.31) | .77 | 0.98 (0.77–1.25) | .88 | 0.97 (0.76–1.24) | .82 |
| NH white (N = 2073) | 1.16 (0.94–1.43) | .16 | 1.10 (0.89–1.35) | .40 | 1.09 (0.87–1.35) | .45 |
| NH black (N = 767) | 1.06 (0.74–1.52) | .74 | 1.04 (0.73–1.49) | .82 | 1.13 (0.78–1.63) | .52 |
| MA (N = 710) | 1.06 (0.67–1.69) | .80 | 0.98 (0.62–1.54) | .91 | 0.92 (0.58–1.47) | .73 |
| ≤65 y (N = 1949) | 1.16 (0.84–1.60) | .37 | 0.96 (0.69–1.34) | .83 | 0.98 (0.70–1.38) | .92 |
| >65 y (N = 1697) | 1.14 (0.91–1.41) | .25 | 1.13 (0.91–1.40) | .28 | 1.10 (0.88–1.38) | .42 |
| PIR < 100% (N = 570) | 0.72 (0.46–1.12) | .15 | 0.71 (0.46–1.10) | .13 | 0.61 (0.39–0.95) | .030 |
| PIR: 100%−200% (N = 906) | 1.08 (0.76–1.55) | .66 | 1.08 (0.76–1.55) | .66 | 1.11 (0.77–1.60) | .57 |
| PIR: >200% (N = 1729) | 1.24 (0.95–1.62) | .11‖ | 1.22 (0.93–1.60) | .15‖ | 1.20 (0.91–1.58) | .19‖ |
| <HS (N = 852) | 0.78 (0.50–1.22) | .28 | 0.74 (0.48–1.16) | .19 | 0.72 (0.46–1.12) | .15 |
| HS (N = 1924) | 1.05 (0.82–1.33) | .71 | 1.02 (0.80–1.29) | .89 | 1.02 (0.80–1.30) | .90 |
| >HS (N = 846) | 1.60 (1.09–2.35) | .017§ | 1.59 (1.08–2.34) | .018 | 1.57 (1.06–2.31) | .023 |
Abbreviations: BMI, body mass index; H. pylori (Hp), Helicobacter pylori; HR, hazard ratio; HS, high school; N, unweighted sample size; NH, non-Hispanic; NHANES, National Health and Nutrition Examination Surveys; PIR, poverty income ratio; MA, Mexican-American.
NOTE. Bold text indicates P<.05. Bold and italic text indicates P<.10.
Cox proportional hazards models with time of follow-up expressed in months since MEC examination. Model 1 was adjusted for age, sex, race, and wave. All models including sampling weights that are specific to NHANES III, phase 1 or combined for the pooled sample. Sample sizes shown are unweighted.
Model 2 is model 1 further adjusted for education and poverty income ratio.
Model 3 is model 2 further adjusted for smoking, weight status, hypertension, diabetes, and dyslipidemia.
P<.05 for interaction term Hp × sex in separate model with Hp and sex retained as main effects.
P<.05 for interaction term Hp × PIR in separate model with Hp and PIR group retained as main effects.
P<.05 for interaction term Hp × EDU in separate model with Hp and EDU group retained as main effects.
Table 3.
H. pylori seropositivity’s association with mortality from Alzheimer’s disease in multiple Cox proportional hazards regression models, overall and stratified by sex, race, age, income, and education groups (pooled sample): NHANES III, phase 1 (1988–1991) combined with NHANES 1999–2000
| Model 1* | Model 2† | Model 3‡ | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Overall (N = 5886) | 1.71 (0.94–3.11) | .08 | 1.60(0.87–2.95) | .13 | 1.56(0.83–2.95) | .17 |
| By gender | ||||||
| Men (N = 2940) | 4.26 (1.69–10.79) | .002§ | 4.08 (1.40–11.88) | .010§ | 4.33 (1.51–12.41) | .006§ |
| Women (N = 2946) | 1.07 (0.48–2.39) | .86 | 0.95 (0.42–2.12) | .90 | 0.89 (0.39–2.05) | .79 |
| By race/ethnicity | ||||||
| NH white (N = 3164) | 2.05 (1.11–3.80) | .022 | 1.89 (1.00–3.57) | .05 | 1.93 (1.01–3.70) | .047 |
| NH black (N = 1167) | 0.26 (0.06–1.11) | .071‖ | 0.24 (0.07–0.86) | .028‖ | 0.30 (0.11–0.83) | .020‖ |
| MA (N = 1286) | 0.44 (0.10–2.06) | .30 | 0.42(0.10–1.79) | .24 | 0.30 (0.08–1.08) | .07 |
| By age group | ||||||
| ≤65 y (N = 3216) | 1.24 (0.21–7.23) | .81 | 0.66 (0.18–2.45) | .54 | 0.57(0.20–1.69) | .32 |
| >65 y (N = 2670) | 1.81 (0.97–3.37) | .06 | 1.85 (0.96–3.55) | .06 | 1.81 (0.93–3.54) | .08 |
| By poverty income ratio | ||||||
| <100% (N = 934) | 0.64 (0.14–2.85) | .56 | 0.48 (0.14–1.66) | .25 | 1.04(0.26–4.81) | .96 |
| 100%−200% (N = 1455) | 0.89 (0.27–2.90) | .85 | 0.89 (0.27–2.97) | .85 | 1.13 (0.33–3.88) | .85 |
| >200% (N = 2714) | 2.29 (0.98–5.36) | .06 | 2.46 (0.97–6.25) | .06 | 2.47 (0.94–6.54) | .07 |
| By education | ||||||
| <HS (N = 1861) | 2.62 (0.70–9.82) | .15 | 2.39 (0.47–12.3) | .30 | 2.23 (0.37–13.48) | .38 |
| HS (N = 2393) | 1.00 (0.45–2.23) | 1.00 | 1.03 (0.47–2.28) | .94 | 0.93 (0.40–2.15) | .87 |
| >HS (N = 1599) | 2.04 (0.57–7.30) | .27 | 2.03 (0.54–7.67) | .29 | 1.91 (0.44–8.26) | .39 |
Abbreviations: BMI, body mass index; H. pylori (Hp), Helicobacter pylori; HR, hazard ratio; HS, high school; N, unweighted sample; NH, non-Hispanic; NHANES, National Health and Nutrition Examination Surveys; MA, Mexican-American.
NOTE. Bold text indicates P<.05. Bold and italic text indicates P<.10.
Cox proportional hazards models with time of follow-up expressed in months since MEC examination. Model 1 was adjusted for age, sex, race, and wave. All models including sampling weights that are specific to NHANES III, phase 1 or combined for the pooled sample. Sample sizes shown are unweighted.
Model 2 is model 1 further adjusted for education and poverty income ratio.
Model 3 is model 2 further adjusted for smoking, weight-status, hypertension, diabetes, and dyslipidemia.
P<.05 for interaction term Hp × sex in separate model with Hp and sex retained as main effects.
P<.05 for interaction term Hp × race in separate model with Hp and race retained as main effects.
Fig. 2.
Hazard rate of Alzheimer’s disease mortality by H. pylori seropositivity status from fully adjusted Cox PH models among (A) Men, (B) Women: NHANES III, phase 1 and NHANES 1999–00 (pooled sample). Analysis time is expressed in months. Smoothed hazard functions are obtained from the fully adjusted model (model 3) among men and women. Abbreviations: H. pylori, Helicobacter pylori; Hp IgG–, H. pylori negative; Hp IgG+, H. pylori positive; NHANES, National Health and Nutrition Examination Surveys; PH, proportional hazards; IgG, Immunoglobulin G.
4. Discussion
To our knowledge, this is the first national cohort study to examine associations of H. pylori seropositivity with incidence of all-cause and AD dementia and with AD dementia mortality. It is also among the few to systematically examine these associations across key sociodemographic groups, including age, sex, race, and SES. Data showed a direct association between H. pylori seropositivity and AD mortality among men (multivariable-adjusted model HR = 4.33, 95% CI: 1.51–12.41, P = .006, pooled sample), with no association detected among women. Importantly, this positive association among men was replicated with outcomes of incident AD and all-cause dementia, with a multivariable-adjusted HR = 1.45 (95% CI: 1.03–2.04, P = .035, pooled sample) and HR = 1.44 (95% CI: 1.05–1.98, P = .022, NHANES III, phase I wave), respectively. Similarly, in the NHANES III subcohort, the association between H. pylori and incident AD/all-cause dementia was restricted to individuals with >high school educational attainment.
Previous studies have been conducted to examine the association between H. pylori infection or eradication and various cognitive outcomes, including AD. While earlier small case-control studies showed positive associations between H. pylori seropositivity and AD and/or mild cognitive impairment occurrence [11,14,15], an intervention study among H. pylori positive AD cases concluded that successful H. pylori eradication can reduce cognitive decline in AD over time. This finding further reinforced the possible causal link between H. pylori and AD [12]. In another intervention study with survival as the end point, the group with successful H. pylori eradication compared to those with unsuccessful eradication had significantly lower mortality risk (HR [95% CI] = 0.29 [0.11–0.73]), after adjusting for baseline age and Mini–Mental State Examination score [13]. Looking further into biomarkers of AD, among 53 AD patients, H. pylori infection was associated with lower Mini–Mental State Examination score (P = .024), higher cerebrospinal fluid phosphorylated Tau protein(181) (P = .014), and tau (P = .021) levels [16]. Two Japanese case-control studies found no association between H. pylori infection and AD or cognitive impairment [17,18]. In one study [18], H. pylori infection was determined by urinary IgG, an unreliable diagnostic method [44]; it failed to match cases and controls by age and sex, and the study sample had high H. pylori infection prevalence (~70%) rendering the study under-powered, and thus requiring larger sample sizes. In view of aforementioned limitations, that study [18] neither confirms the lack of association between H. pylori infection status and AD in the Japanese population nor is it comparable with European studies indicating such associations. (e.g., [11,16]) Lack of age-matching between cases and controls was a limitation for the other Japanese study with null findings as well [17].
Among cross-sectional studies, findings were also suggestive for the most part of a relationship between H. pylori seropositivity and cognitive impairment. These relationships were indicative of an adverse effect, though some were limited to specific sociodemographic groups. Recently, a national cross-sectional study using NHANES III phase 1 data (1988–91) with age-group-specific neuropsychological test batteries and two measures of H. pylori seropositivity (IgG and IgG-CagA) detected worse performance among those aged 60–90 y with H. pylori IgG+versus IgG–on a verbal memory test, with other sex- and race-specific similar associations detected between H. pylori seropositivity and poor performance on tests of verbal memory, psychomotor speed, and orientation [19]. This study and others report a direct relationship between H. pylori and poor cognitive performance among healthy middle-aged adults, with evidence of interaction with sociodemographic and other factors [19,45,46]. For instance, individuals with lower educational attainment, non-white subjects, and women have been found to be more susceptible for the relationship of H. pylori with poorer cognitive outcomes [19,45]. Nevertheless, our previous cross-sectional analyses of NHANES III, phase 1 indicated that H. pylori seropositivity was specifically associated with worse verbal memory performance among men, a replication of our current key study findings [19].
Furthermore, low educational attainment and lower income among several markers of socioeconomic position were linked to myriad poor cognitive outcomes such as incident AD. Proposed underlying mechanisms included “reserve capacity”, “use it or lose it”, and “behavioral mediation”. In the latter case, factors such as smoking and hypertension may be mediating the relationship between SES and cognitive outcomes. Hypertension and other risk factors associated with atherosclerosis can in turn initiate cerebrovascular damage often found in vascular dementia, the second common form of dementia. Nevertheless, vascular damage often coexists with AD pathology, thus making hypertension a relevant mediator in the SES-AD association [47]. Interestingly, in our cohort, after accounting for lifestyle and vascular risk factors, we still found several indications of a higher risk of AD/dementia in H. pylori-infected subjects with higher SES (higher educational and income levels); this finding in a subgroup that is thought to have lower risk warrants replication and further investigations.
As stated earlier, H. pylori seroprevalence is highly dependent on sociodemographic factors, particularly age, sex, race/ethnicity, and SES [19,25,26]. Moreover, H. pylori has been associated with several biological markers that were associated with neurocognitive disorders. Indeed, although exact mechanisms are not understood, it is thought that H. pylori influences cognition through inflammatory pathways. These pathways include accumulated local and inflammatory responses due to gastritis from the infection [46] and nutrient deficiency processes, namely iron deficiency combined with deficiencies in 1-C metabolites (e.g., folate and vitamin B12), leading to elevated serum homo-cysteine concentrations [19]. In fact, H. pylori has been associated with poorer iron status (measured by ferritin and transferrin saturation), lower 1-C metabolite concentrations, and worse antioxidant status [48]. Further national cross-sectional data analyses indicated that H. pylori interacts with folate and inflammatory markers to possibly alter cognitive outcomes [46]. Moreover, H. pylori may contribute to the total infectious burden and inflammatory markers which may increase the risk of cognitive decline and AD [49]. Notably, Toxoplasma gondii interacts with H. pylori to worsen cognitive performance [45]. Combined, these results suggest H. pylori’s involvement in myriad mechanisms influencing cognitive pathology. Although our present study did not distinguish H. pylori seropositivity by CagA status, a previous national study indicated that around 49%–63% of total H. pylori IgG were CagA+. Nevertheless, most positive findings in that study were between total H. pylori IgG status and cognitive performance [19].
Among study strengths are the large nationally representative sample, the inclusion of middle-aged adults (≥45 y), and the assessment of various dementia-related outcomes. Moreover, seroprevalence (~41% H. pylori IgG+) provided ample statistical power for stratification by sociodemographic factors. Limitations included observational study design precluding causal inference, despite ascertainment of temporal relationships. Ultimately, a randomized controlled trial is needed, examining effects of H. pylori eradication on cognitive outcomes. Furthermore, the serological test fails to discriminate between the current and past H. pylori infection, as the antibody itself remains detectable for several months beyond H. pylori eradication [16]. Distinguishing these two states is essential because one key mechanism involves H. pylori inducing immune responses which cross-react with nerve components with shared epitopes (i.e., molecular mimicry) [33]. In fact, it is possible that childhood infection with H. pylori may have initiated the neurodegenerative process independently of the current infection status. Histological analysis of gastric mucosa, an invasive and prohibitive method in population-based studies, is the gold standard for H. pylori detection [12,13]. Finally, residual confounding bias is possible, given the omission of key genetic markers (e.g., ApoE4 status).
In sum, H. pylori seropositivity’s direct association with incidence of all-cause and AD dementia and with AD mortality was restricted to men, and in some cases to higher education or income groups. This sex and SES-specific association was not reported elsewhere and needs further study in terms of underlying mechanisms. Future interventions aiming at eradicating H. pylori should examine sexand SES-stratified effects of this modifiable factor on all-cause and AD incidence or mortality from AD. Assessment of H. pylori seropositivity in those intervention studies should be prospectively carried out using histology, breath test, and stool antigen tests and should evaluate H. pylori eradication outcome, specifically among higher genetic risk groups.
Supplementary Material
RESEARCH IN CONTEXT.
Systematic review: Helicobacter pylori, an infectious agent known for its adverse gastric health effects, was associated with extra-digestive disorders, including atherosclerosis [1], hypertension, and stroke [2], all of which were linked to Alzheimer’s disease through blood-brain barrier impairment [3–5].
Interpretation: Our study is an in-depth assessment of the association between H. pylori infection and key cognitive disorders and outcomes, including Alzheimer’s disease–related mortality, and incident all-cause and Alzheimer’s dementia. This was done by analyzing extensive national data on adults aged 45 y or older which was linked to Medicare and National Death Index registries. We found positive associations between H. pylori infection, and most of these outcomes only among men and individuals of higher socioeconomic status.
Future directions: Future longitudinal studies and intervention studies should examine the impact of H. pylori infection and its eradication onvarious cognitive disorders, stratifying by sex and socioeconomic status.
Acknowledgments
The authors would like to thank Dr. Frances McCarty at the Centers for Disease Control and Prevention, NCHS, Hyattsville, MD for her help with restricted data compilation at the Research Data Center (RDC) and reviewing the output. The authors would also like to thank Mr. Ray Kunz at the RDC in the AHRQ location for supervising the work and sharing output for review during statistical analysis at the RDC.
Source of funding: This research was supported entirely by the Intramural Research Program of the NIH, National Institute on Aging.
Footnotes
The authors have declared that no conflict of interest exists.
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this article are those of the author(s) and do not necessarily represent the views of the Research Data Center, the National Center for Health Statistics, or the Centers for Disease Control and Prevention.
Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jalz.2018.04.009.
References
- [1].Sosa-Ortiz AL, Acosta-Castillo I, Prince MJ. Epidemiology of dementias and Alzheimer’s disease. Arch Med Res 2012;43:600–8. [DOI] [PubMed] [Google Scholar]
- [2].Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: a Delphi consensus study. Lancet 2005;366:2112–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement 2013;9:63–75.e2. [DOI] [PubMed] [Google Scholar]
- [4].Lindeboom J, Weinstein H. Neuropsychology of cognitive ageing, minimal cognitive impairment, Alzheimer’s disease, and vascular cognitive impairment. Eur J Pharmacol 2004;490:83–6. [DOI] [PubMed] [Google Scholar]
- [5].Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 2002; 297:353–6. [DOI] [PubMed] [Google Scholar]
- [6].Turner RS. Biomarkers of Alzheimer’s disease and mild cognitive impairment: are we there yet? Exp Neurol 2003;183:7–10. [DOI] [PubMed] [Google Scholar]
- [7].Helmer C, Pasquier F, Dartigues JF. [Epidemiology of Alzheimer disease and related disorders]. Med Sci (Paris) 2006;22:288–96. [DOI] [PubMed] [Google Scholar]
- [8].Honjo K, van Reekum R, Verhoeff NP. Alzheimer’s disease and infection: do infectious agents contribute to progression of Alzheimer’s disease? Alzheimers Dement 2009;5:348–60. [DOI] [PubMed] [Google Scholar]
- [9].Alzheimer’s Association. 2016 Alzheimer’s disease facts and figures. Alzheimers Dement 2016;12:459–509. [DOI] [PubMed] [Google Scholar]
- [10].Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol 2011;10:819–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kountouras J, Tsolaki M, Gavalas E, Boziki M, Zavos C, Karatzoglou P, et al. Relationship between Helicobacter pylori infection and Alzheimer disease. Neurology 2006;66:938–40. [DOI] [PubMed] [Google Scholar]
- [12].Kountouras J, Boziki M, Gavalas E, Zavos C, Grigoriadis N, Deretzi G, et al. Eradication of Helicobacter pylori may be beneficial in the management of Alzheimer’s disease. J Neurol 2009;256:758–67. [DOI] [PubMed] [Google Scholar]
- [13].Kountouras J, Boziki M, Gavalas E, Zavos C, Deretzi G, Chatzigeorgiou S, et al. Five-year survival after Helicobacter pylori eradication in Alzheimer disease patients. Cogn Behav Neurol 2010; 23:199–204. [DOI] [PubMed] [Google Scholar]
- [14].Kountouras J, Tsolaki M, Boziki M, Gavalas E, Zavos C, Stergiopoulos C, et al. Association between Helicobacter pylori infection and mild cognitive impairment. Eur J Neurol 2007; 14:976–82. [DOI] [PubMed] [Google Scholar]
- [15].Kountouras J, Boziki M, Gavalas E, Zavos C, Deretzi G, Grigoriadis N, et al. Increased cerebrospinal fluid Helicobacter pylori antibody in Alzheimer’s disease. Int J Neurosci 2009;119:765–77. [DOI] [PubMed] [Google Scholar]
- [16].Roubaud-Baudron C, Krolak-Salmon P, Quadrio I, Megraud F, Salles N. Impact chronic helicobacter pylori infection Alzheimer’s disease: preliminary results. Neurobiol Aging 2012;33:1009.e11–1009.e19. [DOI] [PubMed] [Google Scholar]
- [17].Nagga K, Rajani R, Mardh E, Borch K, Mardh S, Marcusson J. Cobalamin, folate, methylmalonic acid, homocysteine, and gastritis markers in dementia. Dement Geriatr Cogn Disord 2003;16:269–75. [DOI] [PubMed] [Google Scholar]
- [18].Shiota S, Murakami K, Yoshiiwa A, Yamamoto K, Ohno S, Kuroda A, et al. The relationship between Helicobacter pylori infection and Alzheimer’s disease in Japan. J Neurol 2011;258:1460–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Beydoun MA, Beydoun HA, Shroff MR, Kitner-Triolo MH, Zonderman AB. Helicobacter pylori seropositivity and cognitive performance among US adults: evidence from a large national survey. Psychosomatic Med 2013;75:486–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chang YP, Chiu GF, Kuo FC, Lai CL, Yang YH, Hu HM, et al. Eradication of helicobacter pylori is associated with the progression of dementia: a population-based study. Gastroenterol Res Pract 2013; 2013:175729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Huang WS, Yang TY, Shen WC, Lin CL, Lin MC, Kao CH. Association between Helicobacter pylori infection and dementia. J Clin Neurosci 2014;21:1355–8. [DOI] [PubMed] [Google Scholar]
- [22].Roubaud Baudron C, Letenneur L, Langlais A, Buissonniere A, Megraud F, Dartigues JF, et al. Does Helicobacter pylori infection increase incidence of dementia? The Personnes Agees QUID Study. J Am Geriatr Soc 2013;61:74–8. [DOI] [PubMed] [Google Scholar]
- [23].Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984;1:1311–5. [DOI] [PubMed] [Google Scholar]
- [24].Kinoshita J Pathogens as a cause of Alzheimer’s disease. Neurobiol Aging 2004;25:639–40. [DOI] [PubMed] [Google Scholar]
- [25].Burucoa C, Axon A. Epidemiology of Helicobacter pylori infection. Helicobacter 2017;22(Suppl 1):1–5. [DOI] [PubMed] [Google Scholar]
- [26].Grad YH, Lipsitch M, Aiello AE. Secular trends in Helicobacter pylori seroprevalence in adults in the United States: evidence for sustained race/ethnic disparities. Am J Epidemiol 2012;175:54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Xu Q, Schett G, Perschinka H, Mayr M, Egger G, Oberhollenzer F, et al. Serum soluble heat shock protein 60 is elevated in subjects with atherosclerosis in a general population. Circulation 2000;102:14–20. [DOI] [PubMed] [Google Scholar]
- [28].Sawayama Y, Ariyama I, Hamada M, Otaguro S, Machi T, Taira Y, et al. Association between chronic Helicobacter pylori infection and acute ischemic stroke: Fukuoka Harasanshin Atherosclerosis Trial (FHAT). Atherosclerosis 2005;178:303–9. [DOI] [PubMed] [Google Scholar]
- [29].D’Andrea MR. Add Alzheimer’s disease to the list of autoimmune diseases. Med Hypotheses 2005;64:458–63. [DOI] [PubMed] [Google Scholar]
- [30].de la Torre JC, Stefano GB. Evidence that Alzheimer’s disease is a microvascular disorder: the role of constitutive nitric oxide. Brain Res Brain Res Rev 2000;34:119–36. [DOI] [PubMed] [Google Scholar]
- [31].Wardlaw JM, Sandercock PA, Dennis MS, Starr J. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke 2003;34:806–12. [DOI] [PubMed] [Google Scholar]
- [32].Xu Z, Li J, Wang H, Xu G. Helicobacter pylori infection and atherosclerosis: is there a causal relationship? Eur J Clin Microbiol Infect Dis 2017;36:2293–301. [DOI] [PubMed] [Google Scholar]
- [33].D’Elios MM, Amedei A, Benagiano M, Azzurri A, Del Prete G. Helicobacter pylori, T cells and cytokines: the “dangerous liaisons”. FEMS Immunol Med Microbiol 2005;44:113–9. [DOI] [PubMed] [Google Scholar]
- [34].Kountouras J, Boziki M, Zavos C, Gavalas E, Giartza-Taxidou E, Venizelos I, et al. A potential impact of chronic Helicobacter pylori infection on Alzheimer’s disease pathobiology and course. Neurobiol Aging 2012;33:e3–4. [DOI] [PubMed] [Google Scholar]
- [35].Talebi Bezmin Abadi A Vaccine against Helicobacter pylori: Inevitable approach. World J Gastroenterol 2016;22:3150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Center for Disease Control and Prevention (CDC). The Third National Health and Nutrition Examination Survey (NHANES III 1988–94) Reference Manuals and Reports (CD-ROM) 1996. Bethesda, MD: Centers for Disease Control and Prevention; 1996. [Google Scholar]
- [37].Gorina Y, Kramarow EA. Identifying chronic conditions in Medicare claims data: evaluating the Chronic Condition Data Warehouse algorithm. Health Serv Res 2011;46:1610–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wener M Helicobacter Pylori IGG Antibodies in Serum by Enzyme Immunoassay. National Health and Nutrition Examination Laboratory Protocol 2008. Hyattsville, MD: National Center for Health Statistics, 2008. Available at: http://www.cdc.gov/nchs/data/nhanes/nhanes_99_00/lab11_met_helicobacter_pylori.pdf. Accessed March 1, 2018. [Google Scholar]
- [39].Centers for Disease Control and Prevention NCfHS. Helicobacter Pylori IgG Antibodies in Serum by Enzyme Immunoassay; 2008. Centers for Disease Control; 2008. [Google Scholar]
- [40].Grundy SM. Hypertriglyceridemia, insulin resistance, and the metabolic syndrome. Am J Cardiol 1999;83:25F–9. [DOI] [PubMed] [Google Scholar]
- [41].Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001;285:2486–97. [DOI] [PubMed] [Google Scholar]
- [42].STATA. Statistics/Data Analysis: Release 14.0 2015. Texas: Stata Corporation; 2015. [Google Scholar]
- [43].Selvin S Statistical Analysis of Epidemiologic Data. 3rd ed 2004. New York, NY: Oxford University Press; 2004. [Google Scholar]
- [44].Leodolter A, Vaira D, Bazzoli F, Schutze K, Hirschl A, Megraud F, et al. European multicentre validation trial of two new non-invasive tests for the detection of Helicobacter pylori antibodies: urine-based ELISA and rapid urine test. Aliment Pharmacol Ther 2003; 18:927–31. [DOI] [PubMed] [Google Scholar]
- [45].Gale SD, Erickson LD, Brown BL, Hedges DW. Interaction between Helicobacter pylori and latent toxoplasmosis and demographic variables on cognitive function in young to middle-aged adults. PLoS One 2015;10:e0116874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Berrett AN, Gale SD, Erickson LD, Brown BL, Hedges DW. Folate and inflammatory markers moderate the association between Helicobacter pylori exposure and cognitive function in US adults. Helicobacter 2016;21:471–80. [DOI] [PubMed] [Google Scholar]
- [47].Beydoun MA, Beydoun HA, Gamaldo AA, Teel A, Zonderman AB, Wang Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC Public Health 2014;14:643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Beydoun MA, Dore GA, Canas JA, Beydoun HA, Zonderman AB. Helicobacter pylori seropositivity’s association with markers of iron, 1-carbon metabolism, and antioxidant status among US adults: a structural equations modeling approach. PLoS One 2015; 10:e0121390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bu XL, Yao XQ, Jiao SS, Zeng F, Liu YH, Xiang Y, et al. A study on the association between infectious burden and Alzheimer’s disease. Eur J Neurol 2015;22:1519–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.