Abstract
The laboratory mouse has become the predominant test species in biomedical research. The number of papers that translate or extrapolate data from mouse to human has grown exponentially since the year 2000. There are many physiological and anatomical factors to consider in the process of extrapolating data from one species to another. Body temperature is, of course, a critical determinant in extrapolation because it has a direct impact on metabolism, cardiovascular function, drug efficacy, pharmacokinetics of toxins and drugs, and many other effects. While most would consider the thermoregulatory system of mice to be sufficiently stable and predictable as to not be a cause for concern, the thermal physiology of mice does in fact present unique challenges to the biomedical researcher. A variable and unstable core temperature, high metabolic rate, preference for warm temperatures, large surface area: body mass ratio, and high rate of thermal conductance, are some of the key factors of mice that can affect the interpretation and translation of data to humans. It is the intent of this brief review to enlighten researchers studying interspecies translation of biomedical data on the salient facets of the mouse thermal physiology and show how extrapolation in fields such as physiology, psychology, nutrition, pharmacology, toxicology, and pathology.
Keywords: Core temperature, Metabolic rate, Thermal conductance, Hypothermia, Extrapolation, Preferred temperature, Behavior
1. Introduction
The laboratory mouse has become the choice test species in biomedical research. This can be surmised easily with a search of published papers in the https://www.ncbi.nlm.nih.gov/pubmed/ data base using the key words mouse versus rat. For example, papers using rats as the test species published in 1980 outnumbered papers using mice by a nearly 2 to 1 ratio (i.e., ~27,000 vs. ~14,000 papers). By 2002 there were approximately equal number of mouse and rat papers published and, since 2003, the number of mouse over rat publications has increased steadily. Indeed, by the beginning of 2016, the number of publications using mice was nearly doubled that of papers using rats (i.e., ~72,000 vs. ~38,000 papers; also see [39]). Moreover, the number of papers that are categorized as a “mouse-human model” or “mouse translational model” has approximately quadrupled from the year 2000 to 2016. In light of this remarkable growth and the likely explosive growth of novel murine models of disease, it behooves researchers to continue to expand their understanding of the mouse and how it’s unique physiology and behavior could affect the translation of biomedical data.
Temperature regulation is a process involving autonomic and behavioral effectors that all biomedical researchers should acknowledge as a critical determinant when translating data from one species to another. Pharmacokinetics and metabolism of drugs and other chemicals is perhaps one of the more indisputable fields where interspecies extrapolation assumes a reasonable similarity in body temperature between species. Considering the temperature-dependence of all biochemical reactions, any significant difference in body temperature would directly impact drug and chemical efficacy and metabolism (for review, [24], 20015). Moreover, a resurgence in understanding the thermal physiology of laboratory mice has been driven primarily by the heavy reliance on mouse models to study mechanisms of obesity and metabolic syndrome [1,10,35]. Overall, mice are being used heavily as a test species in most major disciplines of biomedical research.
A snapshot of the salient physiological and morphological facets of a mouse and human that are tightly linked to thermoregulation is presented in Fig. 1A. The reader is referred to Fig. 1B for further explanation of the thermoregulatory parameters presented in Fig. 1A. The similarities (green boxes) and dissimilarities (red boxes) between the two species are highlighted. Considering the > 3000-fold difference in body mass, many thermoregulatory parameters, including core temperature, the lower critical ambient temperature, skin temperature, and preferred ambient temperature are either equal to or very similar between a typical mouse and nude human. On the other hand, other characteristics that are closely linked to thermoregulation (red-highlighted boxes), including metabolic rate normalized to body mass (i.e., mass-specific metabolic rate), relative mass of brown adipose tissue, surface area:mass ratio, and whole body thermal conductance (i.e., minimal level) differ by at least one order of magnitude between mouse and human. In view of the > 10-fold differences in these parameters, how is the core temperature of mice and humans regulated at nearly the same value? This is achieved with unique physiological strategies employed by each species and it is the primary goal of this paper to highlight and contrast these strategies between mouse and human. Indeed, Maloney et al. [39] commented that the unique thermal physiological and metabolic characteristics of laboratory mice must be considered in the translation of biomedical data from mouse to human.
Fig. 1.
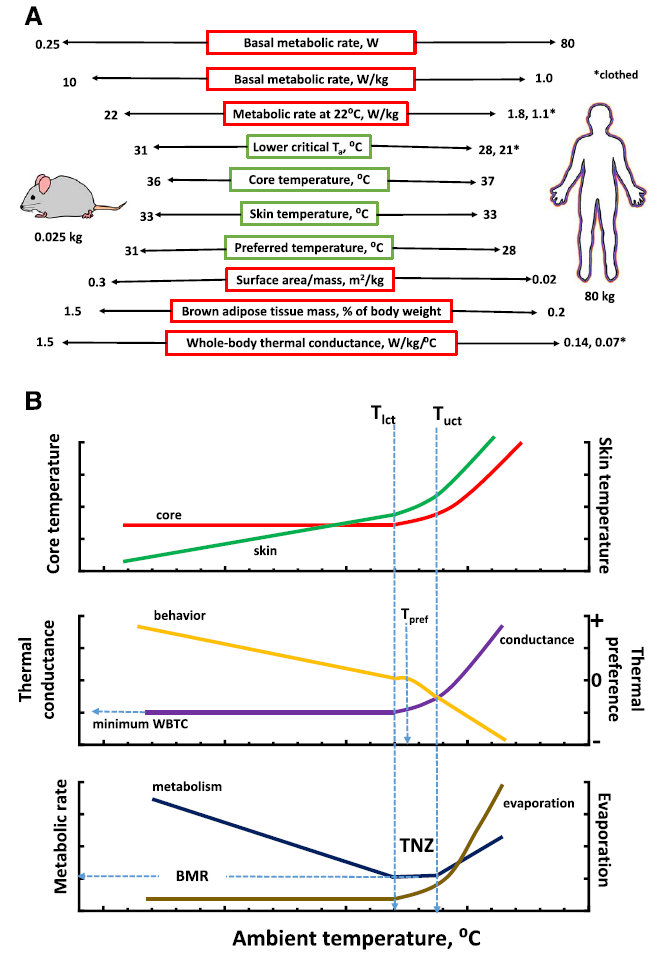
A. Simplified view of the numerical similarities and dissimilarities of basic thermoregulatory, metabolic, and morphometric parameters of a mouse compared to a nude and clothed adult human. Numerical values for mouse and human represent approximate values from the literature. Data with asterisk determined or a clothed adult human with 1.0 clo of insulation. Note that thermal conductance or whole-body thermal conductance was estimated for humans but it is term used primarily in rodent thermal physiological studies and rarely in human thermoregulatory studies. See B for explanation of thermoeffectors. Data and calculations taken from several sources: Hill et al. [30], Gordon [17,25], Schmidt-Nielsen [50], Speakman and Keijer [49]; and Aschoff [2]; BAT weights from [6,8]). B. Diagram illustrating the general trends of core and skin temperature and select thermoeffectors as a function of ambient temperature. Abbreviations: WBTC-whole-body thermal conductance, BMR-basal metabolic rate, Tpref-preferred ambient temperature, TNZ-thermoneutral zone, Tlct-lower critical ambient temperature, Tuct-upper critical ambient temperature. Thermal preference is a qualitative estimate of preference for a warmer (> 0) or cooler (< 0) ambient temperature. Diagram modified from Ingram and Mount [31] and Gordon [17].
Fig. 1B shows a general diagram of the effects of ambient temperature on activation of various thermoeffectors of a homeotherm. Key landmarks of a homeotherms’ thermoregulatory profile, including the lower and upper critical temperature, thermoneutral zone, basal metabolic rate, and preferred temperature are highlighted. Reference to many of these parameters is made frequently in this paper.
This review was prepared to enlighten all researchers studying interspecies translation of biomedical data with particular attention to those who are not necessarily trained in thermal physiology. It is this authors’ intention to present the salient facets of mouse thermal physiology and show how extrapolation of physiological, pharmacological, and related data can be affected. A general approach was selected in the preparation of this review using straightforward graphic and pictorial presentations to illustrate some striking features of the mouse thermoregulatory system. The key features of mice to be presented in this paper are; (i) a seemingly stable thermoregulatory system that is actually subject to marked instability, (ii) reliance on rapid changes in metabolic thermogenesis to maintain thermal stability, (iii) preference for an environment much warmer than that of clothed humans, and (iv) an ability to quickly lower body temperatures as an adaptive response to drugs and toxic chemical.
1.1. Core temperature regulation and variability
The average core temperature of laboratory mice under standard housing conditions is in fact close to that of humans with an average temperature that is just ~1.0 °C below resting human body temperature. However, depending on how the data are analyzed and presented, one could consider the mouse as either an excellent or very poor thermoregulator. Prior to the development of radiotelemetry, colonic and rectal probes were typically used to make single or repeated measures of core temperature of laboratory rodents ([17] for review). Quick, single time-point measurements of rectal or colonic temperature are accurate but give no information on the circadian patterns or dynamic nature of body temperature.
Using radiotelemetry to collect sequential measurements of core temperature of rodents in an undisturbed environment and then averaging over the light or dark phase, provides one with an interpretation of apparent stabilty of the thermoregulatory system (Fig. 2A–D). The data in these graphs are unique because the core temperature of a single mouse, pair-housed with a cage mate in a standard vivarium cage, with nesting material, and averaged over a 7 day period is presented. Most mouse temperature data are collected from individually housed animals in calorimeters or other chambers with no bedding or material for nesting (see [25]). Under these standard vivarium conditions with paired housing, the stability of average core temperature at ambient temperatures of 22 and 30 °C (Fig. 2A) was remarkably consistent. Considering the environmental complexities of the bedding and nesting material, the fact that the thermoregulatory system of the mouse achieves a nearly identical core temperature over an 8 °C change in ambient temperature is noteworthy. A subtle breakdown in stability is seen when temperature is increased from 30 and 32 °C (Fig. 2B). For most of the daytime period, mice at 32 °C maintain a core temperature that is ~0.5 °C above the 22 °C or 30 °C groups. At the onset of the dark phase, the temperature of the 32 °C group rises faster, reaching the core temperature of the 22 °C for a few hours after the start of the dark cycle. There is then some additional separation through the dark phase. At 34 °C, the difference in daytime temperature is much greater but there is a brief period just after lights out when the core temperatures of the 22, 30, 32, and 34 °C groups are equal. A plot of ambient versus mean core temperature during the day and night demonstrates the classic pattern of a homeotherm with a breakdown in thermal homeostasis at ambient temperatures above 30 °C (Fig. 2D). It is of interest to note the differences in the slopes of the lines above 30 °C during the day compared to night. There is a greater rise in core temperature per0C elevation in ambient temperature during the day compared to night. In other words, during the active phase at night, it appears that mice are exerting a greater effort to resist a rise in core temperature when heat stressed. It is also of interest to note that the pattern of the circadian rhythm is preserved at all ambient temperatures but the day-night amplitude is reduced at the warmer temperatures. Abu-Vieiru et al. [1] have performed a thorough analysis of effects of ambient temperature (4–33 °C) on core temperature and metabolic rate of singly-housed male C57BL/6J mice without bedding or nesting material. Under these conditions, daytime core temperature was stable from 18 to 28 °C.
Fig. 2.
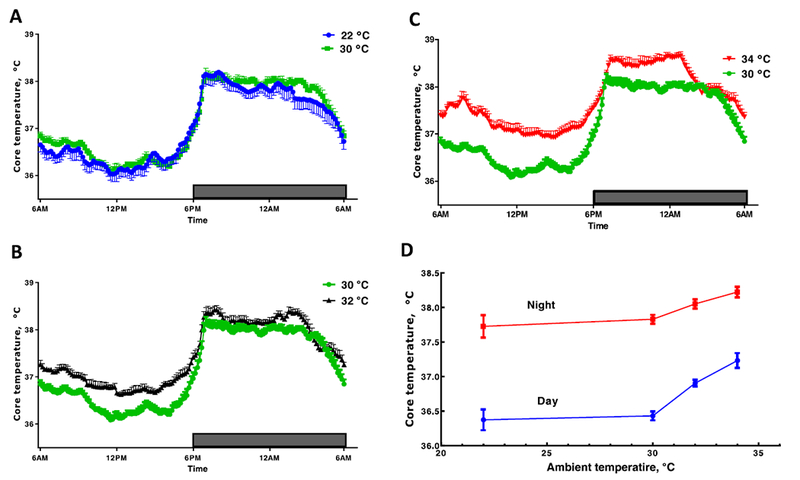
Effects of ambient temperature on stability of core temperature of pairs of female CD-1 mice housed in standard cages with betta chip bedding and provided with two cotton nestlets for nest building (see Fig. 4). Mean core temperatures are compared between ambient temperatures of 22 vs. 30 °C (A), 30 vs. 32 °C (B), and 30 vs. 34 °C (C). D. Plot of ambient temperature versus mean day and night time core temperature. Telemetry data was collected from one of the two mice in each cage. Each line is the mean ± SE of 8 cages of mice with an average of 7 consecutive days of data. Gordon (unpublished).
On the other hand, if raw core temperature data is plotted at 60 s intervals, a marked variability is observed (Fig. 3A). Frequent sampling shows how core temperature of unrestrained and undisturbed mice at a standard laboratory temperature can change abruptly by 3–4 °C over a period of approximately 30 min. The amplitude of the fluctuations can be reduced by raising ambient temperature but instability persists at standard laboratory temperature as well as at thermoneutrality [23]. A frequency distribution of telemetry data of mice and humans illustrates the difference in thermal stability between these species (Fig. 3B). In view of this relative instability of core temperature, this author developed the concept that mice and other relatively small mammals could be termed “average homeotherms”, meaning that they can be classified as homeothermic only when their core temperature is averaged over a relatively long time period [25]. These episodic fluctuations in temperature, metabolism, and other physiological processes are also termed ultradian rhythms. Blessing and Ootsuka [5] in a recent review on this topic note that ultradian rhythms are essential and lead to more efficient utilization of metabolic energy.
Fig. 3.
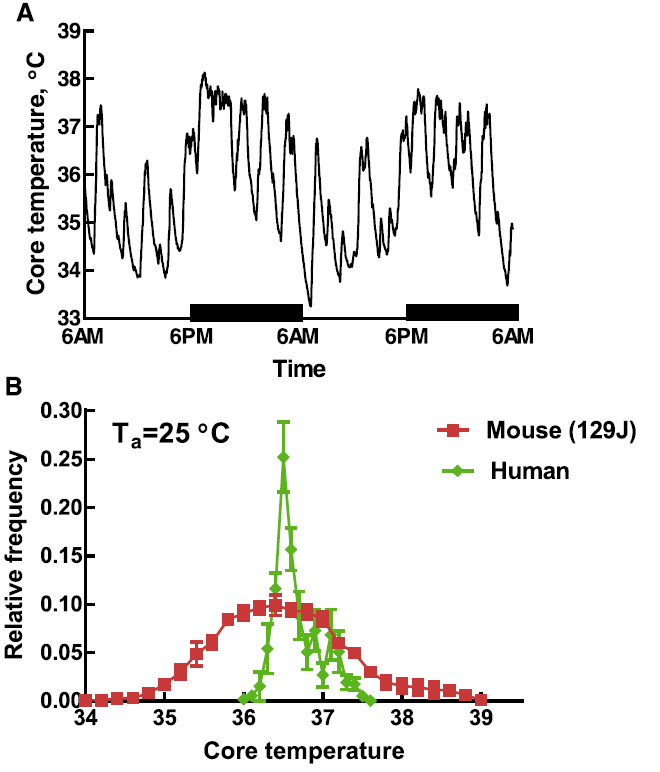
A. Time-course of core temperature of a C57BL/c female mouse maintained at an ambient temperature of 25 °C. Core temperature measured every minute with radio-telemetry. Modified from [25]. B. Frequency distribution of core temperature measured by telemetry for humans and a strain of mice. Data were collected at 60 s intervals for 48 h. Mouse data modified from Gordon [25]. Human data (unpublished, courtesy of Dr. Nigel A. Taylor).
The laboratory rat monitored by telemetry also exhibits episodic fluctuations in core and brain temperature that are apparently linked to the periodic waxing and waning of brown adipose tissue (BAT) thermogenesis as elucidated in recent publications by W.W. Blessing [4,42]. It is apparently not known if a similar mechanism is operative to explain the genesis of the core temperature variations in laboratory mice. Considering the relatively large mass of BAT in mouse and other rodents, one would surmise that BAT thermogenesis is likely linked to the core temperature fluctuations. BAT mass of humans is approximately 10% that of mice but recent studies have indeed shown that it plays a thermogenic role [8]. It is noteworthy that translating BAT function from mouse to human as a means of treating obesity and metabolic disorders has seen a marked growth in recent years [54].
To sum up, the relatively large fluctuations in core temperature of mice should be cause for interest when considering the neural mechanisms of thermoregulation in mice. That is, in viewing the response in Fig. 3A, one must wonder if a constant core temperature is being defended by regulatory mechanisms? When averaged, it appears that temperature is well controlled at a set level during the day and night; however, when data are not averaged, it appears the regulatory mechanisms are far from displaying conventional stability. Moreover, the fluctuations in core temperature may also be viewed as an allosteric as opposed to a homeostatic form of regulation, meaning that the level of regulation is not set but varies depending on environmental circumstances (for review, see [45]).
1.2. Mice are metabolic specialists
In order for a homeotherm to maintain a regulated core temperature that is independent of changes in ambient temperature, total heat loss to the environment must be equal to the total heat production [32]. This classic pattern of thermoregulation is exemplified in Fig. 1B showing a stable core temperature as ambient temperature decreasing below the thermoneutral zone accompanied with a linear increase in metabolic rate. This is a fundamental concept of the heat balance equation and is valid under steady state conditions where core temperature is stable [32]. On the other hand, when considering the thermoeffector responses in states of transition where the organism is subjected to a thermal challenge, the physiological strategy to achieve thermal balance is going to be affected by body mass. As explained below, if a mouse and human were, for example, subjected to an abrupt reduction in ambient temperature, each species will likely recruit thermoeffectors in a unique manner. The mouse would be expected to rely more on metabolic thermogenesis while the human would recruit peripheral vasomotor mechanisms.
The measurement of metabolic heat production by direct or indirect calorimetery is a mainstay method of the thermal biologist (for review, see [33]). Moreover, the concept of a species’ thermoneutral profile, popularized by the seminal study of Scholander et al. [47], who analyzed the effects of temperature on metabolic rate of arctic and tropical birds and mammals, is of paramount importance to make a comparison of thermoregulatory responses between mouse and human. In general, the rise in metabolic heat production per degree °C reduction in ambient temperature below the species’ lower critical temperature, increases in species with smaller body mass and/or reduced insulation. Well-insulated arctic mammals and birds exhibit smaller increases in metabolic rate compared to tropical species with sparse insulation. This concept is exemplified in Fig. 1A showing that the metabolic rate of the mouse nearly doubles while that of the lightly clothed human increases by ~10% when ambient temperature is reduced from 30 to 22 °C.
That the mass-specific basal metabolic rate of mammals increases with a decrease in body mass is a fundamental concept of comparative physiology (e.g., [50]). As shown in Fig. 1A, the basal metabolic rate of a mouse is approximately 10 times that of a human. However, to appreciate the role of metabolism as a thermoeffector in thermoregulation in mouse and human as well as other mammals, one has to consider how these species’ have evolved a particular thermoregulatory strategy that is driven by its metabolic capacity and how this thermogenic capacity affects the development of other thermoeffectors such as peripheral vasomotor tone. Phillips and Heath [44] utilized whole-animal infrared thermography and assessed the role of skin blood flow as a thermoregulatory effector in species ranging in size from mouse to elephant. They developed an index of peripheral vasomotor control and found that this thermoeffector function that controls the transfer of heat from the animal to the environment increases in efficacy and importance with a rise in body size.
Phillips and Heath [44] went on to propose the concept of “metabolic specialist” in small mammals. All endothermic species, of course, rely on a relatively high metabolic rate to achieve thermal homeostasis over a wide range of ambient temperatures. The concept of a metabolic specialist essentially means a small mammal such as a mouse with a relatively large surface area:volume ratio and high thermal conductance (see below) is forced to rely more on metabolic thermo-genesis to regulate core temperature. If one considers that with increasing body size, metabolic rate (normalized to mass) is lower but more stable, then vasomotor control of skin temperature and heat loss from bare surfaces of the body becomes more crucial as a means to achieve stability of the thermal core. A larger mass means greater thermal inertia. This means a greater delay in the rise and dissipation of a heat load. This is not meant to imply that mice and small mammals do not utilize vasomotor mechanisms to control skin temperature. It is clearly operative in mice in the control of thermal conductance as discussed below. To summarize, the reader should not necessarily assume that the mouse thermoregulatory system cannot be used to model human responses but it is important to consider in this species how thermoregulatory effectors are recruited and regulated, especially under conditions of varying ambient temperature.
1.3. Peripheral vasomotor tone, thermal conductance and insulation
The regulation of skin blood flow, conductance of heat from the body to the environment, and insulation are tightly linked thermo-regulatory parameters and are directly affected by ambient temperature (Fig. 1B). Physiological control of the temperature of bare skin through adjustments in peripheral vasomotor tone is a fundamental thermo-regulatory response employed by all mammals and birds. In Fig. 1A it was indicated that mice and humans strive to maintain the same approximate skin temperature of bare surfaces. In fact, there is very little known about the regulation of skin temperature of mice but one can assume, based on their preferred thermal environment, that mice prefer a relatively warm temperature of their bare surfaces of at least 32 °C and possible as high as 36 °C ([20]; also see [25] for review). Preferred skin temperatures of humans vary, of course, by age, gender, health, physical activity, anatomical location, and other factors. In general, a temperature of 32–35 °C is considered ideal (i.e., comfortable) in humans under most circumstances ([7].
In comparing thermoregulation systems of a mouse and human, the differences in surface area/mass and the conductance of heat from the organism to the environment are critical factors. Thermal conductance in dimensions of W/m2/°C, is a measure of the rate at which heat flows across a thermal barrier. In the glossary of thermal physiology, it is defined as the rate of heat transfer between two surfaces when a temperature difference of 1.0 °C is maintained [32]. The transfer of heat from tissue to blood or from peripheral tissues to the surface are examples of thermal conductance. Thermal conductance is not directly measured in animals but is calculated making basic assumptions.
While true thermal conductance could be measured in rodents and other small mammals, a modification of thermal conductance, termed whole-body thermal conductance (WBTC), is the preferred method used by most rodent thermal physiologists studying metabolism and heat exchange as a function of ambient temperature [2,17,40]. The reader should be aware that WBTC is not a term defined in the glossary of thermal physiology [32] but it is nonetheless used frequently in rodent studies:
| (1) |
Assuming steady state where metabolic rate (MR) is equal to heat loss, WBTC is a measure of the rate of heat loss per degree °C change in the difference between core and ambient temperature expressed in units of W/kg/°C, or W/°C. That is, it is measure of the ease of heat transfer from the core to the environment (often normalized to body mass for interspecies comparisons). This measure includes heat loss via radiation, convection, conduction, and evaporation. Below the lower critical ambient temperature (i.e., threshold for activating heat production to defend normothermia), evaporative heat loss is assumed to be a small and constant proportion of the total heat loss (5–10%). At temperatures above the lower critical temperature, evaporative increases and use of the WBTC term has to factor in evaporation. Hence, WBTC is a term that is commonly reported for studies involving ambient temperatures below the lower critical temperature (i.e., measured under state of peripheral vasoconstriction; Fig. 1B). In most rodent studies, WBTC is often reported in units of ml O2/h/g/°C because heat production is measured as oxygen consumption. However, ml O2/h can easily be converted to dimensions of Watts. Further, although WBTC simplifies the complexities of heat transfer between animal and environment, it is very reproducible when measured in controlled thermal environments [40].
The reciprocal of thermal conductance is a measure of the species’ insulation. Small changes in WBTB are used as a measure of a potential physiological, pharmacological, dietary, or genetic effects on peripheral vasomotor tone of mice [1,35]. For example, a 10% decrease in WBTC can be achieved in mice with long-term feeding of a high fat diet [1]. It should also be noted, as is apparent in (Eq. 1), the metabolic rate of a homeotherm at ambient temperatures below the lower critical temperature (e.g., see Fig. 7A) can be predicted by knowing the values of WBTC and ambient temperature (for historical perspective, see [47]).
Fig. 7.

A. A theoretical ambient temperature-metabolism plot for a single mouse with a minimal, resting (or basal) metabolic rate (MR) of 10 W/kg and WBTC of 1.4 W/Kg/°C (I.e., slope of line) and defended core temperature of 36 °C. Dashed line is extrapolation of to an MR = 0 as predicted by the model of [47]. B. Predictive changes in MR from A with a 10% change in WBTC while the defended core temperature is either fixed or allowed to change. When defended core temperature is held at 36 °C, an increase in WBTC (dashed red line) leads to increase in MR and decrease in WBTC (dashed blue line) leads to a reduction in MR. However, if core temperature decreases when WBTC increases (solid blue line), MR is unchanged; or when core temperature increases with decrease in WBTC (solid red line), MR is unchanged. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The rodent thermoregulatory literature is replete with measures of WBTC in rodents and relatively small mammals with a body weight of < 10 kg [2,29]. For laboratory mice, WBTC is 1.0 to 1.5 W/kg/°C depending on body mass and other biological and environmental factors. One of the most well-described concepts of the WBTC is its inverse dependence on body mass [2]. This inverse dependence on weight is exemplified with me WBTC values of select species; 2.3 W/kg/ °C for a 9 g species of Peromyscus, 0.27 W/kg/°C for a 390 g rat, and 0.1 W/kg/°C for a 6.6 kg dog [2].
The method of calculating WBTC (Eq. 1) is typically not used in studies of large mammals, including humans. However, this author has taken the liberty of estimating the WBTC of a nude and clothed human to allow for comparison to that of mice as discussed in Fig. 1A. With this method of comparison, the case can be made that the conductance of heat in the body to the environment for a 1.0 °C change in ambient temperature (below the lower critical ambient temperature) of a mouse is at least one order of magnitude greater than that of a nude adult human. Adding clothing (1 clo) to the human exacerbates the difference. With this simplified calculation of WBTC, one can see how smaller species face a challenge of maintaining an elevated metabolic rate to deal with the relatively rapid loss in body heat.
In regards to peripheral vasomotor tone and extrapolation, one must also consider the obvious morphological differences between mouse and human. The relative proportion of the limbs to the body mass is likely an important factor in extrapolation but rarely considered. The limbs have greater surface area:volume and heat loss is controlled through the arteriovenous anastomoses (AVA’s) of the hands and feet. The arms and legs of an adult human account for approximately 50% of the body weight, whereas the forelimbs and hind limbs of mice account for just 20% of the total mass (M. Serrat, communication). Of course, the tail of mice and rats represents a significant source of heat loss when vasodilated ([16,17] for review) but there is considerably more information on the vasomotor function of the rat (e.g., see [46]). When fully vasodilated, the tail of the rat can dissipate ~25% of the total heat production (for review, [17]). When the tail is vasoconstricted, very little heat is lost through the tail. Regardless of the tail, one can see that the mouse is, relatively speaking, a fur-covered spheroid with very short limbs compared to relatively long-limbed humans.
1.4. Behavioral thermoregulation
A fundamental tenant of thermoregulation of homeotherms is that a relatively stable core temperature is maintained over a wide range of ambient temperatures by employing both autonomic and behavioral thermoeffectors. The innate use of behavior to select a comfortable thermal environment (i.e., thermal preferendum) is a primitive and essential thermoregulatory response of mammals, birds, and many other species of animal life. Moreover, when given the option of choosing between using autonomic or behavioral effectors, behavioral effectors are typically preferred because they are an effective and energetically inexpensive means of optimizing thermal balance.
In the study of homeotherms, thermal physiologists have relied heavily on the thermoneutral profile, the relationship between ambient temperature and metabolic rate, as a means of predicting overall thermal comfort of a homeothermic species. While there are relatively few studies correlating thermoneutral profile with a species’ preferred thermal environment, the consensus would be that the preferred ambient temperature coincides closely with the temperatures just above the lower critical temperature. That is, it is assumed that a homeotherm will select an ambient temperature associated with minimal metabolic expenditure, moderate increase in skin blood flow but without a heat stressed mediated increased in evaporative heat loss. This pattern of thermoeffector responses is depicted in Fig. 1B, showing a preferred ambient temperature coinciding with the metabolic thermoneutral zone.
In rodent thermal physiology, the number of metabolic studies clearly outweighs the number of behavioral thermoregulatory studies [17,25]. However, over the past few decades there has been a growing interest in assessing the optimal ambient temperature to study and house laboratory mice and other laboratory species. Concerns over animal welfare and whether or not the temperatures of animal vivariums are sufficiently warm is one major reason for the growth of research in the area of mouse thermal preference (also see section B. Animal Vivariums below).
Using a variety of methods to study the behavioral thermoregulatory responses, it has become clear that mice generally prefer an environment that is markedly warmer than the ambient temperature of vivariums that are maintained at ~22 °C. One of the most basic and natural means of assessing behavioral thermoregulatory responses is seen in the structure of the nest. This has been studied for many years and a review of the older literature was written by Hart [29]. Mice are often used to study the relationship between thermal stress and nest building behavior [13,25]. As an example of how temperature affects the structure and integrity of mice nests, our laboratory photographed the nests built from two cotton nestlets (Ancare, Bellmore, NY) from pairs of mice whose core temperature data were presented earlier (see Fig. 2). Highly structured nests were built at 22 and 30 °C (Fig. 4). The mice cannot be seen in the 22 °C cage because they are hidden completely under the cotton material. It is interesting to note the relatively well-built nest at a thermoneutral temperature of 30 °C. That is, while this temperature is essentially associated with a minimal metabolic rate, the mice surround themselves with the cotton material, insulating themselves from the ambient air. Raising temperature to 32 °C leads to a slight breakdown in nest structure but the mice are nonetheless making body contact and are surrounded with the cotton material. A temperature of 34 °C is clearly a heat-stress as evidenced by the lack of an organized nest structure.
Fig. 4.
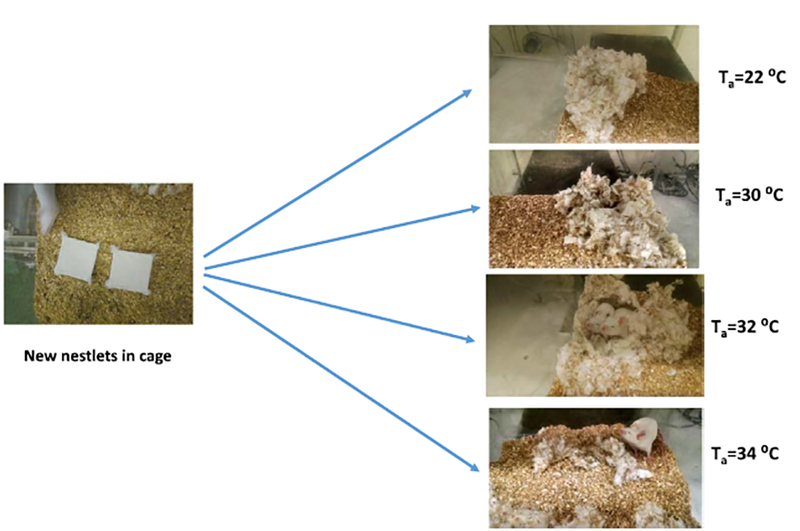
Examples of how the ambient temperature of the vivarium affects the quality of a nest built from cotton nestlets. Nests at 22 and 30 °C are relatively well constructed and mice are essentially protected beneath the cotton. At 32 °C the nest is not as intricate and there is a larger opening. At 34 °C, the nestlets are shredded but not nest is built, suggesting a marked effect of heat stress. Core temperatures of these mice presented in Fig. 2A,B. Gordon (unpublished).
Behavioral thermoregulatory responses are easily studied with a temperature gradient device that allows the rodent to simply move along a gradient of temperatures. Studies in our laboratory and others showed that individual mice placed in a gradient prefer a daytime temperature of 30–32 °C, a temperature equivalent to thermoneutrality (for review, [25]). Constructing a temperature gradient that provided food, water, lighting, and a screen floor for preventing wastes from building up, it was shown that single mice and groups of mice prefer a relatively warm temperature during the daytime and a cooler temperature at night when they were active (Fig. 5). Overall, the daytime and nighttime preferred temperature of mice was significantly higher than that of the standard vivarium temperature [25]. Preferred daytime temperature of mice was estimated to be 8–10 °C warmer than the standard housing temperature.
Fig. 5.

The 24-hour pattern of selected ambient temperature of individual and groups of five female C57Bl/6 mice that were housed in a temperature gradient. Modified from [19].
Temperature gradients are specialized devices that pose a novel environment for rodents. They provide no insulation and are typically made of thermally conductive copper or aluminum. One could argue that the behavior of single mice in a gradient is not representative of the natural thermoregulatory behavior of groups of mice in a covered cage with bedding for burrowing and enrichment. Speakman and Keijer [49] presented a sound argument, based on comparison of thermoregulatory metabolic profiles of mice and humans, that housing mice at standard vivarium temperatures of 20–22 °C for groups of mice and 23–25 °C for individual mice would be equivalent to the thermal environment of a typically clothed human in an indoor environment. However, it should be noted that their presentation emphasized metabolic responses and did not delve far into the behavioral thermoregulatory literature of mice.
Some recent studies suggest that behavioral thermoregulatory studies using temperature gradients discussed above are indeed representative of the behavioral preferences of mice when housed under standard vivarium conditions. Gaskill et al. [11,12] developed a novel thermal choice study utilizing three standard vivarium cages maintained at 20, 25, or 30 °C and interconnected such that groups of three mice could move about freely and select continuously from one of the thermal environments. Under these conditions, the mice spent approximately 55% of the time in the 30 °C cage. The 20 °C cage was occupied ~10% of the time. When provided with enviro-dry nesting material, the time spent at the cooler temperatures increased but the overall time spent at 30 °C was approximately the same as observed without nesting material. Thus, when given bedding and nesting material along with group housing conditions, one sees that the mice still prefer a thermoneutral environment over that of sub-thermoneutral temperatures.
Our laboratory housed groups of female mice in a novel temperature gradient that was designed for long-term housin [26,27]. Groups of three mice were placed in the gradient beginning at 37 days of age and allowed to grow over two months. The mice were observed to behaviorally thermoregulate in the system, preferring very warm floor temperatures during the daytime and moving about to the cooler end of the gradient during the night. Actual preferred temperatures could not be measured continuously as with the past temperature gradient studies. Nonetheless, periodic visual observations showed that the mice preferred a very warm floor temperatures of up to 38 °C during the daytime (Fig. 6). They were observed sleeping side-by-side during the daytime, making contact with each other but not necessarily huddled compared to mice in the isothermal gradient maintained at 22 °C. The mice were provided with nestlets for nest building; however, when housed in a temperature gradient, the mice eventually shredded the nestlets but did not build a nest and appeared to prefer to sleep on the bare, warm aluminum floor. On the other hand, mice housed in the isothermal runway maintained at 22 °C quickly shredded the two nestlets into a flattened pad to insulate themselves from the aluminum floor. It is important to note in this study that the floor is a temperature gradient but the temperature of the air and walls was affected by ambient temperature. Hence, while the mice are sleeping on a very warm floor, the surrounding air and walls are at cooler temperatures that could not be controlled with this type of gradient. Nonetheless, as confirmed in an earlier study using a copper floor temperature gradient [20], this long-term gradient study confirmed that mice prefer very warm floor temperatures when given the opportunity to behaviorally select.
Fig. 6.
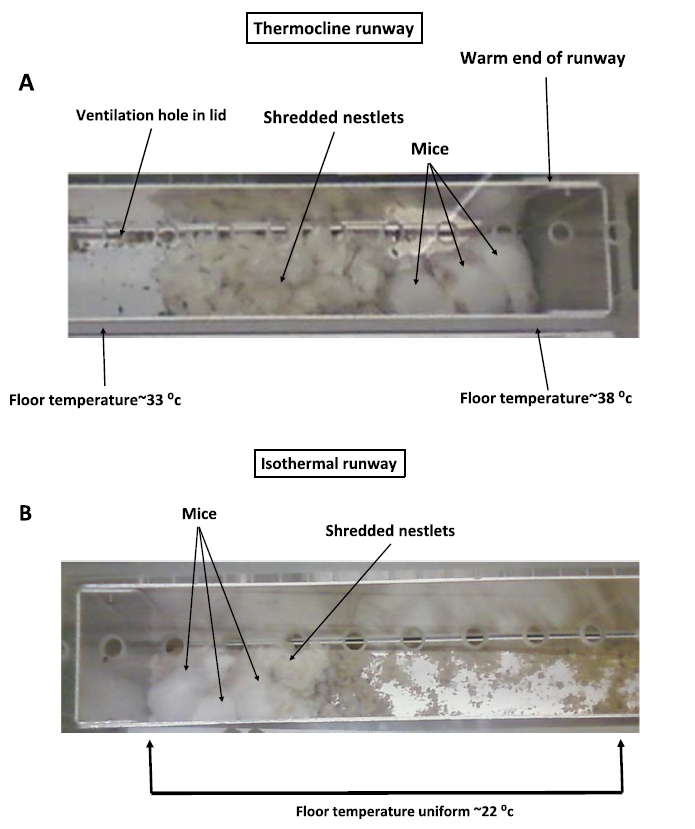
Pictures of groups of three female BALB/c mice housed in a specialized temperature gradient constructed from an aluminum runway with a floor temperature of 22–38 °C (A) or in an identical aluminum runway maintained at an isothermal temperature of 22 °C (B). Pictures were taken during the daytime when mice were sleeping. Note well-built nest of mice in the 22 °C isothermal runway versus poorly constructed nest of mice in the temperature gradient and preference to sleep on the bare aluminum floor. Modified from [26,27]
2. Translating data from mouse to human
Overall, the aforementioned discussion has shown with a general review of the literature that laboratory mice have distinct thermo-regulatory characteristics, including high metabolic rate, high thermal conductance, a variable core temperature, and a preference for relatively warm temperatures. It can be argued that these thermoregulatory characteristics could have a significant impact on most applied studies using mice as the test species. To illustrate the possible impact on translational studies, four areas have been selected: interpreting metabolic rate data, selecting appropriate ambient temperatures for vivariums, the study of obesity and caloric restriction, and interpreting how drugs and toxicants affect thermoregulation.
2.1. Interpreting mouse metabolic data
Commercially manufactured calorimeters are a popular tool to perform a rapid, multi-animal phenotyping of mice and rats. They are being used to characterize and study various pathologies such as obesity, metabolic syndrome, type 2 diabetes, and many others. A measure of metabolic rate may allow one to make a variety of interpretations of a possible effect of an environmental, biological, or genetic manipulation. With mice sequestered in the chamber of a calorimeter, measurement of body temperature is often not practical unless radiotelemetry is used. As mentioned earlier, the best and most accurate way to monitor core temperature in mice is by using radio-telemetry to collect data from non-stressed, undisturbed animals. However, telemetry requires a significant expenditure and labor to surgically implant the transmitters.
This author contends that in metabolic studies of mice as well as other species where monitoring core temperature is not done, there will be an assumption by the investigators that the defended or regulated body temperature is unaffected by the experimental treatment under study. This assumption could lead to errors in the interpretation of the possible effects of the treatment.
There is considerable evidence showing that mice do not defend a normal core temperature when subjected to various environmental, drug, and chemical challenges. It appears that the defended core temperature can go down or up depending on the circumstance or treatment. That is, akin to the classic viewpoint of an elevated body temperature being defended and regulated during a fever, mice and other rodents can respond to defend a lower core temperature.
Before summarizing these effects, a rewriting of Eq. (1) to solve for metabolic rate shows very simply how the defended core temperature can impact the interpretation of metabolic rate data:
That is, metabolic rate below the lower critical temperature is proportional to the difference between ambient and core temperature. Calorimeter studies are typically carried out at standard room temperature of ~22 °C. Hence, any effect of a treatment on metabolism is attributed to (i) an effect of the treatment on thermal conductance (e.g., a drug could induce peripheral vasoconstriction or vasodilation) or (ii) a direct effect of the treatment on shivering or non-shivering thermo-genesis (e.g., a drug stimulating or suppressing BAT thermogenesis). In a calorimeter study, if body temperature is not monitored, most would assume that a constant core temperature is being defended by the mouse thermoregulatory system at a set-point such that the core and ambient temperature difference is a unchanged. With that said, if the treatment causes a marked elevation or reduction in metabolic rate, the investigator would likely conclude that there would be a respective hyperthermia or hypothermia. But the assumption of a defended core temperature would be assumed.
Contrary to our conventional view of the thermoregulatory system of humans and other large mammals, the mouse does not appear to defend a constant core temperature in a similar manner. As discussed earlier, the fluctuations in raw core temperature would make one question the stability of the mouse thermoregulatory system. The core temperature of mice can indeed change rapidly with various biological and environmental challenges. Swoap and Gutila [52] have shown how normal core temperature of mice during ad libitum feeding rapidly shifts to a hypothermic state when food is removed from the cage for at least 6 h. The hypothermia is accompanied by a marked drop in heart rate. The hypothermic response to caloric restriction can be blocked by housing mice at thermoneutral temperatures [37]. Refeeding the mice brings on a rapid recovery of core temperature. It is thought that the hypothermic response is an adaptive mechanism to conserve metabolic fuels during periods of limited food. Mice subjected to heat stroke will become markedly hypothermic for many hours depending upon the severity of the heat stroke [38]. With mild heat stroke, the core temperature can drop to 34 °C for a few hours; with severe heat stroke, core temperature can drop to 20 °C for over 6 h. There are smaller shifts in the apparent defended core temperature when mice are deprived of insulative bedding that would have allowed burrowing [21]. When given ample bedding that permits burrowing, day time core temperature of groups of mice was ~0.7 °C higher than that observed when they were given minimal amounts of bedding. Interestingly, depth of bedding did not have any effect on core temperature at night when the mice were active. It was further found that the type of insulative bedding had little effect on metabolic rate. Instead, the mice lowered their core temperature, a response that reduces the metabolic demand for thermoregulation with the surprising observation that lack of bedding had no effect on metabolic rate. Finally, the defended core temperature of mice administered various toxic chemicals such as ethanol, pesticides, and other toxic chemicals shifts to lower levels, accompanied by a preference for cooler ambient temperatures ([22,26,27] for review). Hypoxia elicits a similar regulated hypothermic response in mice and other rodents [15,18].
Simply stated, if the defended core temperature is not constant, then a treatment-induced effect on metabolic rate may or may not be detected depending on whether core temperature is measured and incorporated into the analysis. This can be demonstrated graphically using a basic temperature-metabolism response for a mouse with a basal or resting metabolism of 10 W/kg, a WBTC of 1.4 W/kg/°C, and a daytime core temperature that would normally be regulated at 36 °C (Fig. 7A). With this model, if a treatment leads to a 10% increase in WBTC and the defended core temperature actually remains at 36 °C, then one would see a significant increase in metabolic rate (dashed red line; Fig. 7B). A 10% decrease in WBTC with a fixed core temperature would lead to a significant decrease in metabolism (dashed blue line). On the other hand, if the mouse lowers its defended core temperature 1.5 °C, then the same 10% increase in WBTC occurs despite no change in metabolic rate. Likewise, lowering WBTC by 10% but with an increase in the defended core temperature to 37.5 °C also corresponds to no change in metabolism.
Making the assumption that the mouse thermoregulatory system is operating to defend a constant core temperature when presented with a variety of chemical, environmental, or genetic challenges must be made with great caution. The example of Fig. 7B illustrates very simply how one can make very careful measurements of metabolic rate while mice are being treated with agents that affect skin blood flow or any number of processes and make an erroneous conclusion that the treatments are having no effect on the thermoregulatory system. Finally, these predictions are made assuming that time of day is not a factor because the defended core temperature is clearly affected by time of day as manifested through the circadian temperature rhythm [25].
2.2. Animal vivariums
From the above discussion on behavioral thermoregulation, it is clear that there is a conflict between the thermal preference of mice housed in vivarium and that of a human wearing PPE (personal protective apparel) (Fig. 8). This pictorial depicts the relationships between core and preferred temperature of a mouse and human. During the daytime, the mouse prefers an ambient temperature that is just 4–6 °C below its core temperature. The human with a core temperature of 37 °C and wearing PPE prefers an ambient temperature that is at least 17 °C below the core (possibly cooler). The difference in slopes of the lines for the mouse and human illustrate the conflict in thermal comfort between the two species. With the vivarium kept at a temperature of 20 °C (Fig. 8A), the thermal state of the human is close to ideal whereas the mouse is cold-stressed. Raising the ambient temperature to 30 °C to approximate the mouse’s daytime preferred temperature ameliorates the cold stress of the mouse but creates an adversely warm environment for the worker in PPE (Fig. 8B).
Fig. 8.
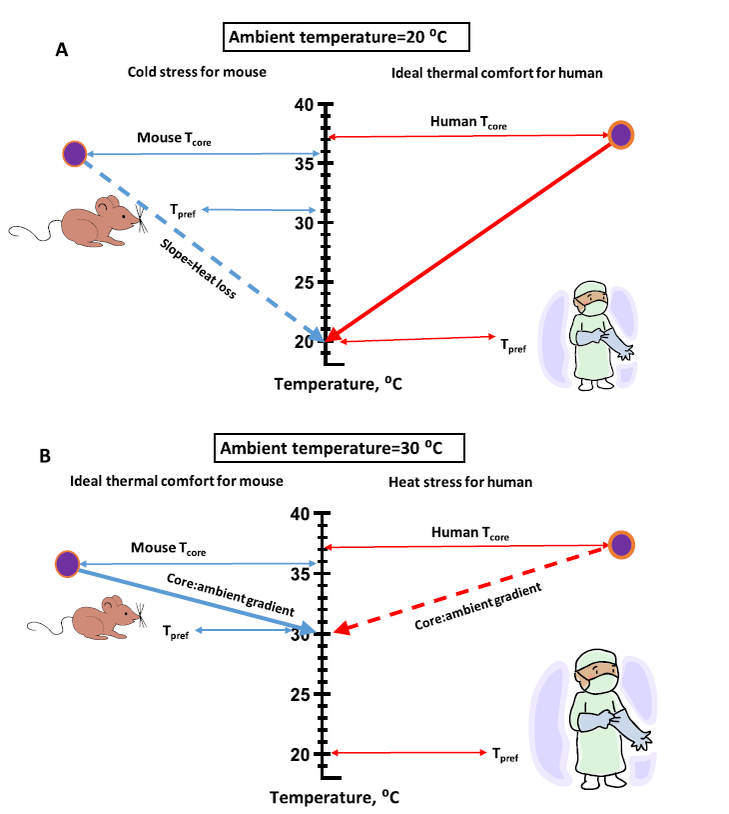
A. A pictorial of the relationships between core and preferred ambient temperature for a mouse and human wearing personal protective equipment (PPE) commonly used in an animal vivarium at a ambient temperature of 20 °C. Under these conditions, the gradient between core and ambient temperature is ideal for the human but a cold stress for the mouse. B. Raising ambient temperature to 30 °C reduces the gradient for both mouse and human creating a situation of ideal thermal comfort for the mouse but relatively uncomfortable heat stress for the human wearing PPE.
Rodent vivarium temperature and relative humidity are essentially set for the comfort of the worker in PPE. It is important to note that the recommended thermal limits for housing mice recommended by the National Research Council have indeed increased recently out of concern for possible cold stress in mice [41]. The current guideline limits the temperature in vivariums to 20–26 °C.
Raising the temperature of the vivarium to provide an environment that matches the preferred temperature for the mouse would be a problem for vivarium workers in PPE. In addition, warmer temperatures accelerate ammonia production of animal wastes in the bedding, raising the time and costs for animal maintenance. Furthermore, because mice prefer cooler temperatures when active at night, raising the ambient temperature to a set level that equates to daytime thermal comfort could translate to thermal stress at night (also see Fig. 9A).
Fig. 9.

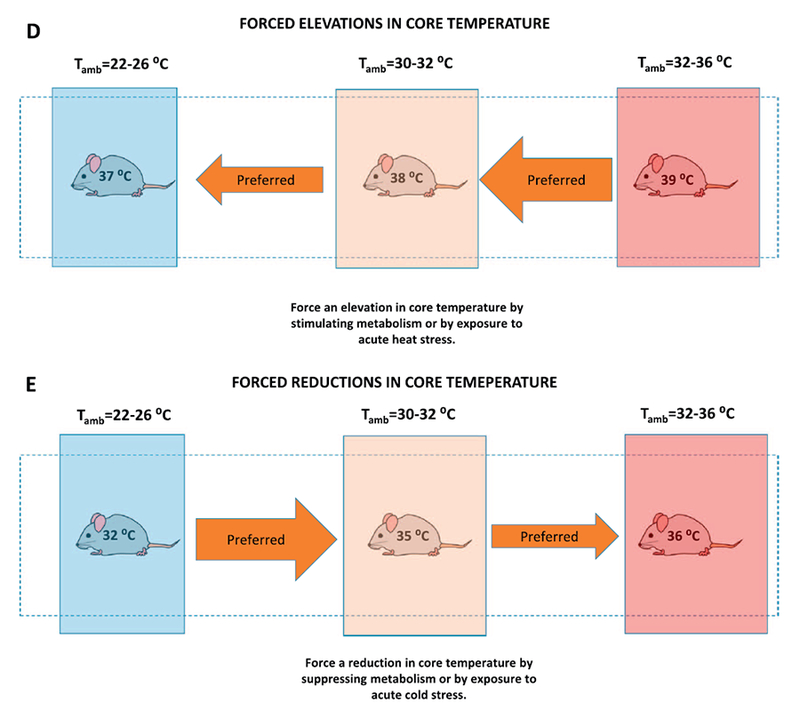
A–E. Depictions of core temperature and behavioral thermoregulatory responses of mice when subjected to various scenarios and treatments. Width of the orange and blue arrows represent relative intensity of behavioral thermoregulatory response to seek a warm or cool temperature, respectively. Black arrows represent forcing mouse from a comfortable to a hot environment. Temperatures on mice indicate the approximate core temperatures. See text for details. See Gordon [14,22]. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
If raising the ambient temperature of the vivarium to match the 24 h thermal preference of mice is not practical, then what can be done to improve conditions of thermal comfort? The recent studies of Gaskill and Garner illustrate the importance of incorporating adequate amounts of bedding and nesting material in mouse cages to improve thermal comfort [11,13]. Our laboratory has recently reported on a device that allows mice to behaviorally thermoregulate in a standard vivarium cage [28]. However, this method requires daily maintenance and would be difficult to implement on a mass scale. The thermal environment of the mouse could also be manipulated with simple changes such as insulative bedding, mouse huts, and increased numbers per cage. Toth et al. [53] has recently assessed how variation in housing density of mice can affect cage temperature, food consumption, and stress hormone responses.
Overall, if the vivarium temperature cannot be manipulated, the researchers have to at least be aware of the potential issues of cold stress in their mice populations. Factors such as age, number of mice per cage, gender, genetic susceptibility, experimental manipulation, and many other factors could affect overall thermal comfort. Moreover, manipulating the bedding and nesting material will have to be considered and implemented to achieve an environment where thermal stress is minimized.
3. Translating obesity and caloric restriction studies
In the field of the etiology of obesity, mice have become the primary test species with the number of published papers rising from 39 in 1990 to over 23,000 in 2016 (PubMed). The thermoregulatory response to overfeeding as well as caloric restriction in mice differs tremendously from that of humans, making the translation of data from mouse to human a challenge. The regulation of caloric intake is complex, involving many physiological and behavioral processes, including temperature regulation. Some of the principal topics covered earlier, preference for warm ambient temperatures, ability to rapidly become hypothermic, and the cold stress from being housed at sub-thermo-neutral temperatures, all have an important role in the translation of data from mouse to human in obesity-related research.
There are many mouse models used in obesity research that involve either overfeeding calorically rich diets and/or using transgenic models such as the ob/ob strain. Overton [43] has written an excellent summary of the potential pitfalls of this research area as it relates to the unique thermoregulatory characteristics of mice. Essentially, the mice housed at a sub-thermoneutral temperature of 22 °C are indeed cold stressed, consuming 50% or more energy than what would be required for basal metabolic needs. Humans experience cold stress infrequently and typically consume the majority of their caloric intake under thermoneutral conditions. One highly cited article noted by Overton [43] is the finding that mice lacking UCP1 developed a propensity for obesity when housed at thermoneutral ambient temperatures as compared to housing at a standard vivarium temperature of 22 °C. It was shown that the cold-stress from housing at this temperature eliminated the obesogenic impact of UCP-1 ablation [9]. The authors went on to propose that housing mice at thermoneutrality “humanized” the mouse model for studying obesity.
This simple finding illustrates how standard housing at 22 °C could impact on translating various obesity findings from mouse to human. In addition, incorporating the mouse’s behavioral thermoregulatory patterns puts an added twist into the study of caloric intake that is rarely discussed. That is, mice prefer relatively warm temperatures in the daytime when they are typically inactive, sleeping, and eating relatively little, whereas, at night they are active, consuming most of their calories, and prefer cooler temperatures. Obesity researchers should consider how the circadian oscillations in preferred ambient temperature and caloric intake could impact on the mouse as a model for obesity research. As discussed by Feldmann et al. [9], while the caloric intake of humans essentially occurs consistently under thermoneutral conditions, laboratory mice are forced to eat and digest under subthermoneutral conditions.
The physiology of caloric restriction has emerged as a key field in aging research as a multitude of studies have shown that chronic caloric restriction increases longevity and generally improves the health of the aged (e.g., for review, see [36]). As noted earlier, due to their large surface area:body mass and high WBTC, mice are able to undergo a marked drop in core temperature when calorically restricted [51]. Mice can easily lower core temperature by over 10 °C within < 12 h after the start of caloric restriction [51]. On the other hand, larger mammals that are calorically restricted can become hypothermic but the depth of hypothermia is limited. The maximum hypothermic response of Brown Norway rats to daily caloric restriction was 2.0 °C [3]. In fact, humans on a prolonged and severed calorically restricted regimen present a core temperature that is just 0.2 °C below that of ad lib fed individuals [48]. These marked differences in hypothermic response exemplify the potential challenges of translating any caloric research data from mouse to human.
3.1. Pharmacology and toxicology of temperature regulation
The mouse often serves as an animal model of drug and chemical sensitivity. Researchers are occasionally aware that their pharmacological studies may often have a marked effect on the core temperature, preferred temperature, and/or thermoeffector functions of mice. This is most apparent at standard room temperatures where the mass-specific metabolic heat production is around 20 times that of the clothed human performing the studies (see Fig. 1A).
How a chemical toxicant or drug affects core temperature and thermoregulatory behavior is summed up in a set of pictorials in Fig. 9 (for review, see [14,22,26,27]). In Fig. 9A, the basic thermal preferences and core temperature of mice during the day and night is summarized as was discussed earlier. These basic patterns of core temperature and thermal preference are translatable to humans although one has to take into consideration the nocturnal activity pattern of mice versus the diurnal pattern of humans. The width of the orange and blue arrows represent the intensity of the mouse’s thermal preference response. The black arrows in Fig. 9A indicate forcing the mouse to a hot environment.
How does behavioral thermoregulation and core temperature of mice respond when drugs or toxic chemicals that interfere with thermoregulation are administered? During a fever as occurs by injection of lipopolysaccharide (LPS) or other pyrogens, there is strong preference for warmer ambient temperatures (Fig. 9B). The width of the arrow in the diagrams is intended to represent the intensity of the likely behavioral thermoregulatory response. Hence, if the mouse is given the LPS at room temperature, the drive to warmer temperatures is very strong. Although not well studied, one might see the mouse preferring even a warmer environment than thermoneutrality as depicted in Fig. 9B. LPS injection is accompanied by an increase in thermogenesis and the evidence would suggest that the response of mice and humans to a fever are comparable.
Considering the facets of thermoregulation highlighted in Fig. 1A of mouse and human that are markedly disparate (highlighted in red), one will find that thermoregulatory responses to agents that lower body temperature are not comparable between the two species (Fig. 9C). As discussed earlier, acute toxic chemicals, hypoxia, large doses of LPS, and other agents will elicit a rapid reduction in the defended core temperature characterized by a preference for cooler temperatures combined with a decrease in metabolic rate, a thermoregulatory state that is termed regulated hypothermia (see [14]). The rapidity and magnitude of the hypothermia is possible in the mouse only because of its ability to rapidly change metabolic rate combined with large surface area:mass ratio and high thermal conductance. If the mouse is heat-stressed and exposed to these agents, there is a marked drive to prefer cooler temperatures. Mice will avoid thermoneutral temperatures and seek a cooler environment during the acute events. This response appears to be adaptive because a lower body temperature ameliorates toxicity of many chemicals and drugs [22,26,27].
Humans and other large mammals can, without a doubt, become hypothermic when treated with various drugs and toxic agents but the response is much slower and drop in core temperature is relatively shallow. With that said, the same chemicals that elicit a marked hypothermia in rodents often elicit a long-term febrile response in humans [22]. Overall, the disparity in developing a hypothermia in response to drugs, chemicals, hypoxia, and other agents is one of greatest challenges facing the study of translating biomedical data from mouse to human. Although not depicted in Fig. 9, it is important to note that a drug or chemical can elicit a change in activity of a thermoeffector such as metabolic rate, skin blood flow, or behavior without necessarily changing core temperature. The study of Kaiyala et al. [34] is one example of such a response where they assessed the effects of inhaled nitrous oxide on thermoregulation of telemetered rats.
The forced changes in body temperature are comparable between mouse and human but the time frame and magnitude of change in body temperature vary (Fig. 9D,E). These final two graphs summarize the expected shifts in thermoregulatory behavior of the mouse if it is subjected to either a forced elevation or reduction in core temperature via a direct manipulation of metabolic thermogenesis with drugs that have no effect on the CNS thermoregulatory centers. These responses can also be elicited by subjecting the mouse to acute heat or cold stress (Fig. 9D,E). It is well known that mice and other rodents are poor thermoregulators when subjected to heat stress compared to humans. This is partially explained by the differences in surface area:mass; a small mass will physically heat faster. Lack of sweating mechanisms is, of course, another key difference between mice and humans [17]. Larger mammals have greater thermal inertia and will resist a drop in core temperature with cold stress. On the other hand, mice and rats can tolerate and survive much greater reductions in core temperature with acute cold stress compared to humans. The key issue of Fig. 9D and E is that the behavioral thermoregulatory patterns of mice and humans to forced reductions in temperature are essentially comparable although mice and rats can survive much lower and prolonged reductions in core temperature [17].
4. Conclusions
This review was written to enlighten researchers to the unique facets of the mouse thermoregulatory system by using a general presentation of graphical and pictorial data. It was this author’s goal to convince researchers to expand their consideration of how the thermal physiology of laboratory mice impacts the translation and extrapolation of pharmacological, physiological, nutritional, and toxicological data. Indeed, the laboratory mouse has unique thermoregulatory characteristics as well as many similarities to larger mammals, including humans (see Fig. 1A). It is the marked differences in metabolic rate, thermal conductance, variable core temperature, and preference for warm temperatures that sets mice apart from humans in the extrapolation of biomedical data.
Acknowledgements
I am most grateful to Drs. Karl Kaiyala, Gustavo Abre-Vieira, and Amir Rezvani for their review of this paper. I thank Dr. A.F. Johnstone for performing the radiotelemetry studies presented in Fig. 2. I am most thankful to Dr. Nigel Taylor for allowing me access to his human study core temperature data.
Footnotes
Publisher's Disclaimer: Disclaimer: The research described in this article has been reviewed by the National Health and Environmental Effects Research Laboratory, U.S. Environmental Protection Agency, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency, nor does the mention of trade names of commercial products constitute endorsement or recommendation for use.
References
- [1].Abreu-Vieira G, Xiao C, Gavrilova O, Reitman ML, Integration of body temperature into the analysis of energy expenditure in the mouse, Mol. Metab 4 (2015) 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Aschoff J, Thermal conductance in mammals and birds: its dependence on body size and circadian phase, Comp. Biochem. Physiol 69A (1981) 611–619. [Google Scholar]
- [3].Aydin C, Gordon CJ, Thermoregulatory, cardiovascular, and metabolic responses to mild caloric restriction in the Brown Norway rat, Physiol. Rep 1 (2) (2013) e00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Blessing W, Mohammed M, Ootsuka Y, Brown adipose tissue thermogenesis, the basic rest-activity cycle, meal initiation, and bodily homeostasis in rats, Physiol. Behav 121 (2013) 61–69. [DOI] [PubMed] [Google Scholar]
- [5].Blessing W, Ootsuka Y, Timing of activities of daily life is jaggy: how episodic ultradian changes in body and brain temperature are integrated into this process, Temperature (Austin) 3 (3) (2016) 371–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chaffee RRJ, Roberts JC, Temperature acclimation in birds and mammals, Annu. Rev. Physiol 33 (1971) 155–202. [DOI] [PubMed] [Google Scholar]
- [7].Ciuha U, Mekjavic IB, Regional thermal comfort zone in males and females, Physiol. Behav 161 (2016) 123–129. [DOI] [PubMed] [Google Scholar]
- [8].Din MU, Raiko J, Saari T, Kudomi N, Tolvanen T, Oikonen V, Teuho J, Sipilä HT, Savisto N, Parkkola R, Nuutila P, Virtanen KA, Human brown adipose tissue [15O]O2 PET imaging in the presence and absence of cold stimulus, Eur. J. Nucl. Med. Mol. Imaging 43 (2016) 1878–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Feldmann HM, Golozoubova V, Cannon B, Nedergaard J, UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality, Cell Metab. 9 (2009) 203–209. [DOI] [PubMed] [Google Scholar]
- [10].Fischer AW, Csikasz RI, von Essen G, Cannon B, Nedergaard J, No insulating effect of obesity, Am. J. Physiol. Endocrinol. Metab 311 (2016) E202–E213. [DOI] [PubMed] [Google Scholar]
- [11].Gaskill BN, Lucas JR, Pajor EA, Garner JP, Working with what you’ve got: changes in thermal preference and behavior in mice with or without nesting material, J. Therm. Biol 36 (2011) 1193–1199. [Google Scholar]
- [12].Gaskill BN, Rohr SA, Pajor EA, Lucas JR, Garner JP, Some like it hot: mouse temperature preferences in laboratory housing, Appl. Anim. Behav. Sci 116 (2009) 279–285. [Google Scholar]
- [13].Gaskill BN, Gordon CJ, Pajor EA, Lucas JR, Davis JK, Garner JP, Heat or insulation: behavioral titration of mouse preference for warmth or access to a nest, Plos One 7 (2012) 32799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gordon CJ, A review of terms and proposed nomenclature for regulated vs. forced changes in body temperature, Life Sci 32 (1983) 1285–1295. [DOI] [PubMed] [Google Scholar]
- [15].Gordon CJ, Fogelson L, Comparative effects of hypoxia on behavioral thermo-regulation regulation in rats, hamsters, and mice, Am. J. Phys 260 (1991) R120–R125. [DOI] [PubMed] [Google Scholar]
- [16].Gordon CJ, Thermal biology of the laboratory rat, Physiol. Behav 47 (1990) 963–991. [DOI] [PubMed] [Google Scholar]
- [17].Gordon CJ, Temperature Regulation in Laboratory Rodents, Cambridge University Press, NY, 1993. [Google Scholar]
- [18].Gordon CJ, The role of behavioral thermoregulation as a thermoeffector during prolonged hypoxia in the rat, J. Thermal Biol 22 (1997) 315–324. [Google Scholar]
- [19].Gordon CJ, Becker P, Ali JS, Behavioral thermoregulatory responses of single-and group-housed mice, Physiol. Behav 65 (1998) 255–262. [DOI] [PubMed] [Google Scholar]
- [20].Gordon CJ, Becker P, Padnos B, A behavioral device to measure the preferred footpad temperature in mice, J. Thermal Biol 25 (2000) 211–219. [Google Scholar]
- [21].Gordon CJ, Effect of cage bedding on temperature regulation and metabolism of group-housed female mice, Comp. Med 54 (2004) 63–68. [PubMed] [Google Scholar]
- [22].Gordon CJ, Temperature and Toxicology: An Integrative, Comparative, and Environmental Approach, CRC Press, Boca Raton, FL, 2005. [Google Scholar]
- [23].Gordon CJ, Quantifying the instability of core temperature in rodents, J. Thermal Biol 34 (2009) 213–219. [Google Scholar]
- [24].Gordon CJ, Response of the thermoregulatory system to toxic insults, Frontiers in Bioscience, Elite ed., 1 2010, pp. 293–311. [DOI] [PubMed] [Google Scholar]
- [25].Gordon CJ, The mouse: an “average” homeotherm, J. Thermal Biol 37 (2012) 286–290. [Google Scholar]
- [26].Gordon CJ, Aydin C, Repasky EA, Kokolus KM, Dheyongera G, Johnstone AFM, Behaviorally mediated, warm adaptation-a physiological strategy when mice behaviorally thermoregulate, J. Thermal Biol 44 (2014) 41–46. [DOI] [PubMed] [Google Scholar]
- [27].Gordon CJ, Johnstone AF, Aydin C, Thermal stress and toxicity, Compr. Physiol 4 (2014) 995–1016. [DOI] [PubMed] [Google Scholar]
- [28].Gordon CJ, Puckett E, Repasky E, Johnstone AF, A device that allows rodents to behaviorally thermoregulate when housed in vivariums, J. Am. Assoc. Lab. Animal Sci 56 (2017) 173–176. [PMC free article] [PubMed] [Google Scholar]
- [29].Hart JS, Rodents, in: Whittow GC (Ed.), Comparative Physiology of Temperature Regulation, Academic Press, NY, 1971, pp. 2–149. [Google Scholar]
- [30].Hill RW, Muhich TE, Humphries MH, City-scale expansion of human thermo-regulatory costs, PLoS One 8 (10) (2013) e76238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ingram DL, Mount LE, Man and Animals in hot Environments, Springer-Verlag, New York, 1975. [Google Scholar]
- [32].IUPS Thermal Commission, Glossary of terms for thermal physiology. Third edition. Revised by The Commission for Thermal Physiology of the International Union of Physiological Sciences, Jap. J. Physiol 51 (2001) 245–280. [PubMed] [Google Scholar]
- [33].Kaiyala KJ, Ramsay DS, Direct animal calorimetry, the underused gold standard for quantifying the fire of life, Comp. Biochem. Physiol. A Mol. Integr. Physiol 158 (2011) 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kaiyala KJ, Butt S, Ramsay DS, Direct evidence for systems-level modulation of initial drug (in)sensitivity in rats, Psychopharmacology 191 (2007) 243–251. [DOI] [PubMed] [Google Scholar]
- [35].Kaiyala KJ, Ogimoto K, Nelson JT, Muta K, Morton GJ, Physiological role for leptin in the control of thermal conductance, Mol. Metab 5 (2016) 892–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Keil G, Cummings E, de Magalhães JP, Being cool: how body temperature influences ageing and longevity, Biogerontology 16 (4) (2015) 383–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Koizumi A, Tsukada M, Wada Y, Masuda H, Weindruch R, Mitotic activity in mice is suppressed by energy restriction-induced torpor, J. Nutr 122 (1992) 1446–1453. [DOI] [PubMed] [Google Scholar]
- [38].Leon LR, Gordon CJ, Helwig BG, Rufolo DM, Blaha MD, Thermoregulatory, behavioral, and metabolic responses to heatstroke in a conscious mouse model, Am. J. Physiol. Regul. Integr. Comp. Physiol 299 (2010) R241–R248. [DOI] [PubMed] [Google Scholar]
- [39].Maloney SK, Fuller A, Mitchell D, Gordon CJ, Overton M, Translating research from animal models: does it matter that our rodents are cold? Physiology 29 (2014) 413–420. [DOI] [PubMed] [Google Scholar]
- [40].McNab BK, On estimating thermal conductance in endotherms, Physiol. Zool 53 (1980) 145–156. [Google Scholar]
- [41].NRC, Guide for the Care and Use of Laboratory Animals, eighth ed., Nat. Res. Council of the National Academy of Science, Washington, DC, 2011. [Google Scholar]
- [42].Ootsuka Y, de Menezes RC, Zaretsky DV, Alimoradian A, Hunt J, Stefanidis A, Oldfield BJ, Blessing WW, Brown adipose tissue thermogenesis heats brain and body as part of the brain-coordinated ultradian basic rest-activity cycle, Neuroscience 20 (164) (2009) 849–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Overton JM, Phenotyping small animals as models for the human metabolic syndrome: thermoneutrality matters, Int. J. Obes. (Lond) 34 (Suppl. 2) (2010) S53–S58. [DOI] [PubMed] [Google Scholar]
- [44].Phillips PK, Heath JE, Dependency of surface temperature regulation on body size in terrestrial mammals, J. Thermal Biol 20 (1995) 281–289. [Google Scholar]
- [45].Ramsay DS, Woods SC, Clarifying the roles of homeostasis and allostasis in physiological regulation, Psychol. Rev 121 (2) (2014) 225–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Romanovsky AA, Ivanov AI, Shimansky YP, Selected contribution: ambient temperature for experiments in rats: a new method for determining the zone of thermal neutrality, J. Appl. Physiol 92 (2002) 2667–2679. [DOI] [PubMed] [Google Scholar]
- [47].Scholander PF, Hock R, Walters V, Johnson F, Irving L, Heat regulation in some arctic and tropical mammals and birds, Biol. Bull 99 (1950) 237–258. [DOI] [PubMed] [Google Scholar]
- [48].Soare A, Cangemi R, Omodei D, Holloszy JO, Fontana L, Long-term calorie restriction, but not endurance exercise, lowers core body temperature in humans, Aging (Albany NY) 3 (4) (2011) 374–379. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Speakman JR, Keijer J, Not so hot: optimal housing temperatures for mice to mimic the thermal environment of humans, Mol. Metab 2 (2012) 5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Schmidt-Nielsen K, Animal Physiology-Adaptation and Environment, Cambridge University Press, New York, 1975. [Google Scholar]
- [51].Swoap SJ, The pharmacology and molecular mechanisms underlying temperature regulation and torpor, Biochem. Pharmacol 76 (7) (2008) 817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Swoap SJ, Gutilla MJ, Cardiovascular changes during daily torpor in the laboratory mouse, Am. J. Physiol. Regul. Integr. Comp. Physiol 297 (2009) R769–R774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Toth LA, Trammell RA, Ilsley-Woods M, Interactions between housing density and ambient temperature in the cage environment: effects on mouse physiology and behavior, J. Am. Assoc. Lab. Anim. Sci 54 (2015) 708–717. [PMC free article] [PubMed] [Google Scholar]
- [54].Wu J, Jun H, McDermott JR, Formation and activation of thermogenic fat, Trends Genet 31 (2015) 232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]


