Summary
Pertussis, a highly contagious infective disease caused by Bordetella pertussis, was in the past very common among newborns and children, causing significant medical, social and economic issues burden, also due to frequent need of hospitalization and high mortality. Following the introduction of vaccines against pertussis, the burden of the disease dramatically decreased, although nowadays, this disease it is still the most widespread among the vaccine preventable ones. First vaccine formulations were composed with whole cell antigen of Bordetella pertussis and were followed by formulations with acellular antigens (PT, FHA, PRN, FIM), that showed to have similar efficacy and less reactogenicity. In particular, all the acellular vaccines, regardless the number of antigenic component included, demonstrated good immunogenicity in clinical trials and high effectiveness in real world evidence studies. Nevertheless, in the recent years it has been notified an increasing number of cases of pertussis.
The most recent evidence demonstrated that for an effective control and prevention of pertussis it is necessary to strengthen vaccination coverage among the whole population, providing primary vaccination to newborns and booster in infancy, adolescence and adulthood every 10 years. Finally, vaccination of women at the third trimester of every pregnancy is the most effective intervention to protect the newborn from pertussis in his first months of life, before developing a protective response after the primary vaccination.
Keywords: Pertussis, Pertussis prevention, Pertussis vaccines
Introduction
Pertussis (P) is a highly contagious infective disease caused by the Gram-negative bacterium Bordetella pertussis (Bp). Until the 1940s, P was extremely common among subjects of pediatric age, especially younger children; from the healthcare, social and economic points of view, it carried a heavy burden in terms of the number of hospitalizations and deaths [1]. Following the introduction of a whole-cell vaccine (wP) in the 1940s, it was thought that the problem of P had largely been solved. Indeed, the frequency of the disease was markedly reduced, at least in areas where the wP was widely used in the pediatric population. In the United States, for example, where over 265,000 cases had been registered in 1934, the incidence of P fell to about 100,000 cases in 1948 and declined further to 1,200-4,000 in the 1980s [2]. Despite this indisputable success, however, the use of wP did not meet with the consensus that would be expected, either among healthcare authorities or among parents. The fact that some of the vaccine formulations available at the time displayed rather low efficacy undoubtedly aroused a certain skepticism. However, what chiefly hindered the systematic introduction of wP into the pediatric vaccination calendar was the fear that its administration might cause potentially severe adverse events. Over time, many of these concerns, such as the fear that the vaccine could cause chronic severe encephalopathy, were shown to be totally unfounded. Nevertheless, the administration of wP did prove to be associated with the onset of significant local reactions and fever in about 50% of vaccinees [3] and of acute systemic manifestations, such as convulsions and persistent crying, in a small, though not negligible, number of subjects [4]. The result of all this was twofold: on the one hand, compliance with vaccination became very low, and vaccination was even not recommended by some healthcare authorities; on the other, efforts were made to develop new vaccines that would be equally efficacious but which would elicit fewer, if any, untoward side-effects.
Subsequently, it was shown that the administration of some components of Bp, such as pertussis toxin (PT), filamentous hemagglutinin (FHA), pertactin (PRN) and fimbrial proteins 2 and 3 (FIM), could induce a protective immune response without eliciting any noteworthy adverse events. This evidence led to the formulation and subsequent diffusion of acellular anti-pertussis (aP) vaccines containing from 1 to 5 of these components. Numerous studies have shown that aP vaccines have similar short-term efficacy to that of wP, but greater safety and tolerability [5-10]. Consequently, these vaccines have been endorsed by the international scientific community and, despite their high cost, they have been incorporated into the vaccination calendars of a great many countries, with high levels of vaccination coverage being achieved.
Nevertheless, a few years after the introduction of aP vaccines, several epidemiological evaluations clearly indicated that the incidence of P was slowly, though steadily, rising, and had even reached higher values than those recorded in periods of widespread wP use [11, 12]. This increase was seen in all pediatric age-groups, though it was quantitatively more evident among older children and adolescents and qualitatively more marked in infants in whom a greater proportion of severe cases was noted. The so-called re-emergence of P inevitably prompted the scientific community to investigate the reasons for this phenomenon. The re-emergence of an infective disease, when appropriate and apparently efficacious preventive measures have already been implemented, may be due to several factors. Thus, efforts were made to ascertain whether the observed rise was real, rather than the result of a different modality of diagnosis or reporting of cases. At the same time, research was undertaken to establish whether the problem was directly or indirectly related to the aP vaccines themselves, as a result either of lower vaccine efficacy than that which had initially been demonstrated, or of a change in the microbial target of the vaccine. Finally, researchers tried to discover whether the efficacy of the various aP vaccines available differed, and whether the possible re-emergence of P was in some way related to a particular commercial preparation. Although these questions have not been completely clarified, the information currently available enables us to draw some conclusions that can, at least in part, explain the re-emergence of P, and hence to propose some possible solutions to the problem. The present analysis briefly summarizes what is currently known about this issue.
Have pertussis cases really increased?
For many years, P was regarded as a typical childhood disease characterized by very specific symptoms – often easily recognizable even by non-experts – especially classic fits of coughing. These were the cases that were reported, which sometimes underwent culture tests of microbiological secretions, and on which epidemiological surveys were based. Over time, however, it emerged that in a non-negligible number of subjects infected by Bp, particularly older children, adolescents and adults, the symptoms were very different: merely a persistent or chronic cough, without respiratory impairment or serious systemic disease. Moreover, it was ascertained that, even in infants, P could have manifestations other than coughing fits, such as, for example, apneic crises [13, 14]. The identification of these cases, which play a key role in the spread of the disease, has inevitably raised the number of cases of P reported and notified to the authorities responsible for epidemiological evaluations, which means that the total number of forms of P diagnosed each year has risen to much higher values than those calculated in the past.
In addition, improvements in laboratory techniques now enable Bp infections to be diagnosed much more easily and rapidly than was hitherto possible by means of classical culture methods. Indeed, current techniques of molecular biology allow Bp to be detected in respiratory secretions and anti-PT antibodies to be detected in saliva within a couple of hours [15-17]. These advances have led to a further increase in the number of cases of P notified.
Nevertheless, some studies have shown that the above-mentioned factors alone are not sufficient to explain the re-emergence of P that has been observed in some countries, albeit to different degrees and in different times. This is clearly demonstrated by one such study, which was conducted by the World Health Organization (WHO) in 19 countries in which data were collected for sufficiently long periods of time on the incidence of P, vaccine administration schedules and vaccination coverage, surveillance methods, the case definition of P, and the type of vaccine used. On applying statistical methods that minimized the impact of greater diagnostic accuracy, it emerged that in five of these countries – Australia, Chile, Portugal, the USA and the UK – the increase in P was real, while in the other 14 countries the rise in the number of cases reported could be explained by cyclical variations in the incidence of the disease and by possible sampling errors. Beyond absolute numbers, the data which seemed to suggest a true quantitative and qualitative increase in P were: the significant rise among infants in severe cases requiring hospitalization or transfer to intensive care or causing death, and the disproportionate increase in forms of P diagnosed in adolescents [18].
The epidemiology of pertussis in Italy
Before the advent of anti-pertussis vaccination, the mean number of notified cases of P in Italy each year was 21,000. By contrast, the latest report issued by the European Centre for Disease Prevention and Control (ECDC), which refers to the period 2011-2015, quotes a mean of 500 cases per year [19]. Thus, in comparison with the pre-vaccination era, the incidence of P in Italy has fallen by 97.6% as a result of both vaccination and the use of aP vaccines (notified cases in 2015 were 503; Tab. I) [19, 20].
Tab. I.
Pertussis cases reported in Italy, 2011-2015.
| Year | 2011 | 2012 | 2013 | 2014 | 2015 |
| Number of cases | 516 | 489 | 523 | 670 | 503 |
From European Centre for Disease Prevention and Control, 2015 19, mod.; Epicentro, 2017 [20], mod.
However, the incidence of P is probably underestimated, especially among adolescents and young adults, in whom the clinical picture is milder than in infants and whose symptoms may be confused with those of other respiratory conditions. Moreover, laboratory techniques, which are essential to confirming the diagnosis, are not frequently implemented. Furthermore, parents are the main source of the contagion of children, in whom the disease tends to be more severe [21, 22]. Notifications of cases of P by age-class in Italy in the period 1996-2009 are shown in Figure 1.
Fig. 1.
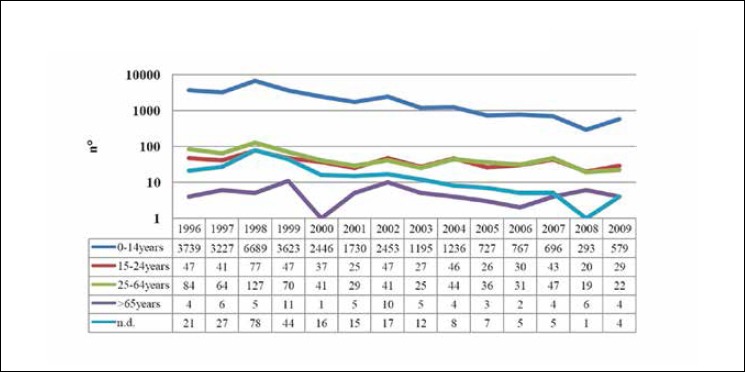
Pertussis: trend in notifications in Italy, 1996-2009 (from Gabutti et al., 2012 [22], mod.).
Short duration of vaccine-induced protection
The re-emergence of P forced the scientific community to seek possible explanations for the phenomenon. Attention was chiefly focused on the duration of vaccine efficacy and on the eventuality that the emergence of genetically mutated Bp strains in the proteins included in the aP vaccines might have blunted, or even nullified, vaccine efficacy.
What aroused the suspicion that aP vaccines might confer only short-term protection was mainly the fact that a large number of cases of P were diagnosed in adolescents, i.e. several years after the primary vaccination. However, it had long been known that neither the disease nor vaccination, regardless of the vaccine used (wP or aP), conferred permanent protection against P. Indeed, the immunity elicited by vaccination lasts from 4 to 12 years, while the protection acquired after natural infection by Bp lasts 7-20 years (Tabs. II, III, IV) [23-36]. For this reason, several countries have for a long time scheduled an anti-P booster vaccination for pre-school children, i.e. aged 5-6 years.
Tab. II.
Studies conducted since the 1990s on the duration of protection induced by the whole-cell pertussis vaccine (wP).
| Author | Year | Subjects | Estimated duration of protection (years) |
Country |
|---|---|---|---|---|
| CDC [24] | 1993 | 225 | 4-6 | USA (Massachusetts) |
| Ramsay [25] | 1993 | 3,150 | 8 | UK |
| Nielsen & Larsen [26] | 1994 | Not known | 10 | Denmark |
| He [27] | 1996 | 3,794 | 5-10 | Finland |
| Van Buynder [28] | 1999 | 15,286 | 5-14 | UK |
| Torvaldsen [29] | 2003 | Not known | 6-9 | Australia |
From Wendelboe AM et al., 2015 [23], mod.
Tab. III.
Studies conducted since the 1990s on the duration of protection induced by acellular pertussis vaccines (aPs).
| Author | Year | Subjects | Number of Bp components | Estimated duration of protection (years) | Country |
|---|---|---|---|---|---|
| Simondon [30] | 1997 | 4,181 | 4 | 4 | Senegal |
| Tindberg [31] | 1999 | 207 | 2 | 10 | Sweden |
| Salmaso [32] | 2001 | 8,432 | 3 | 3 | Italy |
| Lugauer [33] | 2002 | 10,271 | 4 | 6 | Germany |
From Wendelboe AM et al., 2015 [23], mod.
Tab. IV.
Studies conducted since the 1990s on the duration of protection following natural infection by Bp.
| Author | Year | Subjects | Estimated duration of protection (years) |
Country |
|---|---|---|---|---|
| Wirsing [34] | 1995 | 369 | 20 | Germany |
| Miller [35] | 1997 | Not known (review of studies) | 7-10 | UK |
| Versteegh [36] | 2002 | 4 (case series) | 3-12 | Netherlands |
From Wendelboe AM et al., 2015 [23], mod.
The risk of contracting P is directly proportional to the time that has elapsed since the last vaccination. Indeed, it has been reported that children who receive the preschool booster at 4 years of age have a more than 2-fold higher probability of contracting P during the subsequent years of school than those re-vaccinated at 5 years of age [37]. These findings are completely in line with the results of a recent Italian study aimed at ascertaining the etiology of persistent or chronic coughing in children. The data gathered indicated that about 20% of the children and adolescents who had been affected by coughing for no apparent reason for at least 15 days was suffering from P. In over 80% of cases, the subjects had been regularly vaccinated with an aP vaccines and had received the preschool booster. In some cases, moreover, the booster had been administered no more than 2-3 years before the onset of the disease [38].
The different immune response elicited by the various aP vaccines, in comparison with that elicited by natural infection or wP, may explain, at least in part, the different duration of the protection induced. Moreover, although the aP vaccines are effective in preventing the clinical manifestations of P, they are unable to prevent colonization by Bp; they therefore do not reduce the risk of transmission from a colonized subject to a healthy subject [39].
Numerous studies have shown that the levels of antibodies elicited by aP vaccines against the various Bp antigens tend to wane rapidly [40-44]. One of the first studies to investigate this issue was conducted by Esposito et al. [40]. These authors examined 38 children who had regularly received, in the first year of life, the recommended doses of a combined vaccine containing diphtheria, tetanus and hepatitis B vaccines in addition to a 3-component aP vaccine. Analysis of the antibodies against PT, FHA and PRN revealed that, 5 years after the last dose, very few subjects had adequate levels of specific antibodies against all the Bp antigens contained in the vaccine. In addition, in vitro study of the response of the peripheral mononucleated cells to exposure to these antigens revealed that a very small number of subjects tested had marked immunological memory.
The different behavior of the aP and wP vaccines can be explained, at least in part, by the fact that each elicits a substantially different immune response. The wP vaccines induce a response that is very similar, albeit less intense, to that induced by natural infection. In both cases, there is a marked production of IgG1, IgG2 and IgG3 antibodies, which is indicative of a significant Th1 response. In addition, there is a considerable Th17 response. The aP vaccines, by contrast, regardless of the number of components they contain, evoke IgG1 and IgG4 production, but elicit a scant Th17 response; this suggests that the aP vaccines induce a mixed Th1/Th2 response. These differences have been confirmed by studies of the CD4+ response. Indeed, in experimental animals, it has been shown that aP vaccines elicit CD4+ which produce large amounts of interleukin (IL)-4 and IL-5, but only a small quantity of interferon (INF)-γ, a condition that is compatible with a Th2 response [43]. By contrast, the administration of wP is associated with the production of INF-γ and IL-17, which suggests a marked Th1 response [32]. In addition, studies conducted on children have shown that the production of CD4+ cytokines indicative of a Th2 response after primary vaccination is markedly higher in aP vaccinees than in those vaccinated with wP [44].
Although the so-called immunological correlates of protection are not as yet available, all these data seem to indicate that the long-term protection induced by aP vaccines may be lower than that provided by wP, which might explain, at least in part, the reemergence of P.
The appearance of genetically modified bordetella pertussis strains
While the presence of genetically different strains of Bp had already been demonstrated in the period when only wP was in use, the phenomenon became more evident after the introduction of the aP vaccines [47-49]. Despite the lack of official data to correlate this microbiological finding with the rise in cases of P, it is nevertheless possible that the two phenomena are related. Indeed, it does not seem irrational to think that the immunological pressure exerted by the vaccine may have favored the selection of mutated strains, especially as the circulation of these strains has been seen to coincide temporally with the widespread use of aP vaccines and the degree of vaccination coverage reached in the various countries [46, 48]. Mutations of the genes that code for the proteins contained in the various aP vaccines have often been detected. In some cases, such as those of some genetic polymorphisms regarding the genes that code for PT, the variation has proved to result in the production of a greater quantity of PT, thereby determining more severe clinical manifestations in the subjects infected. In other cases, such as when one or more genes have been deleted, the practical effect has not been definitively clarified, though it has been supposed that the very lack of a gene that codes for a vaccine protein may limit vaccine efficacy [49-53].
The most significant data on this issue regard PRN. Bp strains lacking this antigen have been detected almost everywhere in the world, though with different frequency. A low incidence has been found in Finland [53], France [54], Italy [55] and Japan [56], while a high frequency has been found in Australia [57], Israel [58] and the United States [59]. In the US, 640 (85%) of the 753 Bp strains identified in 8 states from March 2011 to February 2013, a period of high incidence of P, were PRN-negative. The hypothesis that deletion of the gene coding for PRN may impair the efficacy of vaccines that contain this antigen also seems to be supported by evidence that PRN-negative Bp strains are able to cause very prolonged infections in experimental animals previously immunized with an aP vaccine containing this protein [60]. However, the problem remains open, as the data collected in the field are conflicting. Indeed, retrospective evaluations of the association between the presence of PRN-poor strains and a higher incidence of P have not always shown a positive correlation between the two variables [61].
The composition of acellular vaccines
All the combined vaccines with Bp antigens contain the PT antigen. The other components of Bp that are sometimes included are FHA, PRN and FIM types 2 and 3. The aP vaccines differ not only in terms of the characteristics of their preparation, such as formulation, combination and concentration in micrograms of the single components, but also with regard to their production modalities, such as the methods of detoxification and purification used. Thus, aP vaccines cannot be compared only on the basis of the number of antigenic components that they contain, not least because the contribution of each antigen to protection is not completely clear [62].
Certainly, the indispensable component, which is always included in all aP vaccines, is PT; this antigen is directly responsible for the development of a protective antibody response following immunization. FHA may be of lesser importance, as it is the strain that is least genetically mutated over time, unlike PRN, the mutations of which have led to the spread of pertactin-resistent strains. By contrast, with regard to the fimbriae, which are also subject to mutations, there is no evidence that they contribute to determining immune protection [19, 22, 62].
Finally, although no correlates or serological indicators of protection regarding P are available, clinical studies have shown that the aP vaccines currently utilized elicit a robust immune response, with post-vaccination antibody levels being higher than on pre-vaccination serological testing [4-10, 23, 25, 30-33, 40, 43, 44, 62].
Efficacy and effectiveness of the various acellular vaccines
Several studies have assessed the efficacy of the aP vaccines, i.e. the direct and specific efficacy of a single vaccine in preventing P in a given clinical trial [63-75]. Unfortunately, however, the possibility of obtaining results that are truly capable of revealing possible differences among the various aP vaccines is very limited; this is not only because long-term evaluations are lacking, but also because the criteria for defining P have, in many cases, been different, meaning that the types of cases enrolled have been different. Moreover, efficacy studies have directly compared the aP vaccines most commonly administered today, such as the hexavalent, pentavalent and quadrivalent vaccines, for example. In addition, given that the trend in pertussis is determined by a multiplicity of factors, such as the duration of the protection induced by vaccination, the administration of boosters in all age-groups, which is sometimes already implemented, and the risk of natural infection due to the ordinary circulation of Bp, it seems somewhat simplistic to carry out efficacy assessments alone. Rather, in order to draw up the most appropriate strategies for the control and prevention of P, it is important to obtain evidence from studies that assess true efficacy in the field, i.e. effectiveness studies that take into account the contribution of all these factors. Indeed, clinical trials are closed, isolated experiments; as such, they do not take into account such factors as the burden and epidemiology of the disease in a given geographical area, and how these vary over time; nor do they consider the actual implementation of vaccination programs in a given country, i.e. whether coverage targets have been reached, whether booster doses are administered, whether vaccination is scheduled for healthcare workers and pregnant women, and so on. Thus, only studies of effectiveness, i.e. the efficacy of vaccination in real life, can yield real-world evidence, and are therefore essential in order to help policy-makers to plan the most appropriate strategy for the control of pertussis in their own countries.
Table V reports the trials which have evaluated the efficacy of aP vaccines and the surveillance studies in which their effectiveness has been assessed.
Tab. V.
Efficacy trials and surveillance studies of the effectiveness of acellular pertussis vaccines (aPs).
| Author or Country | N. antigen components | Efficacy (%) or effectiveness (incidence) |
|---|---|---|
| Greco [7] | 3 | 84% |
| Gustafsson [8] | 2*; 5 | 59%*; 85% |
| Simondon [63] | 2 | 85% |
| Trollfors [75] | 1 | 71% |
| Sweden [65] | 1, 2, 3, 5 | From > 100 cases/100,000 to < 10 cases/100,000 residents |
| Denmark [66] | 1 | From > 100 cases/100,000 to < 10 cases/100,000 residents |
| EU/EEA countries [19] | 1, 2, 3, 5 | From > 100 cases/100,000 to < 10 cases/100,000 residents |
| Non-EU/EEA countries: USA [67], Canada [68], Japan [56] |
1, 2, 3, 5 | From > 100 cases/100,000 to < 10 cases/100,000 residents |
*; 2-component vaccine not registered and never used in national vaccination programs.
EFFICACY STUDIES
Several controlled clinical trials have evaluated the efficacy of aP vaccines in preventing pertussis, according to the definition used in the literature and that proposed by the WHO [74].
In a study by Greco et al. [7], the efficacy of the 3-component aP vaccine was 84% (95% confidence interval [CI] 75.8-89.4). In a trial conducted by Gustafsson et al. [8], in which two aPs were evaluated, the efficacy of the 5-component vaccine was 85% (95% CI 80.6-88.8), while that of the 2-component vaccine was markedly lower: 59% (95% CI 50.9-65.9). This low efficacy score was probably one of the reasons why the 2-component vaccine analyzed in this study was not subsequently registered and was therefore never used in vaccination programs. In a trial conducted by Simondon et al. [63], in which another 2-component vaccine was used, efficacy was 85% (95% CI 66-93); this result was sufficient for approval in the regulatory setting and for implementation in vaccination programs. Finally, in a trial by Trollfors et al., in which a single-component (the PT antigen) vaccine was used, efficacy proved to be 71%; this vaccine is still in use today, especially in northern Europe [65]. In sum, with the exception of the 2-component vaccine that failed to be registered owing to its low efficacy, all the other aP vaccines currently available have displayed high efficacy in the various clinical trials, and have consequently been used in national vaccination programs. These results show that the efficacy of aP vaccines does not depend on the number of Bp antigen components that they contain; rather, it is determined by other factors, which may be the formulation and production modalities and the methods of detoxification and purification.
Zhang et al. [64] conducted a review of the clinical trials (up to 2014) which had compared the efficacy of wP and aP vaccines, these latter containing from 1 to 5 components. Owing to the methodology adopted, this review also included studies involving vaccines that are no longer produced or utilized in national vaccination programs, having been replaced over the years by vaccines with better efficacy profiles. One of the trials included in the review was that of the above-mentioned 2-component vaccine that was never registered, presumably owing to the low efficacy observed [8]. By contrast, the review did not include the trial of the other 2-component vaccine [63] – which is commonly used today – as it had been conducted according to a methodology that did not fall within the inclusion criteria set by the review.
Moreover, as direct efficacy studies of the aP vaccines commonly used today, such as hexavalent and pentavalent vaccines for pediatric vaccinations, are not available, indirect comparisons have been made on the basis of clinical trials of combined vaccines, for both pediatric and adult use, with different valences, giving rise to a heterogeneity bias. A further bias stems from the methodology used in the clinical trials, particularly with regard to the clinical case definition of pertussis. These limitations and biases were identified and described by the authors of the above-mentioned review. Nevertheless, the conclusions regarding the possible differences in efficacy among aP vaccines containing different numbers of antigens, and the differences between these vaccines and wP vaccines, are inevitably distorted.
EFFECTIVENESS STUDIES
Various national epidemiological surveillance studies have evaluated the effectiveness of anti-P vaccination through the analysis of actual experience in the field. In general, all the currently available 1-, 2-, 3- and 5-component aP vaccines have displayed high effectiveness, yielding a marked reduction in the incidence of pertussis, thanks also to the elevated coverage rates achieved. For example, national epidemiological surveillance in Sweden, which began in the year when aP vaccines were introduced (1996), has shown that these vaccines have great effectiveness; indeed, the incidence of P fell from its pre-1996 level of over 100 cases/100,000 residents to below 10 cases/100,000 residents in the period between 2010 and the last measurement in 2016. Moreover, in an analysis stratified by region and type of vaccine used (1-, 2-, 3- and 5-component), no differences in incidence rates emerged; this indicates that P is effectively under control throughout the nation, regardless of the type of aP vaccine used. Notably, from 1996 to today, the primary vaccination coverage rates in infants (3 doses) have always been 97-98%, i.e. above the 95% target for this age-group [65].
In Denmark, a single-component (PT) aP vaccine has been used since 1995; as revealed by national surveillance, pertussis is well controlled. Indeed, up to the last survey in 2013, the incidence of P always remained below 10 cases/100,000 residents, with the exception of 2002, when an epidemic outbreak raised the incidence to 36 cases/100,000. In this case, too, primary vaccination coverage in infants (3 doses) remained particularly high: 90-99% in the period 1995-2005, 58-91% in the period 2006-2013 [66]. In the period 2013-2016 the pertussis incidence in Denmark was 10.8 cases/100,000 inhabitants [19].
In general, as described in the latest ECDC report, in 2015 the incidence of P in the EU/EEA countries was 9 cases/100,000, a similar value to the preceding years. As expected, it emerged from the various national reports that the incidence was higher among children aged < 1 year: 73.1 cases/100,000; 85% of these cases involved infants below 6 months of age. Precisely on account of this latter finding, the ECDC has recommended that the EU/EEA countries increase their commitment to offering vaccination to pregnant women, since an infant up to the age of 6 months has not yet developed the immunity induced by primary vaccination [19].
Surveillance programs conducted in some non-EU/EEA countries, such as the USA [67], Canada [68] and Japan [56], have also revealed the great effectiveness of aP vaccines in preventing and controlling pertussis, with the incidence of the disease declining to less than 10 cases/100,000, regardless of which aP vaccine is used.
Thus, the WHO’s latest position paper on pertussis vaccines states that possible differences among aP vaccines in terms of efficacy, as reported by some trials and reviews, should be interpreted with caution, in that such findings are contradicted by national surveillance programs and studies of real-world evidence, in which all aP vaccines have displayed great effectiveness in preventing and controlling pertussis [69].
Strategy for preventing and controlling pertussis (p)
Immunity to P, whether conferred by vaccination or by natural infection, wanes over time; the need to administer booster vaccine doses is therefore essential. Indeed, although the clinical manifestations of the disease become less severe with age, an infected individual may infect infants in their first months of life, when the disease is clinically more serious.
It has been amply demonstrated that the prevention and control of pertussis depend on the achievement of high vaccination coverage rates in the whole population through the adoption of a vaccination calendar with at least the following features [62, 70, 76]:
scheduled primary vaccination for infants, and administration of a booster dose at pediatric concentration in preschool children;
a booster dose in adolescents and adults (aged 20 years or more) to be repeated every 10 years, with reduced-concentration vaccines in adults.
The main positions and recommendations of official authorities and international experts are described in Table VI [18, 62, 69, 70, 74, 77].
Tab. VI.
Positions and recommendations of official authorities and international experts on pertussis vaccinations.
| WHO, Pertussis vaccines position paper [76] | Surveillance studies in countries where aP vaccines (including 1- and 2-component aPs) are used, have shown high levels of effectiveness in preventing pertussis. All aP vaccines have displayed high levels of effectiveness in preventing pertussis, regardless of the number of Bp antigens they contain. |
| WHO, Pertussis vaccines position paper [69] | Differences in efficacy among aP vaccines reported by trials and reviews must be interpreted with caution, as all aP vaccines have shown high effectiveness in national surveillance programs and studies of real-world evidence |
| WHO SAGE Working Group [18] | There is insufficient evidence to conclude that one type of aP vaccine is superior to another. However, the available data underline the importance of reaching and maintaining high coverage rates and of implementing appropriate vaccination schedules. |
| CDC Pink Book [77] | The efficacy of the various aP vaccines varies from 80 to 85%, while their respective confidence intervals overlap, suggesting that no aP vaccine is significantly more efficacious than the others. |
| Martinon-Torres [70] | To achieve high coverage rates in the population, it is necessary to implement a vaccination strategy that includes both the primary vaccination of infants and booster administration in preschool children, adolescents and adults, with particular emphasis on the vaccination of pregnant women. |
| Gabutti [62] | It is important to bear in mind that the use of the current polyvalent aP vaccines has enabled high coverage rates to be achieved and maintained, regardless of vaccine type and the number of Bp components it contains, which is the key factor in successful intervention against pertussis. |
| Zhang [74] | Reviewing clinical efficacy trials alone may yield a limited picture of the benefits of aP vaccines, not least because of differences among the trials themselves and possible biases. |
In addition, recent studies have highlighted the importance of vaccinating specific groups, such as healthcare workers and pregnant women. Regarding this latter group, vaccination with an adult-formulation vaccine in the 3rd trimester of pregnancy confers the protection of the mother’s antibodies upon the newborn in the first months of life, i.e. before primary vaccination – a period when infection by Bp may be extremely serious. These studies have revealed an effectiveness of over 90%, which means that more than 9 out of 10 cases of pertussis in infants in the first months of life could be avoided if women were vaccinated in the 3rd trimester of pregnancy [71, 72].
Several countries have already drawn up official recommendations that include the above-mentioned interventions. Nevertheless, it is necessary to strengthen the commitment to implement initiatives aimed at raising public awareness and promoting “vaccine confidence” in the population, in order to achieve full adherence to the recommendations and to reach vaccination coverage targets. Italy, for example, has drawn up the National Vaccination Prevention Plan 2017-2019 (PNPV) [73], which not only envisions both the primary vaccination of infants against pertussis and the administration of periodic booster doses at all ages, but also targets specific groups for vaccination, such as healthcare workers and pregnant women. However, some highly sensitive coverage objectives, such as the vaccination of pregnant women, are still far from being reached, and in some regions are not even actively promoted. By contrast, the vaccination coverage of children of preschool age, has either reached or is close to reaching the 95% objective set by the PNPV for this age-group.
Conclusions
On the basis of the clinical trials, effectiveness studies and real-world evidence reported in the literature, the following conclusions may be drawn with regard to all the currently available aP vaccines, regardless of the number of Bp components they contain:
they have proved to be highly safe and immunogenic, eliciting a robust antibody response in vaccines, though the correlates of sero-protection against P are not known;
their efficacy has been demonstrated in clinical trials, i.e. closed experimental studies;
their effectiveness has been demonstrated by epidemiological surveillance and studies of real-world evidence, i.e. actual experience in the field;
in countries where wP vaccines have been replaced by aP vaccines, the control of P has proved highly effective;
strategies for the control and prevention of P must involve the achievement and maintenance of high vaccination coverage rates in the entire population.
Nevertheless, recent evidence has revealed some possible limitations of the currently available aP vaccines, regardless of the number of Bp components they contain:
the fact that the duration of protection is suboptimal means that Bp carriage cannot be eliminated;
there is a higher risk of the onset of P in a non-negligible number of subjects, including those who were last vaccinated only a few years earlier;
booster doses should probably be more numerous and more closely spaced than is currently the case;
the protection elicited is not associated to a Th1/Th17 response, which is probably necessary in order to achieve more efficacious protection;
Bp has developed vaccine resistance as a result of several antigenic mutations.
It is to be hoped that future developments will result in the production of pertussis vaccines whose antigenic formulation also takes into account Bp mutations and the possibility of eliciting a Th1/Th17 immune response. In the meantime, however, the results of effectiveness studies and real-world evidence must be taken into account, in order to work out the most suitable vaccination strategies for each specific epidemiological and geographical context.
In general, in countries where aP vaccines are used, as those in Europe but not only, the following interventions and objectives for the prevention of P should be recommended:
the achievement and maintenance of high vaccination coverage rates at all ages;
primary vaccination of infants in the first year of life: a schedule beginning at 2 months and using combined vaccines, such as hexavalent vaccines, in order to exploit the synergic effect and achieve important results in the prevention of other diseases, too;
administration of booster doses in childhood at 5-6 years of age, in adolescence at 12-18 years and in adulthood every 10 years throughout life, and therefore also in the elderly;
increasing coverage among healthcare workers;
anti-pertussis vaccination during pregnancy, in order to protect the newborn during the first months of life; this would close the gap which exists in many countries between the official recommendations and actual implementation.
Acknowledgements
The author thanks Dr. Bernard Patrick for revising the manuscript.
Funding sources: this research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Conflict of interest statement
Susanna Esposito received research grants and honoraria for participation in Advisory Boards from GSK, Merck and Sanofi.
Authors' contributions
SE conceived, designed and wrote this manuscript.
References
- [1].Centers for Disease Control and Prevention. Pertussis (whooping cough). Sign and symptoms. Available at: www.cdc.gov/pertussis/about/signs-symptoms.html. Accessed on 2018.
- [2].Cherry JD, Brunnel P, Golden G. Report of the Task Force on pertussis and pertussis immunization. Pediatrics 1988;81(Suppl): 933-84. [Google Scholar]
- [3].Cody CL, Baraff LJ, Cherry JD, Marcy SM, Manclark CR. Nature and rates of adverse reactions associated with DTP and DT immunizations in infants and children. Pediatrics 1981;68:650-60. [PubMed] [Google Scholar]
- [4].Jefferson T, Rudin M, DiPietrantonj C. Systematic review of the effects of pertussis vaccines in children. Vaccine 2003;21:2003-14. [DOI] [PubMed] [Google Scholar]
- [5].Trollfors B, Taranger J, Lagergård T, Lind L, Sundh V, Zackrisson G. A placebo-controlled trial of a pertussis-toxoid vaccine. N Engl J Med 1995; 333:1045-50. [DOI] [PubMed] [Google Scholar]
- [6].Storsaeter J, Hallander H, Farrington CP, Olin P, Möllby R, Miller E. Secondary analyses of the efficacy of two acellular pertussis vaccines evaluated in a Swedish phase III trial. Vaccine 1990;8:457-61. [DOI] [PubMed] [Google Scholar]
- [7].Greco D, Salmaso S, Mastrantonio P, Giuliano M, Tozzi AE, Anemona A. A controlled trial of two acellular vaccines and one whole-cell vaccine against pertussis. N Engl J Med 1996;334:341-8. [DOI] [PubMed] [Google Scholar]
- [8].Gustafsson L, Hallander HO, Olin P, Reizenstein E, Storsaeter J. A controlled trial of a two-component acellular, a five-component acellular, and a whole-cell pertussis vaccine. N Engl J Med 1996;334:349-55. [DOI] [PubMed] [Google Scholar]
- [9].Edwards KM, Decker MD. Acellular pertussis vaccines for infants. N Engl J Med 1996;334:391-2. [DOI] [PubMed] [Google Scholar]
- [10].Hallander HO, Gustafsson L. Efficacy and effectiveness of acellular pertussis vaccines: a 20-year Swedish experience. Expert Rev Vaccines 2009;8:1303-7. [DOI] [PubMed] [Google Scholar]
- [11].Centers for Disease Control and Prevention. Vax view. Available at: www.cdc.gov/vaccines/vaxview/index.html. Accessed on 2018.
- [12].European Centre for Disease Prevention and Control. Pertussis - Annual Epidemiological Report 2016]. Available at: https://ecdc.europa.eu/en/publications-data/pertussis-annual-epidemiological-report-2016-2014-data. Accessed on 2018. [PubMed]
- [13].Doyle TJ, Glynn MK, Groseclose SL. Completeness of notifiable infectious disease reporting in the United States: an analytical literature review. Am J Epidemiol 2002;155:866-74. [DOI] [PubMed] [Google Scholar]
- [14].Cherry JD. Pertussis: challenges today and for the future. PLoS Pathog 2013;9:e1003418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].van der Zee A, Schellekens JF, Mooi FR. Laboratory diagnosis of pertussis. Clin Microbiol Rev 2015;28:1005-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kaczmarek MC, Valenti L, Kelly HA, Ware RS, Britt HC. Sevenfold rise in likelihood of pertussis test requests in a stable set of Australian general practice encounters, 2000-2011. Med J Aust 2013;198:624-8. [DOI] [PubMed] [Google Scholar]
- [17].Litt DJ, Samuel D, Duncan J, Harnden A, George RC, Harrison TG. Detection of anti-pertussis toxin IgG in oral fluids for use in diagnosis and surveillance of Bordetella pertussis infection in children and young adults. J Med Microbiol 2006;55:1223-8. [DOI] [PubMed] [Google Scholar]
- [18].World Health Organization. WHO SAGE pertussis working group. Background paper, SAGE; April 2014. Available at: www.who.int/immunization/sage/meetings/2014/april/1_Pertussis_background_FINAL4_web.pdf. Accessed on 2018. [Google Scholar]
- [19].European Centre for Disease Prevention and Control. Pertussis, annual epidemiological report for 2015. Available at: https://ecdc.europa.eu/sites/portal/files/documents/AER_for_2015-pertussis.pdf. Accessed on 2018. [PubMed]
- [20].Epicentro. “I vaccini? Funzionano!”. Settimana europea e mondiale delle vaccinazioni 2017. Available at: www.epicentro.iss.it/temi/vaccinazioni/SettimanaVaccinazioni2017.asp. Accessed on 2018.
- [21].Fedele G, Carollo M, Palazzo R. Parents as source of pertussis transmission in hospitalized young infants. Infection 2017;45:171-8. [DOI] [PubMed] [Google Scholar]
- [22].Gabutti G, Rota MC. Pertussis: a review of disease epidemiology worldwide and in Italy. Int J Environ Res Public Health 2012;9:4626-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wendelboe AM, Van Rie A, Salmaso S, Englund JA. Duration of immunity against pertussis after natural infection or vaccination. Pediatr Infect Dis J 2005;24(Suppl 5):S58-61. [DOI] [PubMed] [Google Scholar]
- [24].Centers for Disease Control and Prevention. Pertussis outbreaks - Massachusetts and Maryland, 1992. MMWR 1993;42:197-200. [PubMed] [Google Scholar]
- [25].Ramsay ME, Farrington CP, Miller E. Age-specific efficacy of pertussis vaccine during epidemic and non-epidemic periods. Epidemiol Infect 1993;111:41-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nielsen A, Larsen SO. Epidemiology of pertussis in Denmark: the impact of herd immunity. Int J Epidemiol 1994;23:1300-8. [DOI] [PubMed] [Google Scholar]
- [27].He Q, Schmidt-Schlapfer G, Just M. Impact of polymerase chain reaction on clinical pertussis research: Finnish and Swiss experiences. J Infect Dis 1996;174:1288-95. [DOI] [PubMed] [Google Scholar]
- [28].Van Buynder PG, Owen D, Vurdien JE, Andrews NJ, Matthews RC, Miller E. Bordetella pertussis surveillance in England and Wales: 1995-7. Epidemiol Infect 1999;123:403-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Torvaldsen S, McIntyre PB. Effect of the preschool pertussis booster on national notifications of disease in Australia. Pediatr Infect Dis J 2003;22:956-9. [DOI] [PubMed] [Google Scholar]
- [30].Simondon F, Preziosi MP, Yam A. Randomized double-blind trial comparing a two-component acellular to a whole-cell pertussis vaccine in Senegal. Vaccine 1997;15:1606-12. [DOI] [PubMed] [Google Scholar]
- [31].Tindberg Y, Blennow M, Granstrom M. Ten-year follow-up after immunization with a two-component acellular pertussis vaccine. Pediatr Infect Dis J 1999;18:361-5. [DOI] [PubMed] [Google Scholar]
- [32].Salmaso S, Mastrantonio P, Tozzi AE. Sustained efficacy during the first 6 years of life of 3-component acellular pertussis vaccines administered in infancy: the Italian experience. Pediatrics 2001;108:E81. [DOI] [PubMed] [Google Scholar]
- [33].Lugauer S, Heininger U, Cherry JD, Stehr K. Long-term clinical effectiveness of an acellular pertussis component vaccine and a whole-cell pertussis component vaccine. Eur J Pediatr 2002;161:142-6 [DOI] [PubMed] [Google Scholar]
- [34].Wirsing von Ko¨nig CH, Postels-Multani S, Bock HL, Schmitt HJ. Pertussis in adults: frequency of transmission after household exposure. Lancet 1995;346:1326-9. [DOI] [PubMed] [Google Scholar]
- [35].Miller E, Gay NJ. Epidemiological determinants of pertussis. Dev Biol Stand 1997;89:15-23. [PubMed] [Google Scholar]
- [36].Versteegh FG, Schellekens JF, Nagelkerke AF, Roord JJ. Laboratory-confirmed reinfections with Bordetella pertussis. Acta Paediatr 2002;91:95-7. [DOI] [PubMed] [Google Scholar]
- [37].Misegades LK, Winter K, Harriman K, Talarico J, Messonnier NE, Clark TA. Association of childhood pertussis with receipt of 5 doses of pertussis vaccine by time since last vaccine dose, California, 2010. JAMA 2012;308:2126-32. [DOI] [PubMed] [Google Scholar]
- [38].Principi N, Litt D, Terranova L, Picca M, Malvaso C, Vitale C. Pertussis-associated persistent cough in previously vaccinated children. J Med Microbiol 2017;66:1699-702. [DOI] [PubMed] [Google Scholar]
- [39].Warfel JM, Zimmerman LI, Merkel TJ. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc Natl Acad Sci USA 2014;111:787-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Esposito S, Agliardi T, Giammanco A, Faldella G, Cascio A, Bosis S. Longterm pertussis-specific immunity after primary vaccination with a combined diphtheria, tetanus, tricomponent acellular pertussis, and hepatitis B vaccine in comparison with that after natural infection. Infect Immun 2001;69:4516e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Witt MA, Katz PH, Witt DJ. Unexpectedly limited durability of immunity following acellular pertussis vaccination in preadolescents in a North American outbreak. Clin Infect Dis 2012;54:1730-5. [DOI] [PubMed] [Google Scholar]
- [42].Weston W, Messier M, Friedland LR, Wu X, Howe B. Persistence of antibodies 3 years after booster vaccination of adults with combined acellular pertussis, diphtheria and tetanus toxoids vaccine. Vaccine 2011;29:8483-6. [DOI] [PubMed] [Google Scholar]
- [43].Tartof SY, Lewis M, Kenyon C, White K, Osborn A, Liko J, Zell E, Martin S, Messonnier NE, Clark TA. Waning immunity to pertussis following 5 doses of DTaP. Pediatrics 2013;131:e1047-52. [DOI] [PubMed] [Google Scholar]
- [44].Klein NP, Bartlett J, Rowhani-Rahbar A, Fireman B, Baxter R. Waning protection after fifth dose of acellular pertussis vaccine in children. N Engl J Med 2012;367:1012-9. [DOI] [PubMed] [Google Scholar]
- [45].Ross PJ, Sutton CE, Higgins S, Allen AC, Walsh K, Misiak A. Relative contribution of Th1 and Th17 cells in adaptive immunity to Bordetella pertussis: towards the rational design of an improved acellular pertussis vaccine. PLoS Pathog 2013;9:e1003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Raeven RH, van der Maas L, Tilstra W, Uittenbogaard JP, Bindels TH, Kuipers B. Immunoproteomic Profiling of Bordetella pertussis Outer Membrane Vesicle Vaccine Reveals Broad and Balanced Humoral Immunogenicity. J Proteome Res 2015;14:2929-42. [DOI] [PubMed] [Google Scholar]
- [47].Hendrikx LH, Schure RM, Oztürk K, de Rond LG, de Greeff SC, Sanders EA. Different IgG-subclass distributions after whole-cell and acellular pertussis infant primary vaccinations in healthy and pertussis infected children. Vaccine 2011;29:6874-80. [DOI] [PubMed] [Google Scholar]
- [48].Brummelman J, Helm K, Hamstra HJ, van der Ley P, Boog CJ, Han WG. Modulation of the CD4(+) T cell response after acellular pertussis vaccination in the presence of TLR4 ligation. Vaccine 2015;33:1483-91. [DOI] [PubMed] [Google Scholar]
- [49].Tubo NJ, Jenkins MK. TCR signal quantity and quality in CD4+ T cell differentiation. Trends Immunol 2014;35:591-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Nicoli EJ, Ayabina D, Trotter CL, Turner KM, Colijn C. Competition, coinfection and strain replacement in models of Bordetella pertussis. Theor Popul Biol 2015;103:84-92. [DOI] [PubMed] [Google Scholar]
- [51].Advani A, Gustafsson L, Ahren C, Mooi FR, Hallander HO. Appearance of Fim3 and ptxP3-Bordetella pertussis strains, in two regions of Sweden with different vaccination programs. Vaccine 2011;29:3438-42. [DOI] [PubMed] [Google Scholar]
- [52].de Gouw D, Hermans PW, Bootsma HJ, Zomer A, Heuvelman K, Diavatopoulos DA, Mooi FR. Differentially expressed genes in Bordetella pertussis strains belonging to a lineage which recently spread globally. PLoS One 2014;9:e84523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Barkoff AM, Mertsola J, Guillot S, Guiso N, Berbers G, He Q. Appearance of Bordetella pertussis strains not expressing the vaccine antigen pertactin in Finland. Clin Vaccine Immunol 2012;19:1703-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Bouchez V, Brun D, Cantinelli T, Dore G, Njamkepo E, Guiso N. First report and detailed characterization of B. pertussis isolates not expressing pertussis toxin or pertactin. Vaccine 2009;27:6034-419. [DOI] [PubMed] [Google Scholar]
- [55].Mastrantonio P, Spigaglia P, van Oirschot H, van der Heide HG, Heuvelman K, Stefanelli P. Antigenic variants in Bordetella pertussis strains isolated from vaccinated and unvaccinated children. Microbiology 1999;145:2069-75. [DOI] [PubMed] [Google Scholar]
- [56].Otsuka N, Han HJ, Toyoizumi-Ajisaka H, Nakamura Y, Arakawa Y, Shibayama K. Prevalence and genetic characterization of pertactin-deficient Bordetella pertussis in Japan. PLoS One 2012;7:e31985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lam C, Octavia S, Ricafort L. Rapid increase in pertactin-deficient Bordetella pertussis isolates, Australia. Emerg Infect Dis 2014; 20: 626–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Bamberger E, Abu Raya B, Cohen L, Golan-Shany O, Davidson S, Geffen Y. Pertussis resurgence associated with pertactin-deficient and genetically divergent bordetella pertussis isolates in Israel. Pediatr Infect Dis J 2015;34:898-900. [DOI] [PubMed] [Google Scholar]
- [59].Martin SW, Pawloski L, Williams M, Weening K, DeBolt C, Qin X. Pertactin-negative Bordetella pertussis strains: evidence for a possible selective advantage. Clin Infect Dis 2015;60:223-7. [DOI] [PubMed] [Google Scholar]
- [60].Hegerle N, Dore G, Guiso N. Pertactin deficient Bordetella pertussis present a better fitness in mice immunized with an acellular pertussis vaccine. Vaccine 2014;32:6597-600. [DOI] [PubMed] [Google Scholar]
- [61].Breakwell L, Kelso P, Finley C, Schoenfeld S, Goode B, Misegades LK. Pertussis vaccine effectiveness in the setting of pertactin-deficient pertussis. Pediatrics 2016;137(5).pii:e20153973. [DOI] [PubMed] [Google Scholar]
- [62].Gabutti G, Azzari C, Bonanni P. Pertussis, current perspectives on epidemiology and prevention. Hum Vaccin Immunother 2015;11:108-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Simondon F, Preziosi MP, Yam A, Kane CT, Chabirand L, Iteman I, Sanden G, Mboup S, Hoffenbach A, Knudsen K, Guiso N, Wassilak S, Cadoz M. A randomized double-blind trial comparing a two-component acellular to a whole-cell pertussis vaccine in Senegal. Vaccine 1997;15:1606-12. [DOI] [PubMed] [Google Scholar]
- [64].Zhang L, Prietsch SOM, Axelsson I, Halperin SA. Acellular vaccines for preventing whooping cough in children. Cochrane Database Syst Rev 2014;9:CD001478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Pertussis surveillance in Sweden. Nineteen-year report. Available at: www.folkhalsomyndigheten.se/contentassets/65ed8f6dbdab4999bc358fcd9b657e77/pertussis-sweden-nineteen-year-report.pdf. Accessed on 2018.
- [66].Dalby T, Andersen PH, Hoffmann S. Epidemiology of pertussis in Denmark, 1995 to 2013. Eurosurveillance 2016;21(36):30334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Skoff TH, Baumbach J, Cieslak PR. Tracking pertussis and evaluating control measures through enhanced pertussis surveillance, emerging infections program, United States. Emerg Infect Dis 2015;21(9):1568-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Smith T, Rotondo J, Desai S, Deehan H. Pertussis surveillance in Canada: trends to 2012. Can Commun Dis Rep 2014;40(3):21-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].WHO. Pertussis vaccines: WHO position paper. Wkly Epidemiol Rec 2015;50(35):433-60. Available at: www.who.int/wer/2015/wer9035.pdf?ua=1. Accessed on 2018. [PubMed] [Google Scholar]
- [70].Martinón-Torres F, Heininger U, Thomson A, Wirsing von König CH. Controlling pertussis: how can we do it? A focus on immunization. Expert Rev Vaccines 2018;17(4):289-97. [DOI] [PubMed] [Google Scholar]
- [71].Skoff TH, Blain AE, Watt J. Impact of the US maternal tetanus, diphtheria, and acellular pertussis vaccination program on preventing pertussis in infants < 2 months of age: a case-control evaluation. Clin Infect Dis 2017;65(12):1977-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Amirthalingam G, Campbell H, Ribeiro S. Sustained effectiveness of the maternal pertussis immunization program in England 3 years following introduction. Clin Infect Dis 2016;63(Suppl 4):S236-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Ministero della Salute. Piano Nazionale di Prevenzione Vaccinale 2017-2019. [Google Scholar]
- [74].WHO meeting on case definition of pertussis: Geneva, 10-11 January 1991. Geneva: World Health Organization; 1991. pp. 4-5. [Google Scholar]
- [75].Trollfors B, Taranger J, Lagergard T. A placebo-controlled trial of a pertussis-toxoid vaccine N Engl J Med 1995;333:1045-50. [DOI] [PubMed] [Google Scholar]
- [76].WHO. Pertussis vaccines position paper 2010. Wkly Epidemiol Rec 2010;40(85):385-400. Available at: www.who.int/wer/2010/wer8540.pdf?ua=1. Accessed on 2018. [PubMed] [Google Scholar]
- [77].Liang JL, Tiwari T, Moro P, Messonnier NE, Reingold A, Sawyer M, Clark TA. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2018;67:1-44. [DOI] [PMC free article] [PubMed] [Google Scholar]


