Summary
Certain pathogenic bacteria and yeast form biofilms on biotic and abiotic surfaces including medical devices and implants. Hence, the development of antibiofilm coating materials becomes relevant. The virulence of those colonizing pathogens can be reduced by inhibiting biofilm formation rather than killing pathogens using excessive amounts of antimicrobials, which is touted as one of the main reasons for the development of drug resistance. Candida albicans is an opportunistic fungal pathogen, and the transition of yeast cells to hyphal cells is believed to be a crucial virulence factor. Previous studies have shown that indole and its derivatives possess antivirulence properties against various bacterial pathogens. In this study, we used various indole derivatives to investigate biofilm‐inhibiting activity against C. albicans. Our study revealed that 7‐benzyloxyindole, 4‐fluoroindole and 5‐iodoindole effectively inhibited biofilm formation compared to the antifungal agent fluconazole. Particularly, 7‐benzyloxyindole at 0.02 mM (4.5 μg ml−1) significantly reduced C. albicans biofilm formation, but had no effect on planktonic cells, and this finding was confirmed by a 2,3‐bis‐(2‐methoxy‐4‐nitro‐5‐sulfophenyl)‐2H‐tetrazolium‐5‐carboxanilide (XTT) assay and three‐dimensional confocal laser scanning microscopy. Scanning electron microscopy analyses revealed that 7‐benzyloxyindole effectively inhibited hyphal formation, which explains biofilm inhibition. Transcriptomic analysis showed that 7‐benzyloxyindole downregulated the expressions of several hypha/biofilm‐related genes (ALS3,ECE1,HWP1 and RBT1). A C. albicans‐infected Caenorhabditis elegans model system was used to confirm the antivirulence efficacy of 7‐benzyloxyindole.
Introduction
Biofilms are microbial cells interwoven in an extracellular polymeric matrix that attach to abiotic and biotic surfaces. Pathogenic bacteria and fungi are protected by this three‐dimensional matrix, which confers them with high tolerance to antimicrobials. (Costerton et al., 1999; Davey and O'Toole G, 2000). Candida albicans is an opportunistic fungal pathogen and causes systemic infections predominantly by contaminating implant devices such as pacemakers, endotracheal tubes, contact lenses, penile implants, intrauterine devices and catheters (Ramage et al., 2005; Sardi et al., 2013). Candida albicans biofilms contain cells in three development stages viz. yeast, pseudohyphae and hyphae. This colony dimorphism in Candida appears to regulate the maturation of biofilms and hyphal transition, the latter of which is considered a crucial virulence factor in Candida infections (Carradori et al., 2016). Hyphal formation in matured biofilms contains high densities of cells in a protected environment, which increases resistance to administered antimicrobials (Williams and Lewis, 2011). Owing to these properties, C. albicans biofilms are thought to be more strongly associated with the emergence of drug resistance than planktonic cells. Commercial antifungals for the treatment of candidiasis are limited to several azoles and polyenes (Tobudic et al., 2010; Taff et al., 2013; Sandai et al., 2016), and thus, small molecule novel antifungal agents are urgently required to prevent C. albicans biofilm formation.
Various studies have demonstrated that extracellular signalling molecules produced by bacteria can mediate quorum sensing (QS), and that QS molecules produced by one organism can modulate the community behaviour of host organisms as well as other organisms. These signalling molecules also direct the transcriptomic outcomes of bacterial genes associated with virulence and adaptive tolerance (Peleg et al. 2010). Several Gram‐positive and Gram‐negative bacteria synthesize indoles as intracellular signalling molecules to control the virulence of pathogenic bacteria, such as, Pseudomonas aeruginosa and enterohaemorrhagic E. coli O157:H7 (Lee et al., 2007, 2011). In Pseudomonas putida, signalling molecules such as indole enhances TtgGHI efflux pump that are relevant for antibiotic resistance (Molina‐Santiago et al., 2014). The previous studies have reported that indole inhibits biofilm formation and suppresses the virulence of bacterial strains such as Staphylococcus aureus, Agrobacterium tumefaciens (Lee and Lee, 2010; Lee et al., 2013, 2015a,b; Lee et al., 2016) and Vibrio cholera (Mueller et al., 2009). Likewise, indole derivatives such as 7‐fluoroindole, 7‐hydroxyindole, 3‐indolyl acetonitrile and 2‐aminobenzimidazoles have been reported to exhibit antimicrobial activities against pathogenic bacteria (Lee et al., 2009, 2011, 2012, 2015a,b; Frei et al., 2012).
Like bacteria, fungi such as Aspergillus sp. and Penicillium sp., produce indole derivatives that have been reported to inhibit C. albicans biofilm formation and hyphal development (Wang et al., 2012; You et al., 2013). Although relatively few reports are available to conclude, indole and indole‐3‐acetonitrile have been shown to suppress biofilm maturation by C. albicans (Jayant et al., 2012; Oh et al., 2012).
In this study, crystal violet and XTT (2,3‐bis(2‐methoxy‐4‐nitro‐5‐sulfo‐phenyl)‐2H‐tetrazolium‐5‐carboxanilide) reduction assays showed efficiency of 7‐benzyloxyindole on biofilm formation by C. albicans. Cell morphology and phenotypic switching of C. albicans biofilm cells were observed by scanning electron microscopy (SEM), and biofilm thicknesses were measured by confocal laser scanning microscopy (CLSM). In addition, transcriptomic studies were performed to determine the antibiofilm and antihyphal effects of 7‐benzyloxyindole in C. albicans. Finally, the effects of 7‐benzyloxyindole were investigated in a C. albicans‐infected Caenorhabditis elegans (a nematode) model.
Results
Effects of indole derivatives on C. albicans biofilm formation
Initially, we investigate whether indole derivatives affect biofilm formation by fluconazole‐resistant C. albicans DAY185 (Manoharan et al., 2017a,b), and cell growth was simultaneously measured in the presence of indole derivatives. Of the 34 commercially available indole derivatives examined, 7‐benzyloxyindole, 4‐fluoroindole and 5‐iodoindole significantly reduced biofilm formation at concentrations of 0.1 and 0.5 mM (Table S1). In particular, 7‐benzyloxyindole and 4‐fluoroindole significantly inhibited biofilm formation in a dose‐dependent manner (Fig. 1). More specifically, 7‐benzyloxyindole significantly inhibited biofilm formation by 63%, 81% and 94% at concentrations of 0.02, 0.05 and 0.1 mM, respectively (Fig. 1A). The commercial antifungal fluconazole (positive control) significantly reduced biofilm formation by 74% at a concentration of 0.1 mM (Fig. 1D). In addition, 4‐fluoroindole and 5‐iodoindole demonstrated wide range inhibition of the growth of C. albicans at the planktonic cell stage (Figs. 1B and C). Interestingly, planktonic cell growth was not affected by 7‐benzyloxyindole at a concentration of 0.1 mM (Fig. 1A), and minimum inhibitory concentrations (MIC) exhibited up to 2 mM against C. albicans. Thus, confirming biofilm inhibition by 7‐benzyloxyindole was due to its antibiofilm activity rather than its antimicrobial activity.
Figure 1.
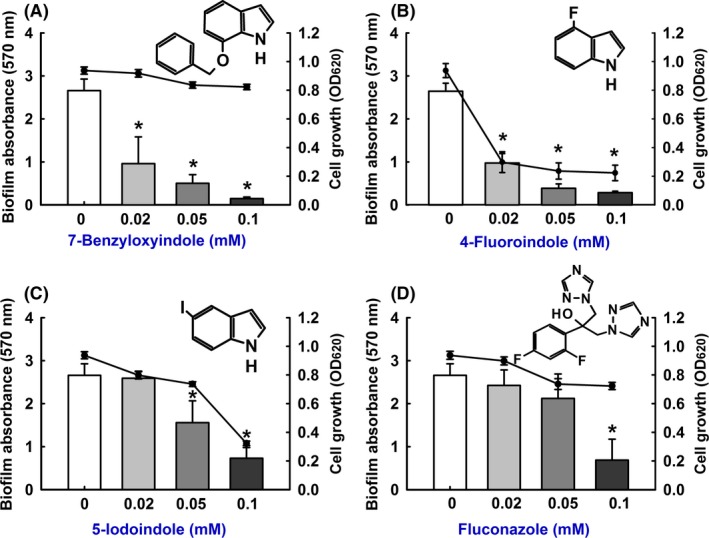
Inhibition of biofilm formation by indole derivatives. The antibiofilm activities of 7‐benzyloxyindole (A), 4‐fluoroindole (B), 5‐iodoindole (C) and fluconazole (D) against C. albicans DAY185 were determined after culturing for 24 h. Two independent experiments were conducted (six wells per sample); error bars indicate standard deviations. *P < 0.05 vs. non‐treated controls. Bars indicate biofilm formation and lines indicate planktonic cell growth.
Effects of 7‐benzyloxyindole on C. albicans metabolic activity
Colorimetric assays are valuable for quantifying the viabilities of eukaryotic cells, and it has been suggested the XTT assay is useful to study fungal biofilm formation and drug resistance (Chandra et al., 2001; Kuhn et al., 2002). Findings from our XTT assay showed that metabolic activity of biofilm and planktonic C. albicans cells was not affected after 7‐benzyloxyindole treatment at 0.02 and 0.05 mM (Fig. 2). As expected, biofilm cell viabilities were significantly reduced by 88% and 96% by 7‐benzyloxyindole at 0.1 or 0.5 mM, respectively. However, planktonic cell viabilities were only slightly affected by 7‐benzyloxyindole at these concentrations (Fig. 2A).
Figure 2.
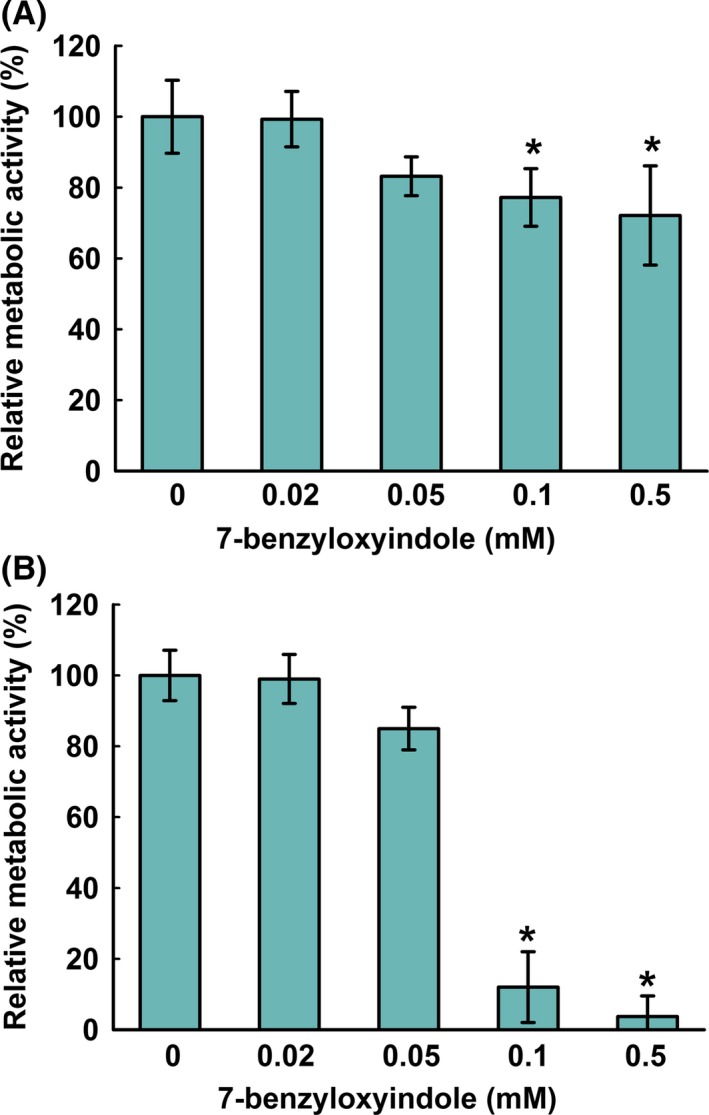
Metabolic activity of 7‐benzyloxyindole against C. albicans. The metabolic activities of C. albicans planktonic cells (A) and biofilms (B) were quantified using an XTT assay in the presence of 7‐benzyloxyindole after incubation for 24 h. Results are presented as mean percentages of metabolic activity versus non‐treated controls. Two independent experiments were conducted (six wells per sample); error bars indicate standard deviations. None indicates non‐treated samples. *P < 0.05 vs. non‐treated controls.
7‐Benzyloxyindole affected C. albicans morphology
To examine the inhibitory effect of 7‐benzyloxyindole on C. albicans morphology, visual microscopy, SEM and CLSM were performed. Initially, the effect of 7‐benzyloxyindole on C. albicans hyphal growth on solid media was examined by cultivating fungal cell colony on PDA agar plate at 37°C. While filament formation on untreated colony was observed after 6 days of incubation, 0.1 mM of 7‐benzyloxyindole was adequate to inhibit filamentation for 10 days (Fig. 3A). Also, SEM analysis showed that 7‐benzyloxyindole was found to substantially suppress hyphal growth in biofilms at concentrations of 0.02 and 0.1 mM on nylon membranes (Fig. 3B), and at 0.02 mM inhibited hyphal cells, which led to an accumulation of pseudohyphae and yeast cells.
Figure 3.
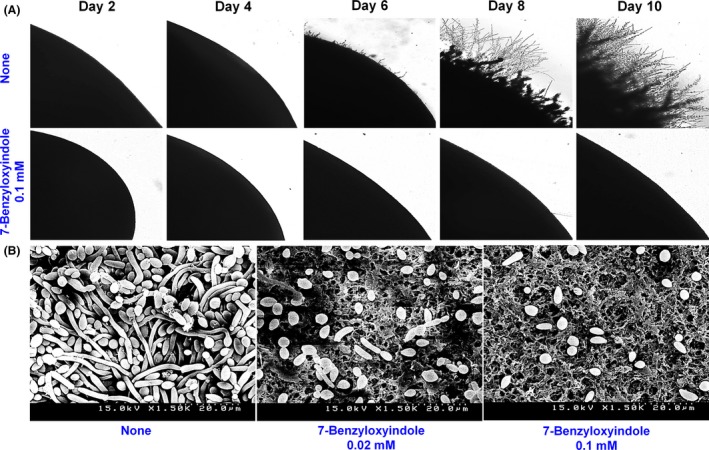
Effects of 7‐benzyloxyindole on C. albicans morphology. C. albicans morphology on solid media (A). C. albicans was streaked on PDA agar plates in the absence or presence of 7‐benzyloxyindole (0.1 mM). Colony morphology was photographed at every two days at 37°C. Inhibition of hyphal growth by 7‐benzyloxyindole was visualized by SEM (B). The scale bar represents 20 μm. At least two independent experiments were conducted. None indicates non‐treated control.
Confocal laser scanning microscopy analysis showed untreated C. albicans formed dense biofilms, and that 7‐benzyloxyindole at 0.1 mM dramatically reduced cellular densities and biofilm thicknesses (Fig. 4), in turn blocking biofilm formation as determined by crystal violet assays. Furthermore, COMSTAT analysis showed 7‐benzyloxyindole at 0.1 mM to significantly reduce biofilm biomass, average thickness and substrate coverage (Fig. 4B). More specifically, biofilm biomass and mean thickness after treatment were reduced by up to ≥ 90% versus untreated controls. Likewise, 7‐benzyloxyindole at 0.1 mM reduced substrate coverage by 82% (Fig. 4B). These results showed 7‐benzyloxyindole to effectively inhibit hyphal formation and biofilm maturation by C. albicans in the liquid medium and on solid plates. These findings suggest 7‐benzyloxyindole to probably downregulate the expression of genes that promote hyphal formation and biofilm maturation.
Figure 4.
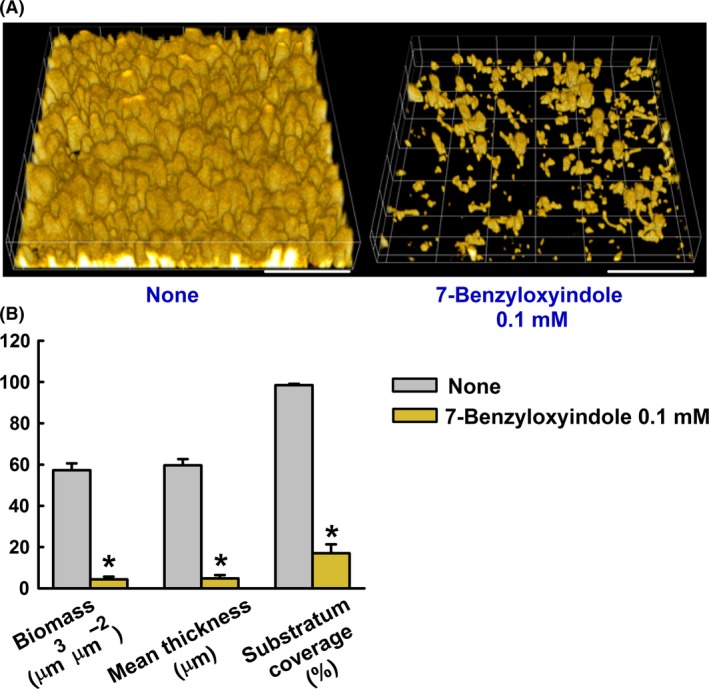
Microscopic observations of the effects of 7‐benzyloxyindole on biofilms. Biofilm formation by C. albicans on polystyrene plates was observed in the presence of 7‐benzyloxyindole at 0.1 mM by confocal laser microscopy (A). Scale bars represent 100 μm. Biofilm formation was quantified by COMSTAT (B) *P < 0.05 vs. non‐treated controls.
Effect of 7‐benzyloxyindole on the expression of hypha‐specific and biofilm‐related genes
Transcriptional levels of hypha‐specific and biofilm‐related genes in C. albicans were quantified by qRT‐PCR. We found 7‐benzyloxyindole at 0.1 mM to significantly reduce the mRNA levels of the hypha‐specific genes HWP1 (3.3‐fold) and RBT1 (3.8‐fold) versus respective non‐treated controls (Fig. 5). Also, HWP1 (fourfold), ALS3 (2.5‐fold), RBT1 (7.1‐fold) and ECE1 (5.5‐fold) levels were reduced significantly after treatment with 7‐benzyloxyindole at 0.2 mM. Interestingly, ALS1, which is involved in biofilm formation, was found to be upregulated by 7‐benzyloxyindole treatment, whereas transcription factor EFG1 to be only slightly affected after the treatment (Fig. 5). Taken together, qRT‐PCR results showed that 7‐benzyloxyindole significantly altered the expression of some hypha‐specific and biofilm‐related genes.
Figure 5.
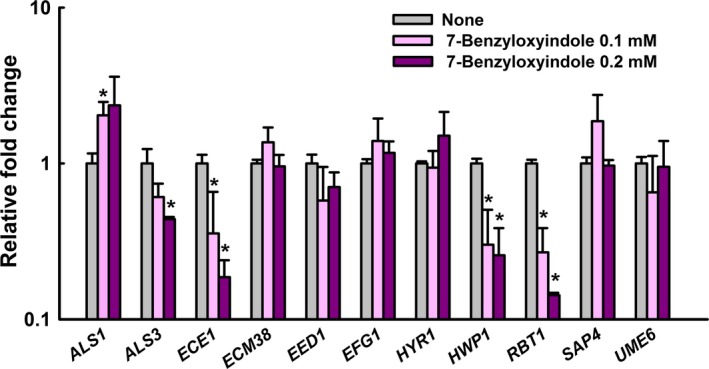
Transcriptional profiles of C. albicans cells treated with or without 7‐benzyloxyindole. C. albicans was cultivated with or without 7‐benzyloxyindole (0.1 mM and 0.2 mM) for 4 h. Transcriptional profiles were measured by qRT‐PCR. Relative expressions represent transcriptional levels after treatment with 7‐benzyloxyindole versus non‐treated controls. Fold changes represents transcription changes in treated C. albicans versus non‐treated controls. The experiment was performed in duplicate. Error bars indicate standard deviations. *P < 0.05 vs. non‐treated controls.
Efficacy of 7‐benzyloxyindole in the nematode Caenorhabditis elegans
In this study, we examined whether 7‐benzyloxyindole could affect Candida virulence in a Caenorhabditis elegans nematode model –an alternative to mammalian models (Tampakakis et al., 2008). Microscopic observations of infected nematodes revealed that C. albicans infection caused 92% fatality in 4 days (Fig. 6A). However, more than 40% of nematodes survived 4 days in the presence of 7‐benzyloxyindole at 0.05 mM, and > 60% survived 4 days in the presence of fluconazole (a commercial antifungal agent) at same concentration (Fig. 6A). To study the toxicity of 7‐benzyloxyindole and 4‐fluoroindole, nematodes without C. albicans infection were exposed to these compounds for 4 days. It was found that 4‐fluoroindole exhibited mild toxicity which is similar to commercial antifungal agent fluconazole at same concentrations (Fig. 6B). Compared to 4‐fluoroindole and fluconazole, 7‐benzyloxyindole showed more toxicity to nematodes. For instance, 55% worms survived at 0.1 mM 4‐fluoroindole, whereas 22% worms survived at same concentration of 7‐benzyloxyindole (Fig. 6B).
Figure 6.
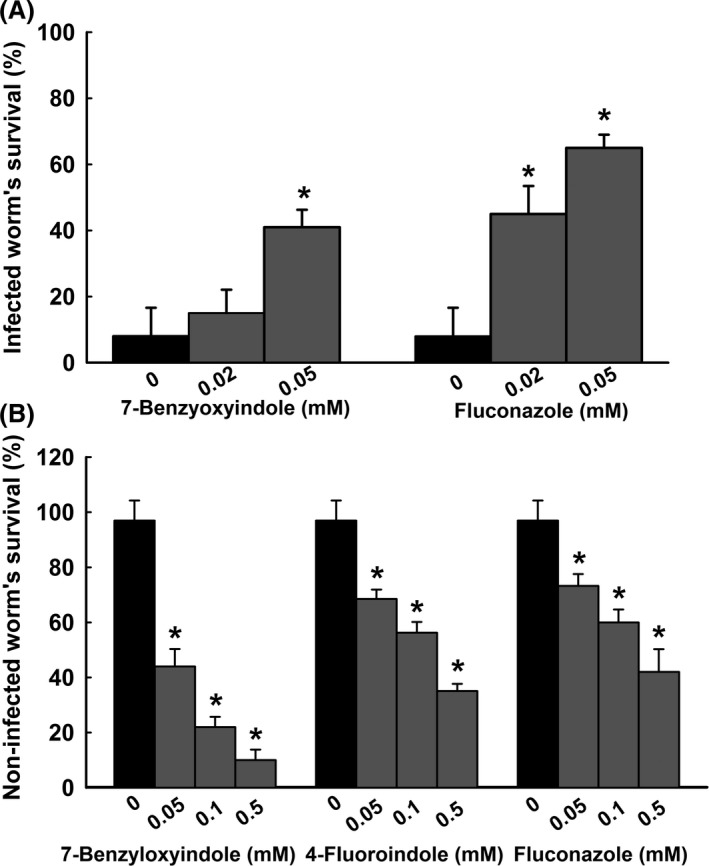
Effects of 7‐benzyloxyindole on C. albicans‐infected C. elegans. The bar graph indicates percentage worm survival after exposure of C. albicans for 4 days to 7‐benzyloxyindole. Fluconazole was used as a positive control (A). The toxicities of 7‐benzyloxyindole, 4‐fluoroindole and fluconazole were studied on non‐infected nematodes survival after 4 days (B). None indicates non‐treated controls. Worm survival was determined based on movement. At least two independent experiments were conducted. Error bars indicate standard deviations. *P < 0.05 vs. non‐treated controls.
Discussion
Increase in the prevalences of multidrug‐resistant Candida strains has encouraged the investigations on the activities of small molecules that play important roles in the inhibition of biofilm formation. Consequently, we searched for new indole derivatives that inhibit biofilm formation rather than only cell growth to reduce the risk of drug‐resistance development. Previously, we have shown that 7‐benzyloxyindole had antivirulence activity and antibiofilm activity against persistent S. aureus infections in vitro (Lee et al., 2013). In the present study, we studied benzyloxy and fluoro‐substituted indoles, and found that benzyloxy group is essential for antibiofilm activity against the fluconazole‐resistant Candida strain (Fig. 1). It has been previously reported that benzyloxy derivatized compounds have beneficial immunological effects in human epithelial cells such as antisickling activity (Abraham et al., 1984; Mahran, 2000). Another study has shown that functional groups of indoles such as indole carboxamide derivatives better inhibited C. albicans biofilms than propenamide derivatives (Olgen et al., 2008).
The metabolic activity experiments showed that C. albicans cells were viable after 7‐benzyloxyindole treatment (Fig. 2), which is consistent with other reports that the small molecules such as indole are not toxic to C. albicans (Olgen et al., 2008; You et al., 2013). Altman (1976) reported mitochondrial succinoxidase, cytochrome P450 and flavoprotein oxidases are primarily responsible for the conversion of XTT to coloured formazan, which is important in fungal research because XTT conversion provides a measure of metabolic activity, which is related to fungal wall biosynthesis. (Kuhn et al., 2002). This findings imply that 7‐benzyloxyindole at concentrations of >0.1 mM reduced metabolic activity due to low levels of C. albicans cells in biofilms (Fig. 2B). The inhibited hyphal formation after 7‐benzyloxyindole treatment could be associated with shortening of hyphal length and accumulation of pseudohyphae, which may be due to phenotypic plasticity, as C. albicans has also been repeatedly shown to undergo morphological changes from the hyphal to yeast form in the presence of environmental stress (Lu et al., 2011; Vediyappan et al., 2013).
Here, 7‐benzyloxyindole treatment downregulated HWP1, ALS3, ECE1 and RBT1 hyphae‐specific and biofilm‐related genes (Fig. 5). It has been reported HWP1 and ALS3 mutants are defective in terms of C. albicans biofilm development (Nobile et al., 2006a,b). Hyphal formation by C. albicans is regulated by the Ras‐cAMP‐Efg1 signalling pathway. In detail, small GTPase RAS1 activates cAMP, which promotes the PKA‐mediated activation of transcription factor EFG1, which in turn regulates hyphae‐specific genes, such as ALS3, HWP1, and ECE1, and thus, modulates hyphal formation (Leberer et al., 2001). RBT1 (repressed by TUP1) encodes cell surface proteins that are regulated by Tup1, which exhibits high similarity to HWP1 (Braun et al., 2001). Here, we suggest 7‐benzyloxyindole inhibits biofilm formation by modulating Ras‐cAMP‐Efg1 signalling pathway genes (HWP1, RBT1 and ECE1), which are strongly associated with long‐term hyphal maintenance, and thus, reducing hyphal development.
Previously, it was shown that hyphal form of C. albicans kills by piercing nematode cuticles, and that the yeast form is non‐lethal (Pukkila‐Worley et al., 2009). Similarly, it was reported that the survival rates of C. albicans‐infected nematodes were increased by treatment with gymnemic acid (Vediyappan et al., 2013), retigeric acid (Chang et al., 2012) or polyphenolic compounds such as magnolol and honokiol (Sun et al., 2015). This implies that 7‐benzyloxyindole could rescue the animals from Candida infection by preventing yeast‐hyphal transition (Fig. 6A). The correlations for toxicities between C. elegans and rodents make the case of inclusion of C. elegans for toxicity assessment (Dengg and van Meel, 2004; Sochova et al., 2006). Our results suggest that tested compounds could use for hyphal inhibition in animals with low dosage. Consistent with previous reports (Berman and Sudbery, 2002; Saville et al., 2003), our study of the indole compounds that we chose led us to speculate that they may be effective against invasive hyphae formation in patients with candidiasis.
In conclusion, the present study indicates indole derivatives such as 7‐benzyloxyindole could be used to control fungal virulence by regulating hyphae‐specific genes and to treat biofilm‐associated infections on medical implant devices and Candidiasis infections.
Experimental procedures
Strains and medium
In this study, C. albicans strain DAY185 was cultured in potato dextrose agar (PDA) and preserved in 1 ml of potato dextrose broth (PDB) supplemented with 30% glycerol at –80°C until use. As previously reported, DAY185 is resistant to the commercial antifungal fluconazole (MIC ~ 512 μg ml−1) (Manoharan et al., 2017a,b). A single colony was inoculated into 25 ml of PDB and incubated for overnight at 37°C. All compounds tested for this study were purchased from Sigma‐Aldrich (St. Louis, MO, USA) and Combi Blocks, Inc. (San Diego, CA, USA) and were dissolved in dimethyl sulfoxide (DMSO), which did not exceed 0.1% (vol/vol) in any experiment. The cell growths and turbidities were measured using spectrophotometer (UV‐160, Shimadzu, Japan) at 620 nm. Overnight C. albicans cells were prepared at the density of 105 CFU ml−1 with the presence of tested compounds in 96‐well polystyrene plates (SPL Life Sciences, Pocheon, Korea) to determine MIC using the Clinical Laboratory Standards Institute dilution method with slight modification (Alastruey‐Izquierdo et al., 2015). The plates were then incubated for 24 h at 37°C and the lowest concentration that inhibited yeast growth by at least 80%, as assessed by spectrophotometry (620 nm) and colony counting was determined as MIC.
Assays for biofilm formation
Candida biofilms were developed on 96‐well polystyrene plates, as previously reported (Lee et al., 2011). Briefly, C. albicans overnight cultures at an initial turbidity of 0.1 at 600 nm were inoculated into PDB (final volume 300 μl) with or without test compounds at varying concentrations and incubated for 24 h without shaking at 37°C. To determine biofilm formation, non‐adherent cells were removed by washing plates three times with H2O, crystal violet staining for 20 min followed by washing three times, and extracting the crystal violet using 95% ethanol. The results were presented as bar graphs as the average of at least six replicates by measuring absorbance at 570 nm.
Biofilm metabolic activity –XTT reduction assay
Biofilm growth was analysed using a XTT [2,3‐bis(2‐methoxy‐4‐nitro‐5‐sulfophenyl)‐2H‐tetrazolium‐5‐carboxanilide sodium salt] reduction assay using established procedures (Ramage et al., 2001; Nett et al., 2011). 300 μl of cell suspension diluted 105 CFU ml−1 was inoculated into PDB with or without 7‐benzyloxyindole at different concentrations for 24 h without shaking at 37°C. The metabolic activities of biofilm cells were measured by mixing freshly prepared XTT and menadione solutions (Sigma‐Aldrich) at 20∶1 (v/v). To each well, XTT‐menadione solution (42 μl) and PBS (158 μl) were added to prewashed biofilms, and incubated at 37°C in the dark for 3 h. The obtained coloured supernatant (100 μl) was transferred to new microtiter plates, and measured by absorbances at 450 nm. Similarly, planktonic cell viability was measured using culture supernatants.
Colony morphology of C. albicans on solid media
A freshly prepared glycerol stock of C. albicans was used to streak on PDA agar plates supplemented with DMSO or 0.1 mM concentration of 7‐benzyloxyindole. The plates were then incubated for 10 days at 37°C, and the temporal colony morphology was photographed at every alternate day using an iRiS™ Digital Cell Imaging System (Logos Bio Systems, Anyang, Korea).
Observations of hyphae by scanning electron microscopy (SEM)
Hyphal formation of C. albicans was observed by SEM, as previously described (Lee et al., 2014). Small pieces (0.5 × 0.5 cm) of nylon filter were placed in each well of 96‐well plates containing 200 μl cells suspension/well at the density of 105 CFU ml−1. Cells were incubated in the absence (untreated) or presence of 7‐benzyloxyindole at 37°C for 24 h without shaking, fixed with glutaraldehyde (concentration 2.5%) and formaldehyde (concentration 2%) for 24 h, and serially postfixed using sodium phosphate buffer and osmium tetroxide, dehydrated using an ethanol series (50, 70, 80, 90, 95 and 100%), and isoamyl acetate. After critical‐point drying, cells on nylon filter were examined under an S‐4200 scanning electron microscope (Hitachi, Tokyo, Japan) at magnifications ranging from × 2000 to ×10 000 and an accelerating voltage of 15 kV.
Confocal laser scanning microscopy of biofilm formation
Candida albicans biofilms were grown in 96‐well plates in the absence or presence of 7‐benzyloxyindole (0.1 mM) without shaking. Planktonic cells were then removed by washing with H2O three times. Carboxyfluorescein diacetate succinimidyl ester (a minimally fluorescent lipophile; Catalog #: C34554; Invitrogen, Molecular Probes, Inc, Eugene, OR, USA)(Weston and Parish, 1990) was used to stain C. albicans cells. The bottom of 96‐well plates was visualized using an (a 488 nm) Ar laser (emission wavelength 500 to 550 nm) under a confocal laser microscope (Nikon Eclipse Ti, Tokyo, Japan). Colour confocal images were constructed using NIS‐Elements C version 3.2 (Nikon Eclipse), and images were obtained with a 20× objective (Kim et al., 2012). For each experiment, two independent cultures were examined for at least 10 random positions. Biofilm formation was quantified by converting colour confocal images (20 image stacks) to grey scale using ImageJ, and COMSTAT biofilm software (Heydorn et al., 2000) was used to calculate biomasses (μm3 μm−2), mean biofilm thicknesses (μm) and substratum coverages (%). For each biofilm image, stack threshold was fixed and divided into four positions and 20 planar images per position were analysed.
RNA isolation and quantitative real‐time PCR (qRT‐PCR)
For the qRT‐PCR assay, 25 ml of C. albicans at an initial turbidity of 0.1 at OD600 was inoculated into PDB broth in 250 ml Erlenmeyer flasks, followed by 4‐h incubation at 37°C with agitation (250 rpm) in the presence or absence of 7‐benzyloxyindole at 0.1 or 0.2 mM. To prevent RNA degradation, RNase inhibitor (RNAlater, Ambion, TX, USA) was added to cells. Total RNA was isolated using hot acidic phenol method (Amin‐ul Mannan et al., 2009), and RNA was purified using a Qiagen RNeasy mini Kit (Valencia, CA, USA). Expression of hyphae‐related genes (ALS1, ALS3, ECE1, ECM38, EED1 EFG1, HYR1, HWP1, RBT1, SAP4 and UME6) was analysed. The specific primers and housekeeping gene (RDN18) used for qRT‐PCR are enlisted in Table S2. The qRT‐PCR method used has been previously described (Lee et al., 2011). SYBR Green master mix (Applied Biosystems, Foster City, CA, USA) and an ABI StepOne Real‐Time PCR System (Applied Biosystems) were used to perform qRT‐PCR. The assays were performed with at least two independent cultures.
Candida infection in the Caenorhabditis elegans model
Caenorhabditis elegans strain N2 Bristol CF512 fer‐15 (b26); fem‐1 (hc17) (Manoharan et al., 2017a,b) was used to perform C. albicans virulence assay using the protocol described by (Manoharan et al., 2017a,b). Briefly, synchronized adults worms were fed on C. albicans lawns for 4 h at 25°C and collected after washing three times with M9 buffer. Approximately 10 worms were then added to each well of 96‐well plates containing PDB medium (300 μl) with or without tested compounds at final concentrations of 0.02 or 0.05 mM. The assay plates were then incubated at 25°C for 4 days without shaking. For toxicity assays, 10 non‐infected worms were pipetted into single wells of a 96‐well dish containing M9 buffer and solutions of the compounds (200 μl) were added to final concentrations of 0.05, 0.1 or 0.5 mM. Plates were then incubated at 25◦C for 4 days without shaking. Three independent experiments were conducted in triplicate. Results were expressed as percentages of alive or dead worms as determined by their response to platinum wire touching after 4 days of incubation. Observations were made using an iRiS™ Digital Cell Imaging System (Logos Bio Systems).
Statistical analysis
All the experiments were conducted, and results are expressed as means of two independent experiment values with standard deviation. The significant differences between treated and non‐treated samples were determined by Student's t test. P values < 0.05 were considered as statistical significance, and indicated by asterisks.
Conflict of interest
None declared.
Supporting information
Table S1. Effects of indole derivatives on C. albicans DAY185 biofilm formation.
Table S2. Sequences of the primers used for quantitative RT‐PCR.
Acknowledgements
We thank Dr. Yong‐Guy Kim for his assistance during the confocal laser scanning microscopic study. We also wish to thank Dr. Vijay Shankar Balakrishnan, Science & Health Journalist/Writer, Marburg, Germany for his careful proofreading.
Microbial Biotechnology (2018) 11(6), 1060–1069
Funding Information
This study was funded by the Yeungnam University research grant.
References
- Abraham, D.J. , Kennedy, P.E. , Mehanna, A.S. , Patwa, D.C. , and Williams, F.L. (1984) Design, synthesis, and testing of potential antisickling agents. 4. Structure‐activity relationships of benzyloxy and phenoxy acids. J Med Chem 27: 967–978. [DOI] [PubMed] [Google Scholar]
- Alastruey‐Izquierdo, A. , Melhem, M.S. , Bonfietti, L.X. , and Rodriguez‐Tudela, J.L. (2015) Susceptibility test for fungi: clinical and laboratorial correlations in medical mycology. Rev Inst Med Trop Sao Paulo 57: 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman, F.P. (1976) Tetrazolium salts and formazans. Prog Histochem Cytochem 9: 1–56. [DOI] [PubMed] [Google Scholar]
- Amin‐ul Mannan, M. , Sharma, S. , and Ganesan, K. (2009) Total RNA isolation from recalcitrant yeast cells. Anal Biochem 389: 77–79. [DOI] [PubMed] [Google Scholar]
- Berman, J. , and Sudbery, P.E. (2002) Candida albicans: a molecular revolution built on lessons from budding yeast. Nat Rev Genet 3: 918–930. [DOI] [PubMed] [Google Scholar]
- Braun, B.R. , Kadosh, D. , and Johnson, A.D. (2001) NRG1, a repressor of filamentous growth in C. albicans, is down‐regulated during filament induction. EMBO J 20: 4753–4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carradori, S. , Chimenti, P. , Fazzari, M. , Granese, A. , and Angiolella, L. (2016) Antimicrobial activity, synergism and inhibition of germ tube formation by Crocus sativus‐derived compounds against Candida spp. J Enzyme Inhib Med Chem 31: 189–193. [DOI] [PubMed] [Google Scholar]
- Chandra, J. , Kuhn, D.M. , Mukherjee, P.K. , Hoyer, L.L. , McCormick, T. , and Ghannoum, M.A. (2001) Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol 183: 5385–5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, W. , Li, Y. , Zhang, L. , Cheng, A. , and Lou, H. (2012) Retigeric acid B attenuates the virulence of Candida albicans via inhibiting adenylyl cyclase activity targeted by enhanced farnesol production. PLoS ONE 7: e41624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton, J.W. , Stewart, P.S. , and Greenberg, E.P. (1999) Bacterial biofilms: a common cause of persistent infections. Science 284: 1318–1322. [DOI] [PubMed] [Google Scholar]
- Davey, M.E. and O'Toole G, A. (2000) Microbial biofilms: from ecology to molecular genetics. Microbiol Mol Biol Rev 64, 847–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengg, M. , and van Meel, J.C. (2004) Caenorhabditis elegans as model system for rapid toxicity assessment of pharmaceutical compounds. J Pharmacol Toxicol Methods 50: 209–214. [DOI] [PubMed] [Google Scholar]
- Frei, R. , Breitbach, A.S. , and Blackwell, H.E. (2012) 2‐Aminobenzimidazole derivatives strongly inhibit and disperse Pseudomonas aeruginosa biofilms. Angew Chem Int Ed Engl 51: 5226–5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydorn, A. , Nielsen, A.T. , Hentzer, M. , Sternberg, C. , Givskov, M. , Ersboll, B.K. , and Molin, S. (2000) Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 146: 2395–2407. [DOI] [PubMed] [Google Scholar]
- Jayant, S.R. , Ravikumar, B.S. , and Mohan, S.K. (2012) Indole, a bacterial signaling molecule, exhibits inhibitory activity against growth, dimorphism and biofilm formation in Candida albicans . Afr J Microbiol Res 6: 6005–6012. [Google Scholar]
- Kim, Y.‐G. , Lee, J.‐H. , Kim, C.J. , Lee, J.C. , Ju, Y.J. , Cho, M.H. , and Lee, J. (2012) Antibiofilm activity of Streptomyces sp. BFI 230 and Kribbella sp. BFI 1562 against Pseudomonas aeruginosa . Appl Microbiol Biotechnol 96: 1607–1617. [DOI] [PubMed] [Google Scholar]
- Kuhn, D.M. , Chandra, J. , Mukherjee, P.K. , and Ghannoum, M.A. (2002) Comparison of biofilms formed by Candida albicans and Candida parapsilosis on bioprosthetic surfaces. Infect Immun 70: 878–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leberer, E. , Harcus, D. , Dignard, D. , Johnson, L. , Ushinsky, S. , Thomas, D.Y. , and Schroppel, K. (2001) Ras links cellular morphogenesis to virulence by regulation of the MAP kinase and cAMP signalling pathways in the pathogenic fungus Candida albicans . Mol Microbiol 42: 673–687. [DOI] [PubMed] [Google Scholar]
- Lee, J.‐H. , and Lee, J. (2010) Indole as an intercellular signal in microbial communities. FEMS Microbiol Rev 34: 426–444. [DOI] [PubMed] [Google Scholar]
- Lee, J. , Jayaraman, A. , and Wood, T.K. (2007) Indole is an inter‐species biofilm signal mediated by SdiA. BMC Microbiol 7: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. , Attila, C. , Cirillo, S.L. , Cirillo, J.D. , and Wood, T.K. (2009) Indole and 7‐hydroxyindole diminish Pseudomonas aeruginosa virulence. Microb Biotechnol 2: 75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J.‐H. , Cho, M.H. , and Lee, J. (2011) 3‐Indolylacetonitrile decreases Escherichia coli O157:H7 biofilm formation and Pseudomonas aeruginosa virulence. Environ Microbiol 13: 62–73. [DOI] [PubMed] [Google Scholar]
- Lee, J.‐H. , Kim, Y.‐G. , Cho, M.H. , Kim, J.A. , and Lee, J. (2012) 7‐Fluoroindole as an antivirulence compound against Pseudomonas aeruginosa . FEMS Microbiol Lett 329: 36–44. [DOI] [PubMed] [Google Scholar]
- Lee, J.‐H. , Cho, H.S. , Kim, Y.‐G. , Kim, J.A. , Banskota, S. , Cho, M.H. , and Lee, J. (2013) Indole and 7‐benzyloxyindole attenuate the virulence of Staphylococcus aureus . Appl Microbiol Biotechnol 97: 4543–4552. [DOI] [PubMed] [Google Scholar]
- Lee, K. , Lee, J.‐H. , Kim, S.I. , Cho, M.H. , and Lee, J. (2014) Anti‐biofilm, anti‐hemolysis, and anti‐virulence activities of black pepper, cananga, myrrh oils, and nerolidol against Staphylococcus aureus . Appl Microbiol Biotechnol 98: 9447–9457. [DOI] [PubMed] [Google Scholar]
- Lee, J.‐H. , Kim, Y.‐G. , Baek, K.H. , Cho, M.H. , and Lee, J. (2015a) The multifaceted roles of the interspecies signalling molecule indole in Agrobacterium tumefaciens . Environ Microbiol 17: 1234–1244. [DOI] [PubMed] [Google Scholar]
- Lee, J.‐H. , Wood, T.K. , and Lee, J. (2015b) Roles of indole as an interspecies and interkingdom signaling molecule. Trends Microbiol 23: 707–718. [DOI] [PubMed] [Google Scholar]
- Lee, J.‐H. , Kim, Y.‐G. , Gwon, G. , Wood, T.K. , and Lee, J. (2016) Halogenated indoles eradicate bacterial persister cells and biofilms. AMB Express 6: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Y. , Su, C. , Wang, A. , and Liu, H. (2011) Hyphal development in Candida albicans requires two temporally linked changes in promoter chromatin for initiation and maintenance. PLoS Biol 9: e1001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahran, M.A. (2000) Quantitative structure‐activity relationship of phenoxy and benzyloxy acid derivatives as antisickling agents. Boll Chim Farm 139: 73–80. [PubMed] [Google Scholar]
- Manoharan, R.K. , Lee, J.‐H. , Kim, Y.‐G. , Kim, S.I. , and Lee, J. (2017a) Inhibitory effects of the essential oils alpha‐longipinene and linalool on biofilm formation and hyphal growth of Candida albicans . Biofouling 33: 143–155. [DOI] [PubMed] [Google Scholar]
- Manoharan, R.K. , Lee, J.‐H. , Kim, Y.‐G. , and Lee, J. (2017b) Alizarin and chrysazin inhibit biofilm and hyphal formation by Candida albicans . Front Cell Infect Microbiol 7: 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina‐Santiago, C. , Daddaoua, A. , Fillet, S. , Duque, E. , and Ramos, J.L. (2014) Interspecies signalling: Pseudomonas putida efflux pump TtgGHI is activated by indole to increase antibiotic resistance. Environ Microbiol 16: 1267–1281. [DOI] [PubMed] [Google Scholar]
- Mueller, R.S. , Beyhan, S. , Saini, S.G. , Yildiz, F.H. , and Bartlett, D.H. (2009) Indole acts as an extracellular cue regulating gene expression in Vibrio cholerae . J Bacteriol 191: 3504–3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nett, J.E. , Cain, M.T. , Crawford, K. , and Andes, D.R. (2011) Optimizing a Candida biofilm microtiter plate model for measurement of antifungal susceptibility by tetrazolium salt assay. J Clin Microbiol 49: 1426–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobile, C.J. , Andes, D.R. , Nett, J.E. , Smith, F.J. , Yue, F. , Phan, Q.T. , et al (2006a) Critical role of Bcr1‐dependent adhesins in C. albicans biofilm formation in vitro and in vivo . PLoS Pathog 2: e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobile, C.J. , Nett, J.E. , Andes, D.R. , and Mitchell, A.P. (2006b) Function of Candida albicans adhesin Hwp1 in biofilm formation. Eukaryot Cell 5: 1604–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh, S. , Go, G.W. , Mylonakis, E. , and Kim, Y. (2012) The bacterial signalling molecule indole attenuates the virulence of the fungal pathogen Candida albicans . J Appl Microbiol 113: 622–628. [DOI] [PubMed] [Google Scholar]
- Olgen, S. , Altanlar, N. , Karatayli, E. , and Bozdayi, M. (2008) Antimicrobial and antiviral screening of novel indole carboxamide and propanamide derivatives. Z Naturforsch C 63: 189–195. [DOI] [PubMed] [Google Scholar]
- Peleg, A.Y. , Hogan, D.A. , and Mylonakis, E. (2010) Medically important bacterial‐fungal interactions. Nat Rev Microbiol 8: 340–349. [DOI] [PubMed] [Google Scholar]
- Pukkila‐Worley, R. , Peleg, A.Y. , Tampakakis, E. , and Mylonakis, E. (2009) Candida albicans hyphal formation and virulence assessed using a Caenorhabditis elegans infection model. Eukaryot Cell 8: 1750–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage, G. , Vande Walle, K. , Wickes, B.L. , and Lopez‐Ribot, J.L. (2001) Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob Agents Chemother 45: 2475–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage, G. , Saville, S.P. , Thomas, D.P. , and Lopez‐Ribot, J.L. (2005) Candida biofilms: an update. Eukaryot Cell 4: 633–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandai, D. , Tabana, Y.M. , Ouweini, A.E. , and Ayodeji, I.O. (2016) Resistance of Candida albicans biofilms to drugs and the host immune system. Jundishapur J Microbiol 9: e37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardi, J.C. , Scorzoni, L. , Bernardi, T. , Fusco‐Almeida, A.M. , and Mendes Giannini, M.J. (2013) Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J Med Microbiol 62: 10–24. [DOI] [PubMed] [Google Scholar]
- Saville, S.P. , Lazzell, A.L. , Monteagudo, C. , and Lopez‐Ribot, J.L. (2003) Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot Cell 2: 1053–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sochova, I. , Hofman, J. , and Holoubek, I. (2006) Using nematodes in soil ecotoxicology. Environ Int 32: 374–383. [DOI] [PubMed] [Google Scholar]
- Sun, L. , Liao, K. , and Wang, D. (2015) Effects of magnolol and honokiol on adhesion, yeast‐hyphal transition, and formation of biofilm by Candida albicans . PLoS ONE 10: e0117695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taff, H.T. , Mitchell, K.F. , Edward, J.A. , and Andes, D.R. (2013) Mechanisms of Candida biofilm drug resistance. Future Microbiol 8: 1325–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tampakakis, E. , Okoli, I. , and Mylonakis, E. (2008) A C. elegans‐based, whole animal, in vivo screen for the identification of antifungal compounds. Nat Protoc 3: 1925–1931. [DOI] [PubMed] [Google Scholar]
- Tobudic, S. , Lassnigg, A. , Kratzer, C. , Graninger, W. , and Presterl, E. (2010) Antifungal activity of amphotericin B, caspofungin and posaconazole on Candida albicans biofilms in intermediate and mature development phases. Mycoses 53: 208–214. [DOI] [PubMed] [Google Scholar]
- Vediyappan, G. , Dumontet, V. , Pelissier, F. , and d'Enfert, C. (2013) Gymnemic acids inhibit hyphal growth and virulence in Candida albicans . PLoS ONE 8: e74189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. , You, J. , King, J.B. , Powell, D.R. , and Cichewicz, R.H. (2012) Waikialoid A suppresses hyphal morphogenesis and inhibits biofilm development in pathogenic Candida albicans . J Nat Prod 75: 707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston, S.A. , and Parish, C.R. (1990) New fluorescent dyes for lymphocyte migration studies. analysis by flow cytometry and fluorescence microscopy. J Immunol Methods 133: 87–97. [DOI] [PubMed] [Google Scholar]
- Williams, D. and Lewis, M. (2011) Pathogenesis and treatment of oral candidosis. J Oral Microbiol 3, 5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You, J. , Du, L. , King, J.B. , Hall, B.E. , and Cichewicz, R.H. (2013) Small‐molecule suppressors of Candida albicans biofilm formation synergistically enhance the antifungal activity of amphotericin B against clinical Candida isolates. ACS Chem Biol 8: 840–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Effects of indole derivatives on C. albicans DAY185 biofilm formation.
Table S2. Sequences of the primers used for quantitative RT‐PCR.


