Abstract
Background
Drug resistance in HIV is the major problem limiting effective antiviral therapy. Computational techniques for predicting drug resistance profiles from genomic data can accelerate the appropriate choice of therapy. These techniques can also be used to select protease mutants for experimental studies of resistance and thereby assist in the development of next-generation therapies.
Results
The machine learning produced highly accurate and robust classification of HIV protease resistance. Genotype data were mapped to the enzyme structure and encoded using Delaunay triangulation. Generative machine learning models trained on one inhibitor could classify resistance from other inhibitors with varying levels of accuracy. Generally, the accuracy was best when the inhibitors were chemically similar.
Conclusions
Restricted Boltzmann Machines are an effective machine learning tool for classification of genomic and structural data. They can also be used to compare resistance profiles of different protease inhibitors.
Keywords: HIV protease, Drug resistance, Machine learning, RBM, Structure-based
Background
Human Immunodeficiency Virus (HIV) is a major pandemic disease [1]. More than 36 million people have been infected and about half of these people receive anti-retroviral therapy [2]. However, retroviruses like HIV mutate rapidly since the conversion from the RNA genome to DNA is error-prone [3]. They readily form quasi-species and distinct viral strains. Therefore, retroviruses can respond effectively to selective pressures such as drug treatment by mutating to evade the antiviral drug. The development of drug resistance in HIV is an ongoing threat to effective long-term therapy.
Machine learning can predict drug resistance from sequence data with high accuracy as shown by tests on genotype-resistance data for HIV protease and reverse transcriptase [4–11]. The critical improvement in the application of machine learning to drug resistance is the inclusion of structural data in the features. We found that using Delaunay triangulation to encode the protein structure [12] is highly effective. The encoding compresses a protein sequence and its corresponding structure into a feature set consisting of 210 components. The set contains the relative frequencies of each kind of amino acid pair from the structure. Yu’s use of compressed encoding in [4] suggested that even fewer features were necessary to encapsulate drug resistance. Therefore, we used Principle Components Analysis (PCA) to explore the remaining redundancy in the data. The availability of a large amount of sequence and resistance data for HIV protease (PR) has proved valuable for method development.
The validity of this approach was verified by experimental studies [13, 14]. Machine learning was used to rigorously select representative highly resistant PR sequences for biochemical and structural characterization. The computationally selected mutant demonstrated several orders of magnitude worse affinity for inhibitors compared to wild type enzyme. The selected mutant had only one mutation in the inhibitor binding site. Therefore, a high level of resistance was achieved almost exclusively by mutations distal from the active site.
Restricted Boltzmann Machines (RBMs) are a generative machine learning algorithm [15, 16]. RBMs only require positive, or in-class, training data, and often generalize more accurately than other approaches. Training the standard algorithm on large datasets is often computationally infeasible. We have developed a highly efficient version of this algorithm [17, 18]. Using a simplified representation of the hidden and visible spins and replacing a numerical estimate of the gradient with an analytic form results in an algorithm that is at least 14 times faster than the conventional algorithm without compromising the accuracy.
Generative machine learning has not been applied to drug resistance in HIV. Therefore, application of this approach to the analysis of drug resistance is of interest. This paper shows that RBMs are as accurate as other machine learning approaches for these data. Additionally, we studied how well RBMs trained on one drug were able to predict resistance for a different drug.
Methods
Datasets and data preparation
Datasets used for the study
The genotype-phenotype datasets were downloaded from the Stanford HIV drug resistance database [19]. Data were used for the HIV protease inhibitors: atazanavir (ATV), nelfinavir (NFV), ritonavir (RTV), indinavir (IDV), lopinavir (LPV), tipranvir (TPV), saquinavir (SQV), fos-amprenavir (FPV) and darunavir (DRV). All the datasets were pre-processed using the methods and the cutoff values described previously in [4]. The threshold for resistance recommended by the database curators was used in this work [19]. The results of the expansion of data for each of the HIV-1 PR inhibitors and proportion of resistant mutants are shown in Table 1.
Table 1.
The results of the expansion for each of the HIV-1 PR inhibitors
| Inhibitor | No. isolates | No. sequences | No. resistant | No. sensitive | Fraction resistant |
|---|---|---|---|---|---|
| SQV | 1722 | 10258 | 4206 | 6052 | 41.0 |
| DRV | 607 | 5973 | 1889 | 4084 | 31.6 |
| LPV | 1444 | 10239 | 5095 | 5144 | 49.8 |
| NFV | 1771 | 10911 | 6170 | 4741 | 56.5 |
| IDV | 1730 | 10537 | 5122 | 5415 | 48.6 |
| ATV | 1141 | 8430 | 4237 | 4193 | 50.3 |
| FPV | 1681 | 10521 | 4405 | 6116 | 41.9 |
| TPV | 847 | 7363 | 2062 | 5301 | 28.0 |
Pre-processing/expansion of the datasets
Wild type HIV PR has a protein sequence of 99 amino acids. Sequences with insertions, deletions, or stop codons were removed. Genomic datasets often include multiple mutations at the same site. In these cases, the data were expanded to multiple sequences with single amino acids at each location to represent a single amino acid sequence for each mutant protein. For example, if one 99-amino acid mutant sequence has two different types of amino acids at one position and another site has three, this one sequence needs to be represented by six unique sequences each differing in only one amino acid substitution. The pre-processing method has been explained in detail in [4]. Each sequence was accompanied by its inhibitor resistance fold values. The relative resistant fold values for each of the inhibitors ranged from 0 to 800-fold resistance. Finally, the expanded datasets with sequences were allotted a unique identifier number to help recover the original sequences and their respective resistance fold change after analysis.
Encoding structure and sequence with Delaunay triangulation
A graph-based encoding system was utilized to represent the sequence and structural information of the protein [6]. The X-ray crystal structure for HIV-1 PR (3OXC) [20] was used as a template for creating the Delaunay triangulation. The structurally adjacent pairs of amino acids were represented as a vector of the 210 unique pairs of 20 standard amino acids. This graph-based encoding of sequence and structure has been proven to be a promising technique for fast and accurate predictions of resistance from sequence in HIV infections [5].
Principal component analysis
Principal Component Analysis (PCA) using Singular Value Decomposition (SVD) was run on all the HIV-1 PR datasets using the Scikit-Learn machine learning library [21]. The datasets for each inhibitor were analyzed using the Pandas data analysis library [22]. The resistance fold values were not included in the PCA calculations since predicting these values is the goal of this work. The results of this analysis are detailed in the “Results” section.
Training the RBM
The mutants with relative resistant fold less than 3.0 were classified as non-resistant (susceptible) and denoted as 0; while those with relative resistant fold of greater than 3.0 were classified as resistant and denoted as 1, as used in [4] and consistent with other analyses of the Stanford HIV resistance database [19]. RBMs work best with bit patterns. These bit patterns were generated by scaling and dividing the range of individual features into equal intervals. Each feature of the data was scaled to the range 0 to 1 based on the maximum and minimum values of that feature. The scaled data were divided into eight intervals encoded with three bits per feature. The testing and training sets were scaled independently.
The RBM was trained using gradient descent with the derivative as shown in Eq. 1. The analytic expression for the expected value of the derivative, shown in Eq. 2 and derived in [17], was used.
| 1 |
| 2 |
In these equations, H and V are hidden and visible (or input) layers respectively, β is the inverse temperature, U is the potential energy, and W are the weights used to define the potential.
During training, the layer that gave the best fit for each new data point was updated with a descent step and the other layers were “anti-trained” with a small ascent step. “Anti-training” improves the convergence and training efficiency of the RBM. Anti-training is only feasible when using an analytic expression for the training gradient. An RBM with 150 units in the hidden layer was trained for each category with a constant step-size of 0.1. A step-size of 0.01 was used for anti-training. An RBM was trained for both resistant and non-resistant classes. Class membership was assigned by the fractional reconstruction error, shown in Eq. 3 as defined in [17].
| 3 |
Five-fold cross validation was used to ensure that the results reflect the error in the models. The models for each fold were trained to convergence with ten iterations and the values for accuracy, positive predictive value, recall, and F from the last iteration were reported.
Results
Classification with an RBM
The classification results are detailed in Table 2 and show a high degree of accuracy. The nearly uniform values of close to 1.0 for accuracy, PPV, recall, and F-score, show that the models reliably predict both resistant and non-resistant classes. These results compare favorably with our earlier results using non-generative machine learning algorithms [4–6, 8].
Table 2.
The accuracy of the machine learning model is shown here
| Inhibitor | Accuracy | PPV | Recall | F |
|---|---|---|---|---|
| Idv | 0.979 | 0.974 | 0.985 | 0.979 |
| Lpv | 0.984 | 0.977 | 0.992 | 0.984 |
| Sqv | 0.969 | 0.963 | 0.986 | 0.974 |
| Tpv | 0.987 | 0.984 | 0.998 | 0.991 |
| Drv | 0.988 | 0.985 | 0.998 | 0.992 |
| Atv | 0.983 | 0.976 | 0.989 | 0.983 |
| Nfv | 0.978 | 0.974 | 0.975 | 0.975 |
| Fpv | 0.988 | 0.984 | 0.998 | 0.991 |
The estimated standard deviation amongst the five folds is <0.013 for all values
Cross-classification with an RBM
RBMs differ from non-generative machine learning methods in an interesting way. It is trivial to train an RBM against one dataset and use it to predict the behavior of another. Table 3 shows the results of a cross-training analysis of resistance data. Each row was trained on one inhibitor and the columns show the accuracy with which that model predicts the resistance for the other inhibitors. The inhibitors generally, but not completely, cross-classify with high accuracy. TPV and DRV seem to have more differences from the other inhibitors.
Table 3.
Cross training reveals similarity between the inhibitors
| Compound | Atv | Drv | Fpv | Idv | Lpv | Nfv | Sqv | Tpv |
|---|---|---|---|---|---|---|---|---|
| Atv | 0.990 | 0.868 | 0.880 | 0.955 | 0.946 | 0.914 | 0.893 | 0.819 |
| Drv | 0.767 | 0.996 | 0.818 | 0.786 | 0.785 | 0.718 | 0.792 | 0.925 |
| Fpv | 0.929 | 0.873 | 0.981 | 0.889 | 0.886 | 0.822 | 0.822 | 0.828 |
| Idv | 0.945 | 0.863 | 0.880 | 0.989 | 0.960 | 0.905 | 0.878 | 0.809 |
| Lpv | 0.939 | 0.892 | 0.877 | 0.963 | 0.988 | 0.891 | 0.865 | 0.837 |
| Nfv | 0.923 | 0.853 | 0.824 | 0.918 | 0.901 | 0.987 | 0.837 | 0.758 |
| Sqv | 0.898 | 0.837 | 0.825 | 0.890 | 0.871 | 0.840 | 0.983 | 0.807 |
| Tpv | 0.723 | 0.929 | 0.765 | 0.729 | 0.728 | 0.655 | 0.732 | 0.993 |
These numbers show the accuracy when a model trained on the compound at the start of the row is used to classify the data from the other inhibitors
The ability of an RBM trained on resistance to one inhibitor to predict the behavior of resistance to another inhibitor shows that the drug resistance of HIV protease does not fully depend on the type of drug. The existence of cross-resistance is well known and our lab has used similar approaches to identify interesting multi-drug resistant mutants for structural study [6, 13, 14].
Principal component analysis
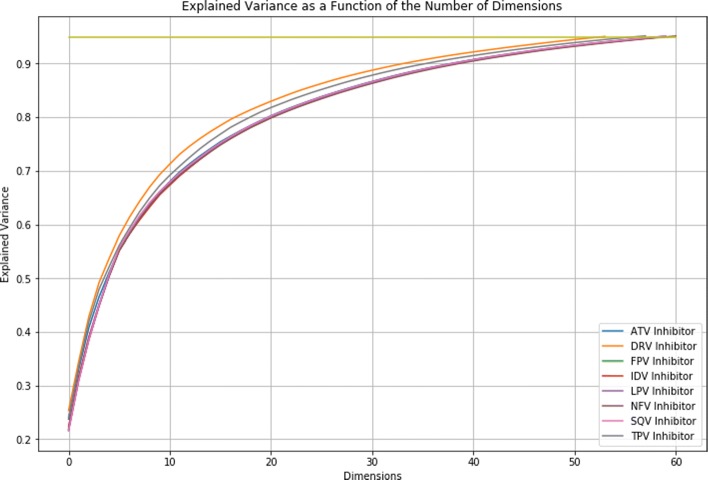
Figure 1 shows the explained variance for each of the datasets as a function of the number of reduced dimensions. As shown in the figure, there is overlap between some of the datasets (some of the plots depict the same data). This suggests that redundancy exists between datasets and not just within a single dataset. The horizontal line in the figure depicts where at least 95% of the explained variance of the datasets is captured. In most cases, the first principal component explained at least 90% of the observed variance. This was true for the ATV, LPV, NFV, and SQV inhibitors. The remaining four inhibitors had an explained variance ratio between 51% and 87% for the first principal component. For all inhibitors except for DRV, 95% of the explained variance for each dataset was captured within 60 dimensions, suggesting that the data could be further compressed while still minimizing the reconstruction error. For DRV, the explained variance could be reduced to 50 dimensions. These results indicate that a more compact encoding for the resistance data exists, consistent with Yu’s results on effectiveness of compressed encoding for machine learning [4].
Fig. 1.
Principal Component Analysis on the HIV-1 PR Datasets. The similarity in the curves indicates that the datasets have a similar underlying structure
Discussion
Classification of resistant mutations of HIV PR
The combination of structure-based encoding and RBMs is an effective technique for the prediction of drug resistance in HIV PR. The five-fold cross validated results in Table 2 clearly demonstrate their success and accuracy. The high values for PPV indicate that the models could be clinically valuable. The use of an RBM is especially interesting because there are essentially no adjustable parameters in the process. Efficient training algorithms allow the RBM to handle large datasets in reasonable times. While these datasets are not quite big data, they are too big for other machine learning programs [7, 23].
Comparison with other methods
We pioneered the approach of using a unified representation of sequence with 3D structural data expressed as a 210-long feature vector for machine learning [4]. This approach gave improved accuracy for predicting drug resistance for HIV protease and reverse transcriptase compared to using sequence data alone.
Another group reported mean R2 values of >0.95 for regression with ANN using a subset of HIVsequences restricted to subtype B with the data filtered to remove rare variants [10]. Their classification accuracy was less impressive. Structural data can also be represented by molecular mechanics calculations on protein-drug complexes. Molecular interaction components calculated between a drug and 36 single mutants of HIV protease were used for SVM classification of resistance and showed improved accuracy over using sequence alone [9]. These results were comparable to our earlier results, but for a much smaller number of sequences. Feature vectors derived from a four-body statistical potential and n-grams were applied in [11]. This approach also used explicit atomic models for the protease and therefore only a few hundred mutants were included for classification and regression. Their reported accuracy was worse than ours.
Our approach preserves structural information using Delauney Triangulation derived from a single protein structure, and is applicable to any mutant, while eliminating the expensive step of calculating molecular properties for models of every mutant structure.
Redundancy in the data.
One of the original motivations for exploring graph-based encoding of protein structures was to remove unnecessary data while retaining the critical features for machine-learning based analysis of structure and function [12]. Earlier work [4], which used compressed encoding, hinted that the redundancy was not completely removed from the data. Our use of PCA on the data demonstrated that further compression is possible because the majority of the variance in the data could be captured with 50–60 dimensions instead of the 210 used in the original representation. This strongly suggests that we may be able to extract patterns of mutations associated with drug resistance from the structural data itself.
Inhibitor specific patterns of drug resistance
Another important difference between generative machine learning and more conventional algorithms is that it is logically consistent to apply generative machine learning across categories. Since the RBM is essentially measuring how well it can reconstruct a given data point, it makes sense to ask whether an RBM trained on one inhibitor such as ATV could reconstruct data for a different inhibitor such as DRV. Examples of two inhibitors, Darunavir and Atazanavir are shown in Fig. 2 which demonstrates the diversity of drug chemistry used to inhibit HIV PR.
Fig. 2.
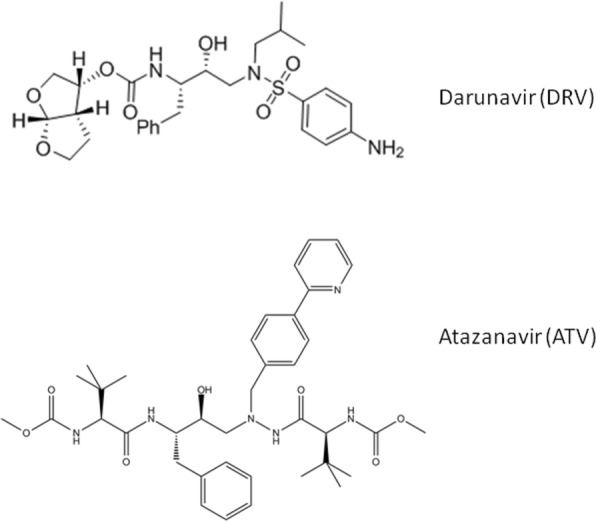
The chemical structures for a sulfonamide-containing (DRV) and non-sulfonamide-containing (ATV) inhibitor are shown here. These demonstrate the variety of chemistry used in inhibitors
The inhibitors segregate into two main classes in the cross-training analysis. Cross-training results in high accuracy for most inhibitors, with the exception of DRV and TPV, which both incorporate sulphonamides. DRV and TPV, predict each other with reasonable accuracy (92.5%), however they show worse prediction for other inhibitors. While this could be due to chemical similarity, it could also be due to these being second generation or salvage inhibitors where the full spectrum of resistance mutations has not had time to evolve.
Accurate cross-prediction is not completely surprising. The inhibitors bind to an active site that is under selective pressure to still recognize its biological substrate. Many of the most highly resistant strains demonstrate multi-drug resistance [6, 13, 14, 24]. Therefore, we expect some level cross-prediction and this work quantifies it.
Conclusion
Generative machine learning algorithms such as the RBM are well-suited to the prediction of drug resistance in HIV PR, and likely will work on other systems as well. The graph-based structure/sequence encoding used in this and related work removes much of the redundancy in the data, but does not remove it all. This result suggests that even more efficient encoding schemes are possible. The RBM was used to analyze similarities in resistance profiles for different clinical inhibitors. The analysis suggests that there are at least two main classes of inhibitors for HIV PR.
Acknowledgements
The research was supported in part by the National Institutes of Health grant GM062920 (ITW & RWH).
Funding
Publication was sponsored by Georgia State University.
Availability of data and materials
The unprocessed datasets can be downloaded from: http://hivdb.stanford.edu/pages/genopheno.dataset.html
About this supplement
This article has been published as part of BMC Bioinformatics Volume 19 Supplement 11, 2018: Proceedings from the 6th Workshop on Computational Advances in Molecular Epidemiology (CAME 2017). The full contents of the supplement are available online at https://bmcbioinformatics.biomedcentral.com/articles/supplements/volume-19-supplement-11.
Abbreviations
- ATV
Atazanavir
- DRV
Darunavir
- FPV
Fos-Amprenavir
- HIV
Human immunodeficiency virus
- HIV PR
HIV protease
- IDV
Indinavir
- LPV
Lopinavir
- NFV
Nelfinavir
- PCA
Principle components analysis
- PPV
Positive Predictive Value
- RBM
Restricted boltzmann machine
- SQV
Saquinavir
- TPV
Tipranavir
Authors’ contributions
All of the authors have read and approved this paper. SDP performed the data preparation, CF performed the PCA, CF and RWH developed and performed the machine learning, and ITW provided the biochemical and structural context.
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shrikant D. Pawar, Email: spawar2@student.gsu.edu
Christopher Freas, Email: cfreas@cs.gsu.edu.
Irene T. Weber, Email: iweber@gsu.edu
Robert W. Harrison, Email: rwh@gsu.edu
References
- 1.Wang Haidong, Wolock Tim M, Carter Austin, Nguyen Grant, Kyu Hmwe Hmwe, Gakidou Emmanuela, Hay Simon I, Mills Edward J, Trickey Adam, Msemburi William, Coates Matthew M, Mooney Meghan D, Fraser Maya S, Sligar Amber, Salomon Joshua, Larson Heidi J, Friedman Joseph, Abajobir Amanuel Alemu, Abate Kalkidan Hassen, Abbas Kaja M, Razek Mohamed Magdy Abd El, Abd-Allah Foad, Abdulle Abdishakur M, Abera Semaw Ferede, Abubakar Ibrahim, Abu-Raddad Laith J, Abu-Rmeileh Niveen M E, Abyu Gebre Yitayih, Adebiyi Akindele Olupelumi, Adedeji Isaac Akinkunmi, Adelekan Ademola Lukman, Adofo Koranteng, Adou Arsène Kouablan, Ajala Oluremi N, Akinyemiju Tomi F, Akseer Nadia, Lami Faris Hasan Al, Al-Aly Ziyad, Alam Khurshid, Alam Noore K M, Alasfoor Deena, Aldhahri Saleh Fahed S, Aldridge Robert William, Alegretti Miguel Angel, Aleman Alicia V, Alemu Zewdie Aderaw, Alfonso-Cristancho Rafael, Ali Raghib, Alkerwi Ala'a, Alla François, Mohammad Rajaa, Al-Raddadi Salem, Alsharif Ubai, Alvarez Elena, Alvis-Guzman Nelson, Amare Azmeraw T, Amberbir Alemayehu, Amegah Adeladza Kofi, Ammar Walid, Amrock Stephen Marc, Antonio Carl Abelardo T, Anwari Palwasha, Ärnlöv Johan, Artaman Al, Asayesh Hamid, Asghar Rana Jawad, Assadi Reza, Atique Suleman, Atkins Lydia S, Avokpaho Euripide Frinel G Arthur, Awasthi Ashish, Quintanilla Beatriz Paulina Ayala, Bacha Umar, Badawi Alaa, Barac Aleksandra, Bärnighausen Till, Basu Arindam, Bayou Tigist Assefa, Bayou Yibeltal Tebekaw, Bazargan-Hejazi Shahrzad, Beardsley Justin, Bedi Neeraj, Bennett Derrick A, Bensenor Isabela M, Betsu Balem Demtsu, Beyene Addisu Shunu, Bhatia Eesh, Bhutta Zulfiqar A, Biadgilign Sibhatu, Bikbov Boris, Birlik Sait Mentes, Bisanzio Donal, Brainin Michael, Brazinova Alexandra, Breitborde Nicholas J K, Brown Alexandria, Burch Michael, Butt Zahid A, Campuzano Julio Cesar, Cárdenas Rosario, Carrero Juan Jesus, Castañeda-Orjuela Carlos A, Rivas Jacqueline Castillo, Catalá-López Ferrán, Chang Hsing-Yi, Chang Jung-chen, Chavan Laxmikant, Chen Wanqing, Chiang Peggy Pei-Chia, Chibalabala Mirriam, Chisumpa Vesper Hichilombwe, Choi Jee-Young Jasmine, Christopher Devasahayam Jesudas, Ciobanu Liliana G, Cooper Cyrus, Dahiru Tukur, Damtew Solomon Abrha, Dandona Lalit, Dandona Rakhi, das Neves José, de Jager Pieter, De Leo Diego, Degenhardt Louisa, Dellavalle Robert P, Deribe Kebede, Deribew Amare, Des Jarlais Don C, Dharmaratne Samath D, Ding Eric L, Doshi Pratik Pinal, Doyle Kerrie E, Driscoll Tim R, Dubey Manisha, Elshrek Yousef Mohamed, Elyazar Iqbal, Endries Aman Yesuf, Ermakov Sergey Petrovich, Eshrati Babak, Esteghamati Alireza, Faghmous Imad D A, Farinha Carla Sofia e Sa, Faro Andre, Farvid Maryam S, Farzadfar Farshad, Fereshtehnejad Seyed-Mohammad, Fernandes Joao C, Fischer Florian, Fitchett Joseph Robert Anderson, Foigt Nataliya, Fullman Nancy, Fürst Thomas, Gankpé Fortuné Gbètoho, Gebre Teshome, Gebremedhin Amanuel Tesfay, Gebru Alemseged Aregay, Geleijnse Johanna M, Gessner Bradford D, Gething Peter W, Ghiwot Tsegaye Tewelde, Giroud Maurice, Gishu Melkamu Dedefo, Glaser Elizabeth, Goenka Shifalika, Goodridge Amador, Gopalani Sameer Vali, Goto Atsushi, Gugnani Harish Chander, Guimaraes Mark D C, Gupta Rahul, Gupta Rajeev, Gupta Vipin, Haagsma Juanita, Hafezi-Nejad Nima, Hagan Holly, Hailu Gessessew Bugssa, Hamadeh Randah Ribhi, Hamidi Samer, Hammami Mouhanad, Hankey Graeme J, Hao Yuantao, Harb Hilda L, Harikrishnan Sivadasanpillai, Haro Josep Maria, Harun Kimani M, Havmoeller Rasmus, Hedayati Mohammad T, Heredia-Pi Ileana Beatriz, Hoek Hans W, Horino Masako, Horita Nobuyuki, Hosgood H Dean, Hoy Damian G, Hsairi Mohamed, Hu Guoqing, Huang Hsiang, Huang John J, Iburg Kim Moesgaard, Idrisov Bulat T, Innos Kaire, Iyer Veena J, Jacobsen Kathryn H, Jahanmehr Nader, Jakovljevic Mihajlo B, Javanbakht Mehdi, Jayatilleke Achala Upendra, Jeemon Panniyammakal, Jha Vivekanand, Jiang Guohong, Jiang Ying, Jibat Tariku, Jonas Jost B, Kabir Zubair, Kamal Ritul, Kan Haidong, Karch André, Karema Corine Kakizi, Karletsos Dimitris, Kasaeian Amir, Kaul Anil, Kawakami Norito, Kayibanda Jeanne Françoise, Keiyoro Peter Njenga, Kemp Andrew Haddon, Kengne Andre Pascal, Kesavachandran Chandrasekharan Nair, Khader Yousef Saleh, Khalil Ibrahim, Khan Abdur Rahman, Khan Ejaz Ahmad, Khang Young-Ho, Khubchandani Jagdish, Kim Yun Jin, Kinfu Yohannes, Kivipelto Miia, Kokubo Yoshihiro, Kosen Soewarta, Koul Parvaiz A, Koyanagi Ai, Defo Barthelemy Kuate, Bicer Burcu Kucuk, Kulkarni Veena S, Kumar G Anil, Lal Dharmesh Kumar, Lam Hilton, Lam Jennifer O, Langan Sinead M, Lansingh Van C, Larsson Anders, Leigh James, Leung Ricky, Li Yongmei, Lim Stephen S, Lipshultz Steven E, Liu Shiwei, Lloyd Belinda K, Logroscino Giancarlo, Lotufo Paulo A, Lunevicius Raimundas, Razek Hassan Magdy Abd El, Mahdavi Mahdi, Mahesh P A, Majdan Marek, Majeed Azeem, Makhlouf Carla, Malekzadeh Reza, Mapoma Chabila C, Marcenes Wagner, Martinez-Raga Jose, Marzan Melvin Barrientos, Masiye Felix, Mason-Jones Amanda J, Mayosi Bongani M, McKee Martin, Meaney Peter A, Mehndiratta Man Mohan, Mekonnen Alemayehu B, Melaku Yohannes Adama, Memiah Peter, Memish Ziad A, Mendoza Walter, Meretoja Atte, Meretoja Tuomo J, Mhimbira Francis Apolinary, Miller Ted R, Mikesell Joseph, Mirarefin Mojde, Mohammad Karzan Abdulmuhsin, Mohammed Shafiu, Mokdad Ali H, Monasta Lorenzo, Moradi-Lakeh Maziar, Mori Rintaro, Mueller Ulrich O, Murimira Brighton, Murthy Gudlavalleti Venkata Satyanarayana, Naheed Aliya, Naldi Luigi, Nangia Vinay, Nash Denis, Nawaz Haseeb, Nejjari Chakib, Ngalesoni Frida Namnyak, de Dieu Ngirabega Jean, Nguyen Quyen Le, Nisar Muhammad Imran, Norheim Ole F, Norman Rosana E, Nyakarahuka Luke, Ogbo Felix Akpojene, Oh In-Hwan, Ojelabi Foluke Adetola, Olusanya Bolajoko Olubukunola, Olusanya Jacob Olusegun, Opio John Nelson, Oren Eyal, Ota Erika, Park Hye-Youn, Park Jae-Hyun, Patil Snehal T, Patten Scott B, Paul Vinod K, Pearson Katherine, Peprah Emmanuel Kwame, Pereira David M, Perico Norberto, Pesudovs Konrad, Petzold Max, Phillips Michael Robert, Pillay Julian David, Plass Dietrich, Polinder Suzanne, Pourmalek Farshad, Prokop David M, Qorbani Mostafa, Rafay Anwar, Rahimi Kazem, Rahimi-Movaghar Vafa, Rahman Mahfuzar, Rahman Mohammad Hifz Ur, Rahman Sajjad Ur, Rai Rajesh Kumar, Rajsic Sasa, Ram Usha, Rana Saleem M, Rao Paturi Vishnupriya, Remuzzi Giuseppe, Rojas-Rueda David, Ronfani Luca, Roshandel Gholamreza, Roy Ambuj, Ruhago George Mugambage, Saeedi Mohammad Yahya, Sagar Rajesh, Saleh Muhammad Muhammad, Sanabria Juan R, Santos Itamar S, Sarmiento-Suarez Rodrigo, Sartorius Benn, Sawhney Monika, Schutte Aletta E, Schwebel David C, Seedat Soraya, Sepanlou Sadaf G, Servan-Mori Edson E, Shaikh Masood Ali, Sharma Rajesh, She Jun, Sheikhbahaei Sara, Shen Jiabin, Shibuya Kenji, Shin Hwashin Hyun, Sigfusdottir Inga Dora, Silpakit Naris, Silva Diego Augusto Santos, Silveira Dayane Gabriele Alves, Simard Edgar P, Sindi Shireen, Singh Jasvinder A, Singh Om Prakash, Singh Prashant Kumar, Skirbekk Vegard, Sliwa Karen, Soneji Samir, Sorensen Reed J D, Soriano Joan B, Soti David O, Sreeramareddy Chandrashekhar T, Stathopoulou Vasiliki, Steel Nicholas, Sunguya Bruno F, Swaminathan Soumya, Sykes Bryan L, Tabarés-Seisdedos Rafael, Talongwa Roberto Tchio, Tavakkoli Mohammad, Taye Bineyam, Tedla Bemnet Amare, Tekle Tesfaye, Shifa Girma Temam, Temesgen Awoke Misganaw, Terkawi Abdullah Sulieman, Tesfay Fisaha Haile, Tessema Gizachew Assefa, Thapa Kiran, Thomson Alan J, Thorne-Lyman Andrew L, Tobe-Gai Ruoyan, Topor-Madry Roman, Towbin Jeffrey Allen, Tran Bach Xuan, Dimbuene Zacharie Tsala, Tsilimparis Nikolaos, Tura Abera Kenay, Ukwaja Kingsley Nnanna, Uneke Chigozie Jesse, Uthman Olalekan A, Venketasubramanian N, Vladimirov Sergey K, Vlassov Vasiliy Victorovich, Vollset Stein Emil, Wang Linhong, Weiderpass Elisabete, Weintraub Robert G, Werdecker Andrea, Westerman Ronny, Wijeratne Tissa, Wilkinson James D, Wiysonge Charles Shey, Wolfe Charles D A, Won Sungho, Wong John Q, Xu Gelin, Yadav Ajit Kumar, Yakob Bereket, Yalew Ayalnesh Zemene, Yano Yuichiro, Yaseri Mehdi, Yebyo Henock Gebremedhin, Yip Paul, Yonemoto Naohiro, Yoon Seok-Jun, Younis Mustafa Z, Yu Chuanhua, Yu Shicheng, Zaidi Zoubida, Zaki Maysaa El Sayed, Zeeb Hajo, Zhang Hao, Zhao Yong, Zodpey Sanjay, Zoeckler Leo, Zuhlke Liesl Joanna, Lopez Alan D, Murray Christopher J L. Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2015: the Global Burden of Disease Study 2015. The Lancet HIV. 2016;3(8):e361–e387. doi: 10.1016/S2352-3018(16)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization HIV Page. http://www.who.int/hiv/data/en/. Accessed 6 June 2018.
- 3.Smyth RP, Davenport MP, Mak J. The origin of genetic diversity in hiv-1. Virus Res. 2012;169(2):415–29. doi: 10.1016/j.virusres.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 4.Yu Xiaxia, Weber Irene, Harrison Robert. Proceedings of the 2013 SIAM International Conference on Data Mining. Philadelphia, PA: Society for Industrial and Applied Mathematics; 2013. Sparse Representation for HIV-1 Protease Drug Resistance Prediction; pp. 342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu X, Weber IT, Harrison R. Prediction of hiv drug resistance from genotype with encoded three-dimensional protein structure. BMC Genomics. 2014;15(5):1. doi: 10.1186/1471-2164-15-S5-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu X, Weber IT, Harrison RW. Identifying representative drug resistant mutants of hiv. BMC Bioinforma. 2015;16(17):1. doi: 10.1186/s12859-014-0430-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durham EEA, Yu X, Harrison RW. FDT 2.0: Improving scalability of the fuzzy decision tree induction tool - integrating database storage. In: 2014 IEEE Symposium on Computational Intelligence in Healthcare and e-health (CICARE): 2014. p. 187–90. 10.1109/CICARE.2014.7007853. [DOI] [PMC free article] [PubMed]
- 8.Shen C, Yu X, Harrison R, Weber IT. Automated prediction of hiv drug resistance from genotype data. BMC Bioinforma. 2016;17(8):278. doi: 10.1186/s12859-016-1114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tingjun H, Wei Z, Jian W, Wei W. Predicting drug resistance of the hiv-1 protease using molecular interaction energy components. Proteins Struct Funct Bioinforma. 2014;82(6):1099. doi: 10.1002/prot.24577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheik Amamuddy O, Bishop NT, Tastan Bishop Ö. Improving fold resistance prediction of hiv-1 against protease and reverse transcriptase inhibitors using artificial neural networks. BMC Bioinforma. 2017;18(1):369. doi: 10.1186/s12859-017-1782-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masso M, Vaisman II. Sequence and structure based models of hiv-1 protease and reverse transcriptase drug resistance. BMC Genomics. 2013;14(4):3. doi: 10.1186/1471-2164-14-S4-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bose P, Yu X, Harrison RW. Encoding protein structure with functions on graphs. In: 2011 IEEE International Conference on Bioinformatics and Biomedicine Workshops (BIBMW). IEEE: 2011. p. 338–44. 10.1109/BIBMW.2011.6112396. [DOI]
- 13.Park JH, Sayer JM, Aniana A, Yu X, Weber IT, Harrison R, Louis JM. Binding of clinical inhibitors to a model precursor of a rationally selected multidrug resistant hiv-1 protease is significantly weaker than that to the released mature enzyme. Biochemistry. 2016;55(16):2390–400. doi: 10.1021/acs.biochem.6b00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agniswamy J, Louis JM, Roche J, Harrison R, Weber IT. Structural studies of a rationally selected multi-drug resistant hiv-1 protease reveal synergistic effect of distal mutations on flap dynamics. PLoS ONE. 2016;11(12):0168616. doi: 10.1371/journal.pone.0168616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinton G. UTML TR 2010–003 A Practical Guide to Training Restricted Boltzmann Machines. Toronto: University of Toronto; 2010. [Google Scholar]
- 16.Salakhutdinov R, Mnih A, Hinton G. Proceedings of the 24th International Conference on Machine Learning. ICML ’07. New York: ACM; 2007. Restricted boltzmann machines for collaborative filtering. [Google Scholar]
- 17.Harrison RW, Freas C. Fuzzy restricted boltzmann machines. In: Melin P, Castillo O, Kacprzyk J, Reformat M, Melek W, editors. Fuzzy Logic in Intelligent System Design. Cham: Springer International Publishing; 2018. [Google Scholar]
- 18.Harrison R, McDermott M, Umoja C. Recognizing protein secondary structures with neural networks. In: 2017 28th International Workshop on Database and Expert Systems Applications (DEXA). DEXA: 2017. p. 62–8. 10.1109/DEXA.2017.29. [DOI]
- 19.Rhee S-Y, Gonzales MJ, Kantor R, Betts BJ, Ravela J, Shafer RW. Human immunodeficiency virus reverse transcriptase and protease sequence database. Nucleic Acids Res. 2003;31(1):298–303. doi: 10.1093/nar/gkg100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The protein data bank. Nucleic Acids Res. 2000;28(1):235–42. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, Blondel M, Prettenhofer P, Weiss R, Dubourg V, et al. Scikit-learn: Machine learning in python. J Mach Learn Res. 2011;12(Oct):2825–30. [Google Scholar]
- 22.McKinney W. Python for High Performance and Scientific Computing. Sebastopol: O’Reilly Media; 2011. pandas: a foundational python library for data analysis and statistics. [Google Scholar]
- 23.Abu-halaweh NM, Harrison RW. Practical fuzzy decision trees. In: 2009 IEEE Symposium on Computational Intelligence and Data Mining: 2009. p. 211–6. 10.1109/CIDM.2009.4938651. [DOI]
- 24.Wensing AMJ, van Maarseveen NM, Nijhuis M. Fifteen years of hiv protease inhibitors: raising the barrier to resistance. Antivir Res. 2010;85(1):59–74. doi: 10.1016/j.antiviral.2009.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The unprocessed datasets can be downloaded from: http://hivdb.stanford.edu/pages/genopheno.dataset.html