In macroautophagy (hereafter autophagy), cells form autophagosomes, transient double-membrane–bound compartments that engulf portions of the cytoplasm for targeted degradation in the lysosome/vacuole to maintain cellular homeostasis under basal and stress conditions. The de novo formation of autophagosomes is driven by hierarchical assembly and function of a sophisticated autophagy protein machinery, which mediates the substantial underlying membrane rearrangements (1, 2). Precursor membranes are nucleated into a single-membrane structure, termed the isolation membrane (IM) or phagophore, which dramatically expands in a cup-shaped manner and encapsulates proximal cargo of diverse size and nature. The closure of the IM results in the formation of an outer and inner vesicle, giving rise to the characteristic double-membrane autophagosome. The outer autophagosomal membrane then fuses with the lysosomal/vacuolar membrane, releasing the inner vesicle and enclosed cargo for degradation. The origin of the membrane lipids and the underlying mechanisms that drive the rapid expansion of IMs are still poorly understood.
The biogenesis of autophagosomes occurs in close association with specialized subdomains of the endoplasmic reticulum (ER), often in close proximity to ER contacts with other organelles (3–6). In mammals, the omegasome, a transient ER subdomain enriched for phosphatidylinsositol-3-phosphate (PI3P), closely enwraps the forming IM (7, 8). The IM in yeast is spatially linked to ER exit sites [(ERESs), ER subdomains dedicated to the generation of COPII transport vesicles] in a nonrandom orientation in which one or two ERESs seem to be tethered to the edge of the expanding IM (3, 9). Thus, physical association of the IM with the ER is an evolutionarily conserved feature of autophagosome formation. However, the identity and nature of potential protein tethers that establish and maintain IM–ER contacts during autophagosome biogenesis have been elusive.
In PNAS, Chowdhury et al. (10) report that the evolutionarily conserved ATG2 proteins adopt extended rod-shaped protein structures with the ability to bind and tether membranes in vitro, suggesting that ATG2 proteins may function as the sought-after tethering factors for autophagy in vivo.
ATG2 is an essential component of the autophagy machinery, and yeast and mammalian cells fail to form autophagosomes in its absence (11–13). However, the function of ATG2 in autophagy has been elusive. ATG2 forms a complex with Atg18/WIPI proteins, members of the PROPPIN protein family that bind to phosphoinositides and recruit ATG2 to the site of autophagosome formation (14). In addition, ATG2 also localizes to lipid droplets and affects their size and distribution (15, 16). Recruitment to forming autophagosomes and to lipid droplets is consistent with an inherent property of ATG2 to bind membranes. To gain insight into the function of ATG2, Chowdhury et al. (10) purified the human ATG2A protein in complex with its binding partner WIPI4. By performing negative-stain single-particle EM analysis, they describe a rod-shaped protein structure for ATG2A of around 200 Å in length and 30 Å in width. The binding partner WIPI4 flexibly connects in a 1:1 stoichiometry to one end, opposite to the N terminus of the extended ATG2A structure. The structural analysis of the yeast Atg2-Atg18 complex by Chowdhury et al. (10) and of the rat ATG2B-WIPI4 complex by Zheng et al. (17) demonstrate that the overall structure of Atg2-Atg18/WIPI complexes is evolutionarily conserved. Performing a detailed analysis, Chowdhury et al. (10) characterize the interactions between ATG2A and WIPI4 and map the N terminus and the CAD domain (a protein domain that contains a highly conserved cysteine–alanine–aspartic acid triad of unknown function) of ATG2A to opposite ends of the rod-shaped protein structure.
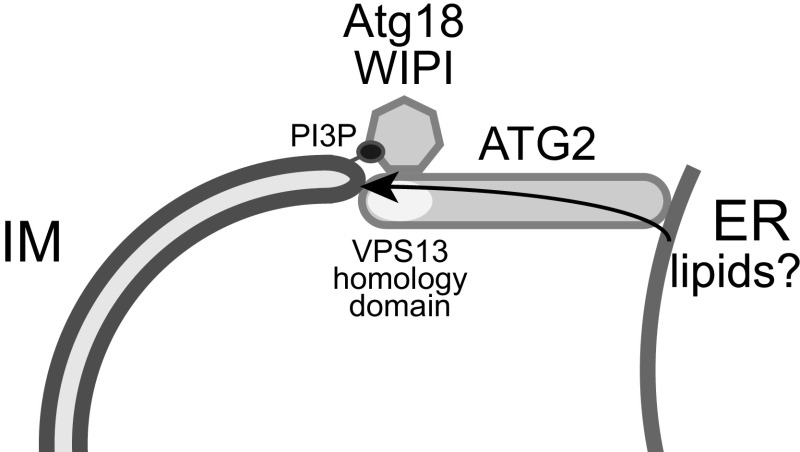
Given its observed membrane association, the extended protein structure of ATG2A with a length of ∼20 nm suggested that the ATG2A-WIPI4 complex can bridge the distances of 10 to 30 nm generally found at membrane contact sites and, thus, might function as a membrane tether (18). Indeed, Chowdhury et al. (10) observed that ATG2A alone is sufficient for homotypic tethering of small unilamellar vesicles by binding the vesicles with its N-terminal and CAD domain-containing tips in vitro. Significantly, they found that the complex of ATG2A and WIPI4 has the ability to specifically mediate membrane tethering of two populations of heterotypic large unilamellar vesicles, one of which contained PI3P, a phosphoinositide that is directly bound by WIPI4 and is essential for autophagosome biogenesis. Thus, these data show that the ATG2A-WIPI4 complex can bridge membranes with different identities, which may be relevant for the association of the IM with the ER in vivo (Fig. 1). This direct demonstration of the membrane tethering function of ATG2 is particularly exciting in light of recent in vivo data which showed that Atg2 plays a critical role in defining ER contact sites with the edge of the cup-shaped IM in coordination with Atg9 and Atg18 in yeast (19). Notably, an Atg2 variant that is defective for the physical interaction with Atg9 promoted the formation of IMs that were enwrapped by ER membranes, consistent with extensive membrane tethering (19).
Fig. 1.
The ATG2-Atg18/WIPI complex may tether forming autophagosomes (IM) to the ER and facilitate lipid transport.
Membrane contact sites are critical regions for lipid transport between different organelles. Recent evidence indicates that protein tethers not only physically connect two membranes but also directly mediate the transport of lipids from one organelle to the other (20, 21). For example, VPS13, a highly conserved protein, dynamically tethers the ER to either mitochondria, late endosomes, or lysosomes/vacuoles dependent on its physical interactions with specific adaptor proteins (20, 22). Very recent work has demonstrated that the N-terminal portion of VPS13 forms a tubular structure containing a hydrophobic cavity, which can solubilize and transport lipids between membranes (20). Excitingly, the N- and C-terminal regions of ATG2 share significant sequence and secondary structure homology with VPS13 (13, 20). In combination with the data from Chowdhury et al. (10), these observations raise the intriguing possibility that ATG2A functions as a membrane tether that, in collaboration with its adaptor protein WIPI4, establishes specific contact sites between the ER and the IM to transport lipids from the ER to the IM and to promote the growth of expanding IMs (Fig. 1). While these exciting hypotheses await thorough experimental testing in vitro and in vivo, the work from Chowdhury et al. and others (10, 17, 19) will likely pave the way toward gaining critical insights into the molecular functions of the ATG2-Atg18/WIPI complexes and the potential role of membrane contact sites in autophagy.
Footnotes
The author declares no conflict of interest.
See companion article on page E9792.
References
- 1.Lamb CA, Yoshimori T, Tooze SA. The autophagosome: Origins unknown, biogenesis complex. Nat Rev Mol Cell Biol. 2013;14:759–774. doi: 10.1038/nrm3696. [DOI] [PubMed] [Google Scholar]
- 2.Kraft C, Martens S. Mechanisms and regulation of autophagosome formation. Curr Opin Cell Biol. 2012;24:496–501. doi: 10.1016/j.ceb.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Graef M, Friedman JR, Graham C, Babu M, Nunnari J. ER exit sites are physical and functional core autophagosome biogenesis components. Mol Biol Cell. 2013;24:2918–2931. doi: 10.1091/mbc.E13-07-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Böckler S, Westermann B. Mitochondrial ER contacts are crucial for mitophagy in yeast. Dev Cell. 2014;28:450–458. doi: 10.1016/j.devcel.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Hamasaki M, et al. Autophagosomes form at ER-mitochondria contact sites. Nature. 2013;495:389–393. doi: 10.1038/nature11910. [DOI] [PubMed] [Google Scholar]
- 6.Nascimbeni AC, et al. ER-plasma membrane contact sites contribute to autophagosome biogenesis by regulation of local PI3P synthesis. EMBO J. 2017;36:2018–2033. doi: 10.15252/embj.201797006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Axe EL, et al. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol. 2008;182:685–701. doi: 10.1083/jcb.200803137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayashi-Nishino M, et al. A subdomain of the endoplasmic reticulum forms a cradle for autophagosome formation. Nat Cell Biol. 2009;11:1433–1437. doi: 10.1038/ncb1991. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki K, Akioka M, Kondo-Kakuta C, Yamamoto H, Ohsumi Y. Fine mapping of autophagy-related proteins during autophagosome formation in Saccharomyces cerevisiae. J Cell Sci. 2013;126:2534–2544. doi: 10.1242/jcs.122960. [DOI] [PubMed] [Google Scholar]
- 10.Chowdhury S, et al. Insights into autophagosome biogenesis from structural and biochemical analyses of the ATG2A-WIPI4 complex. Proc Natl Acad Sci USA. 2018;115:E9792–E9801. doi: 10.1073/pnas.1811874115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shintani T, Suzuki K, Kamada Y, Noda T, Ohsumi Y. Apg2p functions in autophagosome formation on the perivacuolar structure. J Biol Chem. 2001;276:30452–30460. doi: 10.1074/jbc.M102346200. [DOI] [PubMed] [Google Scholar]
- 12.Wang CW, et al. Apg2 is a novel protein required for the cytoplasm to vacuole targeting, autophagy, and pexophagy pathways. J Biol Chem. 2001;276:30442–30451. doi: 10.1074/jbc.M102342200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamura N, et al. Differential requirement for ATG2A domains for localization to autophagic membranes and lipid droplets. FEBS Lett. 2017;591:3819–3830. doi: 10.1002/1873-3468.12901. [DOI] [PubMed] [Google Scholar]
- 14.Proikas-Cezanne T, Takacs Z, Dönnes P, Kohlbacher O. WIPI proteins: Essential PtdIns3P effectors at the nascent autophagosome. J Cell Sci. 2015;128:207–217. doi: 10.1242/jcs.146258. [DOI] [PubMed] [Google Scholar]
- 15.Velikkakath AK, Nishimura T, Oita E, Ishihara N, Mizushima N. Mammalian Atg2 proteins are essential for autophagosome formation and important for regulation of size and distribution of lipid droplets. Mol Biol Cell. 2012;23:896–909. doi: 10.1091/mbc.E11-09-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfisterer SG, et al. Lipid droplet and early autophagosomal membrane targeting of Atg2A and Atg14L in human tumor cells. J Lipid Res. 2014;55:1267–1278. doi: 10.1194/jlr.M046359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng JX, et al. Architecture of the ATG2B-WDR45 complex and an aromatic Y/HF motif crucial for complex formation. Autophagy. 2017;13:1870–1883. doi: 10.1080/15548627.2017.1359381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gatta AT, Levine TP. Piecing together the patchwork of contact sites. Trends Cell Biol. 2017;27:214–229. doi: 10.1016/j.tcb.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Gómez-Sánchez R, et al. Atg9 establishes Atg2-dependent contact sites between the endoplasmic reticulum and phagophores. J Cell Biol. 2018;217:2743–2763. doi: 10.1083/jcb.201710116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar N, et al. VPS13A and VPS13C are lipid transport proteins differentially localized at ER contact sites. J Cell Biol. August 9, 2018 doi: 10.1083/jcb.201807019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawano S, et al. Structure-function insights into direct lipid transfer between membranes by Mmm1-Mdm12 of ERMES. J Cell Biol. 2018;217:959–974. doi: 10.1083/jcb.201704119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bean BDM, et al. Competitive organelle-specific adaptors recruit Vps13 to membrane contact sites. J Cell Biol. July 17, 2018 doi: 10.1083/jcb.201804111. [DOI] [PMC free article] [PubMed] [Google Scholar]