Significance
A recently licensed dengue vaccine requires pre-vaccination screening for dengue virus (DENV)-specific antibodies and was initially recommended for use only in areas with high DENV transmission. However, DENV transmission intensity is not static. We show that the age at which children acquired DENV-specific immunity more than doubled during the observational period (2004–2015) of a pediatric cohort study in Managua, Nicaragua. A high force of infection of DENV in the 1990s could be explained by the introduction of a novel DENV serotype, while a gradual decline in force of infection could be explained by a long-term population demographic transition. Such dynamics make DENV transmission intensity a problematic metric for designing, implementing, and evaluating interventions and for selecting target age groups/sites.
Keywords: dengue virus, force of infection, cohort study, transmission intensity, Nicaragua
Abstract
Dengue virus (DENV) is the most prevalent human vector-borne viral disease. The force of infection (FoI), the rate at which susceptible individuals are infected in a population, is an important metric for infectious disease modeling. Understanding how and why the FoI of DENV changes over time is critical for developing immunization and vector control policies. We used age-stratified seroprevalence data from 12 years of the Pediatric Dengue Cohort Study in Nicaragua to estimate the annual FoI of DENV from 1994 to 2015. Seroprevalence data revealed a change in the rate at which children acquire DENV-specific immunity: in 2004, 50% of children age >4 years were seropositive, but by 2015, 50% seropositivity was reached only by age 11 years. We estimated a spike in the FoI in 1997–1998 and 1998–1999 and a gradual decline thereafter, and children age <4 years experienced a lower FoI. Two hypotheses to explain the change in the FoI were tested: (i) a transition from introduction of specific DENV serotypes to their endemic transmission and (ii) a population demographic transition due to declining birth rates and increasing life expectancy. We used mathematical models to simulate these hypotheses. We show that the initial high FoI can be explained by the introduction of DENV-3 in 1994–1998, and that the overall gradual decline in the FoI can be attributed to demographic shifts. Changes in immunity and demographics strongly impacted DENV transmission in Nicaragua. Population-level measures of transmission intensity are dynamic and thus challenging to use to guide vaccine implementation locally and globally.
The dengue virus serotypes 1–4 (DENV-1–4) are antigenically related flaviviruses that cause the most prevalent mosquito-borne viral disease globally, resulting in up to 96 million dengue cases each year (1). DENV infection induces long-lived immunity against the infecting serotype, as well as immunity to heterologous DENV serotypes that may either provide protection or increase the severity of disease during secondary DENV infection, depending on preexisting antibody level and quality (2–6). This “double-edged sword” feature of dengue disease has been a major challenge to dengue vaccine development.
The currently licensed dengue vaccine, Dengvaxia, is protective in individuals with a history of previous DENV infection (“seropositives”) but can increase risk of severe dengue disease in DENV-naïve individuals (“seronegatives”) (7, 8). In 2016, Dengvaxia was recommended only for areas in which >70% of children age ≥9 years were seropositive for DENV. These recommendations were based on modeling studies using Phase 3 efficacy data showing that in populations with low DENV transmission intensity, which is often measured by the rate of DENV seroconversion in children, vaccination would cause more cases of severe dengue in seronegative individuals than would be averted in seropositive individuals (9–11). Newly released data have shown that Dengvaxia significantly increases the risk of severe dengue in seronegative individuals regardless of age (12). The World Health Organization (WHO) now recommends Dengvaxia only for DENV-seropositive individuals, requiring screening by individual-level serological testing of all potential vaccine recipients (12).
The force of infection (FoI), the rate at which susceptible individuals become infected in a population, is a measure of transmission intensity important for evaluating the impact of vaccination programs and other interventions (13). Initially, for Dengvaxia, knowledge about both the FoI and age-specific DENV seroprevalence was needed to ensure that more individuals benefited than were harmed by vaccination; this information is needed under the current recommendation to select target populations for serologic screening. However, the FoI of DENV exhibits extensive global and local heterogeneity within countries, and even within cities, making vaccination strategies based on transmission intensity problematic (12, 14, 15). In addition, the FoI can vary within a given place over time and by age group. To differentiate age-specific from annual differences in FoI, longitudinal age-stratified incidence data or longitudinal serologic surveys are needed. However, most studies are limited to estimating the FoI from single serosurveys, which requires assuming that transmission is either age-independent or constant over time (13). Estimation of an average FoI may mask important dynamics that could affect the relative risk and benefit of dengue vaccination in a given population.
Several studies have used longitudinal data to estimate annual and age-specific transmission intensity, revealing important changes in DENV transmission over time (16–20). Analysis of longitudinal serologic data demonstrated that the introduction of DENV-3 and later DENV-4 into Iquitos, Peru, resulted in larger epidemics than had been observed for endemic serotypes (20). An observed shift in the age of DENV infection from older to younger ages in Brazil was attributed to the sequential reemergence of DENV serotypes after many years of absence; as older individuals were already immune, only younger individuals not exposed to previously circulating serotypes were susceptible to infection (16). Others have observed a decades-long decline in the FoI of DENV attributable to improved vector control in Singapore (17). Finally, a demographic transition, with declining birth and death rates, was identified as a determinant of declining FoI of DENV in Thailand (18, 19).
Many of the determinants of changes in DENV transmission intensity have also occurred in Managua, Nicaragua. The first reported dengue epidemic in Nicaragua was recorded in 1985, when DENV-1 was introduced after a long absence of dengue from the Americas (21). DENV-2 arrived in Nicaragua in 1991. DENV-4 may have circulated in the early 1990s and was isolated in 1999 from a likely imported case, but there is little evidence that it currently circulates endemically (22). DENV-3 was introduced into Nicaragua in 1994–1995 and in 1998 caused the largest dengue epidemic recorded to date in the country (22–24). Nicaragua has also experienced a demographic shift in the last 70 y; from 1994 to 2015, crude birth rates decreased from 32 births to 20 births per 1000 inhabitants and life expectancy increased from 67 y to 75 y (25–27). Furthermore, the population of Nicaragua grew from 4.1 million in 1990 to 6.1 million by 2015 (25).
Here we estimated the annual and age-specific FoI of DENV in the Pediatric Dengue Cohort Study (PDCS) in Managua, Nicaragua, from 1994 to 2015 and explored different hypotheses for why the FoI changed over time. We fit statistical models to age-stratified seroprevalence data and found years with very high FoI in the 1990s and an underlying trend of decline over time. Using mathematical models, we found that the introduction of a new DENV serotype can explain the high FoI in the late 1990s, whereas a long-term population demographic transition can explain the decline in the FoI.
Results
DENV Serotype Dominance Varied Across Years.
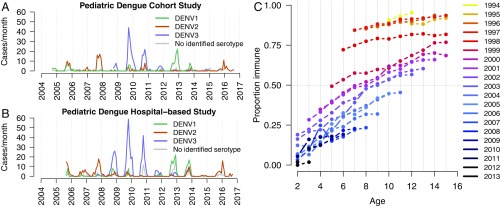
Before the start of the PDCS, a large DENV-3 epidemic occurred from 1994 to 1998, followed by DENV-2 (1999–2001) and DENV-1 (2002–2004) (22–24). Dengue cases were seen throughout the observational period of the PDCS (2004–2015; n = 625). Serotype dominance cycled among DENV-1, DENV-2, and DENV-3, with serotype dominance lasting for 3–4 y (Fig. 1A). DENV-3 was not isolated in either study until 2007; subsequently, DENV3 proceeded to cause large epidemics in the cohort from 2009 to 2011 (Fig. 1A). The dynamics of serotypes observed in the PDCS are similar to the dynamics of dengue cases in the Pediatric Dengue Hospital-Based Study (PDHS; n = 1007) (Fig. 1B).
Fig. 1.
Dengue cases and DENV-specific seroprevalence in Managua, Nicaragua. (A and B) Monthly dengue cases caused by DENV-1, DENV-2, and DENV-3 or DENV that could not be serotyped in the PDCS (A) and the PDHS (B). (C) Age-stratified DENV seroprevalence by birth cohort (children born in the same year) for the PDCS.
DENV Seroprevalence Changed Across Birth Cohorts.
We first evaluated the change in seroprevalence in the PDCS for each birth cohort (i.e., group of children born within the same year), which ranged from 1994 to 2013 (Fig. 1C). Children in cohorts born between 1994 and 1997 reached higher levels of DENV-specific immunity at younger ages than children born in later birth cohorts. The decline in seroprevalence across birth cohorts was relatively gradual, except for the large differences in seroprevalence for those born in or before 1997, those born in 1998, and those born in or after 1999.
The Age at Which Children Became DENV-Seropositive Increased over Time.
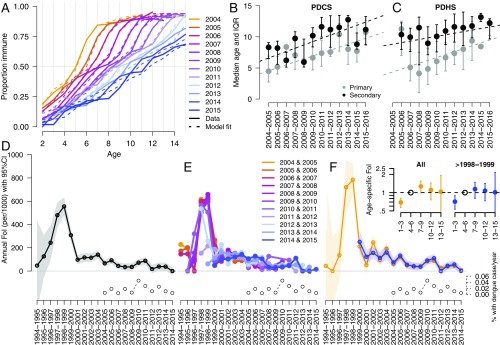
Age-stratified seroprevalence data from the PDCS revealed a significant increase in the age at which children acquired DENV-specific immunity from 2004 to 2015 (Fig. 2A and SI Appendix, Fig. S1 and Table S1; n = 41,302 seroprevalence measurements). The age at which 50% seroprevalence was observed in 2015 was 2.5 times (95% CI, 2.4–2.7) greater than that observed in 2004 (P < 0.001). In 2004, 50% of children age 4.4 y (95% CI, 4.3–4.6) were seropositive, with higher levels of seropositivity in children age >4 y. The age at which children became seropositive to DENV gradually increased over this time, such that by 2015, only 50% of children age 11.2 y (95% CI, 10.8–11.6) were seropositive, with higher levels of seropositivity in children age >11 y.
Fig. 2.
Age at DENV infection in the PDCS and PDHS and estimates of the FoI of DENV in the PDCS. (A) Age-stratified seroprevalence from 2004 to 2015 in the PDCS (shown as thick lines) and the estimates of seroprevalence based on the model with best performance (annual + age model with five age groups, shown as dotted lines). (B and C) Median and interquartile ranges for primary and secondary dengue cases by year in the PDCS (B) and the PDHS (C). Linear regression lines show the relationship of age at primary and secondary dengue across years. All regression models had slopes statistically significantly different from 0 at P < 0.001. (D) Estimates of annual FoI of DENV and 95% confidence intervals based on the annual only FoI model applied to PDCS age-stratified seroprevalence data. (E) Estimates of annual FoI of DENV based on fitting age-stratified seroprevalence data from only 2 y of the PDCS per model. The years used for each model are indicated in the legend. (F) Estimates of the annual and age-specific FoI of DENV based on models that provided the best fit (SI Appendix, Tables S3 and S4) of age-stratified seroprevalence data from all children (orange lines) or only those born after 1998–1999 (blue lines). The percentage of the PDCS subjects who experienced dengue (includes all symptomatic DENV infections) each epidemic season is indicated in the bottom right corners of D–F.
Consistently, the median age of primary and secondary dengue cases also increased over this period in the PDCS (Fig. 2B). The age of primary dengue (n = 299) increased by 0.47 y (95% CI, 0.34–0.61 y) per epidemic season (with epidemic season is defined as July 1 to June 30 of the next year), while the age of secondary dengue (n = 326) increased by 0.53 y (95% CI, 0.41–0.64 y) per epidemic season. This trend was also observed for hospitalized dengue in the PDHS (Fig. 2C), which includes patients from across Managua: primary dengue cases increased in age by 0.47 y per epidemic season (95% CI, 0.31–0.63), n = 464; secondary dengue cases increased in age by 0.25 y per epidemic season (95% CI, 0.15–0.34), n = 543.
There Was a Peak in the FoI of DENV in 1997–1998 and 1998–1999 but an Underlying Gradual Decline in FoI.
We used 12 y of age-stratified seroprevalence data from the PDCS to estimate the FoI (total for all DENV serotypes) for each epidemic season from 1994–1995 to 2014–2015 (Fig. 2D and SI Appendix, Table S2). We found substantial variability in the FoI over this period. A very high FoI was observed in the 1997–1998 (480 per 1000; 95% CI, 371–589) and 1998–1999 (555 per 1000; 95% CI, 478–633) epidemic seasons (Fig. 2D). After 1999, we observed annual fluctuations but an underlying subtle decline in the FoI; after 1999, the maximum FoI occurred in 2010–2011: 88 per 1000 (95% CI, 57–119). As expected, greater FoI values were observed during years in which a larger proportion of the PDCS had symptomatic dengue (Fig. 2D). We also estimated annual FoI using only 2 y of seroprevalence data per model (Fig. 2E) and found that years in which seroprevalence data included children born in or before 1997–1999 show strong agreement that a large epidemic occurred between 1997 and 1999. Annual FoI estimates between 2000 and 2015 fluctuated depending on the seroprevalence data used for the model; however, overall, the FoI declined during this period.
The FoI of DENV Differed by Age.
We also tested for differences in the annual FoI of DENV by age. We extended the annual FoI model to allow for variation in FoI by age group (SI Appendix, Table S3; AIC values and likelihood ratios test values are presented). Because all children born before 1998–1999 were between age 1 and 5 y during the 1997–1999 epidemics, we created models including and excluding their seroprevalence data. The most parsimonious models had five age groups, both when fit to data from all children (AIC = 1004; annual only FoI model, AIC = 1168; SI Appendix, Tables S3 and S4) and when fit to data from only children born after 1998–1999 (AIC = 797; annual only FoI model, AIC = 908; SI Appendix, Tables S3 and S4). These models showed similar dynamics in annual FoI (Fig. 2F) and significantly lower FoI for children age <4 y (Fig. 2F, Inset). These findings suggest that young children experienced a lower FoI of DENV throughout the observational period.
R0 Estimates Were Within Range of Previous Estimates for DENV.
We estimated R0 of DENV using the mean of the FoI estimated from the PDCS [2.49 (95% CI, 1.64–3.42)] (SI Appendix, Method 1), as well as with the annual FoI estimates [2.28 (95% CI, 1.60–2.91)] (SI Appendix, Method 2). These values are within the range of previous R0 estimates for DENV from seroprevalence studies (14).
The Change from Epidemic-to-Endemic DENV Transmission and a Demographic Transition Could Explain the Decline in FoI of DENV.
We hypothesized that the introduction of DENV-3 in the mid-1990s resulted in a sharp spike in the FoI, with the majority of the population acquiring long-lived immunity against DENV-3 and temporary cross-protection against DENV-1 and DENV-2. Although DENV-4 is said to have circulated in the 1990s (22), few RT-PCR–positive DENV-4 infections have been observed since 1999, and thus DENV-4 was not explicitly modeled. Thereafter, primarily young individuals born after the DENV-3 epidemic were susceptible to subsequent DENV-3 infections. This transition from epidemic-to-endemic transmission may explain the high FoI observed from 1997 to 1999. We expect that, as in Thailand (18, 19), the gradual reduction in seropositivity in children born after 1999 is the result of a demographic transition (SI Appendix, Fig. S2) gradually increasing the age at which children acquired DENV-specific immunity.
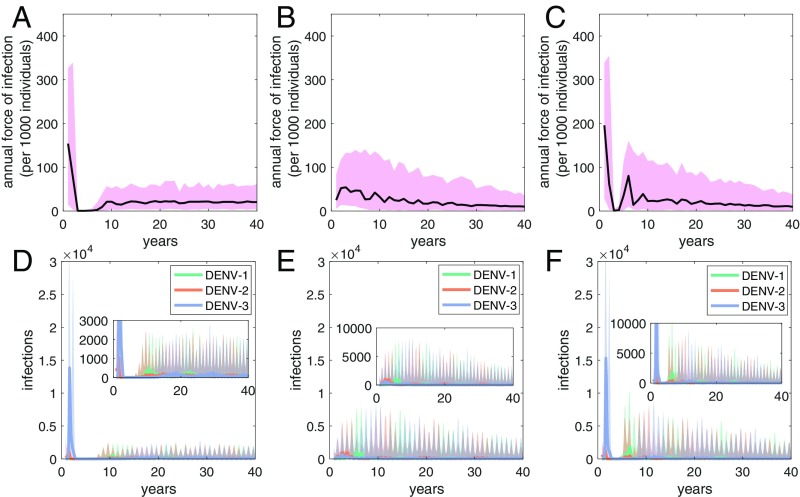
We simulated three mathematical models to determine whether an epidemic-to-endemic transition, a demographic transition, or both could recover the observed patterns in the FoI estimates for the PDCS (Fig. 3). The epidemic-to-endemic transition modeled by the introduction of DENV-3 into the DENV-3 naïve population recovered the dramatically high FoI observed in the late 1990s (Fig. 3A). A trend toward a gradual decline in the FoI was recovered by the demographic transition (Fig. 3B). Therefore, using these models, the epidemic-to-endemic transition and the demographic transition could recover the spike in FoI, followed by the subsequent gradual decrease in FoI (Fig. 3C).
Fig. 3.
Mathematical model simulations. (A and D) Model 1: epidemic-to-endemic transition. (B and E) Model 2: demographic transition. (C and F) Model 3: epidemic-to-endemic transition and demographic transition. (A–C) Simulation estimates of the FoI of DENV. Black lines show median values of FoI simulations. Pink shaded regions show 90% credible intervals of the stochastic simulations. (D–F) Simulation predictions of total numbers of DENV-infected individuals. Bolded lines show median values of FoI simulations. Shaded regions show 90% credible intervals of the stochastic simulations.
Fig. 3 D–F shows the corresponding transmission dynamics of infected individuals for these three models. Model dynamics showed that each serotype predominated for 3–5 y. The epidemic-to-endemic transition resulted in a large simulated DENV-3 epidemic (Fig. 3 D and F), followed by a short period without any new infections for approximately 3 y (Fig. 3 D and F). This period was then followed by large DENV-1 and DENV-2 epidemics, and subsequent endemic dynamics of DENV-1, DENV-2, and DENV-3 cocirculation. The decrease in FoI due to declining birth and death rates resulted in fewer infected individuals overall (Fig. 3 E and F), despite the growing population size underlying this model. Model 1 predicted a period of about 13 y between the introduction of DENV-3 in a DENV-3 naïve population and a subsequent DENV-3 epidemic (Fig. 3D). Inclusion of the demographic transition (Model 3) predicted a shorter period of approximately 11 y between the new DENV-3 epidemic and the subsequent epidemic (Fig. 3F), similar to the 10-y period devoid of DENV-3 infections seen in Nicaragua. When we assumed that tertiary infections were transmissible, the overall dynamics of the models were similar. However, in Models 1 and 3, the magnitude of the initial DENV-3 epidemic and the subsequent DENV-2 and DENV-1 epidemics were larger, and the subsequent DENV-3 epidemic occurred earlier (SI Appendix, Fig. S3).
Discussion
In this study, we estimated the FoI of DENV using annual seroprevalence data from Managua, Nicaragua. We identified two main qualitative changes in the FoI of DENV from 1994 to 2015: (i) a dramatically high FoI in the late 1990s and (ii) a subsequent gradual decrease in FoI. Using simulated mathematical models, we demonstrated that both an epidemic-to-endemic transition and a demographic transition are sufficient to recover the observed dynamics of the FoI of DENV. We also found that younger children experienced a lower FoI of DENV.
Peaks and troughs in estimates of the FoI of DENV were consistent with salient features of the DENV epidemiologic dynamics in the PDCS. The introduction of DENV-3 in the 1990s likely resulted in a high FoI (just over 50% of susceptible individuals were infected in a single year) compared with later years, because a larger fraction of individuals were susceptible to DENV-3. In addition, the second epidemic of DENV-3 in 1998 was larger than when DENV-3 was first introduced into Nicaragua in 1994–1995, consistent with the epidemic dynamics observed when DENV-3 was introduced into Iquitos, Peru (20). It is likely that DENV-3 later reemerged in Nicaragua when enough births occurred to replenish the population susceptible to DENV-3. Estimated values of the FoI showed a small peak in 2009–2010 and 2010–2011, consistent with the reintroduction of DENV-3, which resulted in a large dengue epidemic. The mathematical model including both the epidemic and endemic transition and the demographic transition was able to recover the approximate10-y period in which DENV-3 was absent from Nicaragua.
Estimates of seropositivity in Managua, Nicaragua for children from 2001 to 2003 showed an extremely high seroprevalence, with 80% of children age ≥5 y positive by DENV inhibition ELISA (iELISA) (28). The high seroprevalence was originally attributed to the age at which children began school and thus had more mobility and vector exposure. A later modeling study of these data provided further support for this theory, identifying differences in the FoI experienced by younger and older (age >3.9 y) children (14). In the present study, with additional serologic data from later years, we were able to estimate annual and age-specific FoI over longer periods. While the most dramatic changes in FoI were attributable to the DENV-3 epidemic-to-endemic and demographic transitions, there was still a signal for lower FoI of DENV in young children. Consistent with this observation, when Aedes aegypti-borne chikungunya virus (CHIKV) was introduced to Nicaragua in 2014, seropositivity increased with age in the pediatric population (29), suggesting greater exposure to the vector at older ages.
Our simulations indicate that the gradual decline in FoI can be explained by a demographic transition. While the population size in Nicaragua has been increasing, the decline in birth rates results in lower numbers of young individuals susceptible to infection, and longer average lifespans result in a larger proportion of the population immune to DENV infection. Decreases in birth rates specifically have been shown to reduce the mean transmission rate (30), thereby decreasing FoI and increasing the mean age of infection. In the past 20 y, an increase in the mean age of dengue has been documented in Southeast Asia (18), consistent with shifts toward lower birth rates and longer life-expectancy. Model simulations of a demographic transition also predicted a decline in the total number of DENV infections that outweighed the effect of additional cases due to growing population size. This effect was not detectable in incidence data in the PDCS or PDHS. However, both studies are limited to children, and the incidence of dengue may be changing across the entire population. However, a study in Thailand found that while the FoI of DENV decreased over time, the population size increased, and thus the total number of cases remained constant or increased (19).
The dramatic change in DENV seroprevalence in Managua, Nicaragua over just 12 y highlights the problem with using population-level immunity to guide vaccine implementation to, for instance, lower the incidence of vaccine-associated severe dengue disease, as recommended by the WHO's Strategic Advisory Group of Experts (SAGE) in 2016 for the dengue vaccine Dengvaxia (9). Using the 2016 SAGE criteria, in 2004, Managua would have been deemed an appropriate location for Dengvaxia use. However, seroprevalence declined so rapidly that after 2012, the same population would be overall negatively impacted by Dengvaxia (10, 11). The change in FoI was driven by epidemiologic and demographic factors present in many dengue-affected areas (16–20).
Because vaccination impact is measured in case reduction over 10–30 y, such FOI dynamics can shape the impact and safety of a vaccination program over time (10, 11). Experts no longer consider it feasible or acceptable to use a population-level cut off for seroprevalence and age in recommending Dengvaxia use, and following the reconvening of SAGE in 2018, Dengvaxia is now recommended for use only when individual-level DENV seropositivity can be tested (12). Changes in the FoI of DENV still affect vaccination strategies based on individual screening (12), as the optimal age for screening increases with declining FoI. In countries with declining FoI such as Nicaragua, more adults will continue to develop dengue and possibly severe dengue. Prescreening and vaccination of the fraction of the population that benefits from the dengue vaccines could improve dengue disease outcomes.
This study has several limitations. We had to infer FoI from years when older children were alive, but before enrollment in the cohort, meaning that their seroprevalence data were fit with more free parameters. We did not have complete serotype-specific neutralization data, which others have used to estimate serotype-specific FoI or R0 (13, 20). However, serotype-specific neutralization data do not directly provide information on serotype-specific seroprevalence because of extensive cross-reactivity. Thus, even if such data were available, challenges in estimation of serotype-specific FoI would remain. In addition, because the PDCS and PDHS include only children, we could not reliably estimate FoI from age-specific incidence data, as estimation of the FoI from age-specific incidence data requires incidence for all ages. In addition, while a demographic transition and serotype introduction were sufficient to explain observed trends in the FoI of DENV, we cannot prove that they are causal determinants. Other factors that could affect FoI were not included in our simulation models, such as vector control. Because CHIKV (29) and Zika virus (ZIKV) caused enormous epidemics in the PDCS when they were introduced in 2014–2015 and 2016, respectively, we do not believe that vector control fully accounts for the decline in FoI. However, CHIKV and ZIKV were novel invading pathogens and may have had different transmission dynamics. The effect of vector control and other factors could be investigated in future studies.
Our results suggest that changes in both immunity and demographics over time have substantially impacted DENV transmission intensity in Nicaragua, highlighting how multiple processes need to be considered when enacting effective dengue control policies, particularly dengue vaccination programs.
Materials and Methods
Ethics Statement.
The protocols for the PDCS and the PDHS were reviewed and approved by the Institutional Review Boards of the University of California Berkeley and the Nicaraguan Ministry of Health. Parents or legal guardians of all subjects provided written informed consent. Subjects age ≥6 y provided assent.
PDCS.
The PDCS is an ongoing prospective cohort study consisting of approximately 3,700 children ages 2–14 in District II of Managua, Nicaragua (31). All children enrolled live in the catchment area of the Health Center Socrates Flores Vivas (HCSFV), 17 neighborhoods (15,818 households and 79,090 inhabitants in 2017) in Managua (population 1.4 million), the capital of Nicaragua. Initial enrollment of children age 2–9 y occurred in August 2004. After 2006, children were invited to remain in the study through age 14 y. Every year, a new cohort of 2-y-old children is enrolled in the PDCS, along with children of other ages as needed to maintain the balanced age structure of the study. For the study period of 2004–2015, a total of 6,684 children contributed to annual seroprevalence data (median of 6 y), with 2,826–3,835 children (SI Appendix, Table S1) contributing to seroprevalence data any given year. For all models, we used the ages of children on July 1 of each year, immediately before the start of the dengue epidemic season (DENV is generally transmited from July to February). Ages were rounded to an integer value to bin them by epidemic seasons experienced (some children age 15 y are shown due to rounding). Age groups with <20 children were excluded when estimating the FoI (SI Appendix, Fig. S1 and Table S1).
Age-Stratified Seroprevalence Data.
Each year, a healthy blood sample is collected from all participants in the PDCS and tested for DENV-specific antibodies using a DENV Inhibition ELISA (iELISA), as described previously (6, 28, 32). In brief, serial dilutions of serum or plasma are tested for their ability to block peroxidase-conjugated anti-DENV antibodies from binding a mixture of DENV-1–4 antigens. For each sample, a 50% iELISA titer was estimated relative to a negative control.
Symptomatic Dengue Cases.
PDCS children receive their primary health care at the HCSFV. Participants report to the HCSFV at first sign of fever, and the study team collects acute (days 1–5 since symptom onset) and convalescent (days 14–21) blood samples from children with suspected dengue cases according to the WHO case definition (33) and undifferentiated febrile illnesses, along with clinical information on standardized forms (31). A total of 625 dengue cases were studied.
A separate study, the PDHS, consists of children age 6 mo to 14 y old from across Managua who presented to the National Pediatric Reference Hospital, Hospital Infantil Manuel de Jesús Rivera with suspected dengue (33, 34). Dengue cases between August 2005 and August 2016 for which an acute and convalescent sample was collected were included in this analysis (n = 1,007).
The samples are processed at the Nicaraguan National Virology Laboratory of the Ministry of Health. Dengue cases are identified by testing acute-phase serum/plasma samples for DENV-1–4 using serotype-specific RT-PCR and virus isolation and analyzing paired acute- and convalescent-phase blood samples for seroconversion in the DENV MAC-ELISA and seroconversion or a fourfold or greater rise in antibodies in the DENV iELISA (31, 33). In the PDCS, dengue cases were defined as primary in children without previous DENV infection or antibodies during their time in the cohort and secondary in children with previous DENV infection or antibodies. In the PDHS, primary and secondary dengue cases were defined by convalescent iELISA titers of <2,560 and ≥2,560, respectively (33, 34).
Force of Infection.
We estimated the annual FoI using methods similar to those described by Ferguson et al. (13) using seroprevalence data from the PDCS. We extended this model to estimate age-specific FoI by age group. The models are described in SI Appendix.
Summary Statistics of Seroprevalence and Incidence Data.
We estimated the age at which 50% seroprevalence was observed each year with a two-parameter logistic model. The relationship between age at primary or secondary dengue and epidemic season in the PDCS and PDHS was estimated with linear regression. Slopes with 95% CIs are shown.
Basic Reproduction Number (R0).
We estimated R0 using FoI estimates based on the seroprevalence data (annual only FoI model). We made the simplifying assumption that R0 does not differ by serotype and used a similar approach as that described by Ferguson et al. (13) (SI Appendix).
Mathematical Model Simulations.
We simulated three mathematical models to test whether a transition from serotype introduction to endemic transmission (Model 1), a demographic transition to lower birth rates, increased life expectancy, and increasing population size (Model 2), or a combination of both hypotheses (Model 3) qualitatively reproduced the temporal patterns of the FoI of DENV observed for the PDCS. These models were based on an existing dengue epidemiologic model (35) that assumes lifelong immunity to reinfection with a homologous DENV serotype and temporary cross-protection between heterologous serotypes. To model the epidemic-to-endemic transition, we first simulated a two-serotype model to endemic equilibrium and then simulated the introduction of a third DENV serotype. Only three serotypes were modeled because the literature and available data suggest that DENV-4 does not circulate endemically in Nicaragua. To model the demographic transition, we simulated a three-serotype model with decreasing birth and death rates and increasing population size. Estimates of age-specific population sizes for Nicaragua were acquired from the United Nations website (25) (SI Appendix, Fig. S2). Birth rates (27) and life expectancy (26) were obtained from the World Bank website. To model both hypotheses simultaneously, we simulated a two-serotype model to endemic equilibrium and then simulated the introduction of a third serotype and simultaneously decreased in birth and death rates. Model equations and further details are provided in SI Appendix.
Supplementary Material
Acknowledgments
We thank past and present members of the study team based at the Centro de Salud Sócrates Flores Vivas, the National Virology Laboratory in the Centro Nacional de Diagnóstico y Referencia, and the Sustainable Sciences Institute for their dedication and high-quality work; Hope Biswas for her initial analysis of seroprevalence data; and Michael Baumer for his helpful advice on the FoI models. We also thank the study participants and their families. This research was funded by National Institutes of Health (NIH) Grant P01 AI106695 (to E.H.). The PDCS was supported by NIH Grants P01 AI106695 (to E.H.), U19 AI118610 (to E.H.), and R01 AI099631 (to A.B.), as well as Pediatric Dengue Vaccine Initiative Grant VE-1 (to E.H.) and a FIRST Grant (to E.H.), both from the Bill and Melinda Gates Foundation. The PDHS was supported by NIH Grants AI65359 (to A.B.) and AI62100 (to E.H. and A.B.).
Footnotes
Conflict of interest statement: E.H. served on the Scientific Advisory Board of Sanofi Pasteur during the phase 3 vaccine trials (until June 2015), and her laboratory received research funds from Takeda Vaccines to analyze samples from vaccine recipients.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1809253115/-/DCSupplemental.
References
- 1.Bhatt S, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katzelnick LC, Montoya M, Gresh L, Balmaseda A, Harris E. Neutralizing antibody titers against dengue virus correlate with protection from symptomatic infection in a longitudinal cohort. Proc Natl Acad Sci USA. 2016;113:728–733. doi: 10.1073/pnas.1522136113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buddhari D, et al. Dengue virus neutralizing antibody levels associated with protection from infection in Thai cluster studies. PLoS Negl Trop Dis. 2014;8:e3230. doi: 10.1371/journal.pntd.0003230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halstead SB, O’Rourke EJ. Dengue viruses and mononuclear phagocytes, I: Infection enhancement by non-neutralizing antibody. J Exp Med. 1977;146:201–217. doi: 10.1084/jem.146.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halstead SB. In vivo enhancement of dengue virus infection in rhesus monkeys by passively transferred antibody. J Infect Dis. 1979;140:527–533. doi: 10.1093/infdis/140.4.527. [DOI] [PubMed] [Google Scholar]
- 6.Katzelnick LC, et al. Antibody-dependent enhancement of severe dengue disease in humans. Science. 2017;358:929–932. doi: 10.1126/science.aan6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hadinegoro SR, et al. CYD-TDV Dengue Vaccine Working Group Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med. 2015;373:1195–1206. doi: 10.1056/NEJMoa1506223. [DOI] [PubMed] [Google Scholar]
- 8.Halstead SB. Dengvaxia sensitizes seronegatives to vaccine enhanced disease regardless of age. Vaccine. 2017;35:6355–6358. doi: 10.1016/j.vaccine.2017.09.089. [DOI] [PubMed] [Google Scholar]
- 9.SAGE Working Group on Dengue Vaccines and World Health Organization Secretariat 2016. Background paper on dengue vaccines prepared by the SAGE Working Group on Dengue (World Health Organization, Geneva)
- 10.Flasche S, et al. The long-term safety, public health impact, and cost-effectiveness of routine vaccination with a recombinant, live-attenuated dengue vaccine (Dengvaxia): A model comparison study. PLoS Med. 2016;13:e1002181. doi: 10.1371/journal.pmed.1002181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferguson NM, et al. Benefits and risks of the Sanofi-Pasteur dengue vaccine: Modeling optimal deployment. Science. 2016;353:1033–1036. doi: 10.1126/science.aaf9590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.SAGE Working Group on Dengue Vaccines and World Health Organization Secretariat 2018. Background paper on dengue vaccines (World Health Organization, Geneva)
- 13.Ferguson NM, Donnelly CA, Anderson RM. Transmission dynamics and epidemiology of dengue: Insights from age-stratified sero-prevalence surveys. Philos Trans R Soc Lond B Biol Sci. 1999;354:757–768. doi: 10.1098/rstb.1999.0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imai N, Dorigatti I, Cauchemez S, Ferguson NM. Estimating dengue transmission intensity from sero-prevalence surveys in multiple countries. PLoS Negl Trop Dis. 2015;9:e0003719. doi: 10.1371/journal.pntd.0003719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imai N, Dorigatti I, Cauchemez S, Ferguson NM. Estimating dengue transmission intensity from case-notification data from multiple countries. PLoS Negl Trop Dis. 2016;10:e0004833. doi: 10.1371/journal.pntd.0004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez-Barraquer I, et al. From re-emergence to hyperendemicity: The natural history of the dengue epidemic in Brazil. PLoS Negl Trop Dis. 2011;5:e935. doi: 10.1371/journal.pntd.0000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egger JR, et al. Reconstructing historical changes in the force of infection of dengue fever in Singapore: Implications for surveillance and control. Bull World Health Organ. 2008;86:187–196. doi: 10.2471/BLT.07.040170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cummings DAT, et al. The impact of the demographic transition on dengue in Thailand: Insights from a statistical analysis and mathematical modeling. PLoS Med. 2009;6:e1000139. doi: 10.1371/journal.pmed.1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodríguez-Barraquer I, et al. Revisiting Rayong: Shifting seroprofiles of dengue in Thailand and their implications for transmission and control. Am J Epidemiol. 2014;179:353–360. doi: 10.1093/aje/kwt256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reiner RC, Jr, et al. Time-varying, serotype-specific force of infection of dengue virus. Proc Natl Acad Sci USA. 2014;111:E2694–E2702. doi: 10.1073/pnas.1314933111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kouri G, et al. [Dengue epidemic in Nicaragua, 1985] Rev Inst Med Trop São Paulo. 1991;33:365–371. Spanish. [PubMed] [Google Scholar]
- 22.Guzmán MG, et al. [Dengue in Nicaragua, 1994: Reintroduction of serotype 3 in the Americas] Bol Oficina Sanit Panam. 1996;121:102–110. Spanish. [PubMed] [Google Scholar]
- 23.Harris E, et al. Clinical, epidemiologic, and virologic features of dengue in the 1998 epidemic in Nicaragua. Am J Trop Med Hyg. 2000;63:5–11. doi: 10.4269/ajtmh.2000.63.5. [DOI] [PubMed] [Google Scholar]
- 24.Brathwaite Dick O, et al. The history of dengue outbreaks in the Americas. Am J Trop Med Hyg. 2012;87:584–593. doi: 10.4269/ajtmh.2012.11-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.United Nations Department of Economic and Social Affairs Population by age groups–Both sexes. Available at https://esa.un.org/unpd/wpp/Download/Standard/Population/. Accessed July 22, 2016.
- 26. World Bank. Life expectancy at birth, total (years). Available at https://data.worldbank.org/indicator/SP.DYN.LE00.IN?end=2016&locations=NI. Accessed June 1, 2017.
- 27. World Bank. Birth rate, crude (per 1,000 people), Nicaragua. Available at https://data.worldbank.org/indicator/SP.DYN.CBRT.IN?locations=NI. Accessed June 1, 2017.
- 28.Balmaseda A, et al. High seroprevalence of antibodies against dengue virus in a prospective study of schoolchildren in Managua, Nicaragua. Trop Med Int Health. 2006;11:935–942. doi: 10.1111/j.1365-3156.2006.01641.x. [DOI] [PubMed] [Google Scholar]
- 29.Gordon A, et al. Differences in transmission and disease severity between two successive waves of chikungunya. Clin Infect Dis. 2018:2–32. doi: 10.1093/cid/ciy356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Earn DJ, Rohani P, Bolker BM, Grenfell BT. A simple model for complex dynamical transitions in epidemics. Science. 2000;287:667–670. doi: 10.1126/science.287.5453.667. [DOI] [PubMed] [Google Scholar]
- 31.Kuan G, et al. The Nicaraguan Pediatric Dengue Cohort Study: Study design, methods, use of information technology, and extension to other infectious diseases. Am J Epidemiol. 2009;170:120–129. doi: 10.1093/aje/kwp092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernández RJ, Vázquez S. Serological diagnosis of dengue by an ELISA inhibition method (EIM) Mem Inst Oswaldo Cruz. 1990;85:347–351. doi: 10.1590/s0074-02761990000300012. [DOI] [PubMed] [Google Scholar]
- 33.Gutiérrez G, et al. Evaluation of the diagnostic utility of the traditional and revised WHO dengue case definitions. PLoS Negl Trop Dis. 2013;7:e2385. doi: 10.1371/journal.pntd.0002385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Narvaez F, et al. Evaluation of the traditional and revised WHO classifications of dengue disease severity. PLoS Negl Trop Dis. 2011;5:e1397. doi: 10.1371/journal.pntd.0001397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagao Y, Koelle K. Decreases in dengue transmission may act to increase the incidence of dengue hemorrhagic fever. Proc Natl Acad Sci USA. 2008;105:2238–2243. doi: 10.1073/pnas.0709029105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.