Significance
Many organisms, from plants to humans, have long evolutionary histories with nematode worms. Historically these relationships have been assumed to be detrimental—as with intestinal or blood-borne parasites—or at best innocuous, to their hosts. However, this paradigm has been challenged recently, for example, with findings in mammals that worms can modulate their host’s immune system and thereby thwart autoimmune disease. In our study, we describe a phenomenon wherein sexually transmitted and parentally provided worms benefit their insect hosts, possibly by “engineering” the microbiome present in the maternally constructed chamber where offspring develop. Given nematodes’ association with many insects, particularly those with parental care, this phenomenon may be a more widespread feature of insect health.
Keywords: fitness, microbiome, niche construction, symbiosis
Abstract
A recent accumulation of studies has demonstrated that nongenetic, maternally transmitted factors are often critical to the health and development of offspring and can therefore play a role in ecological and evolutionary processes. In particular, microorganisms such as bacteria have been championed as heritable, symbiotic partners capable of conferring fitness benefits to their hosts. At the same time, parents may also pass various nonmicrobial organisms to their offspring, yet the roles of such organisms in shaping the developmental environment of their hosts remain largely unexplored. Here, we show that the nematode Diplogastrellus monhysteroides is transgenerationally inherited and sexually transmitted by the dung beetle Onthophagus taurus. By manipulating artificial chambers in which beetle offspring develop, we demonstrate that the presence of D. monhysteroides nematodes enhances the growth of beetle offspring, empirically challenging the paradigm that nematodes are merely commensal or even detrimental to their insect hosts. Finally, our research presents a compelling mechanism whereby the nematodes influence the health of beetle larvae: D. monhysteroides nematodes engineer the bacterial and fungal communities that also inhabit the beetle developmental chambers, including specific taxa known to be involved in biomass degradation, possibly allowing larval beetles better access to their otherwise recalcitrant, plant-based diet. Thus, our findings illustrate that nongenetic inheritance can include intermediately sized organisms that live and proliferate in close association with, and in certain cases enhance, the development of their hosts’ offspring.
Parents pass on more to their offspring than just genes. A wide breadth of nongenetic factors—including hormones, epigenetic marks, behavioral variants, and symbionts—can also be faithfully transmitted across generations, providing alternative and parallel mechanisms of inheritance and adaptation (1–3). Symbiosis in particular has emerged as a paradigm model for the inheritance of elements acquired in part from the environment but which are also transmitted across generations, often from host mother to her offspring (4, 5). The microbes associated with insects, for example, can provide them with important ecological functions, such as detoxification of defensive plant compounds (6), synthesis of essential nutrients (7), digestion of plant cell-wall components (8), protection from pathogenic microbes (9, 10), and resistance to heat (11) and desiccation (12). However, in addition to the microbiota of insects, multicellular organisms, such as nematodes (13–16), mites (17–19), and even other insects (20) commonly engage in close associations with insect parents and their offspring, and as such possess the potential to influence the health and fitness of their hosts, acting either in parallel to microbial interactants or potentially through them (21–24).
Nematodes in particular stand out as ubiquitous associates of many insects, yet the nature of their relationship is nearly universally considered detrimental (i.e., parasitic or entomopathogenic), commensal (i.e., phoretic), or on the spectrum between these poles (e.g., necromenic) (25–27). However, evaluating the relationships of nematodes to their invertebrate hosts in natural settings has been challenging, and snapshots based on sampling efforts imply that many nuances of these relationships, especially from the view of the host, may go unnoticed (28). In addition to parasitic or simply phoretic associations, it is alternatively possible that nematodes can benefit their insect hosts, particularly in cases where the nutritional or defensive objectives of nematodes and insects align. Such cases have been described: for example, some pine-sawyer beetles (Monochamus spp.) and the plant-pathogenic nematodes (Bursaphelenchus xylophilus) they harbor both depend on the degradation of tree tissue for nutrition and the detoxification of defensive tree compounds, providing the conditions for an intricate, potentially mutualistic association between nematode and host (29, 30). In principle, cases like this should reveal mechanisms for how animal species, especially those assumed to be commensal or parasitic, may instead be symbiotic mutualists with their hosts.
A compelling mechanism by which nematodes could benefit their insect host is as engineers of the microbial community closely associated with the host. The microbes that are carried with, or modified by, the presence of nematodes may have a crucial role in the health of the associated insect, nematode, or both. In the pine-sawyer beetle example, the insects likely benefit from bacterial species of Serratia and Pseudomonas, which are known to reduce the concentrations of defensive plant terpenes that would be harmful to insects (31, 32), and the growth of these bacteria is dependent on the presence of their nematode associates (22, 33, 34). Likewise, in some dung beetles, which commonly utilize herbivore dung as a food source during both larval and adult stages, it is known that bacteria are transmitted across generations from mother to offspring (35), enhancing larval growth (36). Because brood balls consist largely of nutritionally recalcitrant plant material, dung beetles likely benefit from the enzymatic capacities of microorganisms (37). At the same time, nematodes are well known to form close associations with dung beetles (15, 38, 39), yet whether and how nematodes interact with microbiota in ways that affect host health, development, or fitness is unknown. Here we assess the nature, consequences, and potential mechanisms of such a three-way interaction.
Specifically, we investigated potential interactions between insects, their nematodes, and local microorganisms using the dung beetle Onthophagus taurus. In this system, it is possible to manipulate nematode–insect interactions in a field-like setting, thereby allowing ecological tests of a likely widespread but often intractable type of interspecies interaction. In nature, adult O. taurus provide their offspring with larval nutrition in the form of a consumable “brood ball” constructed from mammalian herbivore (often cow) dung that supports beetle development from egg to adult. Furthermore, O. taurus mothers provide their offspring with a deposit of maternal fecal matter containing a fitness-enhancing microbiome (35, 36). Here we show that symbiotic nematodes may play a critical role in shaping the brood ball environment and resulting host benefits. Using both field animals and a controlled, artificial brood ball system, we (i) identify a species of nematode associated with both the genitalia of adult O. taurus and the developmental chambers of its offspring; (ii) show that the nematode is transmitted both vertically across generations and sexually during copulation between adult beetles; and (iii) show that its presence in the brood ball enhances fitness of host offspring. Finally, our results suggest (iv) a possible mechanism underlying these fitness benefits by showing that the presence of nematodes significantly and reliably alters abundance and composition of the bacterial and fungal communities in the offspring’s developmental environment.
Results and Discussion
Onthophagus Beetles Sexually and Vertically Transmit Diplogastrellus Nematodes.
Field collections of O. taurus from Indiana and North Carolina revealed that both female and male beetles carried the morphospecies Diplogastrellus monhysteroides, specifically in their genitalia (Fig. 1 and SI Appendix, Tables S1 and S2). Using manipulative experiments, we demonstrated that these nematodes are vertically inherited; we transferred nematode-free beetle eggs to either nematode-free or D. monhysteroides-inoculated brood balls, bred the adults (within treatments) that emerged, and assessed their offspring for nematodes. Indeed, all beetle offspring derived from D. monhysteroides-treated parents (n = 9) inherited nematodes; in contrast, all beetle offspring derived from nematode-free parents (n = 12) lacked nematodes. Additionally, D. monhysteroides is sexually transmitted; when we performed another set of crosses to assess female to male transmission, all four previously nematode-free males mated to nematode-positive females acquired D. monhysteroides. Conversely, in the three crosses assessing male-to-female transmission, all previously nematode-free females acquired D. monhysteroides from their male D. monhysteroides-positive partners.
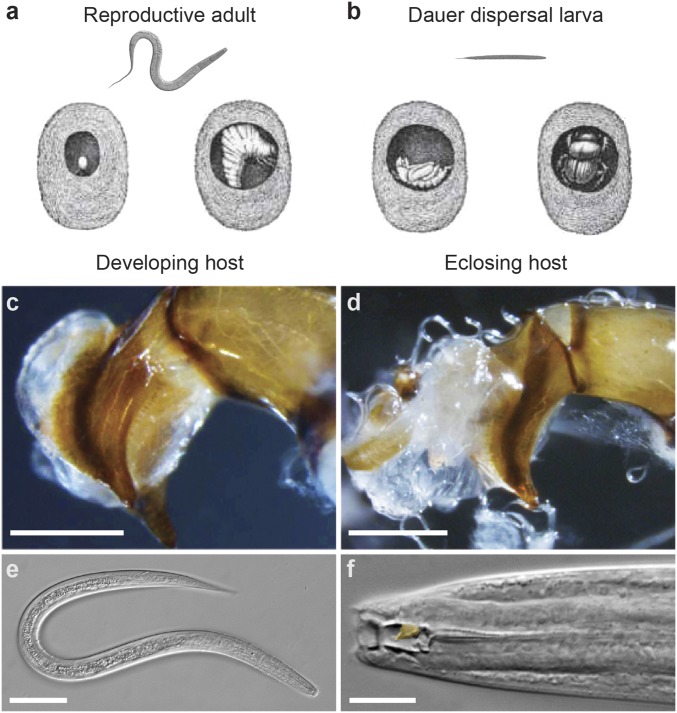
Fig. 1.
D. monhysteroides is closely associated with reproduction of the dung beetle O. taurus. Nematodes are known associates of brood balls, developmental chambers made by beetles from dung to protect and nourish their young. (A) When a beetle deposits an egg in a brood ball, nematodes exit their dauer (J3D) stage and ultimately achieve high population densities in the brood ball as the beetle offspring develops (15). (B) As the beetle larva nears pupation, nematodes again arrest as dauer larvae, thereafter leaving the brood ball with their beetle host. This single species of brood ball-associated nematode was found at high densities on O. taurus genitalia: compare nematode absence and presence in C and D, respectively. (Scale bars for C and D, 0.5 mm.) (E) Dauer of D. monhysteroides, a facultative larval type with a closed mouth and covered in wax that confers physiological resilience in aerial (above-ground) habitats and thus long-distance dispersal with hosts. (Scale bar, 20 μm.) (F) An adult female D. monhysteroides, showing a dorsal tooth (false-colored orange) hypothesized to allow feeding on fungi as well as bacteria (40). (Scale bar, 5 μm.)
Diplogastrellus Nematodes Increase the Fitness of Their Beetle Hosts.
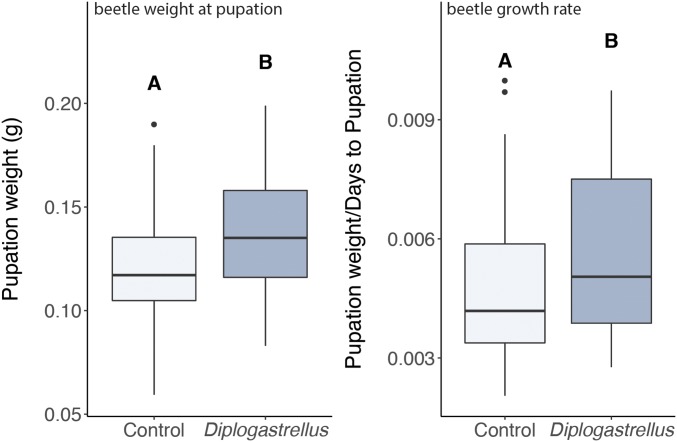
The inclusion of D. monhysteroides in artificial brood balls significantly enhanced O. taurus beetle larval growth (χ2 = 4.09, df = 1, P = 0.04) by enhancing growth rate (χ2 = 4.34, df = 1, P = 0.04). In other words, the beetles were larger at pupation not because they took longer to develop but because they grew more during a given amount of time (Fig. 2). Additionally, we found that this fitness advantage is likely to be conditional in nature, as field-collected beetles varied for the presence of D. monhysteroides (SI Appendix, Supplementary Material and Methods), possibly because the benefits of possessing these nematodes is context-dependent in ways not captured by this study. Taken together, these findings show that D. monhysteroides are conditional mutualists of O. taurus, and that their benefit is conferred during the beetles’ postembryonic development.
Fig. 2.
The presence of Diplogastrellus nematodes enhances overall growth and growth rate of O. taurus larvae. Weight was measured from beetle larvae within 24 h of pupation and growth weight was determined by dividing the pupation weight by the number of days an individual spent as a larva. Lower and Upper box hinges include first and third quartiles; whiskers, 1.5 × the interquartile range. Letters indicate groups with significantly (P < 0.05) different means. Dots represent outliers (data greater than UQ+1.5 × IQD or less than LQ −1.5 × IQD, where UQ = upper quartile, LQ = lower quartile, and IQD = interquartile distance).
Nematodes Dynamically Shift the Relative Abundance of Fungi to Bacteria in the Host Developmental Environment.
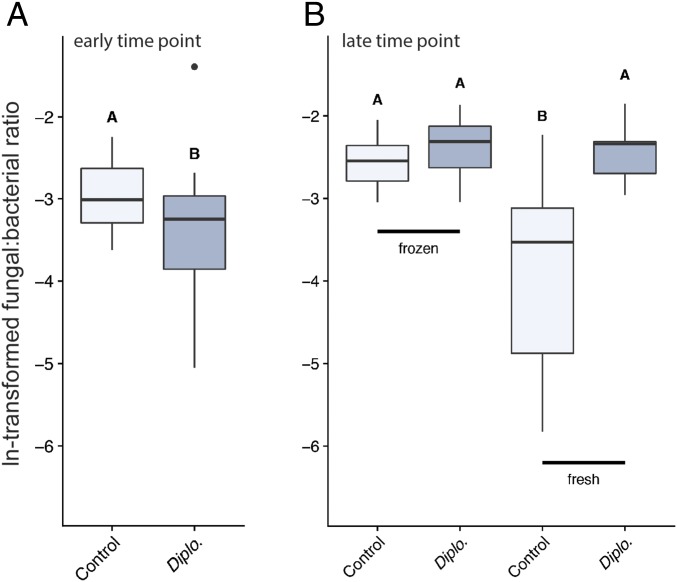
Because Diplogastrellus nematodes likely feed on bacteria, fungi, or both in nature (40), we hypothesized that increased beetle fitness correlated with nematode-induced changes to brood ball microbiota. To assess microbial abundances and communities in the host larval environment, we manipulated nematode presence across two types of dung: dung frozen before the experiments, which eliminates other species of free-living nematodes, and dung collected fresh from a pasture (for late time points only). This two-pronged approach allowed us to assess the influence of D. monhysteroides in the absence of other nematodes, while also allowing us to capture potential D. monhysteroides-mediated differences in more complex microbial communities that might be missed due to the freezing procedure. When we performed these manipulations, we found that inoculation with D. monhysteroides influenced the relative abundances of fungi and bacteria in brood balls, and this effect varied based on the time at which the samples were taken and the type of dung. Specifically, the inclusion of D. monhysteroides resulted in a significantly lower fungi-to-bacteria ratio in the early time-point samples: that is, brood balls sampled 7 d after inoculation with worms (χ2 = 4.99, df = 1, P = 0.03) (Fig. 3A). In contrast, there was a significant interaction effect between the inclusion of D. monhysteroides and dung type on the fungi-to-bacteria ratio in late time-point samples (i.e., 21 d after inoculation; χ2 = 6.93, df = 1, P = 0.01) and a post hoc test revealed that the significant effect was obtained in brood balls constructed from dung collected fresh from a pasture, such that the inclusion of D. monhysteroides resulted in a significantly higher fungi-to-bacteria ratio (t = 3.95, df = 14, P = 0.01) (Fig. 3B). Taken together, our results suggest that the relative abundances of fungi and bacteria in the brood ball can be modified by D. monhysteroides, but that the direction of these effects differs through beetle larval ontogeny.
Fig. 3.
Relative fungal and bacterial abundances are influenced by Diplogastrellus nematodes across time points and dung types. The abundance of fungal and bacterial biomass was estimated by RT-qPCR. Lower and Upper box hinges include first and third quartiles; whiskers, 1.5 × the interquartile range. Letters indicate groups with significantly (P < 0.05) different means. Dot represents an outlier (data greater than UQ+1.5 × IQD or less than LQ −1.5 × IQD, where UQ = upper quartile, LQ = lower quartile, and IQD = interquartile distance). (A) Seven days after inoculation with nematodes, brood balls exhibit a lower abundance of fungal to bacterial biomass relative to controls (P = 0.03). (B) Twenty-one days after inoculation with nematodes, brood balls made from fresh dung have a higher abundance of fungal to bacterial biomass relative to controls (P = 0.01). Diplo., D. monhysteroides.
Nematodes Change Bacterial and Fungal Communities in the Host Developmental Environment.
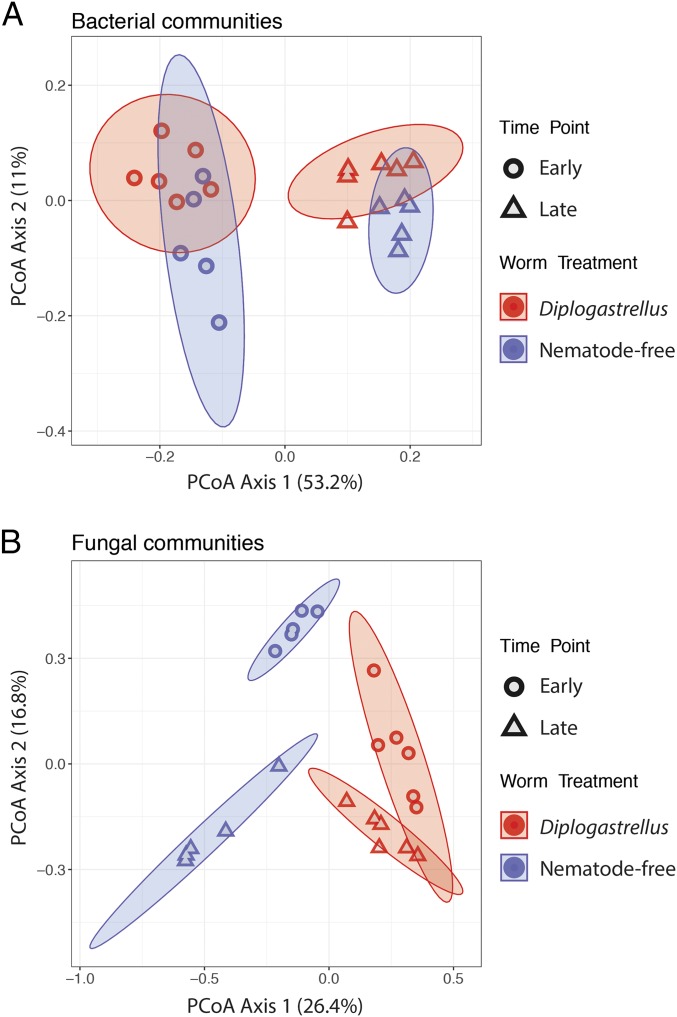
Using the samples derived from dung frozen before the start of the experiment (see previous section), we sought to determine whether the presence of D. monhysteroides influences the bacterial community structure of brood balls constructed in the absence of other nematodes. Significant differences were observed for both early and late time points [F1, 10 = 2.65, P = 0.02 and F1, 9 = 3.16, P < 0.001] when unweighted, but not when weighted UniFrac was used, indicating that the presence of D. monhysteroides influences community membership (what taxa are present) rather than community composition (how much of each taxon is present) (Fig. 4A). The corresponding fungal communities of the same brood balls were similarly significantly affected by the presence of D. monhysteroides, and again the effect was evident at both early and late time points [F1, 10 = 4.38, P < 0.001 and F1, 10 = 5.05, P = 0.01] (Fig. 4B). Thus, our results suggest that both bacterial and fungal communities of brood balls are influenced by the presence of D. monhysteroides nematodes throughout beetle larval ontogeny.
Fig. 4.
Presence of Diplogastrellus nematodes modifies the bacterial and fungal communities of brood balls early and late during beetle development. (A) Brood ball bacterial community composition using the unweighted UniFrac system across different time points and worm treatments. Community composition differed based on the presence of nematodes at both early (P = 0.02) and late time points (P < 0.001). (B) Brood ball fungal community composition using the Bray–Curtis dissimilarity across different time points and nematode treatments. Community composition differed based on the presence of nematode at both early (P = 0.02) and late time points (P = 0.01). Ellipses represent 95% confidence intervals.
Candidate Microbial Taxa for Mediating Nematode-Dependent Fitness Effects on Hosts.
Differential-abundance analyses performed between groups with and without nematodes revealed several bacterial and fungal taxa whose abundance may be selectively changed or regulated by D. monhysteroides (Padj < 0.05) (See SI Appendix, Figs. S3–S5 and Datasets S2–S7). This effect was seen at both early and late time points, as well as in artificial brood balls constructed from either fresh or previously frozen dung. First, we identified candidate bacteria that may contribute to the nutritional needs of O. taurus, particularly microbes that metabolize otherwise inaccessible compounds in a plant-based diet; for example, D. monhysteroides enhanced the abundances of Dysgonomonas and Sphingobacterium (Bacterioidetes: Chitinophagaceae), which as gut flora of wood-feeding beetles (41–46) and termites (47) are known to digest lignocellulose—also a major component of dung and brood balls—for their hosts; likewise, D. monhysteroides modulated the abundances of Nocardioides (Actinobacteria), which include known lignocellulose decomposers (48) associated with termite hosts (49). Furthermore, D. monhysteroides consistently enhanced abundances of bacterial taxa known both to degrade plant biomass and to associate with other insects. Such microbes included members of Firmicutes and Planctomycetes, which contain plant-decomposing species associated with longhorn beetles (45, 50), and Comamonas and Acinetobacter (Proteobacteria), xylanase-expressing bacteria associated with the Colorado potato beetle (46), red turpentine beetles (51), and the cabbage white butterfly (52). In addition to cultivating bacteria that may provide nutritional benefits, D. monhysteroides also mediated levels of potentially harmful bacteria: for example, D. monhysteroides reduced levels of a Desulfovibrio sp., which is a known associate of O. taurus (35) and may provide nutrients to dung beetle larvae, but which also produces toxic hydrogen sulfide as a byproduct (53). Finally, D. monhysteroides consistently regulated abundances of several fungal taxa, which based on the spore-feeding habit of diplogastrid nematodes may have included the early suppression of potentially harmful fungi, although the relatively limited molecular classification of fungi makes their potential effects harder to predict.
Inherited “Macrobionts” Are Engineers of Microbial Communities and Benefit Their Developing Hosts.
Insects are ecologically diverse, and their symbiotic partners—including bacteria, archaea, protists, and fungi (8, 54–56)—are thought to be critical factors underlying their successful colonization of novel habitats (57, 58). In this study, we show that the nematode D. monhysteroides lives and reproduces in close association with the dung beetle O. taurus, is transmitted both sexually during adult copulation and vertically from mothers to their offspring, and enhances the fitness of its larval host. Moreover, our results suggest putative mechanisms, nematode-mediated alterations of bacterial and fungal communities in the brood ball, as enabling these fitness benefits. Together, our results suggest that nongenetic inheritance can include a “macrobiome” of intermediately sized organisms (i.e., small animals) that function as engineers—such as through selective feeding and redistribution—of the microbiome.
The phenomenon we describe herein may constitute functional evidence for what may be a generalizable feature of insect health, particularly in species that show parental niche construction. Although there were previously little functional data documenting the fitness benefits of nematodes to their insect hosts, several examples of putative mutualisms suggest that similar principles may apply more broadly. For example, bark beetles (Scolytidae) commonly harbor nematodes (59, 60), which potentially benefit their hosts by facilitating the degradation of wood (61, 62), and some bark beetle species even have specialized structures for housing nematodes in their wings (63). Pine-wilt nematodes that associate with pine-sawyer beetles may benefit their hosts by carrying bacteria that degrade toxic defensive compounds produced by trees under siege by the beetles (64). In perhaps the most compelling case for nematode-insect symbiosis, female Fergusonina gall flies deposit Ferbusobia nematodes into plant tissues, in which the nematodes appear to induce the galls that sustain the development of the flies’ offspring (65). Intriguingly, a feature common to all of these examples is the presence of a relatively closed “brood chamber”—whether galls, cells in wood galleries, or brood balls—constructed by parents for their offspring. Moreover, other insects known to show parental niche-provisioning specifically carry nematodes in their genitalia, including taxa as disparate as Necrophorus burying beetles (66) and sweat bees (Halictidae) (14, 67). Thus, it is possible in such cases that the postembryonic development of insect host offspring is affected by nematode-dependent modifications to the microbial communities of their developmental environments.
Conclusions
Animals exist in partnerships, such that the health and fitness of animals, including humans, is fundamentally multiorganismal. In particular, maternally transmitted organisms—including insect-associated nematodes, as shown here—form a type of nongenetic, ecological inheritance and, like any other type of inheritance, have the potential to harm, constrain, or chaperone the development of their hosts, depending on circumstances. The results from this work suggest that, in some situations, nematodes are ecological engineers of developmental environments and may be important to a wealth of insect species. Using the model established here, future work can in particular determine how heritable, multileveled symbioses among onthophagine beetles, their nematodes, and their microbes have shaped evolutionary outcomes for an unusually diverse clade of insects.
Materials and Methods
Beetle Collection, Nematode Isolation, and Husbandry.
Adult O. taurus were collected from Maple View Farm in Orange County, North Carolina, with permission from Bob Nutter. Beetles were brought to the laboratory and reared as previously described (68). Briefly, beetles were kept in a sand/soil mixture at a 16:8-h light:dark (L:D) cycle at 25 °C, and fed homogenized (stirred) cow manure twice a week. Dung for breeding, maintenance, and construction of artificial brood balls was collected from Marble Hill Farm in Monroe County, Indiana with permission from Whitney Schlegel. Both Maple View and Marble Hill Farms use avermectins (anthelminthic drugs) intermittently but had not used them within 2 mo before beetle or dung collection. To assess the incidence of D. monhysteroides in our field-collected population, genitalia from 41 individuals were dissected, suspended in M9 solution, and visually inspected. Details on identification and culturing of D. monhysteroides is provided in SI Appendix, Supplementary Materials and Methods.
Manipulation of Nematode Presence in Artificial Brood Balls.
The following methods were used to manipulate nematode presence in the three following experiments: (i) measurement of beetle fitness; (ii) quantification of relative bacterial and fungal abundances; and (iii) comparative microbial profiling of brood balls. Artificial brood balls were created by filling wells (1.5-cm deep, 1.8 cm in diameter) with cow manure that had been previously drained of water to mimic the consistency of brood balls naturally produced by adult beetles (69). Dung was either frozen (at −80 °C for 2 wk, thus eliminating preexisting nematodes) or fresh (i.e., collected the day that eggs were transferred). Frozen dung was used for both measuring beetle fitness and microbial profiling, while fresh dung was used additionally for measuring relative fungal and bacterial abundance (i.e., using real-time, quantitative PCR, RT-qPCR) at a late time point, in particular to determine whether freezing the dung changed the nematode-mediated effects on microbial communities. Furthermore, artificial brood balls were either treated as nonmanipulated controls or inoculated with D. monhysteroides. Finally, for brood balls constructed from frozen dung, samples (∼200 mg) were collected at one of two time points: early or late (7 and 21 d after nematode inoculation, respectively) during brood ball ontogeny. Thus, samples were collected for the following treatments: brood balls constructed from (i) early and (ii) late control dung frozen before the start of the experiment; (iii) early and (iv) late dung frozen before the start of the experiment and inoculated with D. monhysteroides; brood balls constructed from (v) late, control fresh dung; (vi) late fresh dung inoculated with D. monhysteroides.
Diplogastrellus nematodes for inoculation were collected in the following manner: after cleaning adult male O. taurus with sterile water, their aedeagi were dissected and placed in M9 buffer + 0.1% Tween 20 + ampicillin. Tween 20 and ampicillin were included to mechanically and chemically eliminate bacteria from the beetle that might otherwise be transferred with the nematodes to the artificial brood balls, and the effectiveness of these treatments was confirmed by plating treated worms on lysogeny broth plates, which did not yield any bacterial colonies after 24 h of incubation. Because we expected D. monhysteroides dauers to float on the buffer surface, given the presence of wax found on their body surface, dauers were readily collected from the surface of the buffer: after “floating” for 30 min, ∼20 D. monhysteroides dauers were picked onto artificial brood balls.
Because the presence of a developing beetle may influence the behaviors and ecological functions of the nematodes within a brood ball, we generated nematode-free O. taurus eggs for all experimental treatments. We randomly selected 24 colony-raised female beetles and placed them in a cylindrical, light-impermeable ovipositing container filled to a height of 21 cm with sterilized soil (70). After adding ∼200 g of homogenized cow dung, we covered these containers with window screen and perforated black plastic foil. After 5 d, brood balls produced by females were collected and dismantled to harvest eggs. Eggs were rinsed with 0.1% Tween 20 and then sterile water and then transferred to a 2% agar plate for visual inspection to ensure that eggs were completely free of nematodes. Cleaned eggs were then transferred to 12-well plates containing artificial brood balls. Additionally, inspection of brood balls at the end of the experiments confirmed the absence of nematodes throughout development in the nonmanipulated, control brood balls.
Measurement of Beetle Fitness.
Additional experiments were performed with the artificial brood ball system to test whether the inclusion of D. monhysteroides nematodes influenced the growth of O. taurus beetle larvae. Artificial brood balls were constructed from previously frozen dung and nematode inoculations were performed as described above. Each plate received three to six eggs (among separate wells), and each plate became a treatment replicate with all eggs within a plate receiving the same treatment. Each open (lidless) plate was placed in a larger plastic, tight-lidded container, such that nematodes were strongly prevented from traversing wells and completely prevented from contaminating adjacent replicates. These replicate containers were randomized with respect to treatment in a climate-controlled room at 26 °C and a 16L:8D cycle. Four rounds of the experiment were conducted to collect sufficient replication. In total, 72 and 86 beetles from control and D. monhyteroides treated brood balls, respectively, were measured. Control brood balls were checked periodically for nematode contamination (none was detected). Measurements recorded were pupal weight, sex, and days to pupation. To analyze functional data, mixed models were used on natural log-transformed response variables (weight at pupation and growth rate; growth rate was determined by developmental time, i.e., the days between hatching and pupation) using the R package nlme (71). Fixed factors were: (i) nematode treatment; (ii) beetle sex; the interaction of (i) and (ii); and (iii) the experimental round from which the data were collected. The random variable was replicate container. Significance of fixed factors was determined by comparing nested models with likelihood ratio tests.
Assessment of Vertical and Sexual Transmission of D. monhysteroides.
After all experimental animals were collected, males and females were paired within treatments and transferred to individual, shallow containers, such that 10 families each of control and nematode-treated beetles were generated. Pairs were kept and fed in these containers for 1.5 wk, at which point they were transferred as pairs to deep containers that facilitate brood ball creation and burial. Soil in both types of containers had previously been autoclaved at 150 PSI for 30 min to ensure no extraneous nematodes were transferred to the breeding pair. Four families from each of the treatments produced brood balls. After 5 d, brood balls were collected from these deep containers and transferred to 12-well plates without further manipulation; a subset of these individuals were then used for an experiment assessing sexual transmission, and one to four individuals from each family were raised to eclosion to assess vertical transmission. Upon eclosion, beetle genitalia were dissected to determine whether nematodes had been transmitted between generations. Because all adult beetles born during the vertical transmission experiments always had D. monhysteroides if they were treated with D. monhysteroides during development, we assumed that the subset of individuals from the same treatment groups used for the sexual transmission experiment would show the same phenotypes (as D. monhysteroides presence cannot be assessed nondestructively). We thus created two types of crosses for assessing sexual transmission: D. monhysteroides-possessing females to control males (n = 4) and D. monhysteroides possessing males to control females (n = 3). After allowing individuals to mate for 1 wk, they were collected and their genitalia dissected and scored for nematode presence.
DNA Extraction for Comparisons of Bacterial and Fungal Communities.
Artificial brood balls with beetle eggs were generated for RT-qPCR and microbial profiling as described above. Ultimately, three replicate plates per treatment and two brood balls per plate were used for extractions for a total of n = 6 per treatment. Extractions were performed immediately after samples were taken: 7 and 21 d after nematode inoculation for early and late time-point samples, respectively. Early time-point samples were only taken from frozen dung. Six individuals among the fresh dung samples died during development, so these samples were removed from the analysis (three in each of the control and D. monhysteroides treated brood balls, leaving n = 3 for samples constructed from fresh dung). Samples were taken from brood balls in ∼200-mg amounts and were processed with the Qiagen PowerSoil kit. Extracted DNA was stored at −80 °C until further processing for both RT-qPCR and microbial profiling.
Quantification of Relative Bacterial and Fungal Abundances.
The relative abundance of bacterial to fungal biomass can be estimated from environmental samples using taxon-specific, RT-qPCR (72, 73). Further details on bacterial and fungal quantification, including taxon-specific standards (SI Appendix, Table S3) and assessment of absolute values, are provided in SI Appendix, Supplementary Materials and Methods.
Comparative Microbial Profiling of Brood Balls.
Bacterial and fungal taxa were sequenced and statistical analyses of 16 S and ITS rRNA sequences were performed as described in SI Appendix, Supplementary Materials and Methods.
Quantification of Host-Associated Community Identity and Composition.
We calculated unweighted pairwise Unifrac distances (74) among bacterial communities. We used Bray–Curtis dissimilarity to determine whether fungal communities differed between treatments. For statistical analyses, data were placed into three subsets to compare conditions that were most biologically relevant, namely with control vs. D. monhysteroides-treated samples taken at (i) an early time point from frozen dung; (ii) a late time point from frozen dung; and (iii) a late time point from fresh dung. Statistical analyses were performed as described in SI Appendix, Supplementary Materials and Methods.
Identification of Candidate Taxa Regulated by D. monhysteroides in Brood Balls.
To determine which taxa were differentially abundant among samples, sequence data were placed into three subsets to contrast conditions that were determined to be biologically relevant, as described above. Each subset was formatted for the DESeq2 (75) package in R, and DESeq2 was executed using the phyloseq package, as this method for identifying differentially abundant taxa is considered to be more conservative (i.e., in avoiding false positives) than comparing proportions of or rarefying microbial data (76). Lists of bacterial and fungal taxa were trimmed to include only those taxa identified to family level.
Supplementary Material
Acknowledgments
We thank Drs. Whitney and Kipp Schlegel and Mr. Bob Nutter for permission to collect beetles and dung on their farms; Dr. Robin Giblin-Davis for his comments on our manuscript; and the Indiana University Center for Genomics and Bioinformatics, especially Chris Hemmerich, for their assistance. Support for this study was provided by Indiana University (C.C.L.-R. and E.J.R.); National Science Foundation Grants IOS 1120209 and IOS 1256689 (to A.P.M.); and a grant from the John Templeton Foundation (to A.P.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequence reported in this paper has been deposited in the Dryad repository (doi: 10.5061/dryad.2k4m170).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1809606115/-/DCSupplemental.
References
- 1.Bonduriansky R. Rethinking heredity, again. Trends Ecol Evol. 2012;27:330–336. doi: 10.1016/j.tree.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Danchin É, et al. Beyond DNA: Integrating inclusive inheritance into an extended theory of evolution. Nat Rev Genet. 2011;12:475–486. doi: 10.1038/nrg3028. [DOI] [PubMed] [Google Scholar]
- 3.Jablonka E, Raz G. Transgenerational epigenetic inheritance: Prevalence, mechanisms, and implications for the study of heredity and evolution. Q Rev Biol. 2009;84:131–176. doi: 10.1086/598822. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert SF, Bosch TCG, Ledón-Rettig C. Eco-evo-devo: Developmental symbiosis and developmental plasticity as evolutionary agents. Nat Rev Genet. 2015;16:611–622. doi: 10.1038/nrg3982. [DOI] [PubMed] [Google Scholar]
- 5.Bright M, Bulgheresi S. A complex journey: Transmission of microbial symbionts. Nat Rev Microbiol. 2010;8:218–230. doi: 10.1038/nrmicro2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung SH, et al. Herbivore exploits orally secreted bacteria to suppress plant defenses. Proc Natl Acad Sci USA. 2013;110:15728–15733. doi: 10.1073/pnas.1308867110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Douglas AE. Nutritional interactions in insect-microbial symbioses: Aphids and their symbiotic bacteria buchnera. Annu Rev Entomol. 1998;43:17–37. doi: 10.1146/annurev.ento.43.1.17. [DOI] [PubMed] [Google Scholar]
- 8.Warnecke F, et al. Metagenomic and functional analysis of hindgut microbiota of a wood-feeding higher termite. Nature. 2007;450:560–565. doi: 10.1038/nature06269. [DOI] [PubMed] [Google Scholar]
- 9.Brownlie JC, Johnson KN. Symbiont-mediated protection in insect hosts. Trends Microbiol. 2009;17:348–354. doi: 10.1016/j.tim.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Kroiss J, et al. Symbiotic Streptomycetes provide antibiotic combination prophylaxis for wasp offspring. Nat Chem Biol. 2010;6:261–263. doi: 10.1038/nchembio.331. [DOI] [PubMed] [Google Scholar]
- 11.Moran NA, Yun Y. Experimental replacement of an obligate insect symbiont. Proc Natl Acad Sci USA. 2015;112:2093–2096. doi: 10.1073/pnas.1420037112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engl T, et al. Ancient symbiosis confers desiccation resistance to stored grain pest beetles. Mol Ecol. 2018;27:2095–2108. doi: 10.1111/mec.14418. [DOI] [PubMed] [Google Scholar]
- 13.Fuchs AG. Neue an Borken- und Rüsselkäfer gebundene Nematoden, halbparasitische und Wohnungseinmieter: Freilebende Nematoden aus Moos und Walderde in Borken- und Rüsselkäfergänge. Zool Jahrb Abt Syst. 1930;59:506–646. [Google Scholar]
- 14.Giblin RM, Kaya HK. Associations of halictid bees with the nematodes, Aduncospiculum halicti (Diplogasterida: Diplogasteroididae) and Bursaphelenchus kevini (Aphelenchida: Aphelenchoididae) J Kans Entomol Soc. 1984;57:92–99. [Google Scholar]
- 15.Kühne R. Relations between free-living nematodes and dung-burying Geotrupes spp. (Coleoptera: Geotrupini) Fundam Appl Nematol. 1996;19:263–271. [Google Scholar]
- 16.Okumura E, Ishikawa Y, Tanaka R, Yoshiga T. Propagation of Caenorhabditis japonica in the nest of its Carrier insect, Parastrachia japonensis. Zool Sci. 2013;30:174–177. doi: 10.2108/zsj.30.174. [DOI] [PubMed] [Google Scholar]
- 17.Trägårdh I. Die Milben und ihre ökologischen Beziehungen zu den Insekten. Arb Physiol Angew Entomol Berlin-Dahlem. 1943;10:124–136. [Google Scholar]
- 18.Springett BP. Aspects of the relationship between burying beetles, Necrophorus spp. and the mite, Poecilochirus necrophori Vitz. J Anim Ecol. 1968;37:417–424. [Google Scholar]
- 19.Okabe K, Makino S. Parasitic mites as part-time bodyguards of a host wasp. Proc Biol Sci. 2008;275:2293–2297. doi: 10.1098/rspb.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kronauer DJC, Pierce NE. Myrmecophiles. Curr Biol. 2011;21:R208–R209. doi: 10.1016/j.cub.2011.01.050. [DOI] [PubMed] [Google Scholar]
- 21.Koneru SL, Salinas H, Flores GE, Hong RL. The bacterial community of entomophilic nematodes and host beetles. Mol Ecol. 2016;25:2312–2324. doi: 10.1111/mec.13614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng XY, et al. Metagenomic analysis of the pinewood nematode microbiome reveals a symbiotic relationship critical for xenobiotics degradation. Sci Rep. 2013;3:1869. doi: 10.1038/srep01869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cardoza YJ, Moser JC, Klepzig KD, Raffa KF. Multipartite symbioses among fungi, mites, nematodes, and the spruce beetle, Dendroctonus rufipennis. Environ Entomol. 2008;37:956–963. doi: 10.1603/0046-225x(2008)37[956:msafmn]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 24.Rae R, et al. Isolation of naturally associated bacteria of necromenic Pristionchus nematodes and fitness consequences. J Exp Biol. 2008;211:1927–1936. doi: 10.1242/jeb.014944. [DOI] [PubMed] [Google Scholar]
- 25.Bovien P. Some types of association between nematodes and insects. Vidensk Meddelelser fra Dansk Natruhistorisk Foren. 1937;101:1–144. [Google Scholar]
- 26.Poinar GO., Jr . The Natural History of Nematodes. Prentice-Hall; Edgewood Cliffs, NJ: 1971. [Google Scholar]
- 27.Kiontke K, Sudhaus W. 2006 doi: 10.1895/wormbook.1.37.1. Ecology of Caenorhabditis species. WormBook, ed The C. elegans Community. Available at www.wormbook.org. Accessed April 30, 2018. [DOI] [PMC free article] [PubMed]
- 28.Schulenburg H, Félix MA. The natural biotic environment of Caenorhabditis elegans. Genetics. 2017;206:55–86. doi: 10.1534/genetics.116.195511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao L, et al. Chemical signals synchronize the life cycles of a plant-parasitic nematode and its vector beetle. Curr Biol. 2013;23:2038–2043. doi: 10.1016/j.cub.2013.08.041. [DOI] [PubMed] [Google Scholar]
- 30.Zhao L, et al. Ascarosides coordinate the dispersal of a plant-parasitic nematode with the metamorphosis of its vector beetle. Nat Commun. 2016;7:12341. doi: 10.1038/ncomms12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boone CK, et al. Bacteria associated with a tree-killing insect reduce concentrations of plant defense compounds. J Chem Ecol. 2013;39:1003–1006. doi: 10.1007/s10886-013-0313-0. [DOI] [PubMed] [Google Scholar]
- 32.Fürstenberg-Hägg J, Zagrobelny M, Bak S. Plant defense against insect herbivores. Int J Mol Sci. 2013;14:10242–10297. doi: 10.3390/ijms140510242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao BG, Lin F. Mutualistic symbiosis between Bursaphelenchus xylophilus and bacteria of the genus Pseudomonas. For Pathol. 2005;35:339–345. [Google Scholar]
- 34.Zhao BG, Wang HL, Han SF, Han ZM. Distribution and pathogenicity of bacteria species carried by Bursaphelenchus xylophilus in China. Nematology. 2003;5:899–906. [Google Scholar]
- 35.Estes AM, et al. Brood ball-mediated transmission of microbiome members in the dung beetle, Onthophagus taurus (Coleoptera: Scarabaeidae) PLoS One. 2013;8:e79061. doi: 10.1371/journal.pone.0079061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwab DB, Riggs HE, Newton ILG, Moczek AP. Developmental and ecological benefits of the maternally transmitted microbiota in a dung beetle. Am Nat. 2016;188:679–692. doi: 10.1086/688926. [DOI] [PubMed] [Google Scholar]
- 37.Schwab DB, Casasa S, Moczek AP. Evidence of developmental niche construction in dung beetles: Effects on growth, scaling and reproductive success. Ecol Lett. 2017;20:1353–1363. doi: 10.1111/ele.12830. [DOI] [PubMed] [Google Scholar]
- 38.Weller AM, Mayer WE, Rae R, Sommer RJ. Quantitative assessment of the nematode fauna present on Geotrupes dung beetles reveals species-rich communities with a heterogeneous distribution. J Parasitol. 2010;96:525–531. doi: 10.1645/GE-2319.1. [DOI] [PubMed] [Google Scholar]
- 39.Sudhaus W, Rehfeld K, Schluter D, Schweiger J. Interrelationships of nematodes, Coleoptera and Diptera in decomposing cow pats. Pedobiologia (Jena) 1988;31:305–322. [Google Scholar]
- 40.Yeates GW, Bongers T, De Goede RGM, Freckman DW, Georgieva SS. Feeding habits in soil nematode families and genera-an outline for soil ecologists. J Nematol. 1993;25:315–331. [PMC free article] [PubMed] [Google Scholar]
- 41.Mason CJ, Hanshew AS, Raffa KF. Contributions by host trees and insect activity to bacterial communities in Dendroctonus valens (Coleoptera: Curculionidae) galleries, and their high overlap with other microbial assemblages of bark beetles. Environ Entomol. 2016;45:348–356. doi: 10.1093/ee/nvv184. [DOI] [PubMed] [Google Scholar]
- 42.Arias-Cordero E, et al. Comparative evaluation of the gut microbiota associated with the below- and above-ground life stages (larvae and beetles) of the forest cockchafer, Melolontha hippocastani. PLoS One. 2012;7:e51557. doi: 10.1371/journal.pone.0051557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schloss PD, Delalibera I, Handelsman J, Raffa KF. Bacteria associated with the guts of two wood-boring beetles: Anoplophora glabripennis and Saperda vestita (Cerambycidae) Environ Entomol. 2006;35:625–629. [Google Scholar]
- 44.Tagliavia M, Messina E, Manachini B, Cappello S, Quatrini P. The gut microbiota of larvae of Rhynchophorus ferrugineus oliver (Coleoptera: Curculionidae) BMC Microbiol. 2014;14:136. doi: 10.1186/1471-2180-14-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou J, et al. Molecular and biochemical characterization of a novel xylanase from the symbiotic Sphingobacterium sp. TN19. Appl Microbiol Biotechnol. 2009;85:323–333. doi: 10.1007/s00253-009-2081-x. [DOI] [PubMed] [Google Scholar]
- 46.Vilanova C, et al. Bacteria from acidic to strongly alkaline insect midguts: Potential sources of extreme cellulolytic enzymes. Biomass Bioenergy. 2012;45:288–294. [Google Scholar]
- 47.Pramono AK, Sakamoto M, Iino T, Hongoh Y, Ohkuma M. Dysgonomonas termitidis sp. nov., isolated from the gut of the subterranean termite Reticulitermes speratus. Int J Syst Evol Microbiol. 2015;65:681–685. doi: 10.1099/ijs.0.070391-0. [DOI] [PubMed] [Google Scholar]
- 48.Abdulla HM, El-Shatoury SA. Actinomycetes in rice straw decomposition. Waste Manag. 2007;27:850–853. doi: 10.1016/j.wasman.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 49.Menezes L, et al. Food storage by the savanna termite Cornitermes cumulans (Syntermitinae): A strategy to improve hemicellulose digestibility? Microb Ecol. 2018;76:492–505. doi: 10.1007/s00248-017-1128-2. [DOI] [PubMed] [Google Scholar]
- 50.Park D-S, et al. A culture-based study of the bacterial communities within the guts of nine longicorn beetle species and their exo-enzyme producing properties for degrading xylan and pectin. J Microbiol. 2007;45:394–401. [PubMed] [Google Scholar]
- 51.Morales-Jiménez J, Zúñiga G, Villa-Tanaca L, Hernández-Rodríguez C. Bacterial community and nitrogen fixation in the red turpentine beetle, Dendroctonus valens LeConte (Coleoptera: Curculionidae: Scolytinae) Microb Ecol. 2009;58:879–891. doi: 10.1007/s00248-009-9548-2. [DOI] [PubMed] [Google Scholar]
- 52.Robinson CJ, Schloss P, Ramos Y, Raffa K, Handelsman J. Robustness of the bacterial community in the cabbage white butterfly larval midgut. Microb Ecol. 2010;59:199–211. doi: 10.1007/s00248-009-9595-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cypionka H. Oxygen respiration by desulfovibrio species. Annu Rev Microbiol. 2000;54:827–848. doi: 10.1146/annurev.micro.54.1.827. [DOI] [PubMed] [Google Scholar]
- 54.Farrell BD, et al. The evolution of agriculture in beetles (Curculionidae: Scolytinae and Platypodinae) Evolution. 2001;55:2011–2027. doi: 10.1111/j.0014-3820.2001.tb01318.x. [DOI] [PubMed] [Google Scholar]
- 55.Grünwald S, Pilhofer M, Höll W. Microbial associations in gut systems of wood- and bark-inhabiting longhorned beetles [Coleoptera: Cerambycidae] Syst Appl Microbiol. 2010;33:25–34. doi: 10.1016/j.syapm.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 56.Scully ED, et al. Functional genomics and microbiome profiling of the Asian longhorned beetle (Anoplophora glabripennis) reveal insights into the digestive physiology and nutritional ecology of wood feeding beetles. BMC Genomics. 2014;15:1096. doi: 10.1186/1471-2164-15-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moran NA, McCutcheon JP, Nakabachi A. Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet. 2008;42:165–190. doi: 10.1146/annurev.genet.41.110306.130119. [DOI] [PubMed] [Google Scholar]
- 58.Douglas AE. The microbial dimension in insect nutritional ecology. Funct Ecol. 2009;23:38–47. [Google Scholar]
- 59.Rühm W. Die Nematoden der Ipiden. Parasitol Schriftenr. 1956;6:1–435. [Google Scholar]
- 60.Susoy V, Herrmann M. Preferential host switching and codivergence shaped radiation of bark beetle symbionts, nematodes of Micoletzkya (Nematoda: Diplogastridae) J Evol Biol. 2014;27:889–898. doi: 10.1111/jeb.12367. [DOI] [PubMed] [Google Scholar]
- 61.Bleiker KP, Six DL. Dietary benefits of fungal associates to an eruptive herbivore: Potential implications of multiple associates on host population dynamics. Environ Entomol. 2007;36:1384–1396. doi: 10.1603/0046-225x(2007)36[1384:dbofat]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 62.Six DL, Paine TD. Effects of mycangial fungi and host tree species on progeny survival and emergence of Dendroctonus ponderosae (Coleoptera: Scolytidae) Environ Entomol. 1998;27:1393–1401. [Google Scholar]
- 63.Cardoza YJ, Paskewitz S, Raffa KF. Travelling through time and space on wings of beetles: A tripartite insect-fungi-nematode association. Symbiosis. 2006;41:71–79. [Google Scholar]
- 64.Zhao L, Mota M, Vieira P, Butcher RA, Sun J. Interspecific communication between pinewood nematode, its insect vector, and associated microbes. Trends Parasitol. 2014;30:299–308. doi: 10.1016/j.pt.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 65.Giblin-Davis RM, et al. Fergusobia/Fergusonina-induced shoot bud gall development on Melaleuca quinquenervia. J Nematol. 2001;33:239–247. [PMC free article] [PubMed] [Google Scholar]
- 66.Richter S. Phoretic association between the dauerjuveniles of Rhabditis stammeri (Rhabditidae) and life history stages of the burying beetle Nicrophorus vespilloides (Coleoptera: Silphidae) Nematologica. 1993;39:346–355. [Google Scholar]
- 67.McFrederick QS, Taylor DR. Evolutionary history of nematodes associated with sweat bees. Mol Phylogenet Evol. 2013;66:847–856. doi: 10.1016/j.ympev.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 68.Kijimoto T, Costello J, Tang Z, Moczek AP, Andrews J. EST and microarray analysis of horn development in Onthophagus beetles. BMC Genomics. 2009;10:504. doi: 10.1186/1471-2164-10-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shafiei M, Moczek AP, Nijhout HF. Food availability controls the onset of metamorphosis in the dung beetle Onthophagus taurus (Coleoptera: Scarabaeidae) Physiol Entomol. 2001;26:173–180. [Google Scholar]
- 70.Macagno ALM, Zattara EE, Ezeakudo O, Moczek AP, Ledón-Rettig CC. Adaptive maternal behavioral plasticity and developmental programming mitigate the transgenerational effects of temperature in dung beetles. Oikos. March 14, 2018 doi: 10.1111/oik.05215. [DOI] [Google Scholar]
- 71.Pinheiro J, et al. 2018 nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-137. Available at https://CRAN.R-project.org/package=nlme. Accessed April 24, 2018.
- 72.Fierer N, Jackson JA, Vilgalys R, Jackson RB. Assessment of soil microbial community structure by use of taxon-specific quantitative PCR assays. Appl Environ Microbiol. 2005;71:4117–4120. doi: 10.1128/AEM.71.7.4117-4120.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lau JA, Lennon JT. Rapid responses of soil microorganisms improve plant fitness in novel environments. Proc Natl Acad Sci USA. 2012;109:14058–14062. doi: 10.1073/pnas.1202319109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lozupone C, Hamady M, Knight R. UniFrac—An online tool for comparing microbial community diversity in a phylogenetic context. BMC Bioinformatics. 2006;7:371. doi: 10.1186/1471-2105-7-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McMurdie PJ, Holmes S. Waste not, want not: Why rarefying microbiome data is inadmissible. PLOS Comput Biol. 2014;10:e1003531. doi: 10.1371/journal.pcbi.1003531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.