Significance
Mast cell activation contributes to multiple allergic disorders, such as asthma, rhinitis, and atopic dermatitis. Here, we demonstrate that the Raf kinase inhibitor protein (RKIP) functions as an inhibitor of mast cell activation. RKIP negatively regulates the pathogeneses of the mast cell-mediated anaphylactic response and allergic asthma in vivo. Furthermore, the expression of RKIP was significantly down-regulated in peripheral blood from asthma patients. Collectively, our findings not only suggest that RKIP plays an important role in controlling mast cell-mediated allergic responses but also provide insight into therapeutic targets for mast cell-related allergic diseases.
Keywords: RKIP, mast cell, allergic response, asthma, FcεRI signaling
Abstract
The signaling cascades triggered by the cross-linkage of immunoglobulin E (IgE) with its high-affinity receptor (FcεRI) on mast cells contribute to multiple allergic disorders, such as asthma, rhinitis, and atopic dermatitis. Restraint of intracellular signals for mast cell activation is essential to restore homeostasis. In this study, we found that Raf kinase inhibitor protein (RKIP) negatively regulated mast cell activation. RKIP-deficient mast cells showed greater IgE−FcεRI-mediated activation than wild-type mast cells. Consistently, RKIP deficiency in mast cells rendered mice more sensitive to IgE−FcεRI-mediated allergic responses and ovalbumin-induced airway inflammation. Mechanistically, RKIP interacts with the p85 subunit of PI3K, prevents it from binding to GRB2-associated binding protein 2 (Gab2), and eventually inhibits the activation of the PI3K/Akt/NF-κB complex and its downstream signaling. Furthermore, the expression of RKIP was significantly down-regulated in the peripheral blood of asthma patients and in the IgE−FcεRI-stimulated mast cells. Collectively, our findings not only suggest that RKIP plays an important role in controlling mast cell-mediated allergic responses but also provide insight into therapeutic targets for mast cell-related allergic diseases.
Allergic diseases are characterized by increased serum levels of total immunoglobulin E (IgE) and specific IgE against common allergens (1–3). Mast cells are key mediators of IgE-mediated allergic diseases, including asthma, rhinitis, and atopic dermatitis (4). Studies of intracellular regulatory mechanisms in the context of mast cell activation could elucidate the contribution of this mechanism and lead to developments in therapeutic approaches against asthma, rhinitis, etc.
Derived from pluripotent hematopoietic stem cells, mast cells are predominantly localized in the respiratory mucosa, gastrointestinal tract, and connective tissues (5). The aggregation of the high-affinity IgE receptor (FcεRI) on the mast cell plasma membrane, when specific antigens cross-link FcεRI-bound IgE, activates a complex intracellular signaling pathway, resulting in degranulation, cytokine or chemokine production, and eicosanoid release (3, 6). Mast cell degranulation releases preformed proinflammatory chemical mediators, such as histamine and proteases, regulating the early-phase response, or type I immediate hypersensitivity reaction (7). Cytokines and chemokines, as well as some newly synthesized proinflammatory lipid mediators, are secreted in the late stage, mediating a more delayed proinflammatory response (6). The intracellular signaling cascades elicited by the IgE−FcεRI aggregation in mast cells have been studied extensively (5, 8, 9). Briefly, cross-linkage of the IgE−FcεRI complex with an allergen activates a variety of Src family kinases, such as Lyn and Fyn, which are essential for initiating IgE−FcεRI-mediated activation of mast cells. Phosphorylated linker for activation of T cells (LAT)/immunoreceptor tyrosine-based activation motif (ITAM) signalingsomes initiate the principal signaling through the growth-factor receptor-bound protein 2 (Grb2)/phospholipase C-γ1 (PLC-γ1)/SH2 domain-containing leukocyte protein of 76 kDa (SLP-76) axis, leading to the activation of mitogen-activated protein kinases (MAPKs), protein kinase C (PKC) pathways, and calcium flux (Ca2+) (5, 10, 11). Meanwhile, Fyn can directly facilitate a complementary axis, which also helps with lipid mediator production, calcium release, and cytokine production (12). This complementary signaling pathway leads to the phosphorylation of the cytosolic adaptor molecule GRB2-associated binding protein 2 (Gab2) and phosphatidylinositol 3-kinase (PI3K), resulting in the recruitment of serine/threonine kinase 3-phosphoinositide-dependent protein kinase 1 to the plasma membrane, where it subsequently phosphorylates and activates another serine/threonine kinase, Akt. Phosphorylated Akt positively regulates the function of the transcription factor NF-κB and mediates cytokine production (11, 13).
The IgE−FcεRI-mediated activation and subsequent inhibition in mast cells consist of highly ordered, sequential molecular events. An excessive allergic response by mast cells would be harmful to the host. Therefore, the activated signaling is counterbalanced by certain negative regulatory mediators that keep mast cells in a controllable, tightly restricted state and return them to their basal status after activation. Therefore, understanding negative regulatory mechanisms is a prerequisite for acquiring comprehensive knowledge of FcεRI signaling and thereby helps reveal strategies for the treatment of allergic diseases.
Raf kinase inhibitor protein (RKIP) is widely expressed in mammalian tissues such as brain, liver, stomach, spleen, colon, and muscle (14). RKIP is known to act as a tumor suppressor (14). We recently demonstrated that RKIP plays a crucial role in mediating human and mouse colitis by promoting intestinal epithelial cell (IEC) apoptosis (15). Furthermore, we discovered that RKIP contributes to antiviral immune responses (16) and promotes TLR3-induced inflammation (17). Therefore, RKIP functions as a physiological and pathological regulator more than as a tumor suppressor. Here, we have found that RKIP acts as an inhibitory modulator of IgE−FcεRI-mediated mast cell activation by targeting the PI3K p85 subunit. RKIP deficiency significantly exacerbates mast cell-mediated anaphylactic responses and allergic asthma in mice.
Results
RKIP Expression Is Down-Regulated in IgE−FcεRI-Stimulated Mast Cells and in Peripheral Blood from Asthma Patients.
Mast cells have been thought to play a central role in the pathological process of IgE-mediated allergic diseases (5). The gene expression of RKIP in the IgE−FcεRI-activated mast cell was first analyzed. As shown in SI Appendix, Fig. S1A, the mRNA expression of RKIP was significantly reduced in mouse bone marrow-derived mast cells (BMMCs) on stimulation with IgE−FcεRI (after presensitization by anti–dinitrophenyl (DNP)-IgE and activation by DNP-BSA), similar to its expression profile, as shown in the BioGPS gene expression atlas database (SI Appendix, Fig. S1B). Human CD34+-derived mast cells were used to explore whether IgE−FcεRI stimulation affects the expression of RKIP in the human mast cell. As shown in SI Appendix, Fig. S1C, IgE−FcεRI stimulation significantly inhibited the mRNA expression of RKIP in human mast cells. Collectively, these data suggest that RKIP might be involved in IgE−FcεRI-mediated mast cell function.
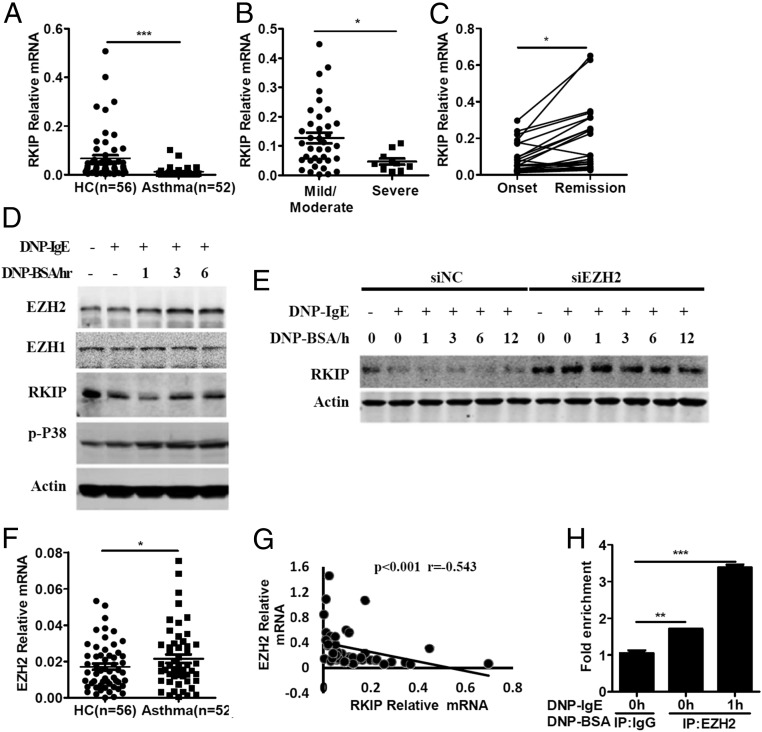
Next, we measured the expression levels of RKIP in peripheral blood from asthma patients and healthy control individuals by real-time PCR. The mRNA level of RKIP was significantly down-regulated in the asthma patients’ peripheral blood (n = 52) compared with the samples from healthy control individuals (n = 56) (Fig. 1A). Furthermore, mRNA expression of RKIP was significantly lower in the severe group than in the mild/moderate group (Fig. 1B). GEO data (GEO accession no. GSE16032) suggest that the expression of RKIP is much higher in the peripheral blood of convalescent asthma patients than in that of patients with exacerbated asthma (SI Appendix, Fig. S1D). We collected peripheral blood samples from patients with asthma at the onset and remission stages of asthma signs and symptoms to further determine the kinetics of RKIP expression during the processing of asthma. As shown in Fig. 1C, RKIP mRNA expression was much higher in the same patient at the remission of asthma symptoms than that at the onset of asthma, indicating that RKIP expression was linked to the occurrence of signs and symptoms in patients with asthma.
Fig. 1.
RKIP expression is down-regulated in FcεRI-stimulated mast cells and peripheral blood from asthma patients. (A) Real-time PCR analysis of RKIP mRNA expression in peripheral blood from asthma patients (n = 52) or healthy control individuals (n = 56). (B) Statistical analysis of the correlation between RKIP expression and severity of asthma in patients: mild/moderate (n = 37) or severe (n = 10). (C) Real-time PCR analysis of RKIP mRNA expression in peripheral blood from children with asthma (n = 26) at the onset and remission of asthma signs and symptoms and statistical analysis of the kinetics of RKIP expression relative to the onset and remission of asthma. (D) Western blot analysis of EZH2, EZH1, and RKIP expression in the IgE−FcεRI-activated BMMCs with indicated antibodies. (E) Western blot analysis of RKIP expression in the EZH2-specific siRNA (siEZH2)- or negative siRNA (siNC)-transfected BMMCs with IgE−FcεRI-mediated stimulation. (F) Real-time PCR analysis of EZH2 mRNA expression in peripheral blood from asthma patients (n = 52) and healthy controls (n = 56). (G) Statistical analysis of the correlation between the RKIP expression and EZH2 expression in peripheral blood from asthma patients (n = 52). (H) Anti-EZH2 antibody was subjected to cell lysate of the IgE−FcεRI-activated WT BMMCs, and the enrichment of EZH2 to proximal E boxes of RKIP promoter was analyzed by real-time PCR. *P < 0.05; **P < 0.01; ***P < 0.001. Data are representative of the three experiments. Data represent the mean and SD.
Previous studies have reported that the enhancer of zeste homolog 2 (EZH2) binds to the proximal E boxes of the RKIP promoter and inhibits RKIP transcription in breast and prostate cell lines (18). We recently found that the enhancer of zeste homolog 1 (EZH1) negatively regulated the expression of RKIP in the IECs (15). Interestingly, IgE−FcεRI treatment enhanced the mRNA and protein expression of EZH2 in mouse BMMCs and human mast cells (Fig. 1D and SI Appendix, Fig. S1 E and F) but inhibited the expression of RKIP (Fig. 1D and SI Appendix, Fig. S1 A and C). However, IgE−FcεRI did not induce any significant effect on EZH1 expression in mouse BMMCs and human mast cells (Fig. 1D and SI Appendix, Fig. S1 G and H). Furthermore, knocking down the expression of EZH2 reversed the down-regulation of RKIP protein levels by IgE−FcεRI stimulation in the BMMCs (Fig. 1E and SI Appendix, Fig. S1I). Consistently, the mRNA level of EZH2, but not EZH1, significantly increased in the peripheral blood of individuals with asthma compared with that of healthy controls (Fig. 1F and SI Appendix, Fig. S1J). Moreover, linear regression analysis showed that the mRNA level of EZH2 was negatively correlated with the mRNA level of RKIP in the peripheral blood of individuals with asthma (Fig. 1G).
We next used a specific antibody against EZH2 to ascertain whether EZH2 can interact with the proximal E boxes of the RKIP promoter in BMMCs as in breast and prostate cell lines (18). As shown in Fig. 1H, enrichment of EZH2 at the proximal E boxes of the RKIP promoter in BMMCs was significantly enhanced compared with an anti-IgG control, and IgE−FcεRI stimulation promoted the binding, indicating that EZH2 may interact with the RKIP promoter and suppress the expression of RKIP in IgE−FcεRI-treated mast cells.
Elevated IgE−FcεRI-Mediated Activation in RKIP-Deficient BMMCs.
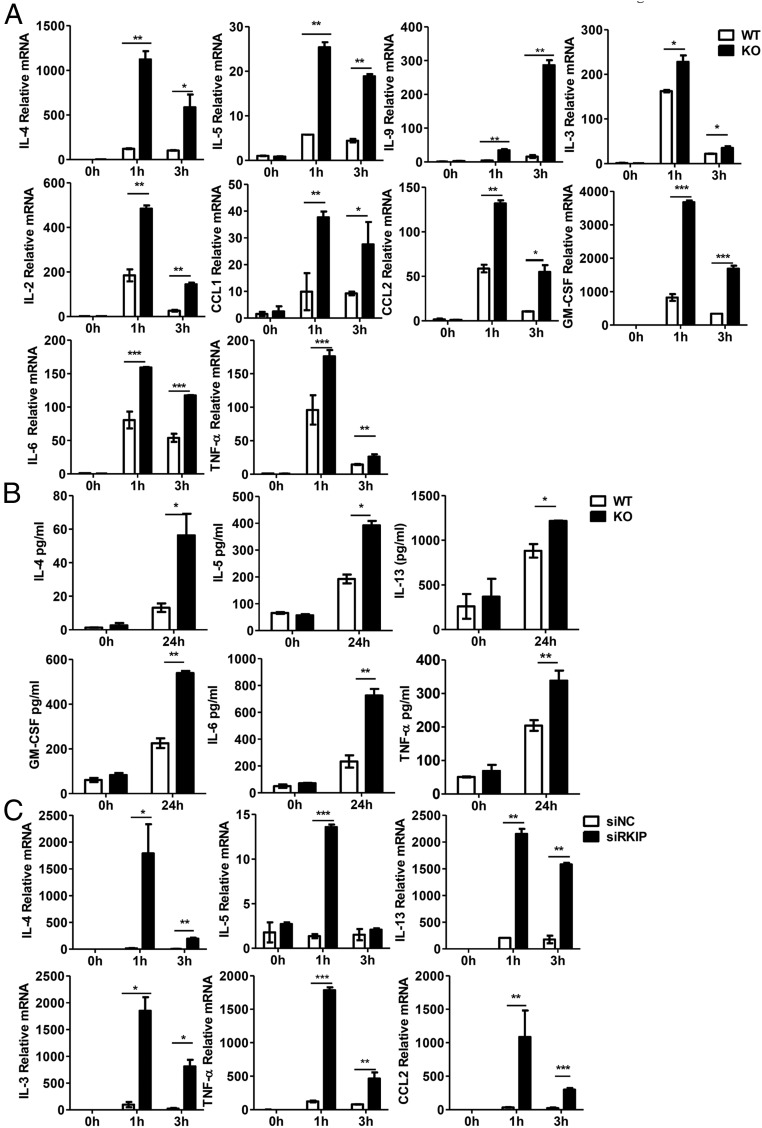
IgE−FcεRI-mediated mast cell activation triggers allergic responses by inducing rapid cell degranulation and secretion of newly synthesized metabolites, cytokines, and chemokines (2, 19) To determine whether RKIP plays a role in the IgE−FcεRI-mediated BMMC response, we measured the gene expression of cytokines and chemokines in RKIP knockout (KO) and wild-type (WT) BMMCs with IgE−FcεRI stimulation. As shown in Fig. 2A, RKIP deficiency greatly increased the expression of cytokines and chemokines on stimulation with IgE−FcεRI. Furthermore, RKIP KO BMMCs exhibited much higher IgE−FcεRI-mediated secretion of the cytokines IL-4, IL-5, IL-13, IL-6, TNF-α, and granulocyte macrophage colony-stimulating factor (GM-CSF) than did the WT BMMCs (Fig. 2B). Meanwhile, RKIP silencing enhanced the IgE−FcεR-induced expression of cytokines and chemokines (Fig. 2C). Consistently, IgE−FcεRI-mediated mRNA expression of cytokines and chemokines was significantly inhibited in Flag-RKIP−overexpressed RBL-2H3 cells (rat-derived basophils cell line) compared with that in control-transfected cells (SI Appendix, Fig. S2A). The overexpression of RKIP in RBL-2H3 cells was confirmed by Western blot (SI Appendix, Fig. S2B). We further explored whether the incubation of mast cells (BMMCs) with recombinant RKIP protein purified from Escherichia coli BL21 stain would affect mast cell activation. To our surprise, the incubation of BMMCs with recombinant RKIP protein significantly inhibited the IgE−FcεRI-mediated proinflammatory cytokine expression in the BMMCs (SI Appendix, Fig. S2C). Western blot assay confirmed the entry of exogenous RKIP protein into the BMMCs (SI Appendix, Fig. S2D). The efficiency of RKIP silencing in BMMCs is shown in SI Appendix, Fig. S2 E and F.
Fig. 2.
RKIP deficiency promotes IgE−FcεRI-mediated cytokine and chemokine production in the BMMCs. (A) WT and RKIP KO BMMCs were presensitized with 1 μg/mL of anti–DNP-IgE overnight and then activated with 100 ng/mL DNP-BSA. The expressions of the cytokines and chemokines were measured by real-time PCR. (B) The production of IL-6, TNF-α, IL-4, IL-5, IL-13, and GM-CSF was measured by ELISA at 24 h. (C) The BMMCs were transfected with negative control siRNA (siNC) or RKIP-specific siRNA (siRKIP) and treated as described in A. The expression of cytokines and chemokines was measured by real-time PCR. *P < 0.05; **P < 0.01; ***P < 0.001. Data are representative of three experiments. Data represent the mean and SD.
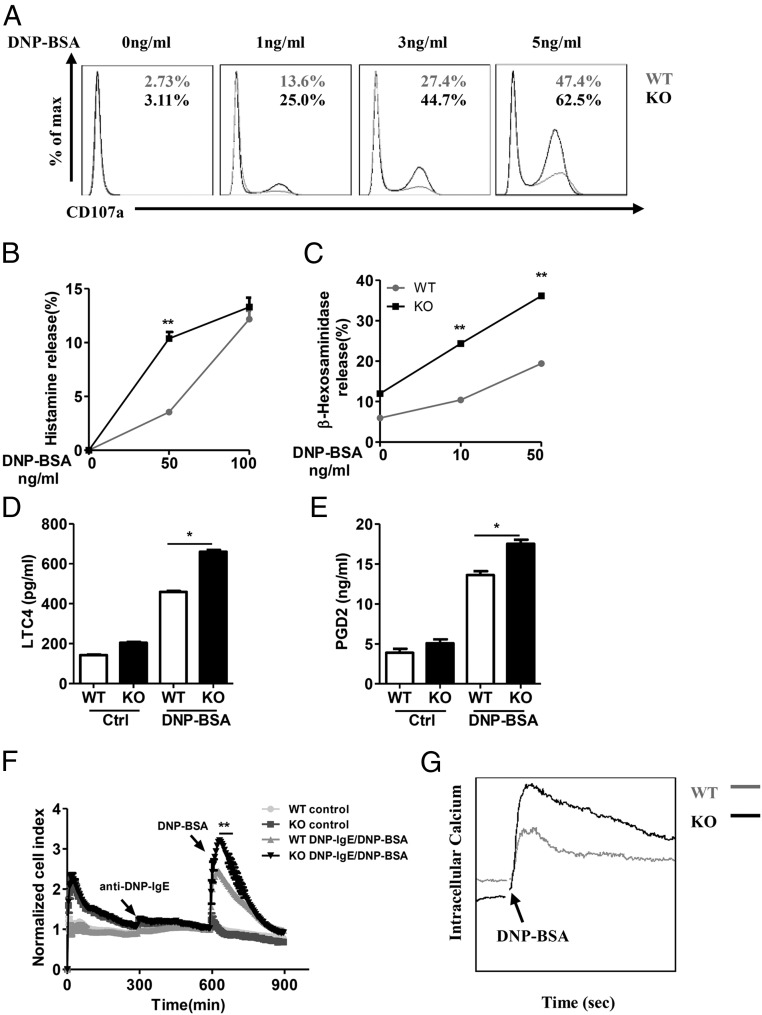
In addition, the loss of RKIP significantly promoted BMMC degranulation, as assessed by surface expression of the degranulation marker CD107a (Fig. 3A). The release of histamine upon IgE−FcεRI activation with suboptimal doses of allergen was higher in the RKIP KO BMMCs than in the WT BMMCs (Fig. 3B). In accordance with these results, RKIP deficiency in mast cells resulted in elevated IgE−FcεRI-elicited release of preformed β-hexosaminidase, leukotriene C4 (LTC4), and prostaglandin D2 (PGD2) (Fig. 3 C–E). Since mast cell degranulation is coupled with cytoskeletal rearrangement, we employed the xCELLigence system (Roche), a microelectronic cell sensor-based technology, for real-time monitoring of the electrical impedance of mast cells, which reflects cytoskeletal rearrangement and degranulation after activation by an allergen (19). As shown in Fig. 3F, with anti–DNP-IgE and DNP-BSA stimulation, both the WT and RKIP KO BMMCs exhibited robust increases in electrical impedance as reflected by the normalized cell index, whereas RKIP deficiency significantly enhanced the normalized cell index, which correlated well with the enhanced degranulation in the RKIP KO BMMCs. Moreover, IgE−FcεRI-elicited Ca2+ flux was significant higher in the RKIP KO BMMCs than that in the WT BMMCs (Fig. 3G). Collectively, these data suggest that RKIP negatively regulates IgE−FcεRI-mediated mast cell activation in vitro.
Fig. 3.
RKIP deficiency promotes IgE−FcεRI-mediated degranulation in mast cells. (A) The WT and RKIP KO BMMCs were presensitized with 1 μg/mL of anti–DNP-IgE overnight and activated with the indicated concentrations of DNP-BSA for 10 min. The BMMCs were incubated with anti-mouse CD107a, c-Kit, or FcεRI antibodies and then subjected to FACS analysis. C-Kit and FcεRI double-positive cells were gated out as BMMCs, and degranulation was assessed by CD107a expression levels on BMMCs. The numbers in the graphs indicate the percentages of CD107a+ cells. (B) WT and RKIP KO BMMCs were presensitized with 1 μg/mL of anti–DNP-IgE overnight and activated with the indicated concentrations of DNP-BSA for 1 h, and the secretion of histamine was determined by ELISA. Degranulation was assessed by histamine release (presented as percentages of total histamine content). (C) BMMCs were presensitized with 1 μg/mL of anti–DNP-IgE IgE overnight and activated with the indicated concentrations of DNP-BSA for 30 min; degranulation was assessed by measuring β-hexosaminidase levels. (D and E) The WT and RKIP KO BMMCs were presensitized with 1 μg/mL of anti–DNP-IgE overnight and activated with 100 ng/mL of DNP-BSA for 1 h; the release of the (D) LTC4 and (E) PGD2 was measured by competitive ELISA (Cayman Chemicals). (F) Measurement of IgE−FcεRI-mediated cell electrical impedance. The WT and RKIP KO BMMCs were seeded onto the fibrinogen-coated surfaces of microelectronic cell sensor arrays integrated into the bottoms of microtiter plates and presensitized with 1 μg/mL of anti–DNP-IgE for 5 h. Subsequently, the cells were stimulated with 100 ng/mL of DNP-BSA, with the impedance being measured every 5 min and then analyzed by xCELLigence (a real-time cell analyzer). (G) Calcium flux was measured at the indicated times after 1 μg/mL of anti–DNP-IgE presensitization and DNP-BSA stimulation in the WT and RKIP KO BMMCs. *P < 0.05; **P < 0.01. Data are representative of the three experiments. Data represent mean and SD.
RKIP Deficiency Aggravates Passive Systemic Anaphylaxis and Passive Cutaneous Anaphylaxis in Vivo.
To investigate the physiological role of RKIP in mast cell activation in vivo, we used mast cell-dependent animal models of passive systemic anaphylaxis (PSA) and passive cutaneous anaphylaxis (PCA) induced by anti–DNP-IgE/DNP-BSA to assess WT and RKIP KO mice (6, 19). Reportedly, no immune defects occur in the RKIP KO mice (14), and no differences occur in the mast cell development between WT and RKIP KO mice (SI Appendix, Fig. S3 A and B). The absolute numbers of mast cells in back skin was comparable in the WT and RKIP KO mice (SI Appendix, Fig. S3A). No significant differences occurred in mast cell morphology or cell surface expression of the c-Kit and FcεRI markers between the WT and RKIP KO BMMCs, as assessed by electron microscopy and flow cytometry (FACS) (SI Appendix, Fig. S3 C and D). Additionally, the gene expression of mouse mast cell proteases 1, 2, 4, 5, and 6 and the sensitivity of mast cells to IL-3−induced signaling (assessed by cellular levels of p-STAT5 and p-Erk1/2) were comparable in the WT and RKIP KO BMMCs (SI Appendix, Fig. S3 E and F). These results suggest that RKIP deficiency has no effect on the development of mast cells.
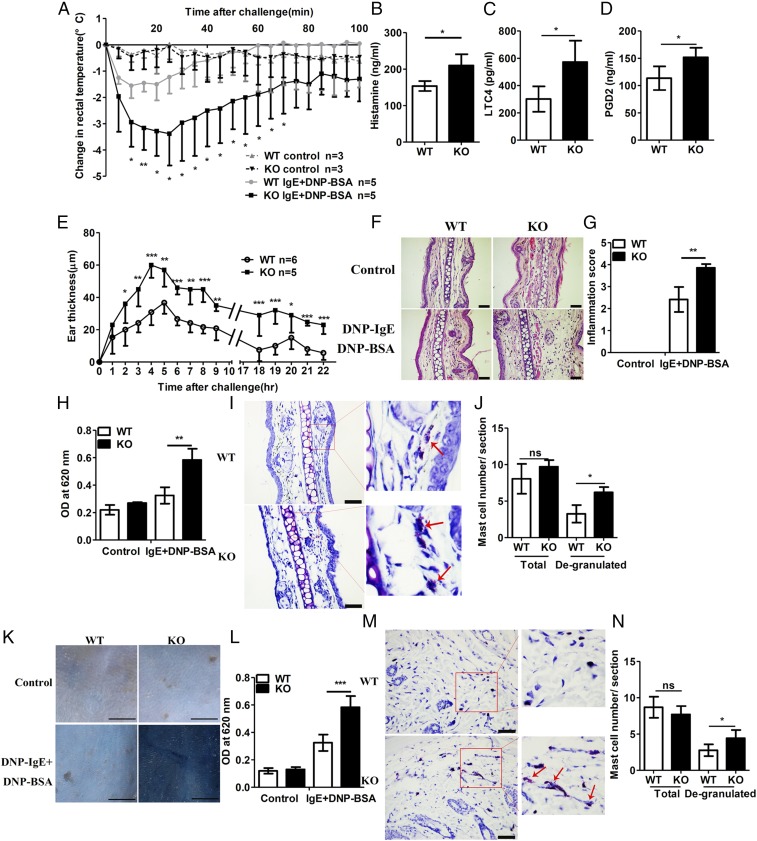
WT and RKIP KO mice were systemically presensitized with anti–DNP-IgE and then i.v.-injected with DNP-BSA to trigger a PSA response. DNP-BSA injection induced significant body temperature drops in both WT and RKIP KO mice, whereas RKIP KO mice showed much greater magnitude and duration of body temperature drops than that shown by the WT mice (Fig. 4A). Consistently, in association with the anaphylactic response, RKIP KO mice had much higher serum levels of histamine, LTC4, and PGD2 at 100 min after antigen challenge than exhibited by the WT mice (Fig. 4 B–D).
Fig. 4.
RKIP deficiency aggravates PSA and PCA in vivo. (A) Mice were injected i.v. with 10 μg of anti–DNP-IgE per mouse and challenged 24 h later with PBS or 100 μg of DNP-BSA per mouse. Rectal temperatures were assessed every 5 min. (B–D) Releases of (B) histamine, (C) LTC, and (D) PGD2 in the serum were measured at 100 min after DNP-BSA activation. (E) Mice were presensitized by intradermal injection of 3 μg of anti–DNP-IgE per ear (left ear) or an identical volume of PBS (right ear), and, 24 h later, the mice were challenged i.v. with 100 μg of DNP-BSA per mouse in PBS/Evans blue. Ear swelling was calculated as the difference between the thickness of the right and left ears (WT n = 6, KO n = 5). (F) H&E staining and (G) inflammatory cell infiltration scores of the histological sections of the right (control) and left ears (DNP-BSA) from mice treated as in E. (Scale bars in F, 50 μm.) (H) Evans blue dye was extracted in formaldehyde from the reaction sites in E and quantified according to the absorbance at 620 nm. (I) Toluidine blue staining and (J) quantification (blind analysis) of mast cells per section of the left ears from mice treated as shown in E. Red arrows indicate degranulated mast cells. (Scale bars in I, 50 μm.) (K) Mice were presensitized intradermally with anti–DNP-IgE or PBS; 2 d later, the mice were challenged with DNP-BSA in PBS/Evans blue, and the skin was examined visually (n = 6). (Scale bars, 10 mm.) (L) Evans blue dye was extracted in formaldehyde from the reaction sites in K and quantified as absorbance at 620 nm. (M) Toluidine blue staining and (N) quantification (blind analysis) of mast cells per section of back skin (DNP-BSA) from mice treated as in K. Red arrows indicate degranulated mast cells. (Scale bars in M, 50 μm.) ns, no significant difference; *P < 0.05; **P < 0.01; ***P < 0.001. Data are representative of three experiments. Data represent mean and SD.
Next, we elicited IgE−FcεRI-mediated PCA reactions to demonstrate the functional role of RKIP in both immediate and late-phase anaphylactic responses. Early-phase PCA and PSA reactions depend largely on the histamine and serotonin secreted by mast cells (20), whereas late-phase PCA reactions are associated with mast cell-derived cytokines in mouse. The ear swelling from inflammatory edema was greater in KO mice than that in WT mice for the entire observation period (Fig. 4E). Consistent with the ear swelling, the degree of inflammatory cell infiltration (Fig. 4 F and G) and the concentration of Evans blue dye (Fig. 4H) in response to IgE−FcεRI-mediated activation were higher in the ears of the KO mice than in the ears of the WT mice. We quantified mast cell degranulation in the ear skin from the KO or WT mice as previously reported (21). Diffuse toluidine blue-positive cells with no clearly defined cell membrane indicate degranulated mast cells. As shown in Fig. 4 I and J, the IgE−FcεRI-mediated mast cell degranulation in the ear of the RKIP KO mice was significantly higher than that in the ear of WT mice. To investigate the role of RKIP in early-phase PCA, 2 d after intradermal injection of anti–DNP-IgE or saline, we injected WT and KO mice with DNP-BSA dissolved in saline containing Evans blue. In saline-presensitized WT and KO mice, dye extravasation at 1 h after the antigen stimulus was comparable in WT and KO skin biopsies. However, Evans blue dye extravasation visualized and quantified in the back skin biopsies of IgE−FcεRI-activated mice was significantly enhanced compared with that of saline-presensitized mice, and the RKIP KO mice exhibited more Evans blue dye extravasation than the WT mice showed after IgE−FcεRI-mediated activation (Fig. 4 K and L). Consistently, the RKIP KO mice showed higher mast cell degranulation in the back skin than the WT mice showed upon IgE−FcεRI stimulation (Fig. 4 M and N). Generally, these data suggest that RKIP deficiency promotes IgE−FcεRI-mediated mast cell activation associated pathology in vivo and therefore enhances anaphylactic responses.
RKIP Deficiency Aggravates IgE−FcεRI-Mediated Allergic Responses in a Mast Cell-Dependent Manner.
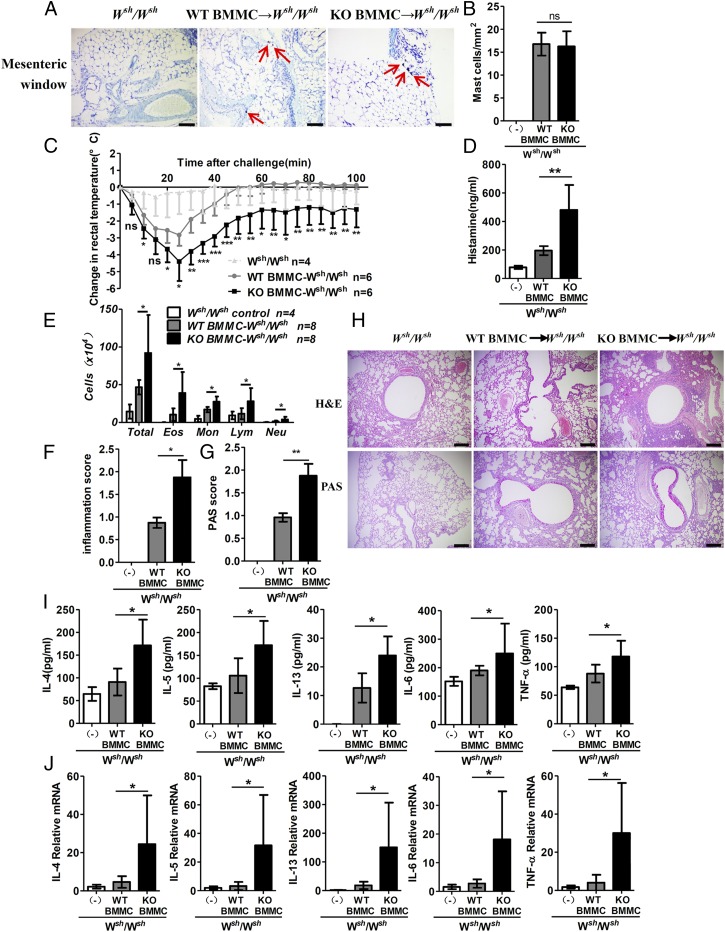
To further confirm whether the in vivo role of RKIP in allergic response is dependent on mast cell activation, we applied a mouse model of PSA in genetically mast cell-deficient KitW-sh/W-sh C57BL/6 mice, reconstituted with WT or KO BMMCs. No differences were observed in the mast cell engraftment between WT BMMC- and KO BMMC-reconstituted mice, as assessed by enumeration of the cells positive for toluidine blue staining in a mesenteric window (Fig. 5 A and B). As expected, after the IgE−FcεRI-mediated activation, the mast cell-deficient Kitw-sh/w-sh C57BL/6 mice reconstituted with the RKIP KO BMMCs showed a significantly enhanced PSA response compared with the mice reconstituted with WT BMMCs, including an amplified body temperature drop and increased histamine release (Fig. 5 C and D). To investigate the role of RKIP in the pathogenesis of mast cell-mediated allergic airway inflammation, we induced ovalbumin (OVA)-mediated mast cell-dependent chronic airway inflammation in Kitw-sh/w-sh C57BL/6 mice reconstituted with WT or RKIP KO BMMCs. The bronchoalveolar fluid (BALF) of Kitw-sh/w-sh mice reconstituted with RKIP KO BMMCs showed more inflammatory cell infiltration, especially eosinophil infiltration, than that of Kitw-sh/w-sh mice reconstituted with WT BMMCs (Fig. 5E). Consistently, KO mast cell-reconstituted Kitw-sh/w-sh mice exhibited enhanced airway inflammation, as evident from hematoxylin and eosin (H&E) and periodic acid−Schiff (PAS) staining scores, compared with the WT mast cell-reconstituted Kitw-sh/w-sh mice (Fig. 5 F and G). Increased peribronchiolar infiltration of inflammatory cells and enhanced hyperplasia of mucus-secreting goblet cells (PAS+) in large bronchioles were observed in the KO mast cell-reconstituted mice, as assessed by H&E and PAS staining, respectively (Fig. 5H). Moreover, KO mast cell-reconstituted Kitw-sh/w-sh mice secreted higher levels of cytokines and chemokines in BALF than secreted by the WT mast cell-reconstituted Kitw-sh/w-sh mice (Fig. 5 I and J). These findings indicate that RKIP-specific deficiency in mast cells leads to exacerbated anaphylactic and allergic responses in vivo.
Fig. 5.
RKIP deficiency in mast cells aggravates IgE-mediated allergic response. Mast cell-deficient mice were given no BMMCs (Wsh/Wsh control; n = 4) or were reconstituted with WT BMMCs (WT BMMC → Wsh/Wsh; n = 6) or RKIP KO BMMCs (KO BMMC → Wsh/Wsh; n = 6). (A) Four months later, tissue mast cells in a mesenteric window were assessed in the indicated chimeric mice by toluidine blue staining. Red arrows indicate mast cells. (Scale bars, 50 μm.) (B) Absolute numbers of mast cells per square millimeter in Wsh/Wsh control mice (n = 4) or chimeric mice (n = 6 per group). Reconstituted mice were presensitized with anti–DNP-IgE overnight and challenged 24 h later with 100 μg of DNP-BSA or PBS in Evans blue dye. (C) Rectal temperatures were assessed every 5 min. (D) Histamine release in serum was measured at 100 min after PBS or DNP-BSA challenge. Wsh/Wsh control (n = 5), WT BMMC → W sh/Wsh mice (n = 8), and KO BMMC → Wsh/Wsh mice (n = 8) were challenged with OVA or PBS in the airway inflammation model and analyzed 24 h after the final challenge. (E) Numbers of total cells (total), eosinophils (Eos), neutrophils (Neu), lymphocytes (Lym), and macrophages (Mac) in the BALF were assessed after Wright−Giemsa staining. (F) Inflammation scores and (G) PAS scores were analyzed as described in Materials and Methods. (H) Sections of lung tissues were stained with H&E and PAS staining. (Scale bars, 200 µm.) (I) The mRNA expression of IL-4, IL-5, IL-13, IL-6, and TNF-α in lung tissue was assessed by real-time PCR (n = 6). (J) Secretion of IL-4, IL-5, IL-13, IL-6, and TNF-α in BALF as measured by ELISA (n = 6). ns, no significant difference; *P < 0.05; **P < 0.01. Data are representative of three experiments. Data represent the mean and SD.
RKIP Deficiency Promotes IgE−FcεRI-Mediated Mast Cell Activation by Enhancing PI3K/Akt/NF-κB Axis Signaling.
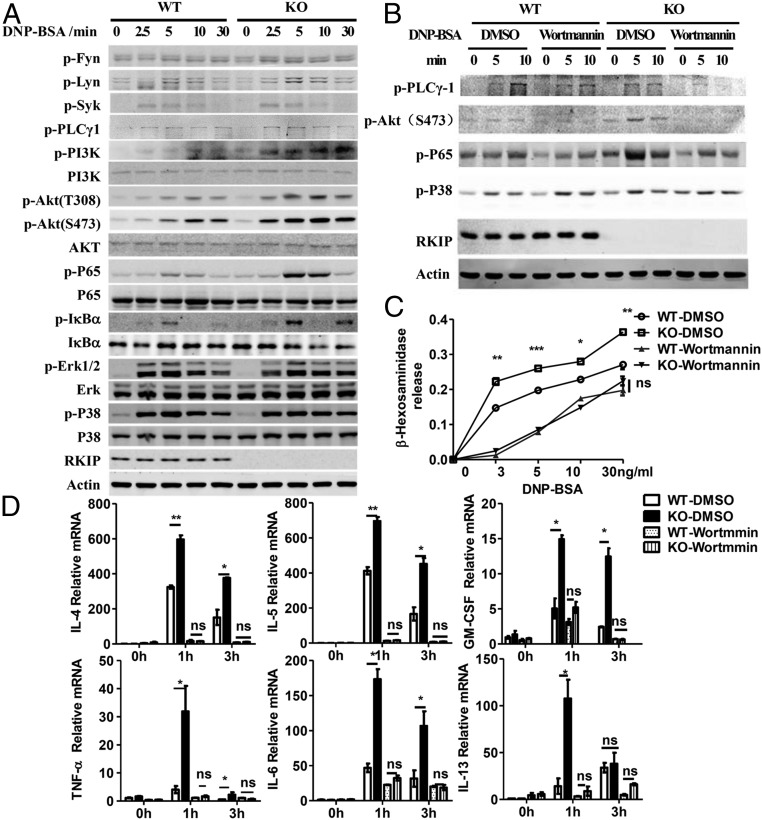
The in vivo and in vitro data suggest that RKIP inhibits mast cell activation and consequent anaphylaxis and allergic disorders. We next investigated the mechanism underlying RKIP inhibition of mast cell activation. Upon interaction between an allergen and the cross-linked IgE−FcεRI complex, the signaling cascades are initiated by activation of the Src family kinases Lyn and Fyn. Activated Lyn, Fyn, and Syk further promote LATs (including LAT1 and LAT2)-dependent Grb2/PLC-γ1/SLP-76/MAPKs/PKCs signaling. However, Fyn can directly phosphorylate another adaptor, Gab2, and further recruits PI3K to phosphatidylinositol 4,5-bisphosphate to activate the PI3K/Akt/IKK/NF-κB pathway (10, 22). WT and RKIP KO BMMCs were presensitized with anti–DNP-IgE and stimulated with DNP-BSA for the lengths of time indicated in Fig. 6A. As shown in the figure, the activation of the FcεRI-proximal tyrosine kinases Lyn, Fyn, Syk, and PLC-γ1 were comparable in the WT and KO BMMCs. IgE−FcεRI-mediated phosphorylation of the MAPKs, such as p38 and ERK1/2, was not altered in the absence of RKIP. However, RKIP deficiency or silencing resulted in a more striking enhancement in the activation of distal signaling, including the phosphorylation of PI3K, Akt, the NF-κB p65 subunit, and IκBα (Fig. 6A and SI Appendix, Fig. S4A). Incubation with recombinant RKIP protein significantly inhibited the IgE−FcεRI-mediated signaling transduction, including phosphorylation of Akt and the NF-κB p65 subunit, whereas it had no effect on the phosphorylation of the MAPKs, such as p38 and ERK1/2, in the mast cells (SI Appendix, Fig. S4B). To further examine whether RKIP KO-mediated mast cell activation depends on the enhancement of the PI3K/Akt pathway, we pretreated the RKIP WT and KO BMMCs with wortmannin, a specific inhibitor of PI3K. As shown in Fig. 6B, the specific PI3K inhibitor not only inhibited Akt activation but also abolished the increased NF-κB p65 phosphorylation in the RKIP KO BMMCs. Furthermore, on suppression of PI3K activity by wortmannin, IgE−FcεRI-mediated degranulation (measured by β-hexosaminidase) (Fig. 6C) and the production of cytokines by KO BMMCs reverted to levels comparable to those in the WT BMMCs (Fig. 6D). Moreover, the PI3K-silenced RKIP KO BMMCs showed signaling activation and cytokine expression comparable to the PI3K-silenced WT BMMCs (SI Appendix, Fig. S4 C and D). Overall, these data suggest that RKIP inhibits mast cell activation by targeting PI3K activation.
Fig. 6.
RKIP deficiency increases FcεRI-mediated PI3K/Akt/NF-κB axis signaling activation in mast cells. (A) Immunoblot analysis in lysates of the WT and RKIP KO BMMCs stimulated by IgE−FcεRI-mediated activation for the indicated times. (B–D) WT and RKIP KO BMMCs were presensitized with 1 μg/mL of anti–DNP-IgE overnight, pretreated with wortmannin (PI3K inhibitor) for 2 h, and then stimulated with 100 ng/mL of DNP-BSA for the indicated times. (B) Immunoblot analysis was performed to identify the total or phosphorylated proteins in lysates using indicated antibodies. (C) The β-hexosaminidase was measured as described in Materials and Methods. (D) The mRNA expression levels of cytokines and chemokines were measured by real-time PCR. ns, no significant difference; *P < 0.05; **P < 0.01; ***P < 0.001. Data are representative of three experiments. Data represent mean and SD.
RKIP Competes with Gab2 to Interact with the PI3K p85 Subunit and Suppresses the Activation of PI3K on IgE−FcεRI Stimulation.
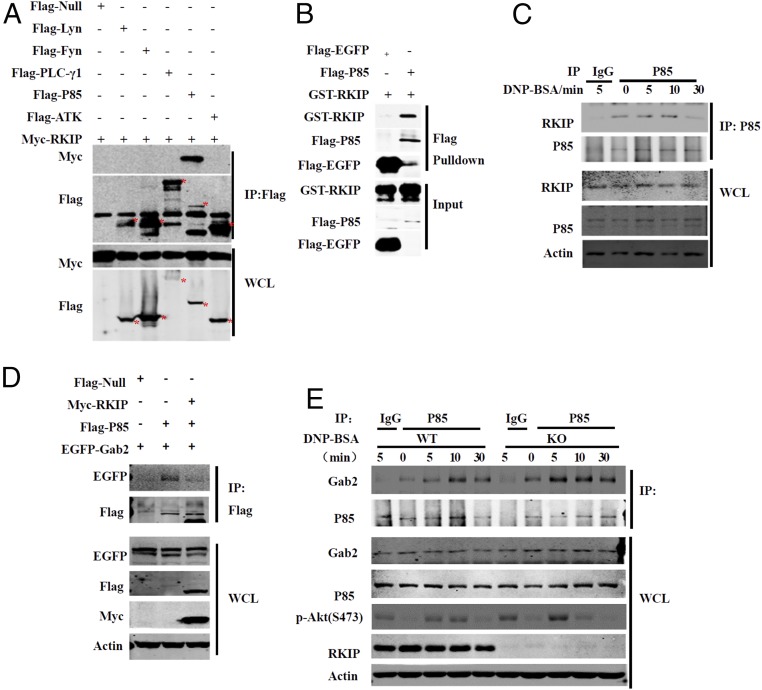
PI3K consists of the p85 regulatory subunit and the p110 catalytic subunit, and both are involved in mast cell activation signaling (23). During FcεRI-mediated mast cell activation, the adapter protein Gab2 binds to the PI3K p85 regulatory subunit and recruits it to the cell membrane, resulting in activation of p85 by Fyn or Syk (5). As shown in Fig. 7A, Myc-RKIP specifically interacted with the PI3K p85 subunit but not with Lyn, Fyn, PLC-γ1, or Akt. In addition, RKIP did not bind to Gab2 (SI Appendix, Fig. S5B) and the PI3K p110 subunit (SI Appendix, Fig. S5C). The Flag-p85 or Flag-EGFP proteins were purified from overexpressed HEK293T cells and incubated with recombinant GST-RKIP protein. As shown in Fig. 7B, RKIP directly interacted with p85. RKIP physically associated with p85 in the mast cells, and IgE−FcεRI stimulation promoted this association in a time-dependent manner, as the interaction peaked at 5 min after DNP-BSA treatment in the BMMCs (Fig. 7C). All PI3K p85 isoforms have an N-terminal Src homology 3 (SH3) domain; two Prorich regions near the N terminus; a BCR homology domain, which binds to phosphorylated tyrosine; and a PI3K p110 binding domain located between two Src homology 2 domains, which form the PI3K p85 core domain (SI Appendix, Fig. S5C) (23). Domain-mapping experiments showed that RKIP interacted with p85 in a manner dependent on the domain of the p85 core (aa 332 to 724) but not on the SH3 domain and P-BH-P domain (aa 1 to 331) (SI Appendix, Fig. S5C). Considering that RKIP has no effect on IgE−FcεRI-mediated Syk or Fyn phosphorylation but affects the PI3K activation, we next explored whether RKIP could inhibit the association of Gab2 with the PI3K p85 regulatory subunit, which is essential for the activation of PI3K. As shown in Fig. 7D, overexpression of RKIP in HEK293T cells inhibited the interaction of p85 with Gab2. Next, we performed coimmunoprecipitation assays in mast cells using an anti-p85 antibody. As shown in Fig. 7E, anti–DNP-IgE/DNP-BSA stimulation induced an interaction between Gab2 and p85, and this interaction was remarkably enhanced in RKIP-deficient mast cells. Collectively, these results suggest that RKIP directly binds to the PI3K p85 subunit and interferes with the association of Gab2 and p85, thereby inhibiting PI3K activation and downstream signaling.
Fig. 7.
RKIP competes with Gab2 to interact with the PI3K p85 subunit. (A) HEK293T cells were transfected with Myc-RKIP and Flag-Null, Flag-Lyn, Flag-PLCγ1, Flag-p85, or Flag-Akt plasmids; then they were immunoprecipitated (IP) with anti-Flag beads (M2 beads) and probed with anti-Myc or anti-Flag antibody. (Lower) Immunoblots analyzing whole cell lysates (WCL) without immunoprecipitation and (Upper) immunoblots analyzing immunoprecipitated flag-tagged proteins. (B) Flag-EGFP or Flag-p85 proteins purified from overexpressed HEK293T cells were mixed with recombinant GST-RKIP protein, and then the proteins were pulled down with M2 beads. (C) The lysates of the IgE−FcεRI-activated BMMCs were IP with anti-p85 or IgG (control) and probed with anti-RKIP or anti-p85 antibody. (D) Immunoblot of the HEK293T cells transfected with EGFP-Gab2 and Flag-p85 with or without Myc-RKIP, then were IP with M2 beads. (E) The lysates of IgE−FcεRI-activated RKIP WT or KO BMMCs were immunoprecipitated with anti-p85 or IgG (control) and probed with anti-Gab2 or anti-p85 antibody. Data are representative of three experiments.
Discussion
Mast cells play a key role in allergic responses and allergic asthma. In this study, we have shown that RKIP functions as an inhibitory modulator of IgE−FcεRI-mediated mast cell activation. RKIP deficiency in mast cells promotes the IgE−FcεRI-mediated secretion of allergic mediators, as well as the signaling transduction. Accordingly, RKIP-deficient mice are more susceptible than WT mice to anaphylactic response and allergic asthma. Furthermore, the expression of RKIP is significantly down-regulated in the IgE−FcεRI-activated mast cells and in asthma patients’ peripheral blood.
RKIP is well established as an inhibitory molecule in oncological processes (14). We recently demonstrated that RKIP is involved in colitis and antiviral innate immune responses, indicating that RKIP functions as an important mediator in immune response (15–17). Here, we found that RKIP deficiency in mast cells does not affect the development of mast cells or the expression of surface markers, including FcεRI and c-Kit, but significantly inhibits the IgE−FcεRI-mediated early-phase mediators, including histamine and β-hexosaminidase, and late-phase mediators, such as type 2 cytokines, proinflammatory cytokines, and other related chemokines (CCL1, CCL2). To our surprise, incubation of mouse mast cells with purified recombinant RKIP protein inhibits mast cell activation. Human mast cells have been reported to engulf and store exogenous IL-17A (24). The molecular mechanisms of RKIP protein uptake by mast cells need to be further explored.
Consistent with in vitro data, RKIP deficiency in mice significantly aggravates mast cell-mediated systemic and local skin allergies. Furthermore, Kitw-sh/w-sh mice reconstituted with RKIP KO mast cells exhibit enhanced PAS response and airway inflammation, accompanied by greatly increased type 2 cytokine production and airway mucus secretion, compared with Kitw-sh/w-sh mice reconstituted with RKIP WT mast cells. These results further demonstrate that RKIP negatively regulates mast cell-mediated hypersensitivity and allergic asthma.
Further experiments showed RKIP expression is down-regulated in IgE−FcεRI-activated mouse mast cells and human CD34+ derived primary mast cells. RKIP mRNA expression of peripheral blood from patients with asthma is significantly more inhibited than that in healthy control individuals. EZH2 has been reported to bind to the E boxes of the RKIP promoter to down-regulate the transcription of RKIP (25). The mRNA and protein expression of EZH2 increased after IgE−FcεRI-mediated activation in BMMCs. Furthermore, silencing of EZH2 in the WT BMMCs completely reversed the IgE−FcεRI-mediated down-regulation of RKIP. Consistently, a chromatin immunoprecipitation assay further confirmed that EZH2 binds to the E boxes of the RKIP promoter in mouse mast cells, indicating that EZH2 may use a similar mechanism to the one it uses in breast and prostate cells to suppress expression of RKIP under IgE−FcεRI-mediated activation. Interestingly, GEO data and our findings show that the expression of RKIP in peripheral blood is negatively correlated with the severity of human asthma. We further determined that RKIP expression was negatively related to the occurrence of signs and symptoms in patients with asthma. Since mature mast cells are not present in blood, reduced RKIP expression in the blood of asthmatic patients suggests RKIP in cells other than mast cells could contribute to human asthma, which needs to be further investigated in the future.
The signal transduction that mediates mast cell activation by IgE−FcεRI is accomplished mainly by phosphorylation of two similar linker proteins, LAT1 and NTAL (LAT2). LATs activate mainly the major activation signaling pathway (Grb2/PLC-γ1/SLP-76/MAPK), whereas a complementary signaling pathway (Gab2/PI3K/Akt/NF-κB) is directly activated by phosphorylated Fyn. RKIP deficiency does not affect the activation of the tyrosine kinases Lyn, Fyn, and Syk or the MAPKs but significantly enhances the phosphorylation of PI3K/Akt and p65. The promoting effect of the RKIP KO on IgE−FcεRI-induced signaling activation, the downstream secretion of cytokines and chemokines, and the release of β-hexosaminidase disappears when specific inhibitors are used to block PI3K activity, indicating that RKIP KO increases the IgE−FcεRI-induced mast cell activation by promoting PI3K activation. Coimmunoprecipitation experiments show that RKIP specifically interacts with the PI3K regulatory subunit p85. We further demonstrate that RKIP competes with Gab2 to interact with p85 and interferes with the association of p85 PI3K and Gab2, dampening the PI3K/Akt/NF-κB signaling activation and the release of downstream mediators.
RKIP was originally identified as a Raf-1 binding protein that inhibits MEK/ERK1/2 phosphorylation (26). However, the activation of ERK1/2 did not differ between the WT and RKIP-deficient BMMCs subjected to the IgE−FcεRI-mediated activation. PKC switches RKIP from a Raf-1 kinase inhibitor to a G protein-coupled receptor regulator by phosphorylating the RKIP at Ser153 (27). We recently demonstrated that RKIP is phosphorylated at Ser109 on infection with virus; this phosphorylation is critical to RKIP’s ability to promote TBK1 activation and antiviral response. Although we have found down-regulated RKIP expression in IgE−FcεRI-activated mast cells, further investigation is needed to determine whether the IgE−FcεRI induced posttranslational modification of RKIP, causing RKIP to function as a PI3K inhibitor instead of a Raf kinase inhibitor.
Together, our findings reveal an important negative regulatory function of RKIP in mast cell activation. RKIP deletion considerably enhances the immunopathological phenotype of mice with mast cell-mediated PSA, PCA, and allergic asthma. Moreover, RKIP expression is down-regulated in asthma patients’ peripheral blood and is negatively correlated with the process of asthma, indicating that the RKIP peptide-mimicking drugs might provide a viable approach to the treatment of allergic asthma.
Materials and Methods
Mice.
RKIP KO (rkip−/−) mice were kindly provided by John Sedivy of Brown University. Rkip−/− mice and their littermates with a C57BL/6 background were used in this study. The progeny of the rkip+/− intercrosses were genotyped by PCR analysis of DNA isolated from the tail. The following three primers were used for the PCR: RKIP KO sense (5′-GAGCCCTGGCCGGTCTCCCTTGTCCCAAACTTT-3′), RKIP WT antisense (5′CACAAAACCAATCTTAAAGAGCCA-3′), and RKIP KO antisense (5′ CCAAAAGGGTCTTTGAGCACCAGAGGACATCCG-3′). Kit w-sh/w-sh mice (B6.Cg-KitW-sh/HNihrJaeBsmGlliJ, Stock No. 012861 | sash) were purchased from The Jackson Laboratory. All of the mice were kept in specific pathogen-free conditions. All animal experiments were performed in accordance with protocols approved by the Scientific Investigation Board of Zhejiang University.
Human Peripheral Blood Samples.
Peripheral blood samples were obtained from healthy controls (n = 56) or children with asthma (n = 52) on admission from Children’s Hospital, Zhejiang University School of Medicine. Peripheral blood samples analyzed in Fig. 1C were obtained from children with asthma (n = 26) at the onset and remission of asthma signs and symptoms on admission from Children’s Hospital, Zhejiang University School of Medicine. The diagnosis and evaluation of asthma severity were made according to the Expert consensus document on diagnosis and treatment of asthma. Detailed patients’ information is listed in Datasets S1 and S2. The study was approved by the ethics committee of the Children’s Hospital, Zhejiang University School of Medicine. Written informed consent was obtained from at least one guardian of each patient before enrollment. The data from the patients were analyzed anonymously.
Statistical Analysis.
Statistical significance of the differences between the two experimental groups was assessed using unpaired two-tailed Student’s t test. The level of statistically significant difference was defined as P < 0.05.
Supplementary Material
Acknowledgments
This work is supported by the National Key Basic Research Program of China Grant 2014CB542101; National Natural Science Foundation of China Grants 31570864, 31700765, and 81871264; and National Postdoctoral Science Foundation of China Grants 2017M612006 and 2017T100433.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.J.G. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1805474115/-/DCSupplemental.
References
- 1.Siegel AM, et al. Diminished allergic disease in patients with STAT3 mutations reveals a role for STAT3 signaling in mast cell degranulation. J Allergy Clin Immunol. 2013;132:1388–1396. doi: 10.1016/j.jaci.2013.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18:693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125(Suppl 2):S73–S80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim DK, et al. DJ-1 regulates mast cell activation and IgE-mediated allergic responses. J Allergy Clin Immunol. 2013;131:1653–1662. doi: 10.1016/j.jaci.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol. 2008;9:1215–1223. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yip KH, et al. The Nedd4-2/Ndfip1 axis is a negative regulator of IgE-mediated mast cell activation. Nat Commun. 2016;7:13198. doi: 10.1038/ncomms13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baba Y, et al. Essential function for the calcium sensor STIM1 in mast cell activation and anaphylactic responses. Nat Immunol. 2008;9:81–88. doi: 10.1038/ni1546. [DOI] [PubMed] [Google Scholar]
- 8.Hwang SL, et al. ERK1/2 antagonize AMPK-dependent regulation of FcεRI-mediated mast cell activation and anaphylaxis. J Allergy Clin Immunol. 2014;134:714–721.e7. doi: 10.1016/j.jaci.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Vennekens R, et al. Increased IgE-dependent mast cell activation and anaphylactic responses in mice lacking the calcium-activated nonselective cation channel TRPM4. Nat Immunol. 2007;8:312–320. doi: 10.1038/ni1441. [DOI] [PubMed] [Google Scholar]
- 10.Gilfillan AM, Rivera J. The tyrosine kinase network regulating mast cell activation. Immunol Rev. 2009;228:149–169. doi: 10.1111/j.1600-065X.2008.00742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J Allergy Clin Immunol. 2006;117:1214–1225; and quiz (2006) 117:1226. doi: 10.1016/j.jaci.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 12.Nishida K. [Role of adaptor molecule Gab2 in mast cell-mediated allergy response] Yakugaku Zasshi. 2013;133:413–418. Japanese. doi: 10.1248/yakushi.12-00227-1. [DOI] [PubMed] [Google Scholar]
- 13.Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006;6:218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- 14.Yesilkanal AE, Rosner MR. Raf kinase inhibitory protein (RKIP) as a metastasis suppressor: Regulation of signaling networks in cancer. Crit Rev Oncog. 2014;19:447–454. doi: 10.1615/critrevoncog.2014012000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin W, et al. Raf kinase inhibitor protein mediates intestinal epithelial cell apoptosis and promotes IBDs in humans and mice. Gut. 2017;66:597–610. doi: 10.1136/gutjnl-2015-310096. [DOI] [PubMed] [Google Scholar]
- 16.Gu M, et al. RKIP and TBK1 form a positive feedback loop to promote type I interferon production in innate immunity. EMBO J. 2016;35:2553–2565. doi: 10.15252/embj.201694060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai R, et al. Raf kinase inhibitor protein preferentially promotes TLR3-triggered signaling and inflammation. J Immunol. 2017;198:4086–4095. doi: 10.4049/jimmunol.1601672. [DOI] [PubMed] [Google Scholar]
- 18.Ubel C, et al. The activating protein 1 transcription factor basic leucine zipper transcription factor, ATF-like (BATF), regulates lymphocyte- and mast cell-driven immune responses in the setting of allergic asthma. J Allergy Clin Immunol. 2014;133:198–206.e9. doi: 10.1016/j.jaci.2013.09.049. [DOI] [PubMed] [Google Scholar]
- 19.Wang D, et al. Tespa1 negatively regulates FcεRI-mediated signaling and the mast cell-mediated allergic response. J Exp Med. 2014;211:2635–2649. doi: 10.1084/jem.20140470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hata D, et al. Involvement of Bruton’s tyrosine kinase in FcεRI-dependent mast cell degranulation and cytokine production. J Exp Med. 1998;187:1235–1247. doi: 10.1084/jem.187.8.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kondeti V, et al. Leukotriene D4 and prostaglandin E2 signals synergize and potentiate vascular inflammation in a mast cell-dependent manner through cysteinyl leukotriene receptor 1 and E-prostanoid receptor 3. J Allergy Clin Immunol. 2016;137:289–298. doi: 10.1016/j.jaci.2015.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barbu EA, Zhang J, Siraganian RP. The limited contribution of Fyn and Gab2 to the high affinity IgE receptor signaling in mast cells. J Biol Chem. 2010;285:15761–15768. doi: 10.1074/jbc.M110.109413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim MJ, Park HR, Shin TY, Kim SH. Diospyros kaki calyx inhibits immediate-type hypersensitivity via the reduction of mast cell activation. Pharm Biol. 2017;55:1946–1953. doi: 10.1080/13880209.2017.1354386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noordenbos T, et al. OP0226 human mast cells engulf and store exogenous IL-17A. Ann Rheum Dis. 2014;73:147. [Google Scholar]
- 25.Ren G, et al. Polycomb protein EZH2 regulates tumor invasion via the transcriptional repression of the metastasis suppressor RKIP in breast and prostate cancer. Cancer Res. 2012;72:3091–3104. doi: 10.1158/0008-5472.CAN-11-3546. [DOI] [PubMed] [Google Scholar]
- 26.Yeung K, et al. Suppression of Raf-1 kinase activity and MAP kinase signalling by RKIP. Nature. 1999;401:173–177. doi: 10.1038/43686. [DOI] [PubMed] [Google Scholar]
- 27.Lorenz K, Lohse MJ, Quitterer U. Protein kinase C switches the Raf kinase inhibitor from Raf-1 to GRK-2. Nature. 2003;426:574–579. doi: 10.1038/nature02158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.