Significance
Free will consists of a desire to act (volition) and a sense of responsibility for that action (agency), but the brain regions responsible for these processes remain unknown. We found that brain lesions that disrupt volition occur in many different locations, but fall within a single brain network, defined by connectivity to the anterior cingulate. Lesions that disrupt agency also occur in many different locations, but fall within a separate network, defined by connectivity to the precuneus. Together, these networks may underlie our perception of free will, with implications for neuropsychiatric diseases in which these processes are impaired.
Keywords: free will, agency, volition, functional connectivity, lesions
Abstract
Our perception of free will is composed of a desire to act (volition) and a sense of responsibility for our actions (agency). Brain damage can disrupt these processes, but which regions are most important for free will perception remains unclear. Here, we study focal brain lesions that disrupt volition, causing akinetic mutism (n = 28), or disrupt agency, causing alien limb syndrome (n = 50), to better localize these processes in the human brain. Lesion locations causing either syndrome were highly heterogeneous, occurring in a variety of different brain locations. We next used a recently validated technique termed lesion network mapping to determine whether these heterogeneous lesion locations localized to specific brain networks. Lesion locations causing akinetic mutism all fell within one network, defined by connectivity to the anterior cingulate cortex. Lesion locations causing alien limb fell within a separate network, defined by connectivity to the precuneus. Both findings were specific for these syndromes compared with brain lesions causing similar physical impairments but without disordered free will. Finally, our lesion-based localization matched network localization for brain stimulation locations that disrupt free will and neuroimaging abnormalities in patients with psychiatric disorders of free will without overt brain lesions. Collectively, our results demonstrate that lesions in different locations causing disordered volition and agency localize to unique brain networks, lending insight into the neuroanatomical substrate of free will perception.
Long the domain of philosophy, free will can be investigated scientifically (1–6). Experiments such as those by Benjamin Libet sparked debate regarding whether free will exists or is an illusion (1–3). This debate remains unsettled, but most researchers agree that we perceive our actions to be freely willed (4–6). Recent investigations have therefore focused on understanding this perception, dividing it into two processes: the intention or motivation to act, referred to as volition (5), and the sense of responsibility for one’s action, referred to as agency (4).
Many approaches have been used to identify brain regions involved in the perception of volition or agency. For example, direct electrical stimulation to some brain regions but not to others can alter free will perception (7–10) while noninvasive brain stimulation can modulate experimental measures of agency and volition (4, 6, 11, 12). Functional neuroimaging can identify brain regions whose activity correlates with volition or agency in normal subjects (13, 14) or is abnormal in patients with “disorders of free will” such as functional movement disorders, psychogenic nonepileptic seizures (PNES), or catatonia (15, 16). Finally, patients with brain lesions in specific locations can experience profound disruptions in volition and agency. For example, patients with akinetic mutism lack the motivation to move or speak (17), while patients with alien limb syndrome feel that their movement is generated by someone else (18). These lesion-induced syndromes are often used as paradigmatic examples of disrupted volition and agency, respectively (15).
Despite these studies, localization of volition and agency in the human brain remains unclear. Alterations in free will perception have been reported following stimulation to a variety of different brain regions (4, 6–12) and neuroimaging correlates of free will perception have been highly heterogeneous across different studies (13, 14). Even focal brain lesions, often considered the gold standard for neuroanatomical localization (19–21), can occur in multiple different brain locations but cause similar disruptions in volition or agency (22, 23).
Lesion network mapping (Fig. 1) is a recently validated technique that identifies regions functionally connected to a lesion location, allowing one to localize symptoms even when lesions occur in different brain locations (24–28). For example, lesions that cause visual hallucinations fall within a single brain network connected to the extrastriate visual cortex, lesions that cause pain fall within a network connected to the posterior insula, and lesions that cause aphasia fall within a network connected to the left inferior frontal gyrus (24). This approach has been validated for 2D approximations of 3D lesions including images of lesions from published articles (24, 26) and has lent insight into complex but poorly understood neuropsychiatric syndromes such as abnormal movements (28, 29), delusions (26), loss of consciousness (27), and criminal behavior (30). A similar approach has been applied to brain stimulation sites in different locations that relieve similar symptoms (31, 32).
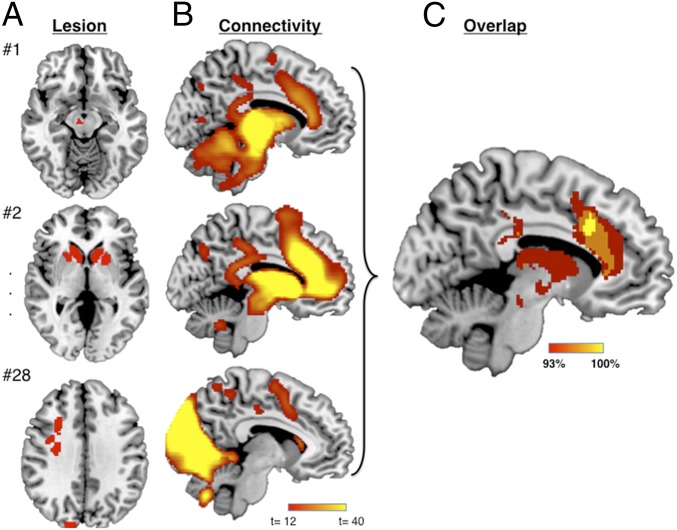
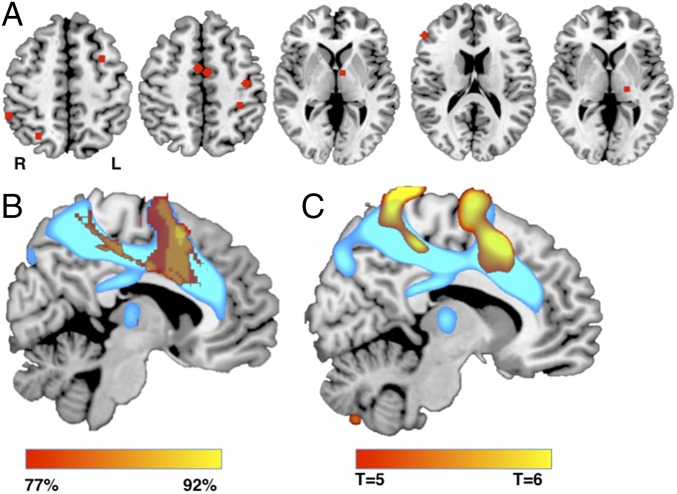
Fig. 1.
Lesion network mapping technique. (A) Three representative lesions causing akinetic mutism or abulia (disordered volition). (B) Network of regions functionally connected to each lesion location across a large (n = 1,000) resting-state functional connectivity dataset. (C) Lesion network overlap map showing regions connected to all or most lesion locations.
Here, we use this network localization approach to determine the neuroanatomical substrate of disordered free will perception. First, we test whether lesions in different brain locations causing akinetic mutism and alien limb are part of the same functionally connected brain network. Second, we test for specificity by comparing our results to those for lesions causing similar physical symptoms, but with intact perception of volition and agency. Finally, we test whether our localizations of volition and agency based on focal brain lesions align with brain stimulation sites altering free will perception and neuroimaging abnormalities in psychiatric patients with disordered free will perception.
Results
Lesion Network Localization of Disordered Volition.
We identified 28 cases where lesions impaired the ability to volitionally initiate movements, causing akinetic mutism or abulia (SI Appendix, Table S1). Lesions were traced onto a standard brain atlas (Fig. 2A and SI Appendix, Fig. S1). Lesion locations were heterogeneous, including the anterior cingulate cortex (ACC) (21% of cases), globus pallidus (29%), thalamus (25%), caudate (18%), and brainstem (11%).
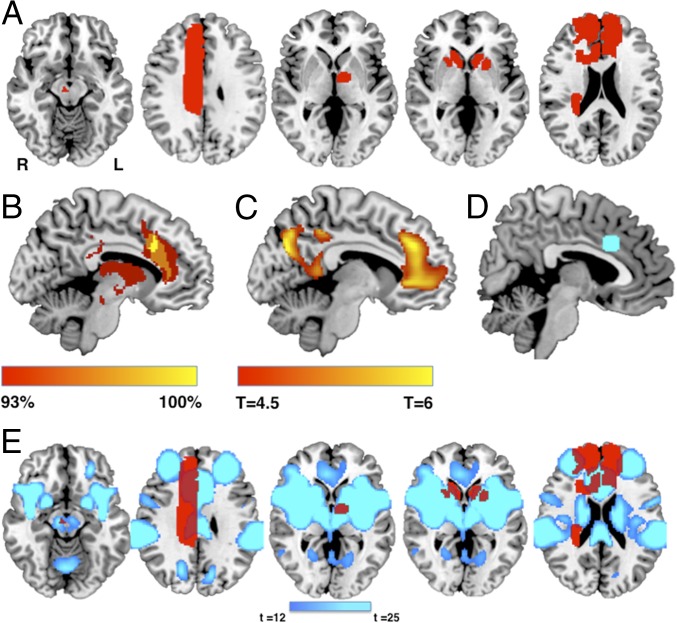
Fig. 2.
Lesion network localization of disordered volition. (A) Five representative lesions (of 28 total) causing akinetic mutism, demonstrating heterogeneity of lesion location. (B) Percentage of lesion locations functionally connected to each brain voxel. (C) t test comparing functional connectivity of lesions causing akinetic mutism vs. lesions causing hemiparesis (voxel-wise few-corrected P < 0.05). (D) Region of interest in the anterior cingulate centered on our peak site of lesion network overlap from B. (E) Functional connectivity with this region of interest defines a brain network (blue) that encompasses heterogeneous lesion locations disrupting volition (red).
Next, we performed lesion network mapping to determine whether these lesion locations were part of a common brain network. Regions functionally connected to each lesion location were identified using a large database (n = 1,000) of resting-state functional connectivity from normal subjects (33). Brain regions that were significantly positively or negatively correlated with each lesion location were identified (Fig. 1B) (24, 30, 32, 34). These lesion network maps were then thresholded at T ≥ 5 [corresponding to whole-brain voxel-wise family-wise error (FWE)-corrected P < 0.05], binarized, and overlapped to identify brain regions significantly connected to all or most lesions causing disordered volition (Fig. 1C). While lesions occurred in different locations, all 28 lesions (100%) were part of a single brain network defined by functional connectivity to the ACC (Fig. 2B and SI Appendix, Table S2).
To assess specificity, we compared the lesion connectivity in patients with akinetic mutism/abulia to that in patients with hemiparesis. Patients with hemiparesis also fail to initiate voluntary movements on their paralyzed side; however, in contrast to patients with akinetic mutism or abulia, patients with hemiparesis retain the urge and motivation to move (i.e., intact volition). We identified 25 lesions causing hemiparesis (35), performed lesion network mapping as above (SI Appendix, Fig. S2), and statistically compared the results to those for lesions that disrupt volition. Lesions causing disordered volition were significantly more connected to the ACC compared with lesions causing hemiparesis, among other regions (Fig. 2C and SI Appendix, Table S3).
To illustrate that lesion locations disrupting volition are part of a common brain network, we computed functional connectivity with our site of peak network overlap in the anterior cingulate [Fig. 2D, Montreal Neurological Institute (MNI) coordinates x = 2, y = 18, z = 32], which defines a spatial network that, by definition, encompasses lesion locations disrupting volition (Fig. 2E).
Lesion Network Localization of Disordered Agency.
We identified 50 cases of brain lesions causing involuntary movements that patients claimed they were not responsible for generating, a clinical syndrome termed alien limb (SI Appendix, Fig. S3 and Table S1). Again, lesion locations were diverse and included the medial frontal cortices (24%), corpus callosum (22%), parietal lobes (36%), and thalamus (8%) (Fig. 3A). While the lesions themselves were spatially diverse, lesion network mapping showed that 45 of the 50 lesions (90%) fell within a single brain network defined by functional connectivity to the precuneus cortex (Fig. 3B and SI Appendix, Table S2).
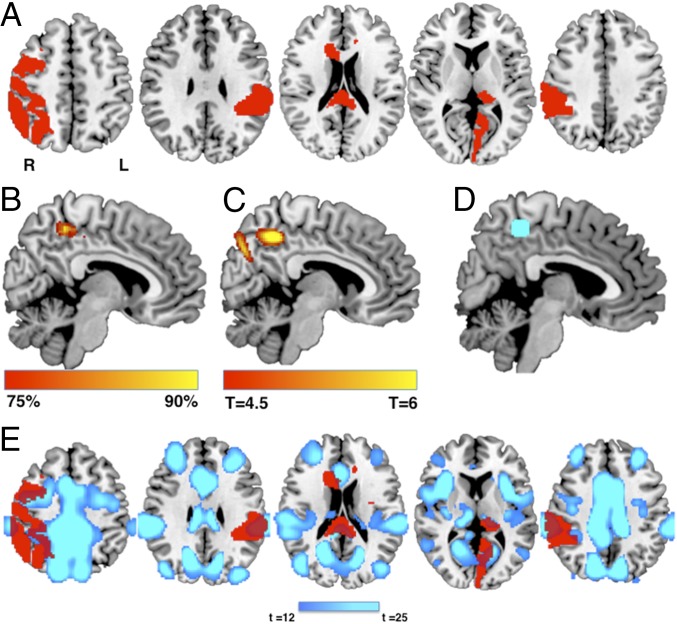
Fig. 3.
Lesion network localization of disordered agency. (A) Five representative lesions (of 50 total) causing alien limb syndrome, demonstrating heterogeneity of lesion location. (B) Percentage of lesion locations functionally connected to each brain voxel. (C) t test comparing functional connectivity of lesions causing alien limb vs. lesions causing hemichorea (voxel-wise few-corrected P < 0.05). (D) Region of interest in the precuneus centered on our peak site of lesion network overlap from B. (E) Functional connectivity with this region of interest defines a brain network (blue) that encompasses heterogeneous lesion locations disrupting agency (red).
To assess specificity, we compared lesions causing alien limb to lesions causing hemichorea (SI Appendix, Fig. S2) (28). In hemichorea, patients have involuntary movements, similar to patients with alien limb; however, patients with hemichorea continue to feel responsible for these movements (i.e., intact agency) (15). We found that connectivity to the precuneus region was specific to lesions causing alien limb compared with hemichorea (Fig. 3C and SI Appendix, Table S3).
For illustration purposes, we computed functional connectivity with our site of peak network overlap in the precuneus (Fig. 3D, MNI coordinates x = 10, y = −40, z = 50), which, by definition, defines a spatial network that encompasses lesion locations disrupting agency (Fig. 3E).
Network Localization of Brain Stimulation Sites Altering Free Will Perception.
To test whether our results, derived from focal brain lesions, align with results of prior brain stimulation studies, we identified 16 stimulation sites altering free will perception based on a systematic literature search [10 direct electrical stimulation sites and 6 transcranial magnetic stimulation (TMS) sites; SI Appendix, Table S4]. We also identified 17 control stimulation sites from the same studies that did not alter free will perception (11 direct neurosurgical stimulation sites and 6 TMS sites). Similar to brain lesions, stimulation sites altering free will perception have been reported across multiple different brain locations (Fig. 4A). However, 15/16 (94%) of these stimulation sites were part of a common functionally connected brain network that overlapped almost exactly with our volition and agency networks derived from focal brain lesions (Fig. 4B). This connectivity pattern was specific for stimulation sites altering free will perception compared with stimulation sites that did not alter free will perception (Fig. 4C). Finally, stimulation sites altering free will perception were significantly more connected to our lesion-derived region of interest for volition (Fig. 2D) and agency (Fig. 3D) compared with stimulation sites that did not disrupt free will [F (2, 30) = 3.69, P < 0.05].
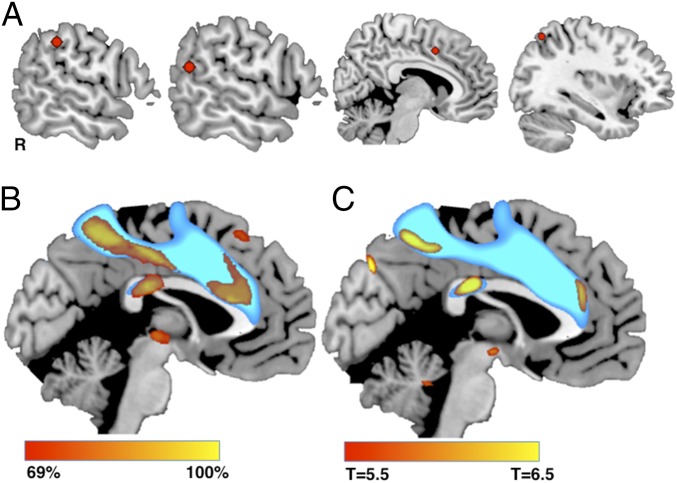
Fig. 4.
Network localization of stimulation locations altering free will perception. (A) Four representative brain stimulation sites (of 16 total) that altered free will perception, demonstrating heterogeneity in stimulation location. (B) Percentage of stimulation sites altering free will perception functionally connected to each brain voxel. (C) t test comparing connectivity of stimulation sites that did vs. did not alter free will perception. Results in B and C are overlaid on the network of voxels connected to our combined volition and agency ROIs derived from focal brain lesions (blue).
Network Localization of Neuroimaging Abnormalities in Psychiatric Disorders of Free Will Perception.
Next, we tested whether our localization of volition and agency based on focal brain lesions was relevant to psychiatric disorders of free will such as motor conversion disorder, PNES, or catatonia. Although these patients do not have focal brain lesions, we identified neuroimaging studies that reported areas of focal atrophy or decreased function in groups of patients with these disorders (motor conversion, n = 6; catatonia, n = 4; PNES, n = 3; SI Appendix, Table S5). Using the neuroimaging coordinates from each study as a “lesion,” we repeated the same analysis we used for lesion locations (Fig. 5A). Neuroimaging coordinates from 85% of studies were functionally connected to a common brain network that aligned well with our network derived from focal brain lesions (Fig. 5B). This connectivity was specific for neuroimaging abnormalities reported in psychiatric patients with disordered free will perception compared with neuroimaging abnormalities from patients without disordered free will perception (Alzheimer’s disease, n = 31, Fig. 5C) (36). Neuroimaging abnormalities in psychiatric disorders of free will were significantly more connected to our lesion-derived region of interest for volition (Fig. 2D) and agency (Fig. 3D) compared with neuroimaging abnormalities from patients without disordered free will perception [F (2, 41) = 10.76; P < 0.001].
Fig. 5.
Network localization of neuroimaging abnormalities in psychiatric disorders of free will perception. (A) Coordinates from five representative neuroimaging studies (of 13 total) reporting abnormalities in patients with psychiatric disorders of free will perception. (B) Percentage of studies whose coordinates were functionally connected to each brain voxel. (C) t test comparing connectivity of coordinates from psychiatric disorders of free will with coordinates from a control disorder not associated with abnormalities in free will. Results in B and C are overlaid on a map of voxels connected to our combined volition and agency ROIs derived from focal brain lesions (blue).
Discussion
Our results show that lesions that disrupt free will perception occur in different brain locations but localize to common brain networks. Specifically, we show that lesions that disrupt volition, causing akinetic mutism or abulia, are part of a common brain network defined by connectivity to the ACC. Lesions that disrupt agency, causing alien limb, are part of a common brain network defined by connectivity to the precuneus. Finally, we show that our lesion-based localization of volition and agency aligns well with brain stimulation sites that disrupt free will perception and neuroimaging abnormalities in psychiatric patients with disordered free will perception.
Lesions Causing Disordered Volition Localize to a Distinct Brain Network Defined by Connectivity to the ACC.
The heterogeneity of lesion-induced akinetic mutism and abulia has led to speculation that disordered volition is a network phenomenon (6, 22, 37). Using brain connectivity with lesion locations, we defined this network and found that it was centered in a specific part of the ACC. The ACC is thought to be involved in the motivation, planning, and control of volitional movements (5, 38) and is the chief neuroimaging correlate of volition in healthy subjects (5, 14). Surgical lesioning of the ACC for depression, obsessive compulsive disorder, or chronic pain is associated with impaired volition, although milder than in patients without these disorders who experience a stroke in this area (39–41). While it remains unknown why neurosurgical lesions lead to milder symptoms, one possibility is that the effects of a lesion are different in psychiatric patients with preexisting dysfunction in the ACC. This is analogous to ablation of the subthalamic nucleus in patients with Parkinson’s disease, which leads to much milder hemiballismus vs. lesions in previously normal persons (42).
Lesions Causing Disordered Agency Localize to a Distinct Brain Network Defined by Connectivity to the Precuneus.
Lesions causing disordered agency (alien limb syndrome) occurred in a network centered in the precuneus. Our peak network overlap site was in the right precuneus, consistent with more common involvement of the left limb in alien limb syndrome in our study (56% of cases) and in prior reports (23, 43, 44). The precuneus has previously been implicated in the normal sense of agency (13, 45), as well as in self-referential processing and visuospatial and motor integration for the body (45).
Interpreting Lesion Network Localization.
Our finding that heterogeneous lesion locations disrupting volition or agency localize to connected brain networks is consistent with results from a growing number of lesion network mapping studies across a variety of neuropsychiatric symptoms (21). The interpretation is similar to that in traditional lesion studies, but rather than localizing lesion deficits to a brain region these studies localize deficits to a brain network. One mechanism that may explain this network localization is functional diaschisis or remote functional effects of a lesion on anatomically intact but connected brain regions (46–49). According to this interpretation, lesion locations functionally connected to the ACC may result in remote functional effects on the ACC, disrupting volition, while lesion locations functionally connected to the precuneus may result in remote functional effects on the precuneus, disrupting agency. Another possibility is that volition requires intact function of a network of brain regions connected to the ACC while agency requires intact function of a network of brain regions connected to the precuneus, and lesions to any of these regions can disrupt volition and agency. According to this interpretation, agency and volition are properties of the entire network, rather than one specific region within that network. These interpretations are not mutually exclusive, and further work is needed to differentiate between them.
One possible concern is that lesion network mapping biases toward finding “hub” regions (e.g., precuneus) that are connected to more regions than nonhub regions (50). However, several pieces of evidence point away from this interpretation. First, previous lesion network mapping studies have often identified nonhub regions, such as extrastriate visual cortex for lesions causing peduncular hallucinosis (24). Second, our results were specific compared with those for lesion locations causing other symptoms, which controls for any potential hub bias. Finally, the precuneus location identified in our study is actually in a “nonhub” region with relatively low global connectivity compared with other brain regions (50).
Network Localization of Brain Stimulation Locations Disrupting Free Will Perception.
Similar to lesion locations causing the same symptom, different brain stimulation sites causing (or relieving) the same symptom may also localize to connected brain networks (31, 32, 51, 52). Several different brain stimulation sites have been reported to alter free will perception, including the ACC (7–9), pre-supplementary motor area (53, 54), and a variety of sites in the lateral parietal cortex (10, 11, 55–57). Unlike in our lesion cases, we did not separate brain stimulation sites into altered volition vs. agency due to a much lower N (14 vs. 78) and the fact that many stimulation effects were an ambiguous combination of the two. Despite this heterogeneity, these stimulation sites shared functional connectivity to a common brain network. More importantly, this network aligned with our network for free will perception derived from focal brain lesions. Convergent findings across two different causal sources of information (brain lesions and brain stimulation) increase our confidence in the current results.
Preliminary Extension of Network Localization to Neuroimaging Abnormalities in Psychiatric Patients.
Many neuropsychiatric diseases without overt brain lesions are conceptualized as disorders of free will. These include functional movement disorders, PNES, and catatonia (15). Our finding that neuroimaging abnormalities in these other disorders are part of the same brain network as focal brain lesions that disrupt volition and agency suggests a common substrate for free will perception. Future studies can address whether neuroimaging abnormalities in other disorders of free will, such as delusions of control and passivity symptoms in schizophrenia, show similar network localization. However, these results should be taken with caution: Unlike lesion network mapping itself, which has been applied and validated across multiple lesion-induced symptoms (24, 26–29, 34), this approach has not been used frequently to study neuroimaging abnormalities in groups of psychiatric patients. Specifically, although we treated these reported imaging abnormalities on structural MRI, fluorodeoxyglucose-PET, and single-photon emission computed tomography (SPECT) as “lesions” in our analysis, the actual dysfunction in these regions is likely to be far more complex. The current results suggest that conceptualizing these abnormalities as functional lesions may have value; however, testing in other symptoms with more established localization is needed.
Limitations Related to Lesion Network Mapping.
There are important limitations of the lesion network mapping technique, many of which have been addressed previously (24, 26, 30). First, accuracy of manual lesion tracing is limited by the quality of published images, and we used 2D lesions based on published images, which may not fully capture the spatial extent of 3D lesions. However, our prior studies have shown that the connectivity of 2D representations of 3D lesions is highly similar to the 3D lesion itself (spatial correlation coefficients >0.9) (24, 26). Moreover, any errors in lesion tracing should bias us against finding consistent network localization across lesions.
Another concern is that lesion network mapping results may depend on the specific connectome dataset used for the analysis. We have previously shown that results do not change when using an age-matched or disease-specific connectome (24, 31). Similarly, results do not change when using alternative connectome processing strategies (24, 31). Finally, we used a large (n = 1,000) normative connectome to determine functional connectivity between different parts of the brain. While this provides a highly accurate representation of group-level connectivity, it is possible that individual patient differences in connectivity would lead to different results. However, obtaining functional connectivity imaging from patients before the occurrence of a brain lesion is not practical, and functional connectivity with the lesion location cannot be computed using data obtained from patients after the lesion has occurred (that tissue is now dead), leaving a large normative connectome as the best practical option.
Because our analysis uses functional connectivity, we cannot determine whether the current results are driven by monosynaptic or polysynaptic connections or the potential directionality of such connections. Moreover, because we use a normative connectome, not functional neuroimaging data from patients themselves, direct physiological effects of the lesions are not measured.
Limitations Related to Defining Disordered Agency and Volition.
An important set of limitations relates to our definition of disordered volition and agency. First, we identified cases of akinetic mutism/abulia and alien limb retrospectively, without standardized patient assessment or recording of symptoms. Important differences between patients were not taken into account, such as the severity of abulia vs. akinetic mutism, or the specific limb affected by alien limb symptoms. This heterogeneity broadens the applicability of the present findings, but increases that chance that more subtle findings may have been missed. Second, it is possible that alien limb and akinetic mutism, clinical syndromes classically used to define abnormal free will perception (15), do not map onto the neural processes we normally associate with “free will” in healthy subjects. For example, one could argue that denying agency in patients with alien limb and involuntary movements is not a disorder of agency, but that continuing to experience agency for involuntary movements in patients with hemichorea is. Similarly, the clinical syndrome of akinetic mutism could result from impaired motivation to make a desired movement, impaired selection of a movement once a desire occurs, and/or from impaired initiation of a desired movement. Our approach cannot differentiate between these impairments, but rather shows common network localization independent of this distinction.
Finally, our study was focused on patients with disorders of free will for movements. However, free will is commonly discussed as it relates to social, legal, and moral responsibility for decisions, not just movement (3, 4). It remains unknown whether the network of brain regions we identify as related to free will for movements is the same as that important for moral decision making (58–60), as prior studies have suggested important differences (30).
Materials and Methods
Patient Cases from the Literature.
To identify patients with disordered volition caused by brain lesions, we searched PubMed for articles with human subjects written in English, using the search terms (“akinetic mutism” or abulia) and (MRI or CT or neuroimaging) and (stroke or hemorrhage or bleed or lesion). Eighty studies were identified. Inclusion criteria included (i) documentation of diminished volitional movements (defined as the presence of spontaneous movements and/or speech in the absence of movement to commands), (ii) focal brain injury due to ischemic or hemorrhagic stroke, and (iii) published structural image (CT or MRI) of high enough quality to be traced onto a standardized brain atlas. Twenty-eight cases fulfilled these criteria and were included. A PubMed search was performed to identify patients with alien limb syndrome using the search terms (“alien limb” or “alien hand”). A total of 266 studies were identified. Inclusion required (i) documentation of movements that patients claimed they were not responsible for, (ii) focal brain lesion due to an ischemic or hemorrhagic stroke, and (iii) published structural image (CT or MRI) of sufficient image quality to trace onto a standardized brain template. Fifty cases met inclusion criteria and were included in the study.
Lesion Localization.
Published images were traced by hand onto a standardized brain atlas (2 × 2 × 2 MNI space), using FMRIB software library as in prior work (24, 26–29). All lesions shown in the original publication were traced for each patient.
Lesion Network Mapping.
Our group recently developed a technique termed lesion network mapping that identifies brain regions functionally connected to lesion locations causing a given neuropsychiatric symptom (24, 26–29, 32, 34). This technique avoids the need to perform functional brain imaging on the patients themselves and has been validated across many different neurological syndromes. Briefly, traced lesions were used as individual seeds in a resting-state connectivity analysis, using data obtained from 1,000 healthy subjects (33). Functional connectivity to each lesion was determined by calculating the correlated time course between each lesion location and every other brain voxel using the resting-state data from each individual normal control, as described in our prior studies using this connectome (32, 34). These correlations for all 1,000 subjects were then combined to calculate a T-score value for every individual voxel. Voxels were thresholded at T > ±5 to create a binarized map of significantly functionally connected regions to each patient’s lesion site (whole-brain voxel-wise FWE-corrected P < 0.05; uncorrected P < 10−6). Finally, maps from each of the patients were combined to form the lesion network mapping overlap for the group, showing the number of patients with lesions functionally connected with each individual voxel.
Comparison with Lesions Causing Similar Neurological Syndromes.
Lesion network mapping results of lesions causing akinetic mutism or abulia were compared with results for lesions causing hemiparesis, which differed according to whether volition was absent (akinetic mutism/abulia) or intact (hemiparesis). Twenty-five lesions causing hemiparesis were obtained from a previously published study (35). Lesion network mapping results of lesions causing alien limb were compared with results for lesions causing hemichorea, which differed according to whether agency was absent (alien limb) or intact (hemichorea). Thirty-nine lesions causing hemichorea were obtained from a previously published study (28). Group differences in lesion network connectivity were calculated using voxel-wise, two-sample t tests implemented in Statistical Parametric Mapping software (SPM12, https://www.fil.ion.ucl.ac.uk/spm/software/spm12/). The search volume included the whole brain. In all analyses, voxel-wise FWE-corrected P values less than 0.05 were considered significant.
Network Localization of Brain Stimulation Locations Disrupting Free Will Perception.
To identify studies where free will perception was disrupted either through direct electrical stimulation during neurosurgical cases or from focal noninvasive brain stimulation using TMS, we searched PubMed for articles with human subjects written in English, using the search terms (“direct electrical stimulation” or “noninvasive brain stimulation” or “transcranial magnetic stimulation”) and (“volition” or “agency”). A total of 119 studies were identified. We limited studies to TMS and excluded transcortical direct current stimulation (tDCS) studies due to the poor neuroanatomical specificity using tDCS. Inclusion required either coordinates for the stimulation location in a standardized brain space or an image of the stimulation location that could be manually traced onto a standardized brain template. We also included active control stimulation sites from these same studies that did not alter free will perception for comparison.
Four-millimeter spherical seeds were created at each stimulation site that did (n = 16) or did not (n = 17) alter free will perception. Functional connectivity with each seed to voxels in the rest of the brain was computed as above across 1,000 normal subjects and analyzed as above to determine whether these different stimulation locations disrupting free will perception were part of the same functionally connected brain network. Group differences in network connectivity between stimulation locations that did vs. did not alter free will were calculated using voxel-wise, two-sample t tests implemented in SPM12 as above, using a FWE-corrected P value less than 0.05.
To test the relationship between stimulation sites that disrupt free will and lesions that disrupt free will we generated 8-mm seed regions centered on the peak lesion network overlap site for akinetic mutism (MNI coordinates x = 2, y = 18, z = 32) and alien limb (MNI coordinates x = 10, y = −40, z = 50). By definition, the functional connectivity networks derived from these seeds encompass the topographic distribution of lesions that disrupt volition or agency, respectively. The functional correlation in blood-oxygen-level-dependent (BOLD) fMRI signal was measured between stimulation sites and each region of interest across the n = 1,000-subject functional connectome. Correlation values were normalized using a Fisher’s r to z transformation. A multivariate analysis of variance (MANOVA) was performed with connectivity strength to the akinetic mutism and alien limb ROIs as dependent variables and free will effect (disrupted vs. not disrupted) as the independent variable.
Network Localization of Neuroimaging Findings in Psychiatric Disorders of Free Will.
We included three psychiatric disorders of free will perception: functional movement disorders, PNES, and catatonia. We included these disorders because all three involved abnormal free will perception for movements. A PubMed search was performed using the search terms (“psychogenic nonepileptic seizures” or “conversion disorder” or “functional neurological disorder” or “catatonia”) and (MRI or SPECT or PET), identifying 319 studies. Neuroimaging studies that compared patients to healthy control subjects and utilized PET, SPECT, or structural MRI with either whole-brain cortical thickness or voxel-based morphometry (VBM) analyses were included. PET/SPECT studies were limited to those focused on blood flow or metabolism.
In each study, coordinates for functional lesions were extracted, defined as atrophy or hypoactivity on functional neuroimaging (at baseline or with volitional movement) in patients with disordered free will vs. control patients. For each individual study, we created 4-mm seeds at all reported coordinates and added these together to create a single, combined seed (Fig. 5A), similar to other techniques for coordinate-based neuroimaging analyses (61, 62). We then treated this combined seed for each study as a lesion and performed an identical procedure as our “lesion network mapping” to determine whether there was common network localization across these different studies.
To test the specificity of our network localization to regions involved in free will, we compared our results with neuroimaging abnormalities in 31 studies of patients with Alzheimer’s disease (36). Group differences in network connectivity were calculated using voxel-wise, two-sample t tests implemented in SPM12 as above, using a FWE-corrected P value less than 0.05.
To test the relationship between neuroimaging abnormalities in psychiatric disorders of free will and lesions that disrupt free will, the functional correlation in BOLD fMRI signal was measured between the locations of neuroimaging abnormalities and the previously defined alien limb and akinetic mutism ROIs across the n = 1,000-subject functional connectome. Correlation values were normalized using a Fisher’s r to z transformation. A MANOVA was performed with connectivity strength to the akinetic mutism and alien limb ROIs as dependent variables and with psychiatric disorders free will vs. Alzheimer’s disease as the independent variable.
Supplementary Material
Acknowledgments
We thank Maurizio Corbetta and Simon Laganiere for sharing lesions for our control analysis. We would also like to thank Mark Hallett for his helpful comments in the preparation of this manuscript. Investigators were supported by funding from the Sidney R. Baer, Jr. Foundation (R.R.D., M.J.B., M.D.F.), the NIH (Grants R01MH113929 and K23NS083741 to M.D.F.), the Nancy Lurie Marks Foundation (M.D.F.), the Dystonia Medical Research Foundation (M.D.F.), the Alzheimer’s Association (R.R.D.), the BrightFocus Foundation (R.R.D.), Academy of Finland (Grant 295580 to J.J.), the Finnish Medical Foundation (J.J.), and Vanderbilt Institute for Clinical and Translational Research (R.R.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1814117115/-/DCSupplemental.
References
- 1.Libet B, Gleason CA, Wright EW, Pearl DK. Time of conscious intention to act in relation to onset of cerebral activity (readiness-potential). The unconscious initiation of a freely voluntary act. Brain. 1983;106:623–642. doi: 10.1093/brain/106.3.623. [DOI] [PubMed] [Google Scholar]
- 2.Libet B. Unconscious cerebral initiative and the role of conscious will in voluntary action. Behav Brain Sci. 1985;8:529–566. [Google Scholar]
- 3.Harris S. 2012 Free Will (Simon and Schuster). Available at https://books.google.com/books/about/Free_Will.html?id=iRpkNcRt1IcC&pgis=1. Accessed July 10, 2015.
- 4.Haggard P. Sense of agency in the human brain. Nat Rev Neurosci. 2017;18:196–207. doi: 10.1038/nrn.2017.14. [DOI] [PubMed] [Google Scholar]
- 5.Haggard P. Human volition: Towards a neuroscience of will. Nat Rev Neurosci. 2008;9:934–946. doi: 10.1038/nrn2497. [DOI] [PubMed] [Google Scholar]
- 6.Hallett M. Physiology of free will. Ann Neurol. 2016;80:5–12. doi: 10.1002/ana.24657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Penfield W, Boldrey E. Somatic motor and sensory representation in man. Brain, 1937;60:389–443. [Google Scholar]
- 8.Kremer S, Chassagnon S, Hoffmann D, Benabid AL, Kahane P. The cingulate hidden hand. J Neurol Neurosurg Psychiatry. 2001;70:264–265. doi: 10.1136/jnnp.70.2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chassagnon S, Minotti L, Kremer S, Hoffmann D, Kahane P. Somatosensory, motor, and reaching/grasping responses to direct electrical stimulation of the human cingulate motor areas. J Neurosurg. 2008;109:593–604. doi: 10.3171/JNS/2008/109/10/0593. [DOI] [PubMed] [Google Scholar]
- 10.Desmurget M, et al. Movement intention after parietal cortex stimulation in humans. Science. 2009;324:811–813. doi: 10.1126/science.1169896. [DOI] [PubMed] [Google Scholar]
- 11.Preston C, Newport R. Misattribution of movement agency following right parietal TMS. Soc Cogn Affect Neurosci. 2008;3:26–32. doi: 10.1093/scan/nsm036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khalighinejad N, Di Costa S, Haggard P. Endogenous action selection processes in dorsolateral prefrontal cortex contribute to sense of agency: A meta-analysis of tDCS studies of ‘intentional binding’. Brain Stimul. 2016;9:372–379. doi: 10.1016/j.brs.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Sperduti M, Delaveau P, Fossati P, Nadel J. Different brain structures related to self- and external-agency attribution: A brief review and meta-analysis. Brain Struct Funct. 2011;216:151–157. doi: 10.1007/s00429-010-0298-1. [DOI] [PubMed] [Google Scholar]
- 14.Rae CL, Hughes LE, Weaver C, Anderson MC, Rowe JB. Selection and stopping in voluntary action: A meta-analysis and combined fMRI study. Neuroimage. 2014;86:381–391. doi: 10.1016/j.neuroimage.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kranick SM, Hallett M. Neurology of volition. Exp Brain Res. 2013;229:313–327. doi: 10.1007/s00221-013-3399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frith CD, Blakemore SJ, Wolpert DM. Abnormalities in the awareness and control of action. Philos Trans R Soc Lond B Biol Sci. 2000;355:1771–1788. doi: 10.1098/rstb.2000.0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cairns H, Oldfield RC, Pennybacker JB, Whitteridge D. Akinetic mutism with an epidermoid cyst of the 3RD ventricle. Brain. 1941;64:273–290. [Google Scholar]
- 18.Goldstein K. Zur Lehre von der motorischen Apraxie [To the theory of motor apraxia] J Psychol Neurol. 1908;11:169–187. [Google Scholar]
- 19.Rorden C, Karnath H-O. Using human brain lesions to infer function: A relic from a past era in the fMRI age? Nat Rev Neurosci. 2004;5:813–819. doi: 10.1038/nrn1521. [DOI] [PubMed] [Google Scholar]
- 20.Adolphs R. Human lesion studies in the 21st century. Neuron. 2016;90:1151–1153. doi: 10.1016/j.neuron.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fox MD. Localizing symptoms to brain networks using the human connectome. N Engl J Med. 2018 doi: 10.1056/NEJMra1706158. in press. [DOI] [PubMed] [Google Scholar]
- 22.Ghoshal S, Gokhale S, Rebovich G, Caplan LR. The neurology of decreased activity: Abulia. Rev Neurol Dis. 2011;8:e55–e67. [PubMed] [Google Scholar]
- 23.Hassan A, Josephs KA. Alien hand syndrome. Curr Neurol Neurosci Rep. 2016;16:73. doi: 10.1007/s11910-016-0676-z. [DOI] [PubMed] [Google Scholar]
- 24.Boes AD, et al. Network localization of neurological symptoms from focal brain lesions. Brain. 2015;138:3061–3075. doi: 10.1093/brain/awv228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sutterer MJ, et al. Canceled connections: Lesion-derived network mapping helps explain differences in performance on a complex decision-making task. Cortex. 2016;78:31–43. doi: 10.1016/j.cortex.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darby RR, Laganiere S, Pascual-Leone A, Prasad S, Fox MD. Finding the imposter: Brain connectivity of lesions causing delusional misidentifications. Brain. 2017;140:497–507. doi: 10.1093/brain/aww288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischer DB, et al. A human brain network derived from coma-causing brainstem lesions. Neurology. 2016;87:2427–2434. doi: 10.1212/WNL.0000000000003404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laganiere S, Boes AD, Fox MD. Network localization of hemichorea-hemiballismus. Neurology. 2016;86:2187–2195. doi: 10.1212/WNL.0000000000002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fasano A, Laganiere SE, Lam S, Fox MD. Lesions causing freezing of gait localize to a cerebellar functional network. Ann Neurol. 2017;81:129–141. doi: 10.1002/ana.24845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darby RR, Horn A, Cushman F, Fox MD. Lesion network localization of criminal behavior. Proc Natl Acad Sci USA. 2018;115:601–606. doi: 10.1073/pnas.1706587115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fox MD, et al. Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proc Natl Acad Sci USA. 2014;111:E4367–E4375. doi: 10.1073/pnas.1405003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horn A, et al. Connectivity predicts deep brain stimulation outcome in Parkinson disease. Ann Neurol. 2017;82:67–78. doi: 10.1002/ana.24974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeo BT, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Darby RR, Fox MD. Reply: Capgras syndrome: Neuroanatomical assessment of brain MRI findings in an adolescent patient. Brain. 2017;140:e44. doi: 10.1093/brain/awx125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegel JS, et al. Disruptions of network connectivity predict impairment in multiple behavioral domains after stroke. Proc Natl Acad Sci USA. 2016;113:E4367–E4376. doi: 10.1073/pnas.1521083113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang J, et al. Voxelwise meta-analysis of gray matter anomalies in Alzheimer’s disease and mild cognitive impairment using anatomic likelihood estimation. J Neurol Sci. 2012;316:21–29. doi: 10.1016/j.jns.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 37.Siegel JS, et al. The circuitry of abulia: Insights from functional connectivity MRI. Neuroimage Clin. 2014;6:320–326. doi: 10.1016/j.nicl.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shenhav A, Botvinick MM, Cohen JD. The expected value of control: An integrative theory of anterior cingulate cortex function. Neuron. 2013;79:217–240. doi: 10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen RA, Kaplan RF, Moser DJ, Jenkins MA, Wilkinson H. Impairments of attention after cingulotomy. Neurology. 1999;53:819–824. doi: 10.1212/wnl.53.4.819. [DOI] [PubMed] [Google Scholar]
- 40.Cohen RA, et al. Alteration of intention and self-initiated action associated with bilateral anterior cingulotomy. J Neuropsychiatry Clin Neurosci. 1999;11:444–453. doi: 10.1176/jnp.11.4.444. [DOI] [PubMed] [Google Scholar]
- 41.Wilkinson HA, Davidson KM, Davidson RI. Bilateral anterior cingulotomy for chronic noncancer pain. Neurosurgery. 1999;45:1129–1134, discussion 1134–1136. doi: 10.1097/00006123-199911000-00023. [DOI] [PubMed] [Google Scholar]
- 42.Barlas O, et al. Do unilateral ablative lesions of the subthalamic nucleu in parkinsonian patients lead to hemiballism? Mov Disord. 2001;16:306–310. doi: 10.1002/mds.1051. [DOI] [PubMed] [Google Scholar]
- 43.Feinberg TE, Schindler RJ, Flanagan NG, Haber LD. Two alien hand syndromes. Neurology. 1992;42:19–24. doi: 10.1212/wnl.42.1.19. [DOI] [PubMed] [Google Scholar]
- 44.Graff-Radford J, et al. The alien limb phenomenon. J Neurol. 2013;260:1880–1888. doi: 10.1007/s00415-013-6898-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cavanna AE, Trimble MR. The precuneus: A review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 46.Carrera E, Tononi G. Diaschisis: Past, present, future. Brain. 2014;137:2408–2422. doi: 10.1093/brain/awu101. [DOI] [PubMed] [Google Scholar]
- 47.Monakow C. 1914. Die Lokalisation im Grosshirn : und der Abbau der Funktion durch kortikale Herde [The Localization in the Cerebrum and the Degradation of the Function by Cortical Foci] (Verlag von J. F. Bergmann, Wiesbaden, Germany) [Google Scholar]
- 48.Geschwind N. Disconnexion syndromes in animals and man. I. Brain. 1965;88:237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- 49.Geschwind N. Disconnexion syndromes in animals and man. II. Brain. 1965;88:585–644. doi: 10.1093/brain/88.3.585. [DOI] [PubMed] [Google Scholar]
- 50.Power JD, Schlaggar BL, Lessov-Schlaggar CN, Petersen SE. Evidence for hubs in human functional brain networks. Neuron. 2013;79:798–813. doi: 10.1016/j.neuron.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72:595–603. doi: 10.1016/j.biopsych.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weigand A, et al. Prospective validation that subgenual connectivity predicts antidepressant efficacy of transcranial magnetic stimulation sites. Biol Psychiatry. 2018;84:28–37. doi: 10.1016/j.biopsych.2017.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moore JW, Ruge D, Wenke D, Rothwell J, Haggard P. Disrupting the experience of control in the human brain: Pre-supplementary motor area contributes to the sense of agency. Proc Biol Sci. 2010;277:2503–2509. doi: 10.1098/rspb.2010.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Douglas ZH, Maniscalco B, Hallett M, Wassermann EM, He BJ. Modulating conscious movement intention by noninvasive brain stimulation and the underlying neural mechanisms. J Neurosci. 2015;35:7239–7255. doi: 10.1523/JNEUROSCI.4894-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chambon V, Moore JW, Haggard P. TMS stimulation over the inferior parietal cortex disrupts prospective sense of agency. Brain Struct Funct. 2015;220:3627–3639. doi: 10.1007/s00429-014-0878-6. [DOI] [PubMed] [Google Scholar]
- 56.Ritterband-Rosenbaum A, Karabanov AN, Christensen MS, Nielsen JB. 10 Hz rTMS over right parietal cortex alters sense of agency during self-controlled movements. Front Hum Neurosci. 2014;8:471. doi: 10.3389/fnhum.2014.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsakiris M, Costantini M, Haggard P. The role of the right temporo-parietal junction in maintaining a coherent sense of one’s body. Neuropsychologia. 2008;46:3014–3018. doi: 10.1016/j.neuropsychologia.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 58.Darby RR, Edersheim J, Price BH. What patients with behavioral-variant frontotemporal dementia can teach us about moral responsibility. AJOB Neurosci. 2016;7:193–201. [Google Scholar]
- 59.Darby RR, Dickerson BC. Dementia, decision making, and capacity. Harv Rev Psychiatry. 2017;25:270–278. doi: 10.1097/HRP.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Darby RR. Neuroimaging abnormalities in neurological patients with criminal behavior. Curr Neurol Neurosci Rep. 2018;18:47. doi: 10.1007/s11910-018-0853-3. [DOI] [PubMed] [Google Scholar]
- 61.Eickhoff SB, et al. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.