Significance
Helminth infection elicits T helper type 2 (Th2) and Treg cells. However, the critical cell subpopulations of Th2 and Treg cells in antihelminth immunity remain unknown. We identified two Th2 cell subpopulations: CXCR6+ST2+ memory Th2 cells and ST2− memory Th2 cells. Although both subpopulations induced the accumulation of eosinophils into the lungs during helminth infection, those induced by CXCR6+ST2+ memory Th2 cells expressed high levels of major basic protein (MBP) and contributed to the reduction of fecundity of helminth. This response was suppressed by ST2+ but not ST2− Treg cells, both of which are induced during helminth infection. We, therefore, identified CXCR6+ST2+ memory Th2 cells as a critical subpopulation to induce accumulation of eosinophils strongly expressing MBP in the lungs.
Keywords: IL-33/ST2, memory Th2 cell subset, Treg, helminth, allergic inflammation
Abstract
Memory T helper (mTh) cells play important roles in the reinfection of pathogens and drive the pathogenesis of diseases. While recent studies have characterized the pathogenic mTh2 cell subpopulations driving allergic inflammation, those that induce immune responses against helminth infection remain unknown. We found that IL-5–producing CXCR6+ST2+CD44+ mTh2 cells play a crucial role in the IL-33–dependent inhibition of the fecundity of helminth, whereas other ST2− mTh2 cells do not. Although both cell types induced the infiltration of granulocytes, especially eosinophils, into the lungs in response to helminth infection, the ST2+ mTh2 cell-induced eosinophils expressed higher levels of major basic protein (MBP), which is important for reducing the fecundity of Nippostrongylus brasiliensis (Nb), than ST2− mTh2 cell-induced ones. Notably, we also found that ST2+ Treg cells but not ST2− Treg cells suppressed CXCR6+ST2+ mTh2 cell-mediated immune responses. Taken together, these findings show that we identified a mechanism against helminth elicited by a subpopulation of IL-5–producing mTh2 cells through the accumulation of eosinophils strongly expressing MBP in the lungs.
Antigen recognition by the T-cell receptor (TCR) drives naïve CD4+ T cells to differentiate into effector T helper (Th) cell subsets, such as Th1, Th2, and Th17 cells, that later become memory T helper type 1 (mTh1), mTh2, and mTh17 cells that orchestrate long-term antigen-specific immune responses (1–3). Recently, based on disparate cytokine production patterns, several functionally distinct mTh2 subpopulations have been identified; Th2 + 1 cells, IL-17–producing Th2 cells, and high IL-5–producing pathogenic T helper type 2 (Tpath2) cells (4–7). Th2 + 1 cells produce IFN-γ in addition to Th2 cytokines, IL-17–producing Th2 cells produce IL-17 and Th2 cytokines, and the high IL-5–producing memory-type Tpath2 cells express ST2, a component of the IL-33 receptor. Tpath2 cells produce large amounts of IL-5 after TCR stimulation (7, 8). Several of these Th cell subpopulations possess effector functions that play crucial roles in the pathogenesis of Th1, Th2, and Th17 cell-mediated inflammatory diseases (3). In comparison with models where the balance of conventional Th cell subsets (Th1, Th2, and Th17) determines certain disease states, we have proposed a “pathogenic Th population disease induction model,” in which the minority presence of unconventional Th cell subsets determines disease (3).
IL-33, a member of the IL-1 family, is released from various cells, including epithelial cells, in response to cellular damage or inflammation (9, 10). Il33 and Il1rl1 are genes well-known to be associated with the severity of asthma symptoms (11). IL-33 stimulation exacerbates allergic airway inflammation and is associated with infiltration of eosinophils into the mucosa (12). The IL-33 receptor consisting of ST2 and IL-1 receptor accessory protein is expressed on various inflammatory cells, including type 2 innate lymphoid cells (ILC2s) and Tpath2 cells (8, 13). IL-33 is important in ILC2 cells for triggering production of IL-5 and IL-13 and also, in Tpath2 cells for chromatin remodeling of the Il5 gene locus and up-regulation of ST2 expression (8).
It has been reported that a subset of Treg cells expresses ST2 (14). Treg cells suppress immune-mediated inflammation (15, 16). ST2+ Treg cells are generated by TCR stimulation in the presence of IL-33 in a process controlled by IRF4, BATF, and PPARγ (17).
Helminth infection is known to induce the generation of Th2 cells and Treg cells (18, 19). In this study, we used the nematode helminth Nippostrongylus brasiliensis (Nb). Nb passes through the lungs before reaching the gut and is expelled within 10 d in mice. Mice acquire and maintain immunity against Nb for over 1 y. Nb induces accumulation of Th2 cells in the lungs that peaked 10 d after infection (18). Helminth-induced Th2 cells produce IL-4, IL-5, and IL-13, which results in elevated serum IgE, eosinophilia, goblet cell hyperplasia, and ultimately, helminth expulsion (20). The type 2 inflammatory immune response induced by helminth infections is similar to that observed in allergic asthma (21). Helminth infection increased IL-33 levels in the lungs (22), such as occurs during asthma pathogenesis, and IL-33 deficiency impairs the expulsion and inhibition of maturation of worms (23). However, the functionally critical subpopulation of mTh2 cells that induces immune responses against helminth remains unknown.
In this study, we identified CXCR6+ST2+ mTh2 cells that help reduce the fecundity of the helminth, Nb, via the accumulation of eosinophils expressing high levels of major basic protein (MBP) in the lung (24). Notably, the reduction of fecundity induced by CXCR6+ST2+ mTh2 cells was suppressed by the ST2+ but not the ST2− Treg cells elicited in Nb infection. We showed a cellular and molecular mechanism underlying the immune response against helminth.
Results
Helminth Infection Induces IL-5–Producing ST2+ mTh Cells.
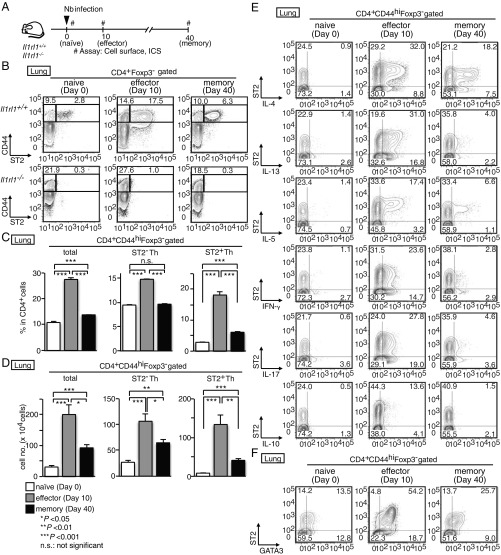
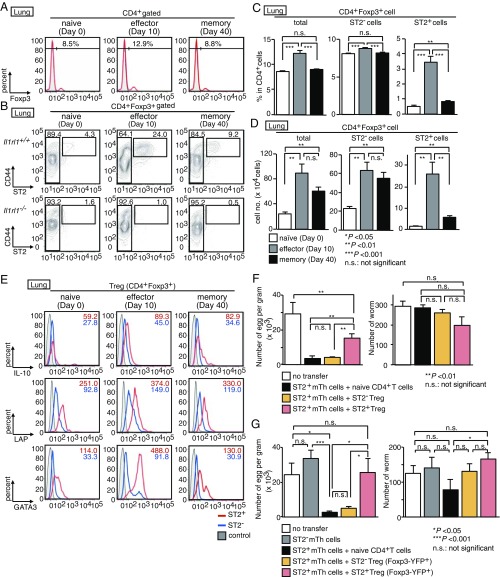
We recently identified a crucial subpopulation of mTh2 cells that promote allergen-induced eosinophilic airway inflammation, named memory-type Tpath2 cells (3). Tpath2 cells selectively express ST2 (a component of the IL-33 receptor). Although it is well-known that Th2 cells play critical roles in helminth infections, whether an analogous ST2+ effector Th2 cell subpopulation also mediates the immune response to helminth remains unknown. To address this question, we analyzed mice infected with the helminth Nb. In this model, the number of Th2 cells in the lungs of wild-type mice reached a peak ∼10 d after infection, correlating with the timing of Nb expulsion from the gut (18, 25). We assessed ST2 expression in Th cells (CD4+CD44hiFoxP3−) from the lungs and spleens of mice infected with Nb for 0 (naïve time point), 10 (effector time point), and 40 d (memory time point), as depicted in Fig. 1A. To avoid false-positive staining, we included the staining of ST2 expression on Th cells of Il1rl1−/− mice as negative control (Fig. 1B). In naïve lungs, less than 5% of Th cells expressed ST2. At the effector time point, expression of ST2 was up-regulated on Th cells in the lungs (Fig. 1B). At the memory time point, the number of ST2+ Th cells in the lungs had declined but remained at least two- to threefold higher than in naïve mice (Fig. 1 C and D).
Fig. 1.
Helminth infection induces IL-5–producing ST2+ mTh cells. (A) Experimental protocols for the infection of Il1rl1+/+ and Il1rl1−/− mice with Nb. #Assay: cell surface, intracellular staining (ICS). (B) CD4+ T cells were isolated from the lungs before and after infection with Nb. Staining profiles of CD44 and ST2 in CD4+Foxp3− T cells are shown with the percentages of cells in each area. (C and D) The percentages (C) and the absolute cell numbers (D) of memory CD4+ T cells (total), ST2− mTh cells (ST2− mTh), and ST2+ mTh cells (ST2+ mTh) in the lungs are shown. Mean values (five mice per group) are shown with SEM. n.s., not significant. *P < 0.05; **P < 0.01; ***P < 0.001. (E and F) Memory CD4+ T cells shown in B were stimulated with phorbol 12-myristrate 13-acetate (PMA) and ionomycin for 6 h. Intracellular staining profiles of ST2, IL-4, IL-13, IL-5, IFN-γ, IL-17, and IL-10 (E) and intracellular staining profile of GATA3 (F) are shown with the percentages of cells in each area. We set positive/negative gates using staining of Il1rl1−/− cells (B) or with isotype controls (E and F). Five independent similar experiments were performed, and similar results were obtained.
Next, we examined the cytokine production profile of Th cells at the effector and memory time points. After Nb infection, the production of Th2 cytokines (IL-4, IL-5, and IL-13) by Th cells in the lungs dramatically increased, particularly among ST2+ cells at the effector and memory time points (Fig. 1E and SI Appendix, Fig. S1 A and B). Although a substantial proportion of ST2− Th cells produced IL-4 and IL-13, IL-5 production was almost exclusively detected within the ST2+ population. Low levels of IFN-γ and IL-17A were detected from both ST2− and ST2+ Th cells. These results indicate that Nb infection induced substantial numbers of IL-5–producing ST2+ Th cells detectable at least until day 40 postinfection. Although less dramatic, a similar pattern of Th2 cytokine production was detected in splenic ST2− and ST2+ Th cells (SI Appendix, Fig. S1 C and D). Moreover, the majority of ST2+ Th cells expressed GATA3 at the effector and memory time points (Fig. 1F and SI Appendix, Fig. S1E).
IL-33 Treatment Enhances ST2 Expression and IL-5 Production in ST2+ mTh Cells.
Next, we investigated the role of IL-33 in ST2− and ST2+ mTh cells (CD4+CD44hiCD25−). Sorted ST2− and ST2+ Th cells recovered at the memory time point (SI Appendix, Fig. S2A) were cultured with or without IL-33 and IL-7 (SI Appendix, Fig. S2B). Culture with IL-33 together with low levels of IL-7 led to significant elevation of IL-5 and IL-13 mRNA expression in ST2+ mTh cells (SI Appendix, Fig. S2C). IL-33 + IL-7 treatment enhanced ST2, IL-5, and IL-13 expression in ST2+ mTh cells (SI Appendix, Fig. S2D). These results indicate that IL-33 + IL-7 treatment can enhance the ability of ST2+ mTh cells to express ST2, IL-5, and IL-13.
Nb-Induced ST2− and ST2+ mTh Cells Exhibit Distinct Gene Expression Profiles.
Next, we used RNA sequence analysis to compare gene expression profiles of Nb-induced lung naïve CD4+ T cells and ST2− and ST2+ mTh cells isolated on day 40 after Nb infection (Fig. 2A). To investigate population-specific gene expression, we selected genes with a difference in expression of over fivefold in one population compared with the other two populations and displayed these gene sets as heat maps. This analysis revealed that ST2− and ST2+ mTh cells possess distinct transcriptional profiles (Fig. 2B). MA plot analysis of transcription factors, cytokines, chemokines, and cell surface molecules (Fig. 2 C, D, and F) revealed that ST2+ mTh cells expressed lower levels of central mTh cell markers, such as Tcf7, Klf2, Ccr7, and Sell, and higher levels of resident mTh cell markers, such as Pparg (26, 27). The expression of Th2 cell-specific genes (Il4, Il5, Il13, Epas1, and Gata3) was higher in ST2+ mTh cells, while Il2, Ifng, Tnf, and Ccl5 were lower. Real-time qPCR analysis confirmed the differential expression of these genes (Fig. 2E). Analysis of cell surface markers revealed CCR7, CD62L, CXCR3, and IL-18Rα to be higher on ST2− mTh cells and CD69, CCR2, and CXCR6 to be higher on ST2+ mTh cells (Fig. 2G). CD69 is a marker of activated cells and also resident memory T cells, while CCR2 and CXCR6 are known to be involved in migration to the lung (28–30).
Fig. 2.
Nb-induced ST2− and ST2+ mTh cells exhibit distinct gene expression profiles. (A) Experimental protocols for the infection of wild-type mice with Nb. #Assay: RNA sequencing. (B) The expression of the genes with a difference in expression of more than fivefold in ST2+ mTh cells compared with the other two groups obtained by RNA sequence analysis. (C and D) MA plots comparing the different expression of transcription factors (C) as well as cytokines and chemokines (D) between ST2+ and ST2− mTh cells. Horizontal line denotes log2[(ST2+ mTh)/(ST2− mTh)], while vertical lines denote (1/2) × log2[(ST2− mTh) × (ST2+ mTh)]. (E) A qRT-PCR analysis of the indicated genes in ST2+ and ST2− mTh cells. The minimum data values were set as one. The mean values (four data per group) are shown with the SEM. n.s., not significant. **P < 0.01; ***P < 0.001. (F) MA plots comparing the differential expression of surface markers between ST2+ and ST2− mTh cells. (G) Staining profiles of indicated markers on ST2+ and ST2− mTh cells are shown with the mean fluorescence intensity of cells in histograms. At least two independent experiments were performed and showed similar results. The mean of FPKM was used for analyses in B–D and F. We set positive/negative gates using staining with isotype controls in G.
Nb-Induced ST2+ mTh Cells Are Required for the Reduction of the Fecundity of Nb Infection.
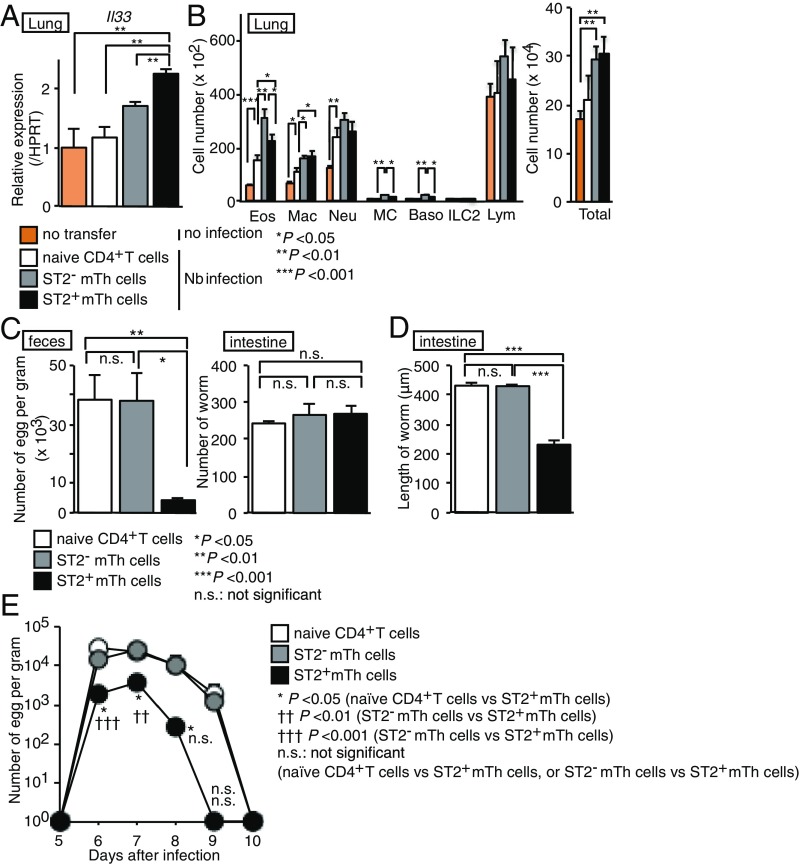
We next sought to investigate the physiological relevance of ST2+ and ST2− mTh cells in the expulsion, maturation, and fecundity of Nb. Naïve CD4+ T cells or ST2+ or ST2− mTh cells purified from BALB/c mice infected 40 d earlier with Nb were adoptively transferred to syngeneic recipients. Four weeks later, adoptively transferred mice were infected with Nb (SI Appendix, Fig. S3). Compared with recipients of naïve Th cells, recipients of ST2+ or ST2− mTh cells up-regulated mRNA level of Il33 in the lungs 2 d after Nb infection when Nb reached the lungs, and we also noted moderate up-regulation of mRNA of Tslp in the lungs of recipients of ST2+ or ST2− mTh cells (Fig. 3A and SI Appendix, Fig. S4A). We observed an increase in the number of lung eosinophils, macrophages, and neutrophils after Nb infection (Fig. 3B, orange bars vs white bars). The numbers of eosinophils and macrophages were elevated in ST2+ or ST2− mTh cell-transferred mice compared with naïve CD4+ T cell-transferred mice 4 d after Nb infection (Fig. 3B, white bars vs. gray or black bars). However, no marked difference was observed between the ST2+ or ST2− mTh recipients (Fig. 3B, gray bars vs. black bars). We next assessed the number of eggs in the feces and the number and size of worms isolated from the small intestines of ST2+ or ST2− mTh recipients 6 d after Nb infection. Compared with recipients of naïve CD4+ T cells and ST2− mTh cells, the recipients of ST2+ mTh cells exhibited dramatically fewer eggs and significantly smaller worms, indicating that the ST2+ mTh cell-mediated immune responses inhibited Nb maturation (Fig. 3C, Left and Fig. 3D). No detectable worms were noted in the skin (days 2 and 6), the lungs (day 6), and small intestine (day 2), and no marked difference was noted in the number of worms isolated from the lungs or small intestine among the three experimental groups (Fig. 3C, Right and SI Appendix, Fig. S4D). A kinetic study of the number of eggs was performed, and an inhibitory effect of ST2+ mTh cells was observed at least on days 5–8 (Fig. 3E). The transfer of ST2+ Th cells isolated from the mice 10 d after Nb infection (effector time point) into naïve mice did not reduce the number of eggs in feces, while those from mice at 40 d after Nb infection (memory time point) did reduce the number of eggs, indicating that ST2+ mTh cells isolated from the mice at 40 d after Nb infection have a memory function against Nb (SI Appendix, Fig. S4 E and F). Collectively, these findings indicate that ST2+ but not ST2− mTh cells reduced the fecundity of Nb.
Fig. 3.
Nb-induced ST2+ mTh cells are sufficient for the reduction of fecundity of Nb. (A–D) Mice were killed 2 (A), 4 (B), or 6 d (C and D) after infection with Nb as described in SI Appendix, Fig. S3. (A) A qRT-PCR analysis of the indicated genes in the lungs of indicated mice. The minimum data values were set as one. The mean values (four data points per group) are shown with the SEM. (B) The absolute cell numbers of eosinophils (Eos), macrophages (Mac), neutrophils (Neu), mast cells (MC), basophils (Baso), and lymphocytes (Lym) in the lungs are shown. The mean values (four mice per group) are shown with the SEM. (C) The absolute numbers of parasite eggs in feces per gram and the number of worms are shown. The mean values (three or four mice per group) are shown with the SEM. (D) The lengths of worms collected from the small intestine of the indicated mice are shown. The mean values (at least 15 worms from four mice per group) are shown with the SEM. n.s., not significant. *P < 0.05; **P < 0.01; ***P < 0.001. (E) The feces were collected from the mice from 5 d after Nb infection as described in SI Appendix, Fig. S3. The absolute numbers of parasite eggs in feces per gram are shown. The mean values (four mice per group) are shown with the SEM. At least two independent experiments were performed with similar results. n.s., not significant. *P < 0.05; ††P < 0.01; †††P < 0.001.
The ST2+ mTh Cell-Mediated Reduction of Fecundity of Nb Depends on IL-33, IL-5, and Eosinophils.
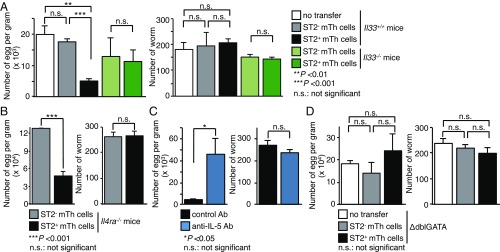
Next, to explore the cellular mechanisms underlying ST2+ mTh cell-dependent immunity against Nb, we assessed the role of IL-33 (SI Appendix, Figs. S5A and S6 A and B). As described above, the adoptive transfer of ST2+ mTh cells into BALB/c mice conferred the ability to suppress the number of fecal Nb eggs 6 d after Nb infection. Strikingly, the transfer of ST2+ mTh cells into IL-33–deficient mice or whole mTh cells from Il1rl1−/− mice into normal Il1rl1+/+ BALB/c mice failed to confer this suppressive effect on the number of eggs recovered from the feces (Fig. 4A and SI Appendix, Fig. S6 A and B). These results suggest that the IL-33–ST2 axis was required for ST2+ mTh cells to induce immune responses against Nb. Furthermore, IL-33 increased the production of IL-13 and IL-5 from ST2+ mTh cells (SI Appendix, Fig. S2). We, therefore, next examined the contribution of IL-13 and IL-5 to the ST2+ mTh2 cell-dependent reduction of fecundity of Nb using Il4ra-deficient mice lacking the expression of types I and II receptors for IL-4 and IL-13 and neutralizing antibody against IL-5. Surprisingly, the transfer of ST2+ mTh cells into Il4ra-deficient mice reduced the number of eggs recovered from the feces compared with ST2− mTh cell-transferred mice, suggesting that IL-4 and IL-13 are dispensable for the ST2+ mTh cell-dependent reaction (Fig. 4B and SI Appendix, Fig. S5B). We next analyzed the role of IL-5 in the ST2+ mTh cell-mediated reduction of fecundity of Nb (SI Appendix, Fig. S5C). Treatment with an IL-5–neutralizing antibody 1 d before Nb infection of ST2+ recipients led to a dramatic increase in the number of fecal eggs compared with control antibody-injected mice (Fig. 4C). IL-5 is known to be an important factor for eosinophil proliferation, activation, and chemoattraction (31). We, therefore, assessed whether eosinophils are involved in the ST2+ mTh cell-dependent reduction of fecundity of Nb using eosinophil-deficient mice: ΔdblGATA mice (32). ΔdblGATA recipients of ST2+ mTh cells did not show a suppressed number of fecal eggs compared with recipients of ST2− mTh cells (Fig. 4D and SI Appendix, Fig. S5D). Although ILC2 cells are known to induce the accumulation of eosinophils via the production of IL-5, the depletion of ILC2 cells did not significantly affect this ST2+ mTh cell-mediated reduction of fecundity of Nb (SI Appendix, Fig. S6 C and D) (13). These results indicate that IL-33, IL-5, and eosinophils are required for ST2+ mTh cell-mediated reduction of fecundity of Nb. Thus, the IL-33–induced IL-5 production from ST2+ mTh cells and the resulting eosinophilic activation play important roles in the reduction of the fecundity of Nb.
Fig. 4.
ST2+ mTh cell-mediated reduction of fecundity of Nb depends on IL-33, IL-5, and eosinophils but not IL-4 and IL-13. (A–D) Mice were killed 6 d after infection with Nb. Experimental protocol is shown in SI Appendix, Fig. S5. The absolute numbers of parasite eggs in feces per gram and the numbers of worms are shown. The mean values (three or four mice per group) are shown with the SEM. At least two independent experiments were performed and showed similar results. n.s., not significant. *P < 0.05; **P < 0.01; ***P < 0.001.
Eosinophils Induced by ST2+ and by ST2− mTh Cells in Nb Infection Exhibit Distinct Gene Expression Profiles.
We found that eosinophils play an important role in the ST2+ mTh cell-dependent immunity against Nb. However, the number of eosinophils accumulated in the lungs by ST2+ mTh cells was almost the same as that accumulated by ST2− mTh cells (Fig. 3). We, therefore, hypothesized that the function of eosinophils induced by ST2+ mTh cells was distinct from that of eosinophils induced by ST2− mTh cells.
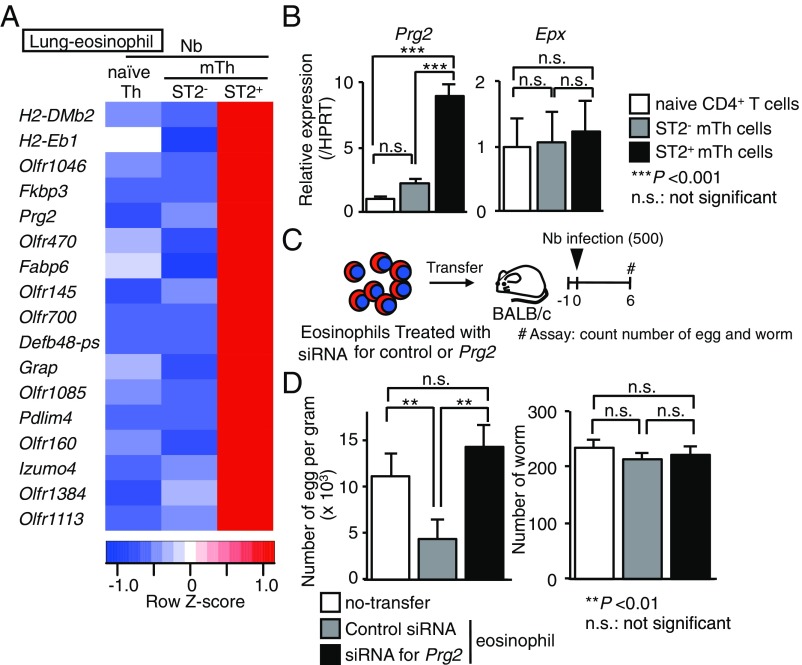
To explore the molecular mechanisms underlying ST2+ mTh cell-dependent immunity against Nb, we compared the gene expression profiles of lung eosinophils in naïve CD4+ T cell- or ST2− or ST2+ mTh cell-transferred BALB/c mice on Nb infection (SI Appendix, Fig. S7) (8). We used heat maps to depict the genes that exhibited differential expression at least twofold higher in a given population than in the other two (Fig. 5A). The ST2+ mTh cell-induced eosinophils selectively expressed 17 genes, including antihelminthic enzyme Prg2 coding MBP (24). This result was confirmed by qRT-PCR (Fig. 5B, Left). We included the expression of Epx as a control (Fig. 5B, Right). Indeed, 10 ng/mL of IL-5 up-regulated the expression of mRNA of Prg2 during eosinophil development (SI Appendix, Fig. S8 A and B). We next examined the functional consequence of MBP in the eosinophils during Nb infection. The expression level of mRNA of Prg2 in eosinophils was almost depleted by the treatment of siRNA for Prg2 (SI Appendix, Fig. S8 C and D). The number of fecal eggs from mice receiving intranasally transferred control siRNA-treated eosinophils was significantly reduced compared with no transfer mice, and the number recovered if MBP-depleted eosinophils was introduced (Fig. 5 C and D). The number of worms was not changed in these experimental settings. These results indicated that eosinophils induced by ST2+ mTh cells and ST2− mTh cells possess distinct gene expression profiles and that ST2+ mTh cells induced eosinophils with high expression of Prg2 mRNA, which is sufficient to reduce the fecundity of Nb in the lungs.
Fig. 5.
Helminth-induced ST2+ mTh2 cells accumulated MBP-expressing eosinophils in the lungs, reducing the fecundity of Nb. (A) The expressions of the genes with a difference in expression of more than fivefold in the sorted eosinophils obtained from the lungs of ST2+ mTh cell-transferred mice compared with the other two groups according to an RNA sequence analysis were selected as depicted in SI Appendix, Fig. S7. The genes listed in two independent experiments are shown. (B) A qRT-PCR analysis of Prg2 and Epx in the sorted eosinophils obtained from the lung of naïve CD4+ T cell-, ST2− mTh cell-, or ST2+ mTh cell-transferred mice. The minimum data values were set as one. The mean values (six data per group) are shown with the SEM. (C) Experimental protocols for the infection of the mice with Nb. #Assay: count numbers of eggs and worms. (D) Mice were killed 6 d after infection with Nb, as described in C. The absolute numbers of parasite eggs in feces per gram and the numbers of worms are shown. The mean values (six mice per group) are shown with the SEM. At least two independent experiments were performed and showed similar results. n.s., not significant. **P < 0.01; ***P < 0.001.
ST2+ Treg Cells Suppress ST2+ mTh Cell-Dependent Immunity Against Nb.
In the colon and adipose tissues, some Treg cells express ST2 (14, 17). Whether these ST2+ Treg cells play a role in helminth infection is not known. We sought to assess whether ST2+ Treg cells occurred in the lungs before and after Nb infection. The frequency of CD4+FoxP3+ Treg cells in the lungs increased after Nb infection (Fig. 6A). We detected a small number of ST2+ Treg cells in the lungs of naïve mice, and these numbers increased after Nb infection at the effector time point (day 10) (Fig. 6 B–D). At the memory time point (day 40), the number of ST2+ Treg cells was elevated in the lungs compared with that at the naïve time point (day 0). FACS analysis revealed that lung ST2+ Treg cells expressed preferentially IL-10 and Latency-associated protein (LAP), a surrogate marker of TGF-β production, compared with ST2− Treg cells (Fig. 6E).
Fig. 6.
ST2+ Treg cells suppress the immune response against helminth induced by ST2+ mTh cells. (A and B) CD4+ T cells were isolated from the lungs of Il1rl1+/+ (A and B) and Il1rl1−/− (B) before and after infection with Nb in Fig. 1A. Staining profiles of Foxp3, CD44, and ST2 expression in lung CD4+ T cells before and after infection with Nb. The percentages of cells in each area are depicted. (C and D) The percentages (C) and numbers (D) of lung Treg cells (total), ST2− Treg cells, and ST2+ Treg cells are shown. Mean values (five mice per group) are shown with the SEM. (E) Treg cells depicted in B were stimulated with phorbol 12-myristrate 13-acetate (PMA) and ionomycin for 6 h and then stained for ST2, IL-10, LAP, and GATA3. The mean fluorescence intensity of cells is shown in histograms. (F and G) Mice were killed 6 d after infection with Nb. The number of parasite eggs per gram of feces and the number of worms are shown. Mean values (three mice per group) are shown with the SEM. At least three independent experiments were performed and showed similar results. We set positive/negative gates using staining of Il1rl1−/− cells (B) or with isotype controls (A and E). n.s., not significant. *P < 0.05; **P < 0.01; ***P < 0.001.
To analyze whether ST2+ or ST2− Treg cells influenced the immunity conferred by adoptively transferred ST2+ mTh2 cells, ST2+ mTh2 cells were cotransferred with ST2+ or ST2− Treg cells compared with naïve CD4 T-cell cotransferred mice. Interestingly, the cotransfer of ST2+ Treg cells but not ST2− Treg cells prepared using CD25 expression (SI Appendix, Fig. S9A) or Foxp3-YFP expression (SI Appendix, Fig. S9B) increased the number of fecal eggs compared with naïve CD4-transferred mice, indicating that ST2+ but not ST2− Treg cells selectively constrained the ST2+ mTh2 cell-mediated immune responses against Nb (Fig. 6 F and G). In addition, on investigating the role of ST2+ Treg cells in NSG mice lacking of T cells, B cells, and ILC2 cells (SI Appendix, Fig. S9C), we noticed that ST2+ mTh2 cells harvested from Nb-infected mice reduced the number of worms in the intestine and that the expulsion of Nb mediated by ST2+ mTh2 cells was significantly inhibited by ST2+ but not ST2− Treg cells (SI Appendix, Fig. S9D). These results indicate that ST2+ mTh2 cells are sufficient for reducing the number of worms in the intestine under lymphodeficient conditions and also support a role for ST2+ Treg cells in suppressing the function of ST2+ mTh2 cells to reduce the fecundity.
Discussion
We identified ST2+ mTh2 cells and ST2+ Treg cells as promoters and repressors, respectively, of immune responses in Nb infection. Nb-induced ST2+ mTh cells produced large amounts of IL-5 and exhibited a transcriptional profile distinct from that of ST2− mTh cells. This study revealed the cellular and molecular mechanisms underlying the ST2+ Th2 cell-mediated immune responses in Nb infection: Nb-induced ST2+ mTh cells caused the inhibition of worm maturation and a reduction of fecundity via a mechanism that was dependent on IL-5 and IL-33 and through the accumulation of eosinophils expressing high levels of MBP, an antihelminthic enzyme (24). The reduction of the fecundity of Nb was suppressed by ST2+ but not ST2− Treg cells. Thus, Nb infection leads to the induction of ST2+ mTh cells capable of inducing immune responses in Nb infection in the lungs.
Recently, it has been reported that Nb infection elicited IL-5 production from Th2 cells resident in peripheral tissues but not in lymphoid organs due to peripheral tissue checkpoint cytokines, including IL-33, IL-25, and TSLP (21). In this study, ST2+ mTh cell-dependent immune responses against Nb were found to require IL-33. TSLP might be involved in this reaction. Nb-elicited ST2+ mTh cells expressed higher levels of Pparg and CD69 and lower levels of Klf2, Tcf7, Ccr7, and Sell compared with ST2− mTh cells. PPARγ modulates cellular metabolism to sustain cell viability and ST2 expression (17). It has been reported that resident memory T cells express higher levels of PPARγ and CD69 compared with central memory T cells (33, 34). Lower Klf2 expression may correlate with lower expression of central memory markers CCR7 and CD62L (26, 35, 36). Nb-induced ST2+ mTh cells exhibited characteristic markers of resident memory T cells; thus, they might respond to IL-33 in peripheral tissues and thereby, acquire their unique transcriptional profile, supporting their ability to produce high levels of IL-5.
Nb-induced ST2+ mTh cells produced higher levels of IL-5 than ST2− mTh cells. IL-5 is reported to be important for the survival, proliferation, migration, and inflammatory response of eosinophils to Toll-like receptor (TLR) ligands (37). In contrast, ST2− mTh cells produced CCL5, a CCR3 ligand that promotes eosinophil chemotaxis (38). Although ST2− and ST2+ mTh cells both induced eosinophil accumulation in the lungs, only ST2+ mTh cells induced the inhibition of the maturation of Nb. Thus, we thought that eosinophils in the lungs may have distinct properties depending on the adoptive transfer of ST2+ vs. ST2− mTh cells. The role of eosinophils against helminth infections has been poorly defined and remains controversial. With regard to Nb infection, eosinophils have been suggested to be associated with a reduction in the fecundity of Nb in primary infection (39). We further revealed that eosinophils contributed to the inhibition of maturation of Nb in secondary infection (SI Appendix, Fig. S10). IL-5 transgenic mice that exhibit eosinophilia are resistant to primary Nb infection (37, 40). These observations suggest that IL-5 is a key factor in eosinophil-dependent immune responses in Nb infection.
Regarding the role of IL-13 in the immune responses against Nb infection, it has been reported that IL-13 is required for the expulsion of adult worms from the small intestine (20, 41). Furthermore, IL-13 produced by T cells was shown to play a minor role in the expulsion of adult Nb worms from the small intestine in primary infection (42). In our experimental settings, the ST2+ mTh2 cells produced both IL-5 and IL-13 and showed a reduction in the fecundity but not the expulsion of the worms in normal mice. Interestingly, however, we detected a decreased number of worms in the intestine of a lymphodeficient NSG mouse model, as shown in SI Appendix, Fig. S9, indicating that ST2+ mTh2 cells have the potential to promote the expulsion of Nb under certain conditions. ST2+ mTh cell-derived IL-13 may play a role in the process of expulsion of Nb.
The number of larvae in the lung 2 d after secondary Nb exposure was slightly decreased when ST2+ mTh cells were transferred as shown in SI Appendix, Fig. S4D. Indeed, similar results were reported by Knott et al. (43). Those authors studied primary and secondary infections using IL-5–deficient and eosinophil-deficient ΔdblGATA mice. During primary infection, they found enhanced numbers of eggs but no marked differences in the number of larvae and worms in the absence of eosinophils. The resistance to secondary infections was impaired in IL-5–deficient and ΔdblGATA mice, with significantly increased numbers of larvae in the lungs 2 d after infection.
We further revealed that eosinophils induced by ST2+ mTh cells in the lungs possess distinct gene profiles from those of eosinophils induced by ST2− mTh cells, including a high expression of MBP. MBP is known to be expressed in eosinophils, and it damages parasites. MBP was highly expressed in the eosinophils in culture with IL-5 in vitro, and the fecundity of Nb was not inhibited by MBP-depleted eosinophils. Our study highlights the fact that the induction of MBP in eosinophils is dependent on IL-33–stimulated IL-5 high-producing ST2+ mTh2 cells.
Helminth-secreted products increase the differentiation of Treg cells to prolong residence within the gut, thereby increasing the number of progeny that can be produced (44). Recently, ST2+ Treg cells were differentiated after TCR stimulation in the presence of IL-33 (14). In this study, we observed an increase in ST2+ Treg cells in the lungs after Nb infection. Thus, ST2+ mTh cells provide inhibition of maturation and reduction of fecundity of helminth, whereas helminths reduce the antihelminth immune response by inducing ST2+ Treg cells. These results suggest that adjuvants that could promote the number of ST2+ mTh2 cells and reduce the number of ST2+ Treg cells would be beneficial in the development of helminth vaccines. Tissue Treg cells not only suppress Th2 responses that expel helminths but also, modulate inflammation to promote host tissue repair (45). Although the molecular mechanisms underlying the suppression of ST2+ mTh2 cell responses by ST2+ Treg cells remain unknown, ST2+ Treg cells and their products are attractive targets for helminth vaccine development.
Comparative RNA sequence analysis of Nb-induced ST2+ mTh cells and ST2− mTh cells revealed that the former selectively expressed two Th2-related transcription factors: Epas1 and Pparg. GATA3 binds to the Epas1 locus (46), and Epas1 expression by effector memory T cells leads to elevated glycolysis (47). PPARγ may contribute to the ST2 expression and functions in ST2+ mTh cells elicited by Nb infection as well as by allergic inflammation (48, 49). Although the respective roles of these Nb infection-specific transcription factors remain unknown, they may contribute to the ability of ST2+ mTh cells to induce the inhibition of maturation and reduction of fecundity of Nb and/or to promote their survival in vivo.
In summary, we have identified a helminth-induced ST2+ mTh2 cell subset that is crucial for the inhibition of worm maturation involving a mechanism dependent on IL-5 and IL-33 that works via the accumulation of eosinophils expressing high level of MBP. This response was suppressed by ST2+ but not ST2− Treg cells. Therefore, this study reveals the immune responses mediated by a subpopulation of IL-5–producing mTh2 cells during helminth infection.
Methods
Mice.
The animals used in this study were backcrossed to BALB/c mice 10 times. Il33−/− mice, Il1rl1−/− mice, and Foxp3-YFP-IRES-Cre mice were provided by Susumu Nakae, The Institute of Medical Science, The University of Tokyo, Tokyo, Japan; Andrew N. J. McKenzie, Medical Research Council, Cambridge, United Kingdom; and Alexander Y. Rudensky, Memorial Sloan Kettering Cancer Center, New York, respectively (10, 50, 51). BALB/c mice were purchased from CLEA Japan. ΔdblGATA mice and Il4ra-deficient mice were purchased from Jakson Laboratory. NSG mice were purchased from Charles River Japan. Animal care was conducted in accordance with the guidelines of Chiba University (#30-63, #30-64) and The Jikei University School of Medicine (2015-102C2).
Antibodies.
Alexa488-conjugated anti-Foxp3 and control rat IgG2b; allophycocyanin (APC)-conjugated anti-CD25, anti-CD69, anti–IL-4 (11B11), anti–IL-5 (TRFK5), anti–IL-10, anti-GATA3, control rat IgG1, IgG2a, and IgG2b; BV421-conjugated anti-ST2 and control rat IgG2a; BV510-conjugated anti-Thy1.1, anti-CD4 (RM4-5), control mouse IgG1 and rat IgG2a; phycoerythrin (PE)-conjugated anti-LAP, anti–IL-17, control mouse IgG1 and rat IgG1; and PE-Cy7–conjugated anti-CD44 and control rat IgG2b were purchased from Biolegend. PE-conjugated anti-CXCR3, APC-conjugated anti-CCR2 and anti-CXCR6, and Alexa Fluor 647-conjugated anti–IL-18Rα were purchased from R and D Systems. PE-conjugated anti–IFN-γ and anti–IL-13 were purchased from BD. APC-conjugated anti-CD62L was purchased from Thermo.
Helminth Infection and ex Vivo Experiments.
Mice were infected with Nb by the s.c. injection of 500 third-stage larvae. To quantity the egg production, feces from individual Nb-infected mice were collected. The eggs were then counted using a phase-contrast microscope. For the isolation of larvae from the skin, the flank skin around the inoculation site was excised. The isolated skin was finely minced with scissors and incubated with simulated gastric juice (0.24% hydrochloric acid and 0.32% pepsin) under constant agitation at 37 °C for 2 h. For the isolation of larvae from the lung, the whole lung was surgically excised. The isolated lung was finely minced with scissors followed by incubation in PBS with a Baermann apparatus at 37 °C for 2 h. For the isolation of adult worms, the small intestine was excised and opened longitudinally followed by incubation under the same conditions as those of the lung samples. For the evaluation of worm length, photomicrographs of worms recovered from the small intestine of infected mice were traced and measured using the ImageJ software program (23). For the in vivo neutralization of IL-5, mice were treated once with an i.p. injection of 200 μg anti–IL-5 (TRFK5) or control rat IgG 1 d before the inoculation.
Real-Time qPCR and RNA Sequence Analyses.
Total RNA was isolated with the TRIzol reagent (Thermo). cDNA was synthesized with an oligo (dT) primer and SuperScript II RT (Thermo). Real-time qPCR was performed with the ABI PRISM 7500 Sequence Detection System as described previously (52). Primers and Roche Universal proves were purchased from Sigma and Roche, respectively. Gene expression was normalized with the Hprt mRNA signal. For cDNA library construction, we used SMARTer Stranded Total RNA-Seq Kit-Pico Input Mammalian (634412; Clontech) according to the manufacturer’s protocol. Sequencing the library fragments was performed on the HiSeq 1500 System (53). For data analysis, read sequences (50 bp) were aligned to the mm10 mouse reference genome (University of California, Santa Cruz; December 2011) using Bowtie 2 (version 2.0.0) and TopHat (version 1.3.2). Fragments per kilobase of exon per million mapped reads (FPKMs) for each gene were calculated using Cufflinks (version 2.0.2).
Flow Cytometric Analyses.
For the flow cytometric analyses, lung samples were treated with reagents of the lung dissociation kit (Miltenyi Biotec). Lung cells and splenocytes were preincubated with anti-CD16/32 on ice for 15 min to prevent the nonspecific binding of irrelevant antibodies. Cells were then stained with the indicated combination of antibodies and analyzed with FACS Verse (BD).
Transfer of mTh Cells.
ST2+ and ST2− CD4+CD44hiCD25− mTh cells, ST2+ and ST2− CD4+CD44hiFoxp3-YFP− mTh cells, ST2+ and ST2− CD4+CD25+ Treg cells, ST2+ and ST2− CD4+Foxp3-YFP+ Treg cells, and CD4+CD44lo naïve CD4+ T cells in spleen and the lungs were purified by the CD4+ T-cell isolation kit (Miltenyi Biotec), AutoMACS (Miltenyi Biotec), and cell sorting (BD Aria III). Those mTh cells (1 × 105 per mouse) or naïve CD4+ T cells (1 × 105 per mouse) with or without ST2+ or ST2− Treg cells (1 × 105 per mouse) were transferred into indicated mice according to the method described (54, 55). Whole CD4+ T cells (5 × 106 per mouse) from spleens of naïve mice were injected i.p. into the NSG mice and nude mice before transfer of mTh cells.
Eosinophils.
Mouse bone marrow was collected, and cells were cultured to differentiate into eosinophils as described previously (56). In brief, bone marrow cells were cultured with 20% FCS (HyClone), 100 IU/mL penicillin (Thermo), 10 μg/mL streptomycin (Thermo), and 2 mM glutamine (Thermo) and supplemented with 100 ng/mL SCF (R and D Systems) and 100 ng/mL FLT3-L (R and D Systems). On day 4, the cells were resuspended with basic medium containing the indicated concentration of IL-5 (Biolegend) only. Over 90% of cultured cells were identified as Siglec-F+ eosinophils by flow cytometric analysis from day 13 after cultivation. Eosinophils were electroporated using a Neon Transfection System (Thermo) with silencer siRNA for a control or Prg2 (Thermo) and then intranasally transferred (1 × 106 per mouse) into naïve BALB/c mice intranasally.
Statistical Analyses.
Data were analyzed with the Excel software program. Comparisons of two groups were calculated with Student’s t tests. Differences with P values <0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank Dr. Mark Bix for his helpful discussion and suggestions during the preparation of the manuscript. We also thank Kaoru Sugaya, Miki Kato, Toshihiro Ito, and Masshardt Yuka for their excellent technical assistance; Yoko Ozawa for animal care; and Naohiro Watanabe for valuable discussion. This work was supported by Ministry of Education, Culture, Sports, Science and Technology Grants-in-Aid for Scientific Research (S) 26221305, Young Scientists (B) 2687072 and 17K15675, and grants from the Practical Research Project for Allergic Diseases and Immunology (Research on Allergic Diseases and Immunology JP18ek0410030) from Japan Agency for Medical Research and Development, Japan Agency for Medical Research and Development (AMED), Takeda Science Foundation, and the Naito Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The RNA sequence datasets reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE120173).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1714731115/-/DCSupplemental.
References
- 1.O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327:1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reiner SL. Development in motion: Helper T cells at work. Cell. 2007;129:33–36. doi: 10.1016/j.cell.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Nakayama T, et al. Th2 cells in health and disease. Annu Rev Immunol. 2017;35:53–84. doi: 10.1146/annurev-immunol-051116-052350. [DOI] [PubMed] [Google Scholar]
- 4.Hegazy AN, et al. Interferons direct Th2 cell reprogramming to generate a stable GATA-3(+)T-bet(+) cell subset with combined Th2 and Th1 cell functions. Immunity. 2010;32:116–128. doi: 10.1016/j.immuni.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Wang YH, et al. A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med. 2010;207:2479–2491. doi: 10.1084/jem.20101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Endo Y, Hirahara K, Yagi R, Tumes DJ, Nakayama T. Pathogenic memory type Th2 cells in allergic inflammation. Trends Immunol. 2014;35:69–78. doi: 10.1016/j.it.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Islam SA, et al. Mouse CCL8, a CCR8 agonist, promotes atopic dermatitis by recruiting IL-5+ T(H)2 cells. Nat Immunol. 2011;12:167–177. doi: 10.1038/ni.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Endo Y, et al. The interleukin-33-p38 kinase axis confers memory T helper 2 cell pathogenicity in the airway. Immunity. 2015;42:294–308. doi: 10.1016/j.immuni.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Martin NT, Martin MU. Interleukin 33 is a guardian of barriers and a local alarmin. Nat Immunol. 2016;17:122–131. doi: 10.1038/ni.3370. [DOI] [PubMed] [Google Scholar]
- 10.Townsend MJ, Fallon PG, Matthews DJ, Jolin HE, McKenzie AN. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J Exp Med. 2000;191:1069–1076. doi: 10.1084/jem.191.6.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gudbjartsson DF, et al. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet. 2009;41:342–347. doi: 10.1038/ng.323. [DOI] [PubMed] [Google Scholar]
- 12.Drake LY, Kita H. IL-33: Biological properties, functions, and roles in airway disease. Immunol Rev. 2017;278:173–184. doi: 10.1111/imr.12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker JA, McKenzie AN. Development and function of group 2 innate lymphoid cells. Curr Opin Immunol. 2013;25:148–155. doi: 10.1016/j.coi.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schiering C, et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014;513:564–568. doi: 10.1038/nature13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 16.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: Mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vasanthakumar A, et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat Immunol. 2015;16:276–285. doi: 10.1038/ni.3085. [DOI] [PubMed] [Google Scholar]
- 18.Voehringer D, Shinkai K, Locksley RM. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity. 2004;20:267–277. doi: 10.1016/s1074-7613(04)00026-3. [DOI] [PubMed] [Google Scholar]
- 19.Pearce EJ, Caspar P, Grzych JM, Lewis FA, Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991;173:159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKenzie GJ, Bancroft A, Grencis RK, McKenzie AN. A distinct role for interleukin-13 in Th2-cell-mediated immune responses. Curr Biol. 1998;8:339–342. doi: 10.1016/s0960-9822(98)70134-4. [DOI] [PubMed] [Google Scholar]
- 21.Van Dyken SJ, et al. A tissue checkpoint regulates type 2 immunity. Nat Immunol. 2016;17:1381–1387. doi: 10.1038/ni.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yasuda K, et al. Contribution of IL-33-activated type II innate lymphoid cells to pulmonary eosinophilia in intestinal nematode-infected mice. Proc Natl Acad Sci USA. 2012;109:3451–3456. doi: 10.1073/pnas.1201042109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hung LY, et al. IL-33 drives biphasic IL-13 production for noncanonical Type 2 immunity against hookworms. Proc Natl Acad Sci USA. 2013;110:282–287. doi: 10.1073/pnas.1206587110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Connell AE, et al. Major basic protein from eosinophils and myeloperoxidase from neutrophils are required for protective immunity to Strongyloides stercoralis in mice. Infect Immun. 2011;79:2770–2778. doi: 10.1128/IAI.00931-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Min B, et al. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med. 2004;200:507–517. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan Y, et al. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature. 2017;543:252–256. doi: 10.1038/nature21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mueller SN, Mackay LK. Tissue-resident memory T cells: Local specialists in immune defence. Nat Rev Immunol. 2016;16:79–89. doi: 10.1038/nri.2015.3. [DOI] [PubMed] [Google Scholar]
- 28.Kimura MY, et al. Crucial role for CD69 in allergic inflammatory responses: CD69-Myl9 system in the pathogenesis of airway inflammation. Immunol Rev. 2017;278:87–100. doi: 10.1111/imr.12559. [DOI] [PubMed] [Google Scholar]
- 29.Wang A, et al. CCL2/CCR2-dependent recruitment of Th17 cells but not Tc17 cells to the lung in a murine asthma model. Int Arch Allergy Immunol. 2015;166:52–62. doi: 10.1159/000371764. [DOI] [PubMed] [Google Scholar]
- 30.Latta M, Mohan K, Issekutz TB. CXCR6 is expressed on T cells in both T helper type 1 (Th1) inflammation and allergen-induced Th2 lung inflammation but is only a weak mediator of chemotaxis. Immunology. 2007;121:555–564. doi: 10.1111/j.1365-2567.2007.02603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kouro T, Takatsu K. IL-5- and eosinophil-mediated inflammation: From discovery to therapy. Int Immunol. 2009;21:1303–1309. doi: 10.1093/intimm/dxp102. [DOI] [PubMed] [Google Scholar]
- 32.Yu C, et al. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J Exp Med. 2002;195:1387–1395. doi: 10.1084/jem.20020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 34.Kim MV, Ouyang W, Liao W, Zhang MQ, Li MO. The transcription factor Foxo1 controls central-memory CD8+ T cell responses to infection. Immunity. 2013;39:286–297. doi: 10.1016/j.immuni.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carlson CM, et al. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature. 2006;442:299–302. doi: 10.1038/nature04882. [DOI] [PubMed] [Google Scholar]
- 36.Schenkel JM, et al. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014;346:98–101. doi: 10.1126/science.1254536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenberg HF, Dyer KD, Foster PS. Eosinophils: Changing perspectives in health and disease. Nat Rev Immunol. 2013;13:9–22. doi: 10.1038/nri3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125(2) Suppl 2:S73–S80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang L, Appleton JA. Eosinophils in helminth infection: Defenders and dupes. Trends Parasitol. 2016;32:798–807. doi: 10.1016/j.pt.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shin EH, et al. Protective roles of eosinophils in Nippostrongylus brasiliensis infection. Int Arch Allergy Immunol. 1997;114:45–50. doi: 10.1159/000237717. [DOI] [PubMed] [Google Scholar]
- 41.Minutti CM, et al. Epidermal growth factor receptor expression licenses type-2 helper T cells to function in a T cell receptor-independent fashion. Immunity. 2017;47:710–722.e6. doi: 10.1016/j.immuni.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sullivan BM, et al. Genetic analysis of basophil function in vivo. Nat Immunol. 2011;12:527–535. doi: 10.1038/ni.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knott ML, et al. Impaired resistance in early secondary Nippostrongylus brasiliensis infections in mice with defective eosinophilopoeisis. Int J Parasitol. 2007;37:1367–1378. doi: 10.1016/j.ijpara.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 44.Grainger JR, et al. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway. J Exp Med. 2010;207:2331–2341. doi: 10.1084/jem.20101074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith KA, et al. Low-level regulatory T-cell activity is essential for functional type-2 effector immunity to expel gastrointestinal helminths. Mucosal Immunol. 2016;9:428–443. doi: 10.1038/mi.2015.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hosokawa H, et al. Functionally distinct Gata3/Chd4 complexes coordinately establish T helper 2 (Th2) cell identity. Proc Natl Acad Sci USA. 2013;110:4691–4696. doi: 10.1073/pnas.1220865110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doedens AL, et al. Hypoxia-inducible factors enhance the effector responses of CD8(+) T cells to persistent antigen. Nat Immunol. 2013;14:1173–1182. doi: 10.1038/ni.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen T, et al. PPAR-γ promotes type 2 immune responses in allergy and nematode infection. Sci Immunol. 2017;2:eaal5196. doi: 10.1126/sciimmunol.aal5196. [DOI] [PubMed] [Google Scholar]
- 49.Nobs SP, et al. PPARγ in dendritic cells and T cells drives pathogenic type-2 effector responses in lung inflammation. J Exp Med. 2017;214:3015–3035. doi: 10.1084/jem.20162069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oboki K, et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci USA. 2010;107:18581–18586. doi: 10.1073/pnas.1003059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rubtsov YP, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 52.Obata-Ninomiya K, et al. The skin is an important bulwark of acquired immunity against intestinal helminths. J Exp Med. 2013;210:2583–2595. doi: 10.1084/jem.20130761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Onodera A, et al. Spatial interplay between polycomb and trithorax complexes controls transcriptional activity in T lymphocytes. Mol Cell Biol. 2015;35:3841–3853. doi: 10.1128/MCB.00677-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mottet C, Uhlig HH, Powrie F. Cutting edge: Cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 55.Griseri T, Asquith M, Thompson C, Powrie F. OX40 is required for regulatory T cell-mediated control of colitis. J Exp Med. 2010;207:699–709. doi: 10.1084/jem.20091618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dyer KD, et al. Eosinophils from lineage-ablated Delta dblGATA bone marrow progenitors: The dblGATA enhancer in the promoter of GATA-1 is not essential for differentiation ex vivo. J Immunol. 2007;179:1693–1699. doi: 10.4049/jimmunol.179.3.1693. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.