Significance
Cell–cell contact-mediated responses play a key role in coordinating cell migration. The conventional view has emphasized repulsive responses, based primarily on studies of head-on collisions. Using micropatterned substrates to facilitate various modes of collision, we report that cells migrate toward their neighbors upon contact with tails. This response, referred to as contact following of locomotion (CFL), is found with both epithelial and mesenchymal cells and is reminiscent of the behavior of Dictyostelium discoideum during stream formation. Pharmacological studies implicate the Wnt signaling pathway, and suggest that CFL is necessary for collective migration. CFL may thus represent a critical aspect of diverse biological phenomena that involve collective migration, such as wound healing, tissue development, and metastasis.
Keywords: cell migration, contact inhibition of locomotion, collective migration, front–rear polarity, Wnt signaling
Abstract
Contact inhibition of locomotion (CIL), the repulsive response of cells upon cell–cell contact, has been the predominant paradigm for contact-mediated responses. However, it is difficult for CIL alone to account for the complex behavior of cells within a multicellular environment, where cells often migrate in cohorts such as sheets, clusters, and streams. Although cell–cell adhesion and mechanical interactions play a role, how individual cells coordinate their migration within a multicellular environment remains unclear. Using micropatterned substrates to guide cell migration and manipulate cell–cell contact, we show that contacts between different regions of cells elicit different responses. Repulsive responses were limited to interaction with the head of a migrating cell, while contact with the tail of a neighboring cell promoted migration toward the tail. The latter behavior, termed contact following of locomotion (CFL), required the Wnt signaling pathway. Inhibition of the Wnt pathway disrupted not only CFL but also collective migration of epithelial cells, without affecting the migration of individual cells. In contrast, inhibition of myosin II with blebbistatin disrupted the migration of both individual epithelial cells and collectives. We propose that CFL, in conjunction with CIL, plays a major role in guiding and coordinating cell migration within a multicellular environment.
Migration represents one of the most crucial functions for animal cells. Cell migration is required for physiological processes such as embryonic development and wound repair, and for pathological processes such as cancer metastasis. While an extensive body of knowledge is available about the migration of single cells, many biological phenomena involve cell migration within a multicellular environment, where cell–cell interactions may affect the behavior and facilitate concerted migration toward a common destination (1–3).
Through physical or chemical interactions, cell–cell contact acts as a major means for cells to communicate, affecting a wide variety of phenomena such as migration, differentiation, and morphogenesis (1–3). The effect of cell–cell contact on migration was first described in the classic studies of Abercrombie. Known as contact inhibition of locomotion (CIL) (4–7), migrating cells in 2D cultures avoid moving on top of other cells and migrate away from each other upon contact (8, 9). CIL is believed to be crucial to tissue morphogenesis as part of the mechanisms for formation of ordered multicellular structures such as cell sheets (10–13). In addition, the loss of CIL is believed to allow the invasion of cancer cells into other tissues (4, 14), and the invasion process itself may involve the migration of collectives of cancerous cells (12, 15, 16).
Despite decades of documentation, the understanding of contact-mediated responses of cell migration remains deficient in many ways. First, CIL is drawn primarily from the observation of head-on collisions on 2D surfaces, while responses to the contact of other regions of a polarized cell have not been clearly documented (17). Second, CIL as a repulsive response cannot account for many behaviors of a multicellular environment. It is particularly difficult for CIL to explain collective migration, where cells follow rather than repel each other to migrate in concert (18, 19). Questions therefore arise as to whether the site of cell–cell contact may determine the outcome of collision, and whether such context-dependent responses may provide a more comprehensive understanding of collective migration.
Contact following, a phenomenon observed in the slime mold Dictyostelium, was proposed to account for their collective migration (20). During the formation of fruit bodies, cells repeatedly extend their pseudopodia toward the retracting end of the preceding cells (11, 21), which leads to the formation of a stream of cells connected in a tail-to-head manner. It was pointed out that this process is unlikely to be driven by mechanical forces via cell adhesion or chemotaxis but involves active movement and coordination (21, 22). While a parallel process for animal cells represents an attractive mechanism to complement CIL in collective migration, such behavior has yet to be demonstrated.
To address these questions, we have designed micropatterned substrates to achieve two purposes. The first was to trap cells temporarily at intersections, which facilitates collisions between different regions of cells to allow the detection of contact site-dependent responses. The second was to create a simplified experimental model of collective migration, where multiple cells migrated in single file, to facilitate the dissection of mechanism. These approaches have allowed us to demonstrate a tail-following behavior of cultured epithelial cells, similar to what was described for Dictyostelium (23). This behavior, termed contact following of locomotion (CFL), may play a key role in epithelial collective migration based on their shared pharmacological sensitivities.
Results
Contact Inhibition of Locomotion Accounts for Only Some of the Responses to Cell–Cell Contact.
Most experiments were conducted with NRK-52E epithelial cells, which were migratory as single cells, albeit at a slow average speed of 0.22 µm/min. We used elastic polyacrylamide substrates micropatterned with gelatin strips 30 µm wide to confine migration. Cell polarity was determined based on persistent migration along these strips and on distinct head–tail morphology, and confirmed with the localization of nonmuscle myosin II-B (NMII-B), which is known to concentrate toward the tail (SI Appendix, Fig. S1) (24, 25). CIL was observed upon head-on collision of individual cells moving toward each other (88.9%, n = 51), where both cells reversed the direction of migration (SI Appendix, Fig. S2).
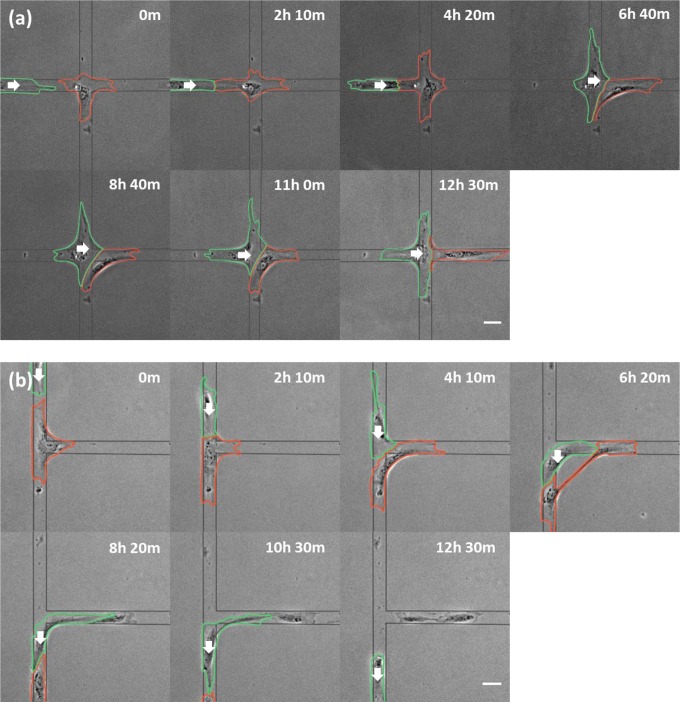
To facilitate collisions at different sites between pairs of cells, cells were plated on crisscrossing strips 30 µm in width that formed X or T intersections (Fig. 1). While cells migrated persistently along linear strips, most cells (66%) stalled at intersections through the end of the observation period (up to 28 h) in the absence of cell–cell contact. These cells extended short-lived processes into branches, resulting in random migration around the intersection with a speed similar to that along strips. Stalling ended upon contact with an approaching migrating cell; the majority of stalled cells began migration out of the intersection within 2 h (88.2% for X; 88.7% for T intersections; Fig. 1, Table 1, and Movies S1 and S2). Stalled cells preferred to leave the X intersection along the branch opposite the approaching cell (72.0%, n = 82), rather than along other available branches. In addition, the pairs of cells maintained tail-to-head contact, suggesting a tail-following behavior by the approaching cell.
Fig. 1.
Migration of stalled NRK-52E cells after contacting the head of an approaching cell. Phase-contrast images of a cell stalled at an X (A; red outline) or T (B; red outline) intersection show the initiation of migration after interacting with the head of an approaching cell (green outlines). White arrows indicate the direction of migration of the approaching cell. Substrate micropattern is indicated by gray lines. Note both cells on the T micropattern break into two pieces, forming a pair of anuclear cytoplasts along the horizontal branch. Elapsed times are shown above each panel. (Scale bars, 50 µm.)
Table 1.
Repulsive response of NRK-52E cells to head contact
| Setting | Approaching cell maintaining original polarity, % | Stalled cell retreating from the approaching neighbor, % |
| X intersection | 90.3 ± 3.1 (n = 93) | 88.2 ± 3.4 (n = 93)** |
| T intersection | 82.3 ± 4.9 (n = 62) | 88.7 ± 4.1 (n = 62)*** |
| Double seeding | 69.4 ± 3.7 (n = 160) | 73.1 ± 3.5 (n = 160)*** |
In all three settings tested (left column), a migratory cell approaches a stalled or spreading cell to make contact. The majority of stalled or spreading cells migrate away from the approaching cell following the same direction (right column), while the majority of approaching cells continue to migrate along the original direction (middle column). Significance was determined using a one-sample binomial test against the null hypothesis of equal probability of entering any branch. **P < 0.005, ***P < 0.0005.
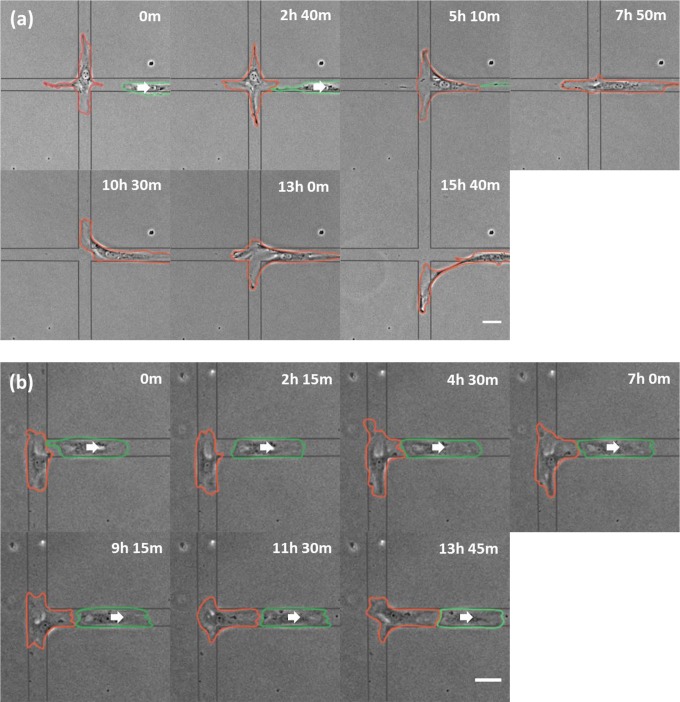
While most approaching cells continued to migrate past the interaction as described above, a small fraction reversed direction after contact with the stalled cell (9.7% for X; 17.7% for T intersections; Fig. 1 and Table 1). In cases where the tail of a retreating cell contacted the stalled cell, migration of the stalled cell ensued toward the tail and out of the intersection within 2 h (69.4% for X; 73.3% for T intersections; Fig. 2, Table 2, and Movies S3 and S4).
Fig. 2.
Migration of stalled NRK-52E cells following contact with the tail of a neighboring cell. Phase-contrast images of a cell stalled at an X (A; red outline) or T (B; red outline) intersection show the initiation of migration after interacting with the tail of an adjacent cell (green outlines). White arrows indicate the direction of migration of the retreating cell. Elapsed times are shown above each panel. Substrate micropattern is indicated by gray lines. (Scale bars, 50 µm.)
Table 2.
Attractive response of NRK-52E cells to tail contact
| Setting | Retreating cell maintaining original polarity, % | Stalled cell following the retreating neighbor, % |
| X intersection | 96.8 ± 2.3 (n = 62) | 69.4 ± 5.9 (n = 62)** |
| T intersection | 97.8 ± 2.2 (n = 45) | 73.3 ± 6.7 (n = 45)* |
| Double seeding | 92.1 ± 1.7 (n = 267) | 71.2 ± 2.8 (n = 267)*** |
In all three settings tested (left column), a cell migrates away from a stalled or spreading cell in contact. The majority of stalled or spreading cells migrate toward the retreating cell (right column), while the majority of retreating cells continue to migrate along the original direction (middle column). Significance was determined using a one-sample binomial test against the null hypothesis of equal probability of entering any branch. *P < 0.05, **P < 0.005, ***P < 0.0005.
Stalled cells exhibited no front–rear polarization relative to the micropattern, as visualized with immunostaining of NMII-B (87.2%, n = 39; SI Appendix, Fig. S1). Following the interaction with a neighboring polarized cell, the majority of stalled cells were found to polarize either away from an approaching cell (81.8%, n = 9; SI Appendix, Fig. S3) or toward a retreating cell (83.3%, n = 10; SI Appendix, Fig. S4).
These observations suggest that contact with the tail of a neighboring cell has an effect opposite that from head contact, promoting migration toward the tail. This response parallels what was observed with Dictyostelium during stream formation, and may be referred to as contact following of locomotion.
The Phenomenon of CFL Is Not Limited to NRK-52E Cells.
While CIL was discovered with 3T3 fibroblasts, many other cell types exhibit CIL, such as epithelial cells and neural crest cells (5, 13, 26). To determine if CFL as observed with NRK-52E cells also applies to other cell lines, we seeded Madin–Darby canine kidney (MDCK) epithelial cells and NIH 3T3 fibroblasts on the micropatterns described above. Both showed the same responses at intersections as NRK-52E cells (Table 3). Cells were stalled at X intersections for an extended period of time in the absence of cell contact (∼5.8 h for MDCK, ∼7.8 h for 3T3). Upon contact with the head of an approaching cell, the stalled cell initiated migration away from the intersection within 1.4 h (83.6% for MDCK, 86.3% for 3T3). The majority of approaching cells continued to migrate in their original direction (85.7% for MDCK, 78.4% for 3T3; Movies S5 and S6), following the tail of the cell displaced from the intersection. In cases where a stalled cell contacted the tail of a retreating cell, the stalled cell initiated migration to follow the tail within 1.5 h (75.7% for MDCK, 70% for 3T3) while the retreating cell continued to migrate in its original direction (90.5% for MDCK, 95% for 3T3; Movies S7 and S8). These results suggest that, like CIL, CFL is not restricted to specific cell lines or types.
Table 3.
Responses of MDCK epithelial cells and NIH 3T3 fibroblasts to head or tail contact
| Cell line | Retreating from an approaching neighbor, % | Following a retreating neighbor, % |
| MDCK epithelial cells | 83.6 ± 3.7 (n = 91)*** | 75.7 ± 5.0 (n = 74)*** |
| NIH 3T3 fibroblasts | 86.3 ± 4.9 (n = 51)*** | 70.0 ± 7.3 (n = 40)* |
The majority of cells stalled at an X intersection initiated migration within 1.5 h upon contact with a migrating cell, either away from an approaching cell or toward a retreating cell. Numbers indicate the percentage of cells stalled at an X intersection that initiated directional migration upon contact with a migrating cell. Significance was determined using a one-sample binomial test against the null hypothesis of equal probability of entering any branch. *P < 0.05, ***P < 0.0005.
Cell–Cell Contact Promotes Symmetry Breaking of Spreading Cells in a Site-Dependent Manner.
Symmetry breaking is the initiation of directional cell migration after substrate adhesion and symmetric spreading (27). Given the observations of CIL and CFL, we suspected that cell–cell contact may facilitate symmetry breaking along a direction determined by the site of contact.
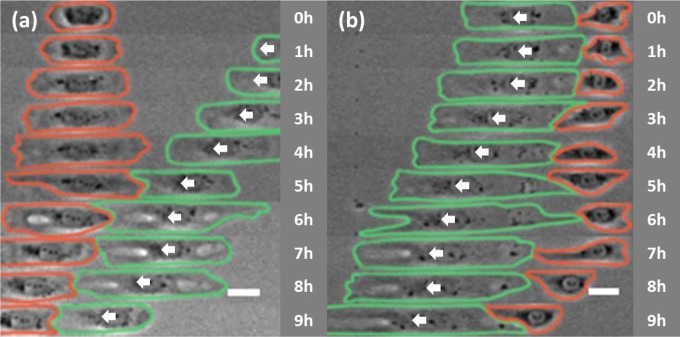
Isolated NRK-52E cells typically spent 4 h to adhere and spread symmetrically, and 4 additional hours to break symmetry (28). To determine the effect of cell–cell contact on symmetry breaking, we seeded cells in two rounds onto linear strips (termed double seeding), such that cells plated earlier were migrating directionally as the cells plated later started to spread. We found that symmetry breaking took place within ∼1.8 h upon contact with a migrating cell. Contact with the head of an approaching cell caused spreading cells to migrate away from the head (94.3%; Fig. 3A and Table 1), while contact with the tail of a retreating cell caused spreading cells to migrate toward the tail (86.1%; Fig. 3B and Table 2). These results reinforce the notion of contact site-dependent stimulation of cell polarization and migration.
Fig. 3.
Facilitation of symmetry breaking by site-dependent cell–cell contact. NRK-52E cells are seeded in two rounds 2 h apart. Phase-contrast images show a stationary cell from the second round of seeding (red) making contact with the head (A) or tail (B) of an adjacent cell from the first round of seeding (green). Symmetry breaking took place soon after contact, causing the stationary cell to migrate away from the approaching cell (A) or follow the retreating cell (B). In both cases, the pair of cells migrated as a collective in a tail-to-head manner. White arrows indicate the direction of migration of the cell from the first round of seeding. Elapsed times are shown to the right. (Scale bars, 30 µm.)
Functional Role of Myosin II Contractility and Wnt Signaling Pathways in Contact Responses.
Previous literature suggested that cell-generated contractile forces may play a role in contact-dependent cell–cell communications (29–31). We therefore probed the involvement of myosin II-dependent contractility in CFL using the small-molecule inhibitor blebbistatin (32). Treatment of NRK-52E cells with 50 μM blebbistatin caused severe disruptions to cell shape and migration persistence, precluding further assessment of contact-mediated responses.
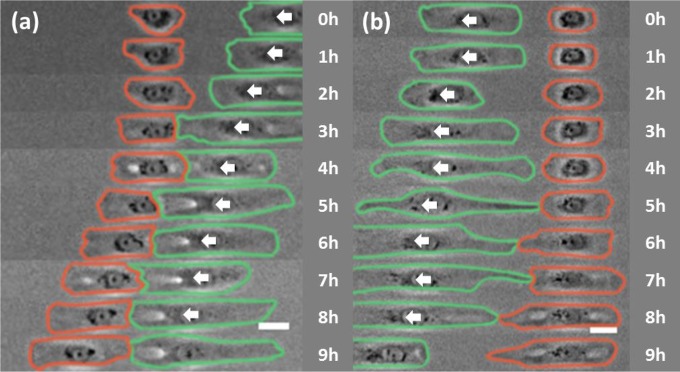
The development of cell polarity with regard to CIL and CFL may be related to planar cell polarity (23), which in turn involves a noncanonical Wnt pathway (33–36). To investigate whether Wnt pathways may be involved in CFL, we used the small-molecule inhibitor NSC 668036 to prevent activation of Dishevelled (Dvl) by Frizzled (Fzd), a key component of the Wnt signaling pathways (37). We found that the morphology, migration speed, and persistence of individual cells were unaffected by NSC 668036 (SI Appendix, Table S1). In contrast, NSC 668036 disrupted CFL as detected at X intersections, such that only 35.1% of stalled cells migrated toward the tail of a retreating cell out of the intersection (compared with 69.4% for untreated cells; Table 4 and Movie S9). The failure of CFL was caused primarily by the inability to initiate migration following contact. Similarly, only 48.0% of cells stalled at a T intersection migrated toward the tail of a retreating cell (compared with 73.3% untreated; Table 4 and Movie S10). Additionally, NSC 668036 disrupted symmetry breaking in response to tail contact, as seen in the double-seeding experiment (50.0%, compared with 71.2% untreated; Fig. 4B and Table 4). Interestingly, NSC 668036 did not seem to affect the formation and migration of cell pairs following CIL (Fig. 4A and Table 4). Treatment with a second small-molecule inhibitor of Dvl activation, 3289-8625 (38), caused inhibition of CFL without affecting CIL, similar to NSC 668036 (SI Appendix, Fig. S5 and Table S2 and Movie S11). These results suggest that Wnt signaling pathways are required for CFL.
Table 4.
Responses of NRK-52E cells to head or tail contact after Dvl inhibition
| Retreating from an approaching neighbor | Following a retreating neighbor | |||
| Setting | Control, % | NSC 668036, % | Control, % | NSC 668036, % |
| X intersection | 88.2 ± 3.4 (n = 93) | 78.6 ± 4.5 (n = 84)NS | 69.4 ± 5.9 (n = 62) | 35.1 ± 8.0 (n = 37)††† |
| T intersection | 88.7 ± 4.1 (n = 62) | 94.7 ± 3.7 (n = 38)NS | 73.3 ± 6.7 (n = 45) | 48.0 ± 10.2 (n = 25)† |
| Double seeding | 73.1 ± 3.5 (n = 160) | 81.2 ± 2.8 (n = 197)NS | 71.2 ± 2.8 (n = 267) | 50.0 ± 3.0 (n = 288)††† |
NRK-52E cells treated with 50 µM NSC 668036 maintain the ability to migrate away from the head of approaching neighbors (CIL), but lose their ability to migrate toward the tail of retreating neighbors (CFL) in all of the three experimental settings. Significance was determined using a two-sample t test against control. †P < 0.05, †††P < 0.0005; NS, not significant.
Fig. 4.
Inhibition of CFL, but not CIL, during cell contact-mediated symmetry breaking by the Dvl inhibitor NSC 668036. (A) Phase-contrast images show a spreading NRK-52E cell from the second round of seeding (red), making contact with and migrating away from the head of an approaching cell (green) in the presence of 50 µM NSC 668036. (B) Phase-contrast images of a spreading cell from the second round of seeding (red) making contact with and failing to follow the tail of a retreating cell (green) in the presence of 50 µM NSC 668036. White arrows indicate the direction of migration of the cell from the first round of seeding. Elapsed times are shown to the right. (Scale bars, 30 µm.)
Collective Migration Requires Wnt Signaling Pathways.
The tail-following behavior of CFL represents an attractive mechanism for collective migration. To investigate the role of CFL and Wnt pathways in collective migration, we confined linear collectives of five or six cells along micropatterned strips (27, 39, 40). These trains showed unidirectional migration while maintaining cell–cell contact, sharing many attributes of migrating monolayer sheets without the complex migration pattern of the conventional scratch wound assays (15, 16). Except for the trailing cell, centrosomes were positioned ahead of the nucleus for cells in these trains (SI Appendix, Fig. S6). Furthermore, interior cells continued to migrate forward for 2 h after the leader cell reached the end of a strip (SI Appendix, Fig. S6), suggesting that these cells migrated independent of the forward protrusion of the leading cell. As with single cells and cell pairs, the migration speed and morphology of cells in collectives were unaffected by NSC 668036 or 3289-8625.
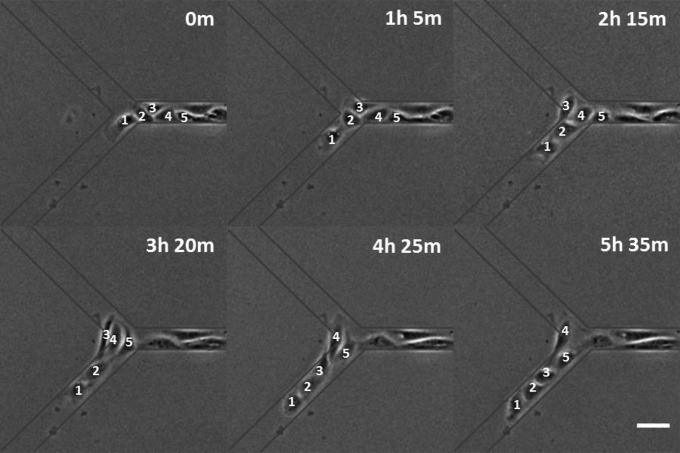
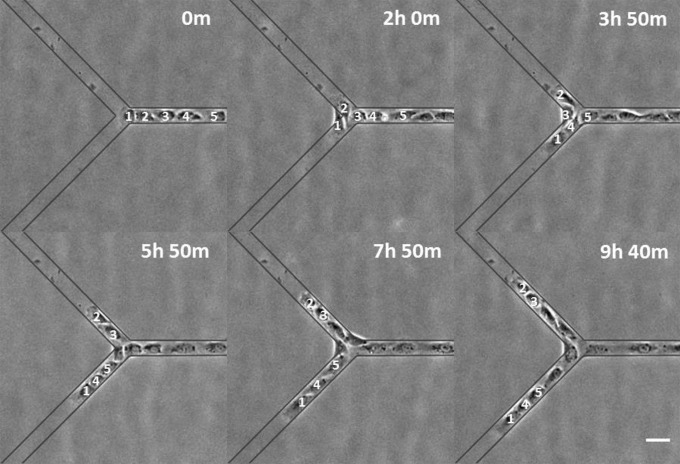
To test if collectives were actively maintained by cell–cell contact rather than confinement of the pattern and neighboring cells, cell trains were placed on Y-branched micropatterns to determine if cells in a collective chose to maintain tail-to-head contact or migrate toward free space as during wound repair. We found that the majority of cells followed their predecessor into the same branch (73.8%, n = 43; Fig. 5 and Movie S12). However, in the presence of NSC 668036, the majority of cells failed to follow their predecessors (33.3% of cells followed their predecessor, n = 17, P < 0.0005, compared with control; Fig. 6 and Movie S13), causing the train to branch into two. Cells also failed to follow their predecessors in the presence of 3289-8625 (33.6% of cells followed their predecessor, n = 29, P < 0.0005, compared with control). The shared sensitivity of CFL and collective migration to Dvl inhibitors suggests that CFL acts to promote collective migration.
Fig. 5.
Collective migration as tested at a Y junction. Most NRK-52E cells within a train follow their predecessors to enter the same branch. The first five cells of the train are annotated for tracking purposes. Elapsed times are shown in the upper right. Micropattern is indicated by gray lines. (Scale bar, 50 µm.)
Fig. 6.
Response of collective migration to Dvl inhibition. Phase-contrast images show a train of NRK-52E cells splitting between the two branches of a Y junction upon overnight treatment with 50 µM NSC 668036. The first five cells are annotated for tracking purposes. Substrate micropattern is indicated by gray lines. Elapsed times are shown in the upper right. (Scale bar, 50 µm.)
CIL has also been previously implicated in facilitating collective migration (26). We therefore investigated how CIL might contribute to collective migration by examining the response of individual cells upon head-on collision with a train. The individual cell exhibited CIL and reversed its direction. As a result, its new tail became connected to the head of the train, which caused the incorporation of the cell into the train as its new leader (SI Appendix, Fig. S7). Thus, collectives may use a combination of CIL and CFL to both maintain the collective and incorporate additional cells.
Discussion
Contact-Mediated Regulation of Cell Migration.
Coordination of cell migration is important for generating and maintaining order within a multicellular organism. CIL, referring to the migration away from neighbors upon head-on collision (4, 13, 19, 26, 41–44), has served as the main paradigm in the understanding of cell–cell contact-mediated coordination of cell migration (5, 6, 9, 13, 42–44). Its involvement in collective migration was previously implicated based on mutation and inhibition studies (26). However, it has been reported that CIL does not take place consistently upon head–tail contact between cells (41). Moreover, the repulsive response of CIL alone cannot explain how cells may coordinate their migration within collectives, which raises the possibility that other modalities of contact-mediated interactions may play a complementary role in regulating cell migration.
Using micropatterned substrates to facilitate collisions between different sites of cells, we found that while CIL is the primary response to head–head collisions, an opposite response of tail following, termed CFL, took place when a cell contacted the tail of a neighbor. We further showed that, like CIL, CFL represented a general phenomenon shared by multiple lines of epithelial cells and fibroblasts. Indeed, CFL is similar to CIL in several key facets. Both are contact-dependent responses that influence directional migration and polarity between pairs of cells. Furthermore, both are responses of individual cells, and may help govern the more complex behaviors of higher-order structures.
While the mechanism of CIL is only starting to be revealed (4, 26, 42, 43), our results raise an equally important question as to what may be responsible for the difference in response between head–head and head–tail contacts, which may involve both physical and chemical interactions. While it has been suggested that adhesive cells can react to the presence of neighboring cells through forces propagated across a compliant substrate (45), our use of relatively stiff substrates, where the strain due to traction forces is expected to be undetectable at 10 μm from the cell boundary, argues against a major role of substrate-mediated force transmission in CIL and CFL. With regard to direct forces between cells, pushing forces are expected upon contact with a protruding front, while pulling forces are expected upon contact with a retracting tail. This difference has been hypothesized to cause opposite responses of retraction or protrusion (46, 47).
Additionally, CIL and CFL may involve chemical factors transmitted between cells. An increasing number of proteins, including Vangl2, Merlin, Fat2, Lamellipodin, and Dachsous, have been found to localize differentially at the head or tail of cells undergoing directional migration (29, 48–50), raising the possibility that contact with the head or tail of a neighbor may elicit differential downstream signaling responses. Consistent with this mechanism, we showed that CFL requires the Wnt signaling pathways, using the specific small-molecule inhibitors NSC 668036 and 3289-8625 against its key component Dishevelled. The similarity of effects induced by two small-molecule inhibitors with different molecular structures argues against the possibility of off-target effects. The involvement of Wnt, in particular the noncanonical Wnt pathway for establishing planar cell polarity, represents an appealing possibility given its implicated role in cell polarity and contact-mediated responses (34–36, 45, 51, 52). Downstream activation of Rho GTPases may then regulate actin–myosin–dependent functions required for cell migration (4, 43).
A number of studies have shown front–rear asymmetrical accumulation of proteins involved in Wnt pathways, such as proteins in the Fzd and Van Gogh-like families (34–36, 51, 52), which may account for the differential responses to head or tail contact in a manner similar to the role of the cAMP pathway in the contact following of Dictyostelium (23, 53). Although the Wnt signaling pathway is distinct from that of cAMP-response pathways, a similar paradigm may be applicable to the regulation of cell migration and polarity in CFL (54).
Effects of CIL and CFL on Collective Cell Migration.
Much of cell migration in a multicellular organism involves cell collectives, such as during development, wound repair, and cancer metastasis. How individual cells interact and follow each other in collectives remain relatively unexplored. CIL alone, as a repulsion mechanism, is unable to explain the formation and maintenance of collectives. In the present study, we showed that a process similar to the contact following previously described with Dictyostelium (11, 20–23) exists in mammalian cells. This behavior may represent the missing link for the understanding of collective migration.
We took advantage of micropatterned substrates for studying the migration of small linear collectives of epithelial cells, which overcame the complexity of conventional models based on the repair of monolayers after scratch wound (15, 16). In addition, micropatterned Y junctions allowed us to test the robustness of collective migration and rule out passive ushering for collectives confined within a narrow strip. We showed that most cells follow their predecessors through Y junctions, suggesting that tail following takes precedence over cells’ general preference for free space, as seen during monolayer wound repair. These linear collectives degenerated into two branches upon the inhibition of Wnt signaling pathways with the small-molecule Dvl inhibitors NSC 668036 or 3289-8625. The shared sensitivity to Dvl inhibition between CFL and collective migration suggests that CFL is responsible for maintaining tail-to-head contact and coordinating the directional migration of cell collectives.
The involvement of Wnt signaling pathways does not contradict previous studies that emphasized the role of cadherin family proteins in collective cell migration (10, 29–31), which may mediate cell–cell interactions upstream of the Wnt signaling pathways. While myosin II-dependent contractile forces may play a role in collective migration (3, 29–31), responses to blebbistatin suggested a more general role in maintaining cell shape and migration persistence, which are also required for collective migration. In contrast, Wnt signaling pathways likely operate through a mechanism more specific to collective migration, given the lack of apparent effects of the inhibitors on single-cell migration.
We observed CIL and CFL in both NRK-52E epithelial and NIH 3T3 mesenchymal cells. However, the lack of prominent collective migration in NIH 3T3 cells suggests that CIL and CFL are necessary but not sufficient for collective migration. Other factors such as cell–cell adhesions may play complementary roles. Furthermore, factors in the microenvironment may promote collective migration of mesenchymal cells, as demonstrated by the collective invasive migration of cancerous cells (3, 12, 15, 16). The differences between mesenchymal and epithelial cells may help elucidate other components in collective migration.
In addition to the maintenance of collective migration by CFL, our observations suggest how CIL and CFL may work together to build cell collectives. Within the collective, a combination of CIL and CFL would cause cells to migrate unidirectionally and keep them from colliding with each other. Furthermore, as a collective makes head-on contacts with individual cells, CIL would cause the single cell to reverse its direction, causing its tail to face the front of the collective. The ensuing CFL would then cause the individual cell to incorporate into the collective and become its new leader cell.
In summary, we have discovered that, in addition to the repulsive response of CIL to head contact, contact with the tail of a migrating cell elicits an attractive response of CFL. The discovery of CFL for mammalian cells allows a more complete understanding of the initiation and maintenance of collective cell migration, beyond what is possible through CIL, contractility, and cadherin-mediated adhesions. These studies may particularly benefit computational models which utilize contact-mediated cell–cell interactions to model collective cell migration (55, 56). Finally, our experimental system maintains key aspects of collective migration while minimizing the complexity of a conventional system based on wound repair of monolayers, to facilitate the detection of conditions that affect the collective behavior.
Materials and Methods
Cell culture, reagents, micropatterning, microscopy, and statistics are described in detail in SI Appendix, Materials and Methods. Manual analysis was carried out using ImageJ (NIH) and custom software (SI Appendix, Materials and Methods).
Supplementary Material
Acknowledgments
This work was supported by NIH Grant R01GM118998.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1807543115/-/DCSupplemental.
References
- 1.Kornberg TB, Roy S. Communicating by touch—Neurons are not alone. Trends Cell Biol. 2014;24:370–376. doi: 10.1016/j.tcb.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perez-Moreno M, Jamora C, Fuchs E. Sticky business: Orchestrating cellular signals at adherens junctions. Cell. 2003;112:535–548. doi: 10.1016/s0092-8674(03)00108-9. [DOI] [PubMed] [Google Scholar]
- 3.Haeger A, Wolf K, Zegers MM, Friedl P. Collective cell migration: Guidance principles and hierarchies. Trends Cell Biol. 2015;25:556–566. doi: 10.1016/j.tcb.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Roycroft A, Mayor R. Molecular basis of contact inhibition of locomotion. Cell Mol Life Sci. 2016;73:1119–1130. doi: 10.1007/s00018-015-2090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Middleton CA. Contact inhibition of locomotion in cultures of pigmented retina epithelium. Exp Cell Res. 1972;70:91–96. doi: 10.1016/0014-4827(72)90185-1. [DOI] [PubMed] [Google Scholar]
- 6.Huttenlocher A, et al. Integrin and cadherin synergy regulates contact inhibition of migration and motile activity. J Cell Biol. 1998;141:515–526. doi: 10.1083/jcb.141.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaughan RB, Trinkaus JP. Movements of epithelial cell sheets in vitro. J Cell Sci. 1966;1:407–413. doi: 10.1242/jcs.1.4.407. [DOI] [PubMed] [Google Scholar]
- 8.Abercrombie M, Heaysman JE. Observations on the social behaviour of cells in tissue culture. II. Monolayering of fibroblasts. Exp Cell Res. 1954;6:293–306. doi: 10.1016/0014-4827(54)90176-7. [DOI] [PubMed] [Google Scholar]
- 9.Guo WH, Frey MT, Burnham NA, Wang YL. Substrate rigidity regulates the formation and maintenance of tissues. Biophys J. 2006;90:2213–2220. doi: 10.1529/biophysj.105.070144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Theveneau E, et al. Collective chemotaxis requires contact-dependent cell polarity. Dev Cell. 2010;19:39–53. doi: 10.1016/j.devcel.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weijer CJ. Collective cell migration in development. J Cell Sci. 2009;122:3215–3223. doi: 10.1242/jcs.036517. [DOI] [PubMed] [Google Scholar]
- 12.Friedl P, Locker J, Sahai E, Segall JE. Classifying collective cancer cell invasion. Nat Cell Biol. 2012;14:777–783. doi: 10.1038/ncb2548. [DOI] [PubMed] [Google Scholar]
- 13.Davis JR, et al. Emergence of embryonic pattern through contact inhibition of locomotion. Development. 2012;139:4555–4560. doi: 10.1242/dev.082248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abercrombie M. Contact inhibition and malignancy. Nature. 1979;281:259–262. doi: 10.1038/281259a0. [DOI] [PubMed] [Google Scholar]
- 15.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445–457. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 16.Rørth P. Collective cell migration. Annu Rev Cell Dev Biol. 2009;25:407–429. doi: 10.1146/annurev.cellbio.042308.113231. [DOI] [PubMed] [Google Scholar]
- 17.Abercrombie M. Contact inhibition in tissue culture. In Vitro. 1970;6:128–142. doi: 10.1007/BF02616114. [DOI] [PubMed] [Google Scholar]
- 18.Rørth P. Fellow travellers: Emergent properties of collective cell migration. EMBO Rep. 2012;13:984–991. doi: 10.1038/embor.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carmona-Fontaine C, et al. Complement fragment C3a controls mutual cell attraction during collective cell migration. Dev Cell. 2011;21:1026–1037. doi: 10.1016/j.devcel.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaffer BM. The acrasina. Adv Morphog. 1962;2:109–182. [Google Scholar]
- 21.Umeda T, Inouye K. Possible role of contact following in the generation of coherent motion of Dictyostelium cells. J Theor Biol. 2002;219:301–308. doi: 10.1006/jtbi.2002.3124. [DOI] [PubMed] [Google Scholar]
- 22.Inouye K. 1977. Analysis of collective movements of slime mould cells. Master’s thesis (Kyoto University, Kyoto)
- 23.Dormann D, Weijer G, Parent CA, Devreotes PN, Weijer CJ. Visualizing PI3 kinase-mediated cell-cell signaling during Dictyostelium development. Curr Biol. 2002;12:1178–1188. doi: 10.1016/s0960-9822(02)00950-8. [DOI] [PubMed] [Google Scholar]
- 24.Vicente-Manzanares M, Newell-Litwa K, Bachir AI, Whitmore LA, Horwitz AR. Myosin IIA/IIB restrict adhesive and protrusive signaling to generate front-back polarity in migrating cells. J Cell Biol. 2011;193:381–396. doi: 10.1083/jcb.201012159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vicente-Manzanares M, Koach MA, Whitmore L, Lamers ML, Horwitz AF. Segregation and activation of myosin IIB creates a rear in migrating cells. J Cell Biol. 2008;183:543–554. doi: 10.1083/jcb.200806030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carmona-Fontaine C, et al. Contact inhibition of locomotion in vivo controls neural crest directional migration. Nature. 2008;456:957–961. doi: 10.1038/nature07441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Guo WH, Wang YL. Microtubules stabilize cell polarity by localizing rear signals. Proc Natl Acad Sci USA. 2014;111:16383–16388. doi: 10.1073/pnas.1410533111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takemura T, Kondo S, Homma T, Sakai M, Harris RC. The membrane-bound form of heparin-binding epidermal growth factor-like growth factor promotes survival of cultured renal epithelial cells. J Biol Chem. 1997;272:31036–31042. doi: 10.1074/jbc.272.49.31036. [DOI] [PubMed] [Google Scholar]
- 29.Das T, et al. A molecular mechanotransduction pathway regulates collective migration of epithelial cells. Nat Cell Biol. 2015;17:276–287. doi: 10.1038/ncb3115. [DOI] [PubMed] [Google Scholar]
- 30.Vedula SRK, Ravasio A, Lim CT, Ladoux B. Collective cell migration: A mechanistic perspective. Physiology (Bethesda) 2013;28:370–379. doi: 10.1152/physiol.00033.2013. [DOI] [PubMed] [Google Scholar]
- 31.Trepat X, et al. Physical forces during collective cell migration. Nat Phys. 2009;5:426–430. [Google Scholar]
- 32.Straight AF, et al. Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 33.Devenport D. The cell biology of planar cell polarity. J Cell Biol. 2014;207:171–179. doi: 10.1083/jcb.201408039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vladar EK, Antic D, Axelrod JD. Planar cell polarity signaling: The developing cell’s compass. Cold Spring Harb Perspect Biol. 2009;1:a002964. doi: 10.1101/cshperspect.a002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strutt H, Strutt D. Long-range coordination of planar polarity in Drosophila. BioEssays. 2005;27:1218–1227. doi: 10.1002/bies.20318. [DOI] [PubMed] [Google Scholar]
- 36.Fanto M, McNeill H. Planar polarity from flies to vertebrates. J Cell Sci. 2004;117:527–533. doi: 10.1242/jcs.00973. [DOI] [PubMed] [Google Scholar]
- 37.Shan J, Shi DL, Wang J, Zheng J. Identification of a specific inhibitor of the dishevelled PDZ domain. Biochemistry. 2005;44:15495–15503. doi: 10.1021/bi0512602. [DOI] [PubMed] [Google Scholar]
- 38.Grandy D, et al. Discovery and characterization of a small molecule inhibitor of the PDZ domain of Dishevelled. J Biol Chem. 2009;284:16256–16263. doi: 10.1074/jbc.M109.009647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang SS, Guo WH, Kim Y, Wang YL. Guidance of cell migration by substrate dimension. Biophys J. 2013;104:313–321. doi: 10.1016/j.bpj.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo WH, Wang YL. A three-component mechanism for fibroblast migration with a contractile cell body that couples a myosin II-independent propulsive anterior to a myosin II-dependent resistive tail. Mol Biol Cell. 2012;23:1657–1663. doi: 10.1091/mbc.E11-06-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Desai RA, Gopal SB, Chen S, Chen CS. Contact inhibition of locomotion probabilities drive solitary versus collective cell migration. J R Soc Interface. 2013;10:20130717. doi: 10.1098/rsif.2013.0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Becker SF, Mayor R, Kashef J. Cadherin-11 mediates contact inhibition of locomotion during Xenopus neural crest cell migration. PLoS One. 2013;8:e85717. doi: 10.1371/journal.pone.0085717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Batson J, Astin JW, Nobes CD. Regulation of contact inhibition of locomotion by Eph-ephrin signalling. J Microsc. 2013;251:232–241. doi: 10.1111/jmi.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stramer BM, Dunn GA, Davis JR, Mayor R. Rediscovering contact inhibition in the embryo. J Microsc. 2013;251:206–211. doi: 10.1111/jmi.12045. [DOI] [PubMed] [Google Scholar]
- 45.Reinhart-King CA, Dembo M, Hammer DA. Cell-cell mechanical communication through compliant substrates. Biophys J. 2008;95:6044–6051. doi: 10.1529/biophysj.107.127662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong S, Guo WH, Wang YL. Fibroblasts probe substrate rigidity with filopodia extensions before occupying an area. Proc Natl Acad Sci USA. 2014;111:17176–17181. doi: 10.1073/pnas.1412285111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barlan K, Cetera M, Horne-Badovinac S. Fat2 and Lar define a basally localized planar signaling system controlling collective cell migration. Dev Cell. 2017;40:467–477.e5. doi: 10.1016/j.devcel.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Findlay AS, et al. The core planar cell polarity gene, Vangl2, directs adult corneal epithelial cell alignment and migration. R Soc Open Sci. 2016;3:160658. doi: 10.1098/rsos.160658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arata M, Sugimura K, Uemura T. Difference in Dachsous levels between migrating cells coordinates the direction of collective cell migration. Dev Cell. 2017;42:479–497.e10. doi: 10.1016/j.devcel.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 51.Taylor J, Abramova N, Charlton J, Adler PN. Van Gogh: A new Drosophila tissue polarity gene. Genetics. 1998;150:199–210. doi: 10.1093/genetics/150.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vinson CR, Conover S, Adler PN. A Drosophila tissue polarity locus encodes a protein containing seven potential transmembrane domains. Nature. 1989;338:263–264. doi: 10.1038/338263a0. [DOI] [PubMed] [Google Scholar]
- 53.Parent CA, Blacklock BJ, Froehlich WM, Murphy DB, Devreotes PN. G protein signaling events are activated at the leading edge of chemotactic cells. Cell. 1998;95:81–91. doi: 10.1016/s0092-8674(00)81784-5. [DOI] [PubMed] [Google Scholar]
- 54.Miao Y, et al. Altering the threshold of an excitable signal transduction network changes cell migratory modes. Nat Cell Biol. 2017;19:329–340. doi: 10.1038/ncb3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Camley BA, et al. Polarity mechanisms such as contact inhibition of locomotion regulate persistent rotational motion of mammalian cells on micropatterns. Proc Natl Acad Sci USA. 2014;111:14770–14775. doi: 10.1073/pnas.1414498111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kulawiak DA, Camley BA, Rappel WJ. Modeling contact inhibition of locomotion of colliding cells migrating on micropatterned substrates. PLoS Comput Biol. 2016;12:e1005239. doi: 10.1371/journal.pcbi.1005239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.