Significance
Here, by real-time monitoring with single-molecule fluorescence microscopy the size-dependent catalytic process of individual Au clusters at single-turnover resolution, we study the size-dependent catalytic behaviors of gold (Au) clusters at the single-cluster level, and then observe the strong unique size effect on the catalytic properties of individual Au clusters, in both catalytic product formation and dissociation processes. Such a unique size effect on the nanocatalysis could be attributed intrinsically to the size-dependent electronic structure of Au clusters, leading to a more comprehensive understanding of the catalytic mechanism of Au particles.
Keywords: single-molecule nanocatalysis, gold clusters, single-molecule fluorescence microscopy, size dependence, quantum effect
Abstract
Atomically precise metal clusters have attracted increasing interest owing to their unique size-dependent properties; however, little has been known about the effect of size on the catalytic properties of metal clusters at the single-cluster level. Here, by real-time monitoring with single-molecule fluorescence microscopy the size-dependent catalytic process of individual Au clusters at single-turnover resolution, we study the size-dependent catalytic behaviors of gold (Au) clusters at the single-cluster level, and then observe the strong size effect on the catalytic properties of individual Au clusters, in both catalytic product formation and dissociation processes. Surprisingly, indicated by both experiments and density functional theory (DFT) calculations, due to such a unique size effect, besides observing the different product dissociation behaviors on different-sized Au clusters, we also observe that small Au clusters [i.e., Au15(MPA)13; here, MPA denotes 3-mercaptopropionic acid] catalyze the product formation through a competitive Langmuir–Hinshelwood mechanism, while those relatively larger Au clusters [e.g., Au18(MPA)14 and Au25(MPA)18] or nanoparticles catalyze the same process through a noncompetitive Langmuir–Hinshelwood mechanism. Such a size effect on the nanocatalysis could be attributed intrinsically to the size-dependent electronic structure of Au clusters. Further analysis of dynamic activity fluctuation of Au clusters reveals more different catalytic properties between Au clusters and traditional Au nanoparticles due to their different size-dependent structures.
Due to the limited resources of gold (Au) on earth, we need to minimize the usage of Au by extracting its highest possible catalytic activity on the Au atom basis (1–4). To achieve this, significant work has been done to examine size-dependent catalysis of Au nanoparticles (5–7). Compared with traditional ensemble methods, the single-molecule, single-particle method can give deeper insight by removing ensemble averaging, thus uncovering heterogeneous and dynamic behaviors of individual nanoparticles that are often hidden in ensemble-averaged measurements (8–13). For this reason, in recent years, the method of single-molecule nanocatalysis based on single-molecule fluorescence microscopy has been used extensively to investigate the size-dependent catalytic kinetics and dynamic behaviors of single metal nanoparticles (e.g., platinum, palladium, and gold) at the single-molecule, single-particle level (6, 14–17). Atomically precise Au clusters with well-defined size and structure could perfectly match with the single-molecule fluorescence microscopy technique to uncover several challenging fundamental issues in Au nanocatalysts. In particular, ultrasmall Au clusters often feature unique size effects (18) due to their size-dependent electronic structures (19, 20) and work functions (21), etc. The catalytic properties of Au clusters are highly dependent on (and sensitive to) their sizes due to the size-sensitive electronic structures (such as charge density profile), which are distinctly different from their larger counterparts, traditional Au nanoparticles with a large numbers of Au atoms. However, little is known about the size effect on catalytic properties of Au clusters at the single-molecule, single-particle level, although the synthesis of such atomically precise Au clusters with different sizes has been well developed in the cluster community (5, 22–24).
To study the size effect on catalytic properties of Au clusters with precise number of atoms (at atomic precision), here we chose three different sized thiolate-stabilized Au clusters—Au15(MPA)13, Au18(MPA)14, and Au25(MPA)18—as model catalysts (MPA denotes 3-mercaptopropionic acid). A gold-catalyzed fluorogenic reaction (i.e., reduction of nonfluorescent resazurin to fluorescent resorufin) was chosen as a probe to study the catalytic kinetics and dynamics of individual Au clusters of different sizes, leveraging on the powerful single-molecule fluorescence microscopy. Strong size-dependent catalytic behaviors of Au clusters were observed in both catalytic product formation and dissociation processes. The size-dependent catalytic activities and mechanisms could be attributed to the size-dependent adsorption behaviors of the substrate and product molecules on Au clusters, which are induced by the size-dependent electronic structures of Au clusters.
Results and Discussion
Catalytic Kinetics of Individual Clusters in Different Sizes.
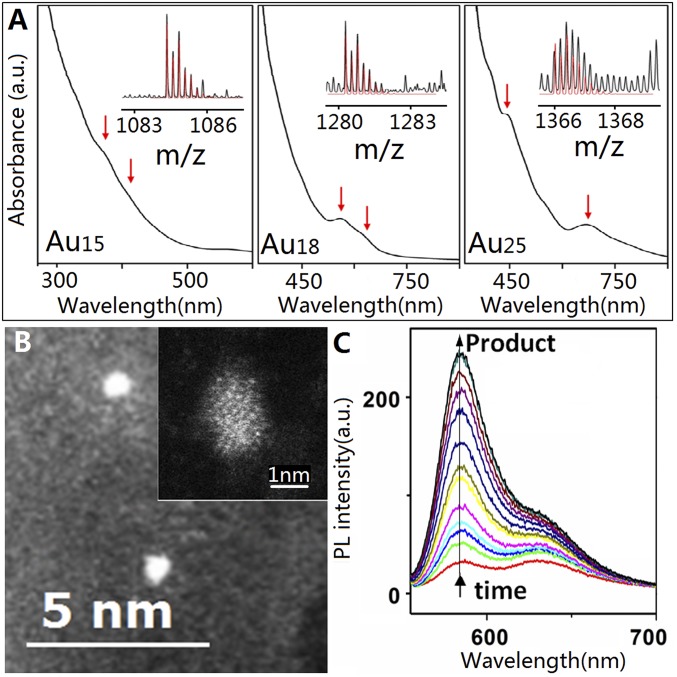
Water-soluble Au clusters protected by MPA with different sizes [e.g., Au15(MPA)13, Au18(MPA)14, and Au25(MPA)18] were synthesized according to a reported protocol (25). UV-Vis absorption and electrospray ionization (ESI) mass spectra (Fig. 1A and SI Appendix, Fig. S1) confirmed the successful synthesis of Au15(MPA)13, Au18(MPA)14, and Au25(MPA)18 with high purity (hereafter denoted as Au15, Au18, and Au25 clusters, respectively). TEM images (Fig. 1B) further support the ultrasmall size of Au clusters used in this study. Similar to the traditional Au nanoparticles (6, 14), the as-prepared Au clusters can effectively catalyze the reduction of nonfluorescent resazurin to fluorescent resorufin by the reductant hydroxylamine (NH2OH), as shown in Fig. 1C (14). Control experiments further indicate that only the gold atoms rather than the ligand MPA are the active components for the catalysis. The remarkable stability of ligand MPA on Au clusters or the Au clusters on slide during the catalysis was also confirmed by the X-ray photoelectron spectroscopy (XPS) and size analysis before and after the long-term (6 h) ensemble catalytic process (SI Appendix, Fig. S2).
Fig. 1.
Characterization of Au clusters. (A) UV-Vis absorption spectra and ESI-MS spectra (black lines in Insets) of the as-synthesized Au15 (Left), Au18 (Middle), and Au25 (Right) clusters; the red arrows indicate characteristic absorption peaks of the corresponding Au nanoclusters. The red lines in the Insets are the simulated isotope patterns of [Au15(MPA)13 − 5 H + Na]4−, [Au18(MPA)14 − 9 H + 5 Na]4−, and [Au25(MPA)18 − 7 H + Na]5−. (B) Typical TEM image of Au25 clusters. (Inset) Typical high-angle annular dark-field imaging–TEM image of one Au25 cluster. (C) Fluorescence spectra of resazurin reduction by NH2OH catalyzed by Au25 clusters (λex = 532 nm; [resazurin] = 10 µM; [NH2OH] = 20 mM). The arrow indicates fluorescence increase of the product with time.
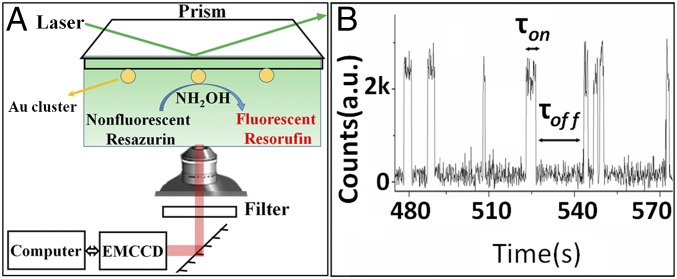
In a typical single-molecule experiment setup (Fig. 2A and SI Appendix, Scheme S1), the as-prepared Au clusters were first dispersed sparsely (SI Appendix, Fig. S3) on a quartz slide surface, followed by an overnight flowthrough of pure water to remove any extra MPA from the cluster surface before flow in the reactant solution. By detecting the fluorescence of individual resorufin molecule formed locally on the surface of individual Au clusters (SI Appendix, Fig. S4), we can monitor the catalytic behaviors of individual Au clusters at single-turnover resolution under ambient reaction conditions. Fig. 2B shows a typical fluorescence trajectory during the catalytic reaction, reflecting the product formation process (τoff) and dissociation process (τon) on an individual Au cluster. The digital nature of the stochastic off–on fluorescence bursts and the consistent height of the on level are characteristics of single-molecule fluorescence detection. τoff is the time before each burst, and it is the time for the formation of each fluorescent product on an individual Au cluster. τon is the time required for the dissociation of each fluorescent product from the Au cluster surface after its formation. Each off–on cycle corresponds to a single turnover of catalytic process. According to a reported study (14), 〈τoff〉−1 and 〈τon〉−1 are the time-averaged product formation rate and dissociation rate of a single particle, respectively (〈〉 denotes averaging) (26, 27). Therefore, the fluorescence turnover trajectories, such as the one shown in Fig. 2B, would allow us to study the catalytic kinetics and dynamics of an individual Au cluster. It should be noted that, under the laser intensity and the flow rate of solution used in the present study (14, 28), photobleaching or blinking of the fluorescent product resorufin is insignificant, compared with its short residence time on a particle. A continuous observation of such digital signals from individual clusters in a long-time window (>5 h) indicates a strong binding of individual clusters on slide. Further control experiments indicate that the strong binding or interaction between the slide and clusters only leads to negligible effect on the catalytic activity of clusters (SI Appendix).
Fig. 2.
Single-turnover detection of single Au cluster catalysis. (A) Experimental design using total internal reflection fluorescence microscopy for single-molecule, single-particle measurement. (B) A typical fluorescence turnover trajectory of a single Au25 cluster with 100-ms imaging rate. a.u., arbitrary units.
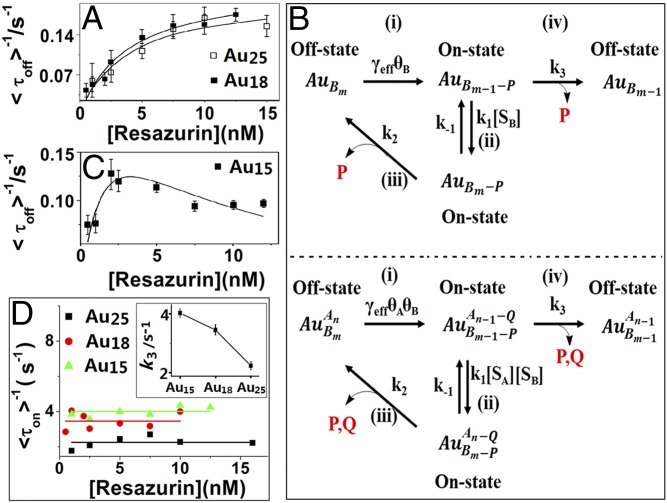
To study the size-dependent catalysis of an individual Au cluster, by simply fixing the concentration of reductant NH2OH (denoted as A), we obtained the average product formation rates and dissociation rates at different resazurin (denoted as B) concentrations. Interestingly, as shown in Fig. 3A, the product formation rates on both Au18 and Au25 clusters show saturated behavior with the concentration increase of resazurin, indicating a noncompetitive Langmuir−Hinshelwood mechanism (Fig. 3B, Top). This mechanism is described quantitatively by the following equations (6, 14, 29, 30):
Fig. 3.
Kinetic study of different-sized Au clusters. (A and C) Resazurin concentration titrations of <τoff>−1 on Au25 (A), Au18 (A), and Au15 (C) with 20 mM NH2OH. Each data point is averaged over the turnover trajectories of >50 clusters, with SEM as the error bar. The solid lines are fittings with Eqs. 1 (A) and 3 (C), respectively, with parameters summarized in SI Appendix, Table S1. (B, Top) Noncompetitive Langmuir−Hinshelwood mechanism of catalysis on a single nanoparticle. (B, Bottom) Competitive Langmuir−Hinshelwood mechanism of catalysis on a single nanoparticle. [A], the substrate NH2OH concentration; [B], the substrate resazurin concentration; Au, Au nanoparticle; P, the product resorufin; Q, the product from NH2OH. γeff represents the combined reactivity of all surface catalytic sites of a nanoparticle. k1, k−1, k2, and k3 are the rate constants at each steps. θ is the fraction of catalytic sites that are occupied by substrates. (D) Resazurin concentration dependence of <τon>−1 of Au15, Au18, and Au25. The solid lines are the fittings with constants. Inset is the size dependence of k3.
Product formation rate:
| [1] |
and the product dissociation rate:
| [2] |
where [SB] is the resazurin concentration; γeff is the rate constant representing the intrinsic reactivity per cluster for the catalytic conversion reaction; αB is the adsorption equilibrium constant of resazurin; k2 and k3 are the rate constants for the indirect and direct dissociation of the product, respectively; G1 = k1/(k−1 + k2).
With the size decrease of Au clusters, unexpectedly, the product formation rate on Au clusters with only 15 Au atoms (Au15) first increases with the substrate concentration (Fig. 3C), and then decreases after a maximum point. Similar behavior was also observed from the dependence of product formation rate on the reductant (NH2OH) concentration as shown in SI Appendix, Fig. S5. As the decrease of product formation rate at high reactant concentration is not due to the gradual deactivation of Au clusters during the catalysis (SI Appendix, Fig. S6), all of these results suggest unambiguously a two-site competitive Langmuir−Hinshelwood mechanism (Fig. 3B, Bottom) between the two reactants, as described quantitatively by the following equations (29, 30):
Product formation rate:
| [3] |
and the product dissociation rate:
| [4] |
where [SA] is the NH2OH concentration; αA and αB is the adsorption equilibrium constant of NH2OH and resazurin, respectively; G = []k1/(k−1 + k2).
However, the obtained dissociation rate on clusters is independent of the substrate concentration (Fig. 3D), which is different from the observed substrate concentration-dependent product dissociation behavior on traditional Au nanoparticles (6). Such independence of substrate concentration observed here on Au clusters could be for the following two reasons (14): (i) k2 = k3; and (ii) k1 = 0. Due to the limited surface atoms or active sites on individual Au cluster for the substrate and/or product adsorption, after the adsorption of substrate molecules on the surface of an individual Au cluster reaches an equilibrium state, the binding of more substrate molecules would be hindered because of the steric hindrance effect without the release of substrate or product molecules, which means k1 = 0 or the product molecules formed on the Au cluster surface could only be dissociated directly without the effect of substrate molecules. Taken together, the right side of Eqs. 2 and 4 can be simplified as k3, which is the rate constant of direct dissociation of the product.
By fitting the experimental data of the product formation rates and dissociation rates using the above equations and k1 = 0 (Fig. 3 A, C, and D), the corresponding kinetic parameters for the product formation process (γeff, αA, and αB) and dissociation process (k3) on different-sized Au clusters were obtained, and these data are included in SI Appendix, Table S1. Interestingly, these parameters clearly suggest a strong size-dependent relationship. In particular, γeff increases with the size decrease of Au clusters, which is consistent with an ensemble-level observation on the reported polyvinyl pyrrolidone-stabilized Au clusters (31); however, this result is contrary to a previous observation on individual Au nanoparticle (6). In addition, both the adsorption equilibrium constant (αB) of the dye molecules and the direct product dissociation rate constant (k3; Fig. 3D, Inset) decrease with the size increase of Au clusters, which is also contrary to a previous observation on Au nanoparticles (6). The decrease trend with the size increase indicates a strong adsorption of reactant resazurin and a weak adsorption of product resorufin molecules on small Au clusters.
To provide more insights on the size-dependent catalytic activity of Au clusters, the diameters (d in nanometers) of ligand-protected Au clusters (with size smaller than 2 nm) were calculated according to a reported equation: n = 59 atoms/nm3 × 4/3 × π × (d/2)3 nm3 (32), where N is the number of metal atoms per cluster (SI Appendix). As shown in SI Appendix, Table S1, the effective product formation rate constant per cluster (γeff) increases with the size decrease of Au clusters, which is contrary to the observation from Au nanoparticles (6). In addition, the intrinsic catalytic activity per surface area or active site [γeff/S, S = 4π × (d/2)2, the surface area of a single cluster; SI Appendix, Table S1] also increases with the size decrease of Au clusters, which agrees well with the observation from Au nanoparticles (6). For the Au clusters used in this study, the higher catalytic reactivity of smaller clusters is accompanied with a stronger substrate adsorption to (larger αB) and a faster product desorption from (larger k3) the catalytic sites of Au clusters (33). However, the apparently different size dependence of γeff between Au clusters and nanoparticles is probably for the following reason: γeff ∝ nT∙(γeff/S), where nT is the total number of active sites on a Au cluster or nanoparticle surface. For a nanoparticle with large number of surface atoms, the 2- to 3-nm decrease of size only leads to a small increase of γeff/S but results in a significant decrease of the number of surface active sites (nT); therefore, the result is the decrease of γeff or nT∙(γeff/S) with the size decrease of nanoparticles. While for the Au clusters with size <2 nm (SI Appendix, Table S1), the value of γeff/S is very sensitive to the size (d) of clusters. For example, with a tiny decrease of size, such as from Au18 to Au15, the value of γeff/S remarkably increases, while the value of nT only decreases slightly, therefore leading to the increase of γeff or nT∙(γeff/S) with the size decrease of clusters.
The above results show that the catalytic properties of clusters vary with size apparently. Further control experiments based on another type of Au clusters [Au25(MHA)18; MHA, 6-mercaptohexanoic acid; SI Appendix, Fig. S7 and Table S1] indicate that the observed difference of catalytic properties among clusters is not due to the ligand effect, instead, according to previous work (18, 19), such a difference could be attributed to the unique size or quantum electronic effect on the catalysis of Au clusters. However, the observed difference between small Au clusters and large nanoparticles could be attributed in part to their structural difference due to the fact that the atomic arrangements of small Au clusters are different from those of large nanoparticles.
Density Functional Theory Calculation for Cluster Size-Dependent Adsorption of Substrate and Product.
Furthermore, to figure out why Au clusters with different sizes could catalyze the product formation process in different pathways, or why the reductant NH2OH could affect the adsorption of resazurin on the surface sites of small clusters, we carried out density functional theory (DFT) (SI Appendix) calculation to understand the adsorption of reactants and products on different-sized Au clusters. The data summarized in SI Appendix, Table S2 (first column), suggest that the intrinsic binding affinity of reactant resazurin is stronger on smaller-sized Au clusters. Such predicted size-dependent absorption of the reactant resazurin on Au clusters is consistent with the size-dependent adsorption equilibrium constant of the reactant (αB) obtained experimentally from the single-molecule nanocatalysis, as shown in SI Appendix, Table S1. To further understand why the product formation on relatively larger clusters of Au18 and Au25 follows a noncompetitive Langmuir−Hinshelwood mechanism (Fig. 3A), while the catalysis of product formation on small Au15 clusters follows a competitive Langmuir−Hinshelwood mechanism, we studied the effect of reductant NH2OH on the adsorption of reactant resazurin (SI Appendix). Surprisingly, the results (SI Appendix, Table S2) indicate that the presence of NH2OH can weaken the adsorption of resazurin on small Au15 clusters, confirming the observed competitive mechanism on Au15 cluster (Fig. 3C). In contrast, NH2OH shows negligible effect on the adsorption of resazurin on Au18 and Au25 clusters, confirming the observed noncompetitive mechanism (Fig. 3A). The distinct difference of the catalytic mechanism between Au15 and Au18 clusters is probably due to the huge difference between their surface structures. As for the adsorption energy of product resorufin on Au clusters with different numbers of NH2OH molecules around, interestingly, as shown in SI Appendix, Table S2, the adsorption energy of product decreases with the size decrease of Au clusters, which is consistent with the size-dependent k3 shown in SI Appendix, Table S1. Such consistence indicates that the direct dissociation step of product resorufin can be affected by the reductant rather than the substrate resazurin, probably due to the unique surface structure of Au clusters.
Quantum Size Effect on Catalysis of Individual Clusters.
According to a previous study (34), the specific catalytic activity of Au clusters could be attributed to the well-known quantum size effect. Wood derived an expression for the variation of metal work function as a function of the size (d in nanometers) of metal (35, 36):
| [5a] |
where is the bulk metal work function (5.10 eV for Au). Based on this, the variation in Fermi level energy () of metals with the size of metal particles could be expressed as follows (35, 36):
| [5b] |
The energy spacing or gap () between adjacent levels or electronic states could then be expressed by the following equation (37):
| [6a] |
where N [=59 atoms/nm3 × 4/3 × π × (d/2)3 nm] is the number of metal atoms per ligand-protected Au cluster (with size smaller than 2 nm) (32). Substituting in Eq. 6a with Eq. 5b and considering as positive, the relationship between and d can be expressed as follows:
| [6b] |
Accordingly, the δ values of Au clusters with different sizes were obtained, as shown in SI Appendix, Table S1. It shows that the energy gap of Au clusters decreases with the size increase of Au clusters.
Recently, Häkkinen and coworkers (38) observed a positive linear correlation between the energy gap (δ) and the adsorption energy or binding ability of O2 on Au clusters. Based on it, we hold the opinion that the adsorption equilibrium constant (α) of reactants on Au clusters could probably be expressed as follows:
| [7] |
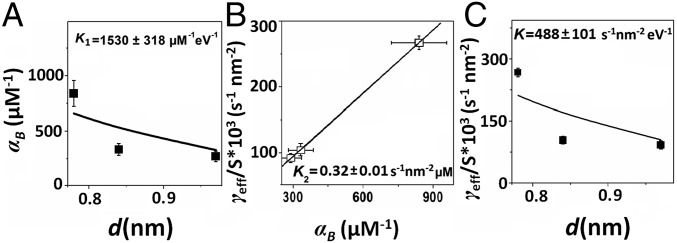
K1 is the proportional factor between α and δ. It indicates that the adsorption or binding ability of O2 on clusters decreases with the increasing of the cluster size (d), which is consistent with the experimental observation of resazurin adsorption (αB; SI Appendix, Table S1) on Au clusters, but contrary to the observed resazurin adsorption on Au nanoparticles (SI Appendix, Table S1) (6). Such differences between Au clusters and nanoparticles could be mainly attributed to the unique size effect on catalysis of clusters (38). As for the resazurin adsorption (αB) on Au clusters (SI Appendix, Table S1), by fitting the d-dependent αB with Eq. 7 (Fig. 4A), the value of K1 was obtained to be 1,530 ± 318 μM−1⋅eV−1.
Fig. 4.
Size-dependent catalytic kinetics of Au clusters. (A) Size dependence of the adsorption equilibrium constant of resazurin (αB), where the solid line is the fittings according to Eq. 7. (B) The correlation analysis between the effective rate constant per surface area (γeff/S) and the adsorption equilibrium constant of resazurin (αB), where the solid line is the linear fitting. (C) Size dependence of the effective rate constant per surface area (γeff/S), where the solid line is the fitting according to Eq. 8.
However, it was further found that the correlation shown in Eq. 7 does work for some adsorbates (such as O2 and resazurin) but not for all on Au clusters. Actually, it has been reported that the CO adsorption ability increases monotonically with Au cluster size due to the more favorable LUMO (M)–σ(CO) interaction for larger clusters (39). Interestingly, such CO adsorption on Au clusters is consistent with the observed adsorption of product resorufin on Au clusters indicated by the dissociation rate (large k3) of resorufin from Au clusters (SI Appendix, Table S1). While the difference of adsorption properties observed between substrate resazurin and product resorufin on the same set of Au clusters probably could be attributed to the different electronic interactions between clusters and these two types of molecules, while such different electronic interactions could be further attributed to the structural/compositional differences between substrate resazurin and product resorufin.
Moreover, as SI Appendix, Table S1 shows, the catalytic activity and the adsorptions of both substrate and product molecules correlate with the work function (Wd) (obtained from Eq. 5a) of clusters in the same manners as that with the energy gap (δ) due to the similar size (d) dependences of Wd (Eq. 5a) and δ (Eq. 6b).
Moreover, for the Au clusters used in this study, unexpectedly, Fig. 4B shows a linear correlation between the catalytic reactivity (γeff/S, the catalytic rate constant per surface area) and substrates binding (αB) to the Au clusters, with a slope of K2 = 0.32 ± 0.01 s−1·nm−2·μM, the proportional factor between α and γeff/S. Thus, the relationship between γeff/S and δ or d can be expressed as follows:
| [8] |
where K = K2∙ K1. By fitting the d-dependent γeff/S with Eq. 8 (Fig. 4C), we obtained the proportionality constant K (488.3 ± 101.4 s−1·nm−2·eV−1), and then K2 = K/K1 = 0.32 s−1·nm−2·μM, which is exactly the same as that obtained from Fig. 4B (K2 = 0.32 ± 0.01 s−1·nm−2·μM). These data confirm the reliability of the linear relationship between the energy gap and adsorption energy (38), and the linear relationship between the catalytic rate constant and binding ability of substrate to Au clusters (Fig. 4B). Both of these two linear relationships observed on Au clusters are due to the unique size effect on the catalysis of Au clusters (37).
As for the higher catalytic activity of smaller Au clusters (shown in SI Appendix, Table S1), besides the contribution from the quantum size effect as discussed above, a ligand effect was also observed. The Au 4f7/2XPS spectra (SI Appendix, Fig. S8A) on different clusters show clear positive shift of binding energy with the size decrease. Due to the positive correlation between the binding energies and sulfur (S) contents (Au25 with S 41 wt%, Au18 with S 43 wt%, and Au15 with S 46 wt%) on different clusters (SI Appendix, Fig. S8B), the positive shift of binding energy with the size decrease could be attributed to the stronger interaction between Au and sulfur in the form of Au–S bonding. It has been known that the higher binding energy of metal nanocatalysts usually corresponds to higher catalytic activity (40); therefore, the higher catalytic activity of smaller Au clusters observed in this study could be partially attributed to the ligands effect (41, 42).
Size-Dependent Catalytic Dynamics of Individual Clusters.
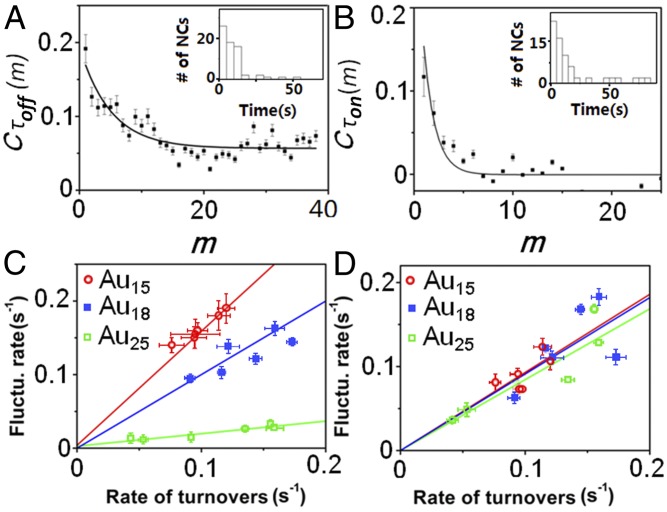
Similar to previous nanoparticle studies (6, 14), to study the catalytic dynamics of individual Au clusters, we further determined the activity fluctuations of individual Au clusters. The activity fluctuations could be reflected by the reaction rate variations in the τoff reaction (the catalytic product formation), the τon reaction (the product dissociation), or both. To study the activity fluctuations of τoff and τon processes, we extracted the sequences of individual τoff and τon from each turnover trajectory, and then calculated their autocorrelation functions, (43, 44). Here, τ is either τoff or τon, m is the turnover index number in the sequence, and . With activity fluctuations, the values of Cτ(m) are positive and show a decay behavior with the decay time constant being the fluctuation correlation time. Fig. 5 A and B show exemplary Cτoff(m) and Cτon(m) of a single Au15 cluster. The exponential decay behaviors of Cτoff(m) and Cτon(m) directly demonstrate the activity fluctuations in the catalytic product formation and dissociation reaction, respectively. For the single cluster shown in Fig. 5 A and B, the exponential decay constant of Cτoff(m) is moff = 5.0 ± 0.4 turnovers, and that of Cτon(m) is mon = 1.2 ± 0.2 turnovers. With an average turnover time of ∼11.2 s for this particular cluster, the activity fluctuation correlation time for its τoff and τon reactions is ∼56 and ∼13 s, respectively. These two correlation times are the timescales of the catalysis-induced dynamic conformation restructuring of the clusters (45, 46). The activity fluctuation of individual clusters could be attributed to small-scale dynamic conformation restructuring, similar to that of nanoparticles (6, 14). While for Au clusters with small number of atoms here, changing adsorbate–surface interactions during catalysis can induce a dynamic conformation reconstruction, resulting in oscillatory kinetics due to the different activities of different surface structures. Moreover, compared with the distribution of fluctuation correlation time of Au nanoparticles (the width of the time distribution is about 400 s) (6, 14), the distributions (Fig. 5 A and B, Insets) of the fluctuation correlation time of Au clusters are much narrower (the width of the time distribution is about 170 s), indicating that individual clusters have more consistent restructuring timescales, probably due to their much narrower size distribution compared with Au nanoparticles.
Fig. 5.
(A and B) Autocorrelation function of the τoff (A) and τon (B) from the turnover trajectory of a single Au15 cluster at 10 nM resazurin. The solid lines are the exponential fits with decay constants of moff = 5.0 ± 0.4 turnovers and mon = 1.2 ± 0.2 turnovers. (Inset) Histograms of the fluctuation correlation times for τoff and τon reactions at 10 nM resazurin. (C and D) Dependence of the activity fluctuation rate (the inverse of fluctuation correlation time) of the τoff reaction (C) and the τon reaction (D) on the rate of turnovers. Each data point is an average from >50 trajectories. Error bars are SEM. The solid lines are linear fits.
Furthermore, the catalysis-induced activity fluctuations or surface restructuring were also observed from the positive correlations between the activity fluctuation rates (i.e., the inverse of fluctuation correlation times) and the turnover rates (Fig. 5 C and D). It shows that the activity fluctuation rates of both τoff and τon reactions increase with the increase of turnover rates, following a linear correlation approximately. Interestingly, the slope of such linear correlation increases with the size decrease of Au clusters for both τoff and τon reactions. Such size dependence indicates that the catalysis of smaller Au clusters can induce a faster dynamic surface restructuring, which is consistent with the observation on Au nanoparticles (6). Moreover, Fig. 5 C and D further suggest that the slope of the τoff reaction (Fig. 5C) increases faster with the size decrease than that of the τon reaction (Fig. 5D), indicating that τoff reaction-induced activity fluctuations or surface restructuring is more sensitive to the size of Au clusters than τon reaction-induced activity fluctuations or surface restructuring, which is contrary to the observation on Au nanoparticles (6). Furthermore, as shown in Fig. 5 C and D, the activity fluctuation rates were extrapolated linearly to zero rate of turnovers for all three Au clusters. The positive intercepts approximate the rates of spontaneous (compared with the catalysis-induced restructuring) dynamic surface restructuring of individual Au clusters in an aqueous environment, but the small values (approximately zero) of these intercepts indicate that the spontaneous dynamic surface restructuring of Au clusters could be neglected, compared with the catalysis-induced restructuring (Fig. 5 C and D). Such observation indicates that the surface of Au clusters is more stable than the surface of Au nanoparticles in aqueous environment since the remarkable spontaneous surface restructuring has been observed on Au nanoparticles (6). Such differences observed above between Au clusters and nanoparticles could be partially attributed to the strong stabilization of MPA ligands to the surface Au atoms.
It should be noted that the size-dependent catalytic properties of Au clusters revealed here intrinsically could be attributed to their size-dependent structures, such as size-sensitive electronic structure (charge density profile). The tiny fluctuation of the electronic structure induced by the size variation of clusters can hugely affect the interaction between the clusters and the substrate or product molecules.
Conclusions
The size-dependent catalytic activity of Au clusters under ambient solution conditions was studied at the single-molecule, single-cluster level. Atomically precise Au clusters with different sizes show distinct catalytic kinetics and mechanisms with different substrate and product adsorption ability. In particular, the smaller Au15 cluster follows a competitive Langmuir−Hinshelwood mechanism with stronger substrate binding ability and weaker product binding ability, while the larger ones, Au18 and Au25 clusters, follow a noncompetitive Langmuir−Hinshelwood mechanism with weaker substrate binding ability and stronger product binding ability. DFT calculation suggests that such size-dependent catalytic activities and catalytic mechanisms could be attributed to the different adsorption behaviors of substrate and product molecules on clusters with different sizes, which are intrinsically induced by the size-dependent electronic structures of the Au clusters. The analysis of dynamic activity fluctuations and surface reconstruction of the Au clusters revealed more different catalytic properties between Au clusters and traditional Au nanoparticles due to the unique size-dependent properties of clusters. The knowledge obtained here at the single-cluster level provides fundamental insights into the catalytic behaviors of cluster catalysts, which are complementary to, and often inaccessible in ensemble-averaged measurements.
Experimental Procedures
The MPA-protected Au clusters were synthesized according to a reported protocol (25). All single-molecule nanocatalysis was carried out in home-built flow cells, which were formed by double-sided tapes sandwiched between a quartz slide (Technical Glass) and a coverslip (Gold Seal). The solution was flowed into the cell with a flow rate of 10 μL·min−1. A continuous-wave circularly polarized 532-nm laser was focused onto an area of about 90 × 50 μm2 on the sample to directly excite the fluorescence of the product resorufin. The fluorescence of resorufin was collected by a water-immersion objective, filtered by two filters and projected onto an EMCCD camera (Andor Technology), which is controlled by Andor IQ software.
Supplementary Material
Acknowledgments
This work was funded by the National Natural Science Foundation of China Grants U1601211, 21633008, 21733004, 21721003, and 21433003; K. C. Wong Education Foundation and Science and Technology Innovation Foundation of Jilin Province for Talents Cultivation Grants 20160519005JH and L20142200005; and the Jilin Youth Foundation Grants 20160520137JH.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1805711115/-/DCSupplemental.
References
- 1.Haruta M, Kobayashi T, Sano H, Yamada N. Novel gold catalysts for the oxidation of carbon-monoxide at a temperature far below 0 °C. Chem Lett. 1987;2:405–408. [Google Scholar]
- 2.Haruta M, Yamada N, Kobayashi T, Iijima S. Gold catalysts prepared by coprecipitation for low-temperature oxidation of hydrogen and of carbon-monoxide. J Catal. 1989;115:301–309. [Google Scholar]
- 3.Somorjai GA. The development of molecular surface science and the surface science of catalysis: The Berkeley contribution. J Phys Chem B. 2000;104:2969–2979. [Google Scholar]
- 4.Bergeret G, Gallezot P, Ertl G, Knözinger H, Weitkamp J. Handbook of Heterogeneous Catalysis. VCH; Weinheim, Germany: 1997. [Google Scholar]
- 5.Tong X, et al. Intact size-selected Au(n) clusters on a TiO2(110)-(1 × 1) surface at room temperature. J Am Chem Soc. 2005;127:13516–13518. doi: 10.1021/ja052778w. [DOI] [PubMed] [Google Scholar]
- 6.Zhou X, Xu W, Liu G, Panda D, Chen P. Size-dependent catalytic activity and dynamics of gold nanoparticles at the single-molecule level. J Am Chem Soc. 2010;132:138–146. doi: 10.1021/ja904307n. [DOI] [PubMed] [Google Scholar]
- 7.Fenger R, Fertitta E, Kirmse H, Thünemann AF, Rademann K. Size dependent catalysis with CTAB-stabilized gold nanoparticles. Phys Chem Chem Phys. 2012;14:9343–9349. doi: 10.1039/c2cp40792b. [DOI] [PubMed] [Google Scholar]
- 8.Chen P, et al. Spatiotemporal catalytic dynamics within single nanocatalysts revealed by single-molecule microscopy. Chem Soc Rev. 2014;43:1107–1117. doi: 10.1039/c3cs60215j. [DOI] [PubMed] [Google Scholar]
- 9.Chen P, et al. Single-molecule fluorescence imaging of nanocatalytic processes. Chem Soc Rev. 2010;39:4560–4570. doi: 10.1039/b909052p. [DOI] [PubMed] [Google Scholar]
- 10.Orrit M, Ha T, Sandoghdar V. Single-molecule optical spectroscopy. Chem Soc Rev. 2014;43:973–976. doi: 10.1039/c4cs90001d. [DOI] [PubMed] [Google Scholar]
- 11.Janssen KPF, et al. Single molecule methods for the study of catalysis: From enzymes to heterogeneous catalysts. Chem Soc Rev. 2014;43:990–1006. doi: 10.1039/c3cs60245a. [DOI] [PubMed] [Google Scholar]
- 12.De Cremer G, Sels BF, De Vos DE, Hofkens J, Roeffaers MBJ. Fluorescence micro(spectro)scopy as a tool to study catalytic materials in action. Chem Soc Rev. 2010;39:4703–4717. doi: 10.1039/c0cs00047g. [DOI] [PubMed] [Google Scholar]
- 13.Chen T, Zhang Y, Xu W. Size-dependent catalytic kinetics and dynamics of Pd nanocubes: A single-particle study. Phys Chem Chem Phys. 2016;18:22494–22502. doi: 10.1039/c6cp02719a. [DOI] [PubMed] [Google Scholar]
- 14.Xu W, Kong JS, Yeh Y-TE, Chen P. Single-molecule nanocatalysis reveals heterogeneous reaction pathways and catalytic dynamics. Nat Mater. 2008;7:992–996. doi: 10.1038/nmat2319. [DOI] [PubMed] [Google Scholar]
- 15.Chen P, Xu W, Zhou X, Panda D, Kalininskiy A. Single-nanoparticle catalysis at single-turnover resolution. Chem Phys Lett. 2009;470:151–157. [Google Scholar]
- 16.Tachikawa T, Yamashita S, Majima T. Evidence for crystal-face-dependent TiO2 photocatalysis from single-molecule imaging and kinetic analysis. J Am Chem Soc. 2011;133:7197–7204. doi: 10.1021/ja201415j. [DOI] [PubMed] [Google Scholar]
- 17.Wang N, Tachikawa T, Majima T. Single-molecule, single-particle observation of size-dependent photocatalytic activity in Au/TiO2 nanocomposites. Chem Sci. 2011;2:891–900. [Google Scholar]
- 18.Tyo EC, Vajda S. Catalysis by clusters with precise numbers of atoms. Nat Nanotechnol. 2015;10:577–588. doi: 10.1038/nnano.2015.140. [DOI] [PubMed] [Google Scholar]
- 19.Kappes MM, Radi P, Schar M, Schumacher E. Probes for electronic and geometrical shell structure effects in alkali-metal clusters. Photoionization measurements on KxLi, KxMg and KxZn (x < 25) Chem Phys Lett. 1985;119:11–16. [Google Scholar]
- 20.Kappes MM, Kunz RW, Schumacher E. Production of large sodium clusters (Nax, x < 65) by seeded beam expansions. Chem Phys Lett. 1982;91:413–418. [Google Scholar]
- 21.Bergeron DE, Castleman AW, Jr, Morisato T, Khanna SN. formation of Al13I−: Evidence for the superhalogen character of Al13. Science. 2004;304:84–87. doi: 10.1126/science.1093902. [DOI] [PubMed] [Google Scholar]
- 22.Böhme DK, Schwarz H. Gas-phase catalysis by atomic and cluster metal ions: The ultimate single-site catalysts. Angew Chem Int Ed Engl. 2005;44:2336–2354. doi: 10.1002/anie.200461698. [DOI] [PubMed] [Google Scholar]
- 23.Yu Y, et al. Identification of a highly luminescent Au22(SG)18 nanocluster. J Am Chem Soc. 2014;136:1246–1249. doi: 10.1021/ja411643u. [DOI] [PubMed] [Google Scholar]
- 24.Ye R, Zhukhovitskiy AV, Deraedt CV, Toste FD, Somorjai GA. Supported dendrimer-encapsulated metal clusters: Toward heterogenizing homogeneous catalysts. Acc Chem Res. 2017;50:1894–1901. doi: 10.1021/acs.accounts.7b00232. [DOI] [PubMed] [Google Scholar]
- 25.Yu Y, et al. Scalable and precise synthesis of thiolated Au10-12, Au15, Au18, and Au25 nanoclusters via pH controlled CO reduction. Chem Mater. 2013;25:946–952. [Google Scholar]
- 26.Xu WL, Kong JS, Chen P. Single-molecule kinetic theory of heterogeneous and enzyme catalysis. J Phys Chem C. 2009;113:2393–2404. [Google Scholar]
- 27.Xu WL, Shen H, Liu GK, Chen P. Single-molecule kinetics of nanoparticle catalysis. Nano Res. 2009;2:911–922. [Google Scholar]
- 28.Chen T, Zhang Y, Xu W. Single-molecule nanocatalysis reveals catalytic activation energy of single nanocatalysts. J Am Chem Soc. 2016;138:12414–12421. doi: 10.1021/jacs.6b05600. [DOI] [PubMed] [Google Scholar]
- 29.Satterfield CN. Heterogeneous Catalysis in Practice. McGraw-Hill; New York: 1980. [Google Scholar]
- 30.Han KS, Liu G, Zhou X, Medina RE, Chen P. How does a single Pt nanocatalyst behave in two different reactions? A single-molecule study. Nano Lett. 2012;12:1253–1259. doi: 10.1021/nl203677b. [DOI] [PubMed] [Google Scholar]
- 31.Tsunoyama H, et al. Size-controlled synthesis of gold clusters as efficient catalysts for aerobic oxidation. Catal Surv Asia. 2011;15:230–239. [Google Scholar]
- 32.Jin R. Quantum sized, thiolate-protected gold nanoclusters. Nanoscale. 2010;2:343–362. doi: 10.1039/b9nr00160c. [DOI] [PubMed] [Google Scholar]
- 33.Strizhak PE. Nanosize effects in heterogeneous catalysis. Theor Exp Chem. 2013;49:2–21. [Google Scholar]
- 34.Valden M, Lai X, Goodman DW. Onset of catalytic activity of gold clusters on titania with the appearance of nonmetallic properties. Science. 1998;281:1647–1650. doi: 10.1126/science.281.5383.1647. [DOI] [PubMed] [Google Scholar]
- 35.Wood DM. Classical size dependence of the work function of small metallic spheres. Phys Rev Lett. 1981;46:749. [Google Scholar]
- 36.Phala NS, Steen Ev. Intrinsic reactivity of gold nanoparticles: Classical, semi-empirical and DFT studies. Gold Bull. 2007;40:150–153. [Google Scholar]
- 37.Kubo R. Electronic properties of metallic fine particles. I. J Phys Soc Jpn. 1962;17:975–986. [Google Scholar]
- 38.Lopez-Acevedo O, Kacprzak KA, Akola J, Häkkinen H. Quantum size effects in ambient CO oxidation catalysed by ligand-protected gold clusters. Nat Chem. 2010;2:329–334. doi: 10.1038/nchem.589. [DOI] [PubMed] [Google Scholar]
- 39.Lee TH, Ervin KMJ. Reactions of copper group cluster anions with oxygen and carbon monoxide. J Phys Chem. 1994;98:10023–10031. [Google Scholar]
- 40.Ruan M, Sun X, Zhang Y, Xu W. Regeneration and enhanced catalytic activity of Pt/C electrocatalysts. ACS Catal. 2015;5:233–240. [Google Scholar]
- 41.Tsunoyama H, Ichikuni N, Sakurai H, Tsukuda T. Effect of electronic structures of Au clusters stabilized by poly(N-vinyl-2-pyrrolidone) on aerobic oxidation catalysis. J Am Chem Soc. 2009;131:7086–7093. doi: 10.1021/ja810045y. [DOI] [PubMed] [Google Scholar]
- 42.Chaki NK, Tsunoyama H, Negishi Y, Sakurai H, Tsukuda T. Effect of Ag-doping on the catalytic activity of polymer-stabilized au clusters in aerobic oxidation of alcohol. J Phys Chem C. 2007;111:4885–4888. [Google Scholar]
- 43.Lu HP, Xun L, Xie XS. Single-molecule enzymatic dynamics. Science. 1998;282:1877–1882. doi: 10.1126/science.282.5395.1877. [DOI] [PubMed] [Google Scholar]
- 44.Witkoskie JB, Cao J. Single molecule kinetics. I. Theoretical analysis of indicators. J Chem Phys. 2004;121:6361–6372. doi: 10.1063/1.1785783. [DOI] [PubMed] [Google Scholar]
- 45.Imbihl R, Ertl G. Oscillatory kinetics in heterogeneous catalysis. Chem Rev. 1995;95:697–733. [Google Scholar]
- 46.Newton MA, Belver-Coldeira C, Martínez-Arias A, Fernández-García M. Dynamic in situ observation of rapid size and shape change of supported Pd nanoparticles during CO/NO cycling. Nat Mater. 2007;6:528–532. doi: 10.1038/nmat1924. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.