Significance
Axon degeneration is caused by multiple chemotherapeutic agents and is a hallmark of chemotherapy-induced peripheral neuropathy (CIPN). CIPN is associated with the loss of the essential metabolite nicotinamide adenine dinucleotide (NAD+), a vital metabolite involved in energy homeostasis, but whether NAD+ loss or the accumulation of an NAD+ precursor, nicotinamide mononucleotide (NMN), is responsible for axon degeneration is controversial. We found that axon degeneration caused by vincristine, a widely used chemotherapeutic agent, could be ameliorated by forcing peripheral neurons to use an NAD+ biosynthetic pathway that bypasses NMN formation. This strategy provides an approach for preventing CIPN.
Keywords: chemotherapy-induced peripheral neuropathy, axon degeneration, NAD+, nicotinamide mononucleotide, nicotinic acid riboside
Abstract
Axon degeneration, a hallmark of chemotherapy-induced peripheral neuropathy (CIPN), is thought to be caused by a loss of the essential metabolite nicotinamide adenine dinucleotide (NAD+) via the prodegenerative protein SARM1. Some studies challenge this notion, however, and suggest that an aberrant increase in a direct precursor of NAD+, nicotinamide mononucleotide (NMN), rather than loss of NAD+, is responsible. In support of this idea, blocking NMN accumulation in neurons by expressing a bacterial NMN deamidase protected axons from degeneration. We hypothesized that protection could similarly be achieved by reducing NMN production pharmacologically. To achieve this, we took advantage of an alternative pathway for NAD+ generation that goes through the intermediate nicotinic acid mononucleotide (NAMN), rather than NMN. We discovered that nicotinic acid riboside (NAR), a precursor of NAMN, administered in combination with FK866, an inhibitor of the enzyme nicotinamide phosphoribosyltransferase that produces NMN, protected dorsal root ganglion (DRG) axons against vincristine-induced degeneration as well as NMN deamidase. Introducing a different bacterial enzyme that converts NAMN to NMN reversed this protection. Collectively, our data indicate that maintaining NAD+ is not sufficient to protect DRG neurons from vincristine-induced axon degeneration, and elevating NMN, by itself, is not sufficient to cause degeneration. Nonetheless, the combination of FK866 and NAR, which bypasses NMN formation, may provide a therapeutic strategy for neuroprotection.
Axon degeneration is an early feature of chemotherapy-induced peripheral neuropathy (CIPN), a severe and often permanent condition caused by anticancer agents that perturb microtubule depolymerization, such as taxanes (paclitaxel), vinca alkaloids (vincristine, vinblastine), and proteasome inhibitors (bortezomib) (1). The asymmetrical shape of peripheral neurons, with axons that can extend for meters, renders these cells particularly sensitive to insults that impair fast axonal transport. The numbness, pain, and heat/cold hyperalgesia associated with CIPN can be extremely troubling to patients, potentially limiting the use of otherwise effective chemotherapeutic agents (2).
In many ways, CIPN is reminiscent of Wallerian degeneration, a self-destructive process involving the distal portion of transected axons characterized by a beaded appearance, retraction, and axonal disintegration (3). Initially attributed to a failure to transport essential nutrients, Wallerian degeneration is now thought to result from an intrinsic axon destruction pathway. A key finding in this more recent understanding was the discovery of a spontaneously occurring mouse mutation, Wallerian degeneration slow (Wlds), which prevented degeneration of transected axons (4). Molecular characterization of Wlds revealed a fusion protein containing the nicotinamide adenine dinucleotide (NAD+) biosynthetic enzyme nicotinamide mononucleotide adenylyltransferase 1 (NMNAT1) linked to a sequence that mislocalizes this normally nuclear protein to the cytoplasm (5, 6). The rescue engendered by Wlds is consistent with the observation that NMNAT2, the predominant cytoplasmic isoform of the enzyme, becomes depleted in axons after transection because of its short half-life (7). This depletion was predicted to result in decreased levels of axonal NAD+ (8), an idea supported by the findings that protection from degeneration could be achieved by providing the NAD+ precursors, nicotinamide mononucleotide (NMN) or nicotinamide riboside (NR), augmenting activity of nicotinamide phosphoribosyltransferase (NAMPT) (9), or increasing NMNAT expression (3) (Fig. 1A).
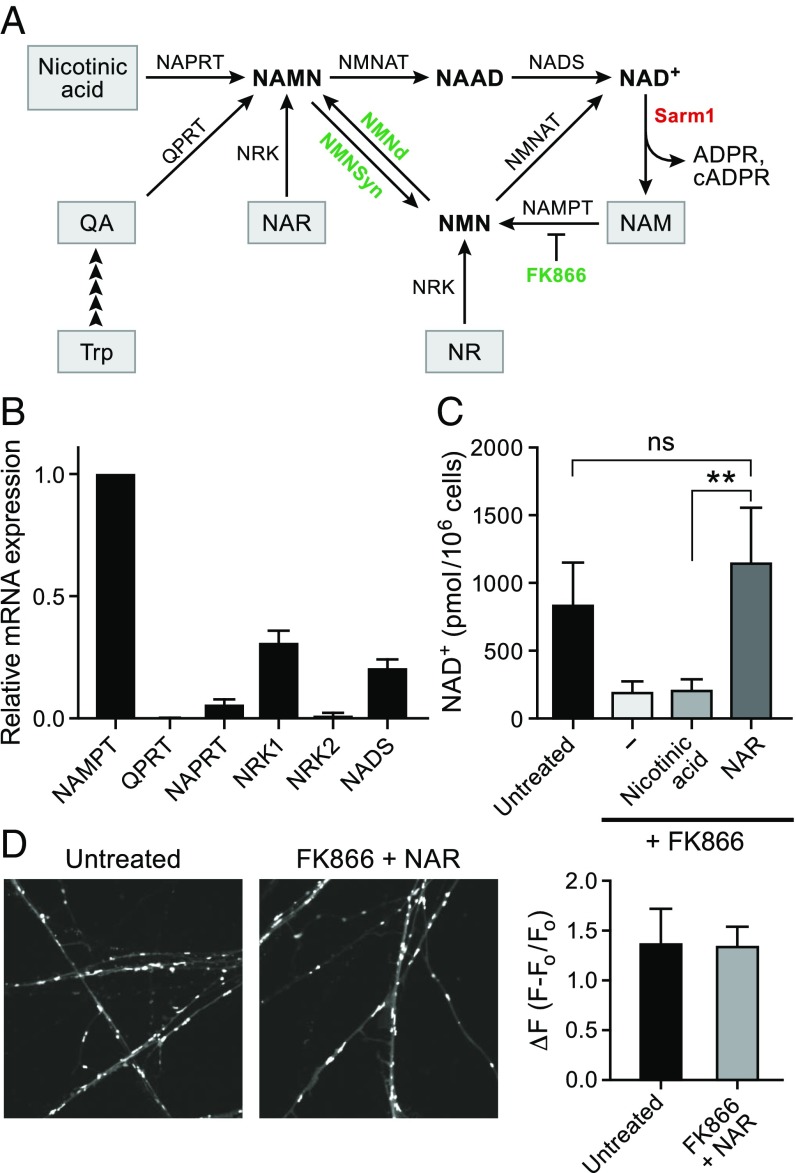
Fig. 1.
NAR maintains NAD+ synthesis in DRG neurons that lack NAPRT. (A) Diagram of NAD+ synthesis pathways. The de novo pathway converts tryptophan (Trp) to quinolinic acid (QA) and then, via quinolinic acid phosphoribosyltransferase (QPRT), to NAMN. NAMN is converted to NAAD and, ultimately, NAD+, via NMNAT and NAD+ synthetase (NADS). NAMN can also be produced from nicotinic acid and NAR via NAPRT and NRK, respectively. NAM and NR are converted to NMN by NAMPT and NRK1 and NRK2, as indicated, and NMNAT converts NMN to NAD+. FK866 is a specific inhibitor of NAMPT. NMNd and NMNsyn are bacterial enzymes that convert NMN to NAMN and NAMN to NMN, respectively. Axon injury activates SARM1, which hydrolyzes NAD+ to produce ADPR, cADPR, and NAM. (B) Relative levels of NAD+ biosynthetic enzymes in DRG neurons determined by RT-qPCR (mean ± SD; n = 3; P < 0.005 for each gene compared with NAMPT). (C) Levels of NAD+ in DRG neurons 16 h after treatment with 100 nM FK866 and 500 µM nicotinic acid or NAR (mean ± SD; n = 3; **P < 0.005). (D) Representative images (Left) and quantitation of tetramethylrhodamine methyl ester staining (Right) 24 h after exposure to the indicated agents. F0 represents the signal intensity after addition of the uncoupling agent FCCP.
A second key observation was the finding that an intrinsic axon degeneration pathway depended on the prodegenerative protein, SARM1 (10). Initially identified in Drosophila, the SARM1 ortholog in mammalian neurons is both necessary and sufficient for axonal degeneration (11). Moreover, CIPN, as well as other models of Wallerian degeneration such as posttraumatic brain injury, can be rescued by the loss of SARM1 (12). The finding that SARM1 contains an intrinsic NAD+ hydrolase activity supported the idea that NAD+ plays a central role in maintaining axonal integrity after injury (13). Whether NAD+ loss and the associated defects in energy metabolism are the primary effectors of axonal degeneration is controversial, however. NAD+ hydrolysis mediated by in vitro translated SARM1 generates other bioactive molecules, namely, ADP ribose (ADPR) and cyclic ADP ribose (cADPR) (14), both of which can cause potentially deleterious elevations in intracellular Ca2+ (15).
Although there is considerable evidence supporting the idea that loss of NAD+ contributes to axon degeneration, other studies suggest a different model; namely, that axonal injury and the subsequent loss of NMNAT2 activity increases levels of the NMNAT2 substrate NMN, and that it is the elevated level of NMN that triggers degeneration. Support for this idea includes the findings that a degree of protection was afforded by FK866, an NAMPT inhibitor, and that reducing NMN levels in neurons by expressing Escherichia coli NMN deamidase (NMNd), a bacterial enzyme that converts NMN to its deamidated form, nicotinic acid mononucleotide (NAMN), protected axons as well as Wlds or eliminating SARM1 (16, 17). Reconciling these two models has been difficult because elevating NMN levels by increasing NAMPT expression has also been shown to be protective (9).
Although inducing expression of genes such as Wlds and NMNd were effective in preventing Wallerian degeneration, these approaches have limited potential for clinical translation. We sought to take advantage of the well-characterized pathways for NAD+ synthesis to develop a pharmacological approach for CIPN treatment, aiming to identify a small molecule or combination of small molecules that would eliminate NMN accumulation while maintaining NAD+ levels. The three classical pathways for NAD+ biosynthesis use tryptophan (the de novo pathway), nicotinic acid (NA; the Preiss-Handler pathway), and nicotinamide (NAM; the NAM salvage pathway). The de novo and Preiss-Handler pathways converge at the level of NAMN, whereas the NAM salvage pathway and the recently identified NR kinase pathway (18) go through NMN. We hypothesized, therefore, that use of the former two pathways, which do not produce NMN, might be beneficial for preventing CIPN. As a model for CIPN, we examined axon degeneration in dorsal root ganglion (DRG) neurons treated with the chemotherapeutic agent, vincristine. We found that the de novo and Preiss-Handler pathways are not functional in DRGs, and that these neurons relied on the NAM salvage pathway for NAD+ synthesis. To reroute the pathway for NAD+ synthesis to one that avoided NMN, we employed a deamidated form of NR, nicotinic acid riboside (NAR) (19). Similar to NR, NAR is converted to its product, in this instance NAMN, through the actions of the widely expressed NR kinases, NRK1 and NRK2 (18, 20). Of note, nerve injury has been shown to induce expression of the NRK gene products (9, 20), potentially increasing the efficacy of NAR in supporting NAD+ synthesis.
Although NAR treatment delayed the axon degeneration caused by vincristine, it was only marginally more effective than NR and did not reduce NMN levels below those in DRGs treated with vincristine alone. To decrease NMN production further, we blocked NAMPT activity with the inhibitor FK866. This combination of NAR and FK866 reduced NMN levels and protected the neurons as well as NMNd. In addition, we showed that expressing the Francisella tularensis NMN synthetase (NMNsyn), an enzyme that converts NAMN to NMN, reversed this protection. The effects of FK866 and NAR administered in combination provide the rationale for a therapeutic strategy for preventing CIPN.
Results
NAR Can Maintain NAD+ Synthesis in DRG Neurons.
The principal routes for NAD+ synthesis in mammalian cells are depicted in Fig. 1A. To determine which NAD+ biosynthetic pathways are functional in DRG neurons, we performed RT-qPCR analysis of mRNA isolated from rat E15 DRGs maintained for 6 d in culture (DIV6) and treated with deoxyfluorouridine to minimize glial contamination. We found that NA phosphoribosyltransferases (NAPRT) and quinolinic acid phosphoribosyltransferases were \expressed at low levels compared with NAMPT, with NRK1 and NAD+ synthetase at intermediate levels (Fig. 1B). Treatment of cells with NA, NR, or FK866 did not change the expression pattern (SI Appendix, Fig. S1A). These results suggested that most NAD+ production in DRG neurons occurs through the NAM salvage pathway. Moreover, because of the relatively low expression of NAPRT, we predicted that NA would not be able to serve as an effective NAD+ precursor. In contrast, NAR, which requires only NRK to produce NAMN, should be able to produce NAD+ in the absence of NAPRT. To test this hypothesis, we used quantitative targeted NAD+ metabolomics (21) to ascertain whether NA or NAR could support NAD+ levels in DRG neurons treated with the NAMPT inhibitor, FK866. As expected, NAD+ levels fell in DRGs treated with FK866 and were not restored by NA (Fig. 1C). In contrast, NAR restored NAD+ levels to normal even when NAMPT was inhibited. To test whether this pharmacological rerouting of NAD+ synthesis pathways could sustain mitochondrial function, we examined mitochondrial membrane potential using tetramethylrhodamine methyl ester. No diminution in tetramethylrhodamine methyl ester staining was evident in DRGs treated with NAR and FK866 (Fig. 1D), and axon integrity was maintained (SI Appendix, Fig. S1B). Taken together, these experiments indicate that although NAD+ synthesis in DRG neurons normally occurs via NAM salvage, NAD+ production can be redirected to a deamidated pathway by combining NAR and FK866, bypassing the putatively neurotoxic NMN intermediate.
NR and NAR Delay Vincristine-Induced Axon Degeneration.
Chemotherapeutic drugs are thought to mediate axon degeneration by activating SARM1, leading to a loss of NAD+ and production of cADPR and ADPR (Fig. 1). Many of these drugs, particularly those that interfere with microtubule polymerization and depolymerization, reduce levels of axonal NMNAT2, which is predicted to increase NMN accumulation in addition to contributing to the depression of NAD+. We confirmed these changes by measuring NAD+ and NMN levels in E15 DRG neurons treated with 50 nM vincristine at DIV6. After 16 h of vincristine treatment, NMN levels increased from undetectable to 14.5 ± 6.8 pmol/106 cells and NAD+ fell to about 40% of control levels (Fig. 2A). The observation that the alternative NR kinase substrate, NAR, produces NAMN rather than NMN (19) led us to test whether NAR might be more effective than NR in protecting axons from the effects of vincristine. Further, we thought these agents might help sort out whether the NMN increase or NAD+ decrease is responsible for degeneration. For these experiments, we treated DRG neurons with 500 µM NR or NAR at the same time that vincristine was administered and assayed for NMN and NAD+ 16 h later. As expected, NMN levels in DRGs treated with vincristine were significantly higher after NR than NAR, whereas both treatments restored NAD+ to the basal level. It was not possible to follow NMN or NAD+ levels at later times because the axons displayed significant degeneration.
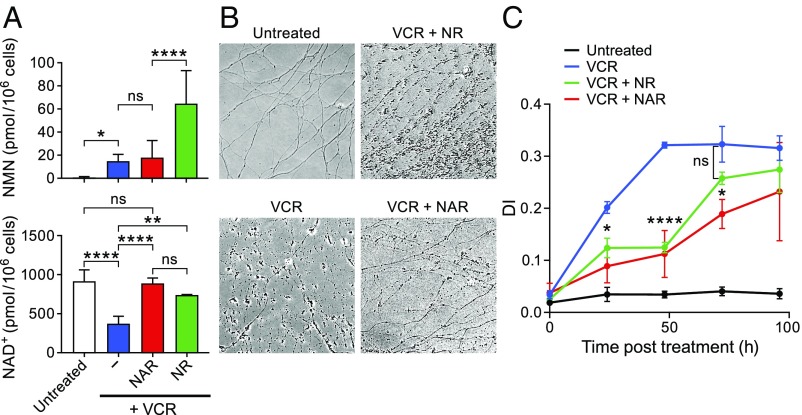
Fig. 2.
NR and NAR delay but do not prevent vincristine-induced axon degeneration. (A) Levels of NMN (Top) and NAD+ (Bottom) in DRG neurons treated with 50 nM vincristine. At the same time as vincristine on DIV6, 500 µM NR or NAR were added, and cells were collected for LC-MS 16 h later. (B) Representative images of DRG axons 72 h after vincristine treatment. NAR or NR were added at same time as vincristine. (C) DI (Methods) at various times after treatment. For A, statistics were determined using one-way ANOVA. ns, not significant; *P < 0.05; **P < 0.005; ***P < 0.0005; ****P < 10−4 denote differences using Bonferroni’s multiple comparisons test (mean ± SD; n ≥ 3 for each treatment). For C, statistics were determined using two-way ANOVA; asterisks denote P values between indicated treatments, using Bonferroni’s multiple comparisons test (n = 12 for each time).
To examine how these treatments affected axon degeneration, we treated cells with NR or NAR at the time of vincristine exposure and monitored axon degeneration at various times after treatment, shown in representative images 72 h after treatment (Fig. 2B) and in determination of the degeneration index (DI; Fig. 2C and SI Appendix, SI Materials and Methods). Both treatments delayed degeneration compared with vincristine alone, but NAR was only slightly more effective than NR despite the difference in NMN accumulation. By 96 h, no protection was evident after either agent. Taken together, these studies showed that restoration of NAD+ levels afforded by NR and NAR only partially protected against axon degeneration. Consistent with the idea that NMN appears to be a result of NMNAT2 depletion (17), NAR treatment did not reduce the vincristine-induced accumulation of NMN, possibly accounting for the limited degree of protection. In addition, consistent with the reversibility of the NMNAT reaction (22), some NMN accumulation was also seen in DRGs treated with NAR and FK866 in the absence of vincristine (SI Appendix, Fig. S2).
Combining NAR with FK866 Prevents Axon Degeneration.
Previous studies showed that E. coli NMNd protected primary neuron cultures from Wallerian degeneration, presumably by converting NMN to NAMN (17). This protection was reminiscent of that provided by Wlds, which has been shown to lower NMN levels in injured axons (16). In an effort to recapitulate the actions of NMNd and Wlds pharmacologically, we tested whether rerouting NAD+ synthesis from the NAM salvage pathway to the deamidated pathway could protect axons from degeneration as well as these genetic approaches. We first examined effects of FK866, which blocks formation of NMN from NAM. FK866 eliminated vincristine-induced NMN production, but at the expense of drastically reducing levels of NAD+ (Fig. 3A). To augment NAD+ production, we added 500 µM NAR to the regimen. Interestingly, NAD+ levels after treatment with vincristine, FK866, and NAR were approximately the same as after vincristine alone, and still substantially lower than levels in untreated neurons. However, this combination significantly depressed NMN accumulation compared with vincristine alone (note difference in scale between Figs. 2 and 3). For comparison, we showed that a lentivirus expressing E. coli NMNd completely eliminated NMN accumulation.
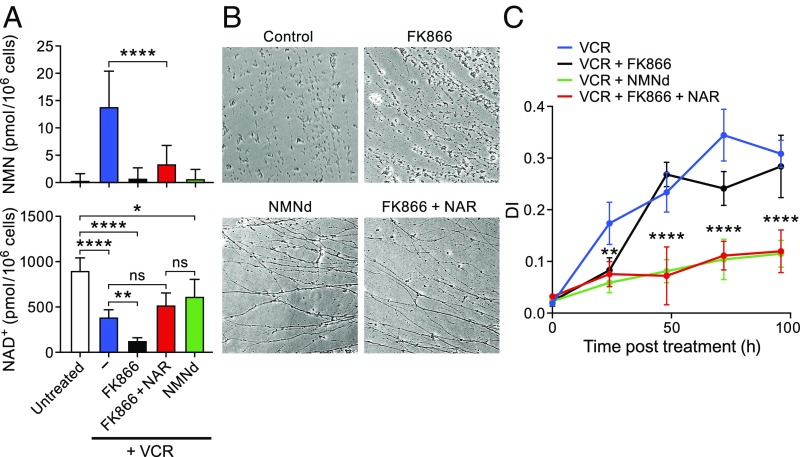
Fig. 3.
Combination of FK866 and NAR protects axons from degeneration. (A) NMN (Top) and NAD+ (Bottom) levels in DRGs exposed to 50 nM vincristine and treated with the indicated compounds or transduced at DIV2 with a lentivirus expressing NMNd. NAR and FK866 were added at the same time as vincristine on DIV6, and cells were collected for LC-MS analysis 16 h later (mean ± SD; n ≥ 3 for each treatment). (B) Representative images of DRG axons at 72 h posttreatment. Control represents neurons treated with vincristine alone. (C) Quantitation of axon degeneration at various times after vincristine administration. Asterisks denote P values as earlier (n = 12 for each time).
We then tested the combination of NAR and FK866 in axonal protection assays, treating DRG neurons with both agents at the time of vincristine administration. As shown in representative images (Fig. 3B) and DI assays (Fig. 3C), the combination of NAR and FK866 protected neurons as well as NMNd expression. Taken together, these experiments indicate that substantial protection from vincristine-induced axon degeneration can be achieved by simultaneously blocking NMN accumulation and providing an alternative source for NAD+. The FK866 and NAR combination did not increase NAD+ levels back to normal, however, suggesting that the key determinant for axon protection is reducing NMN accumulation. Thus, a depressed level of NAD+, when combined with low levels of NMN, is not sufficient for axon degeneration.
F. tularensis NMNsyn Reverses Protection Afforded by NAR and FK866.
To test the idea that the combination of NAR plus FK866 protected against axon degeneration by depressing NMN levels, we infected DRGs with a lentivirus expressing NMNsyn. This enzyme functions in a manner opposite to NMNd, converting NAMN to NMN. In vincristine-treated neurons, the depression in NMN accumulation achieved by treatment with FK866 and NAR (compare lanes 1 and 2 of Fig. 4A) was reversed by expression of NMNsyn, and NAD+ levels were restored (Fig. 4A, lane 3). NMNsyn, when combined with NAR, increased NMN to the level detected in DRG neurons treated with vincristine and elevated NAD+ to levels above normal. In degeneration assays of DRGs treated with vincristine, NMNsyn largely reversed the protective effect of NAR plus FK866 (Fig. 4 B and D), whereas NMNsyn administered with NAR did not cause degeneration when vincristine was absent (Fig. 4C). Taken together, these experiments indicate that shunting NAD+ synthesis from a pathway through NAMN to one that involves NMN eliminates the protection from vincristine-induced degeneration. In the absence of vincristine, however, the rise in NMN was insufficient to induce degeneration if NAD+ levels were elevated. Consistent with a previous study (23), NR alone similarly increased NMN levels, elevated NAD+, and did not cause degeneration (SI Appendix, Fig. S3). In contrast, blocking the salvage pathway via FK866 treatment caused degeneration after 72 h (SI Appendix, Fig. S3B).
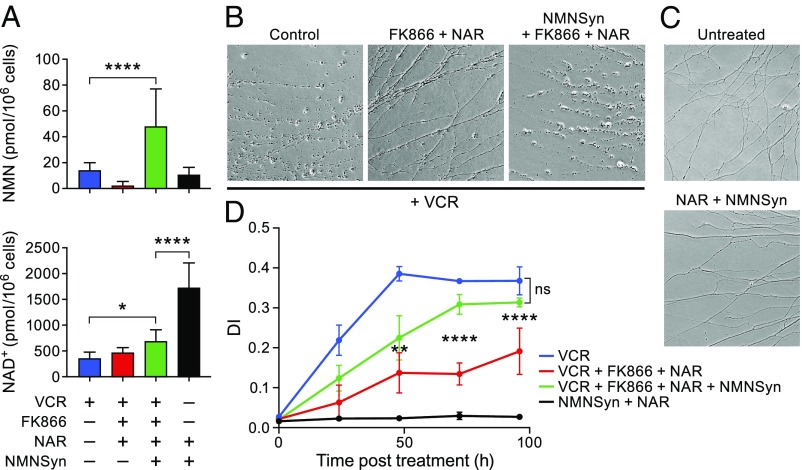
Fig. 4.
NMNsyn reverses protection afforded by FK866 plus NAR. (A) NMN (Top), and NAD+ (Bottom) levels in DRGs transduced with a lentivirus expressing F. tularensis NMNsyn or control on DIV2. Cells were cultured for 5 d after infection and treated with 50 nM vincristine, FK866, and NAR, as indicated (mean ± SD; n ≥ 3 for each treatment). (B) Representative images of DRG neurons at 72 h posttreatment with the agents indicated. (C) Representative images of NMNSyn-expressing DRG neurons at 72 h post-NAR treatment. (Axons were not treated with vincristine.) (D) Quantitation of axon degeneration. Asterisks denote P values as earlier (n = 12 for each time).
The idea behind using NMNd was to reduce levels of the potentially neurotoxic NMN, but this manipulation should also increase flux through the deamidated NAD+ synthesis pathway (Fig. 1A). NAR uses this pathway as well, and should similarly increase levels of deamidated NAD+ precursors. It is possible that some of these deamidated intermediates could contribute to the axon protective effects of NMNd or the FK866 plus NAR combination by blocking SARM1 activity. To test whether flux through the deamidated pathway was augmented, we quantified levels of nicotinic acid adenine dinucleotide (NAAD), the final step in the deamidated pathway before NAD+ production (24). As anticipated, levels of NAAD were increased in DRGs treated with vincristine and NAR, NMNd, or the FK866 plus NAR combination (SI Appendix, Fig. S4). NMNsyn eliminated the NAAD accumulation mediated by FK866 plus NAR.
SARM1 contains an amino-terminal autoinhibitory domain that prevents its activity under basal conditions. Removal of this inhibitory domain reveals an enzymatic activity that allows the hydrolysis of NAD+ to produce ADPR and cADPR (14). To test whether a deamidated NAD+ precursor such as NAAD might interfere with SARM1 activity, we purified epitope-tagged SARM1, either full-length or the active SARM1 fragment, from HEK293 cells and tested its ADPR cyclase activity in vitro. No interference with NAD+ hydrolysis or cADPR generation was detected (SI Appendix, Fig. S5) in the presence of NAAD, suggesting that NAAD does not function as a SARM1 inhibitor.
Discussion
Wallerian degeneration has served as a model of multiple neurological conditions, but perhaps its most common manifestation is in CIPN, a frequent adverse effect of many commonly used chemotherapeutic agents. Insights into the mechanisms underlying Wallerian degeneration provide the hope of developing agents capable of preventing or reversing this serious condition. Genetic studies in Drosophila and mouse models led to the discovery of two key determinants of Wallerian degeneration; namely, Wlds (4) and SARM1 (10). The finding that loss of SARM1 phenocopies Wlds suggested that the two might intersect on a common pathway, and this idea was supported by the observations that Wlds contains an NAD+ biosynthetic enzyme and SARM1 has an NAD+ hydrolase activity. Regardless of whether SARM1 promotes axon degeneration by depleting NAD+ or by generating ADPR and cADPR, pharmacologic agents capable of inhibiting SARM1 would be good candidates for CIPN prevention. Unfortunately, no such agents have been developed. Our approach was to address the consequences of Wlds and SARM1 action; namely, effects on the NAD+ metabolome.
There is general agreement that axon injury leads to a loss of NMNAT2 and a decrease in NAD+ (3). How this relates to axon degeneration is controversial, however. Although considerable evidence supports the idea that NAD+ loss, and perhaps the generation of bioactive NAD+ metabolites, are major factors (9, 13), some studies have suggested that build-up of the NMNAT2 substrate NMN is more important (16, 17). Obviously, these two models lead to opposing predictions; in the first, it should be beneficial to increase NAD+ production by providing NAD+ precursors, whereas in the second, efforts should be devoted to depressing elevated NMN levels. In this report, we attempt to reconcile the two models and provide a pharmacologic approach to preventing CIPN. We call this approach NAR addiction because it renders DRG neurons dependent on an exogenous precursor not normally used in NAD+ synthesis that feeds into the normally vestigial deamidated pathway.
Consistent with the second model, using quantitative targeted metabolomics, we observed a persistent elevation of NMN levels after vincristine treatment. Unlike a previous study that showed the elevated NMN levels decreasing to baseline at 6 h after axotomy (23), we found that the levels remained elevated for 24 h. This discrepancy could be a result of different types of injury, axotomy versus vincristine, improved internal standards, or that we quantify whole-cell NMN levels as opposed to those in axons alone. NR, commonly used to prevent conditions associated with NAD+ loss (20, 25, 26), protected against the vincristine-induced decrease in NAD+, but generated even higher levels of NMN. In an effort to reduce NMN formation pharmacologically, we treated DRGs with NAR, a precursor of NAMN that feeds into the deamidated pathway (Fig. 1). The Preiss-Handler pathway normally depends on NAPRT, the NA-dependent equivalent of NAMPT. NAPRT mRNA levels in DRG neurons were found to be extremely low, however, rendering this pathway nonfunctional. NAR, similar to NR, requires only phosphorylation by NRK1/NRK2 to generate the next intermediate in the NAD+ biosynthesis pathway. Both precursors restored NAD+ levels to normal, but NAR caused a substantially smaller increase in NMN. Still, NAR did not eliminate the vincristine-induced increase in NMN, which might account for the residual propensity for degeneration. We suggest that this NMN is generated from the activity of NAMPT, which produces NMN from nicotinamide, and from the bidirectional activity of NMNAT. Notably, our results indicated that restoring NAD+ levels to normal was not sufficient for full protection.
Consistent with observations of others (17), we found that E. coli NMNd greatly depressed NMN levels in neurons treated with vincristine and protected neurons from degeneration. In an effort to reduce NMN levels pharmacologically, we used a combination of agents that simultaneously blocked the NAM salvage pathway while supporting NAD+ production through the deamidated pathway. In the presence of vincristine, FK866 greatly depressed NAD+ levels, as expected from the block of two salvage pathway enzymes, and this decrease was partially rescued by adding NAR. The combination of FK866 and NAR was as protective as E. coli NMNd in axon degeneration assays, however. NAD+ levels in neurons treated with vincristine, NAR, and FK866 were no greater than those observed after vincristine alone. This suggests that the loss of NAD+ does not fully account for vincristine-induced axon degeneration.
As a further test of the idea that NAR plus FK866 protected axons by eliminating production of NMN, we examined the effects of F. tularensis NMNsyn, whose activity is the opposite of the E. coli NMNd. NMNsyn shunts the Preiss-Handler intermediate NAMN to NMN, allowing NAR to feed into the salvage pathway at the level of NMN. NMNsyn severely limited the axonal protection afforded by FK866 and NAR, apparently by reestablishing neurotoxic levels of NMN. Because NAD+ levels in neurons expressing NMNsyn returned to baseline, these studies suggest that the NMN increase, rather than NAD+ loss, was responsible for degeneration. One caveat of our studies is that in addition to depressing NMN levels, NAR increased levels of the deaminated NAD+ precursor, NAAD. However, we found that NAAD did not reduce cADPR generation by purified, full-length SARM1 or a SARM1 fragment lacking the autoinhibitory domain, suggesting that NAAD inhibition of SARM1 activity is unlikely.
It has been suggested that SARM1 inhibitors might be useful for axon protection, but to date, no SARM1 inhibitors have been developed. The agents we have used target pathways known to be affected by SARM1 and are readily available. It could be imagined that elevating cellular NAD+ content might promote growth of transformed cells, but the combination of NAR with FK866 did not lead to supraphysiological NAD+ levels, and neurons treated with this combination appeared normal (SI Appendix, Fig. S1) and maintained normal mitochondrial ion gradients.
Taken together, our results largely support the idea that NMN promotes axon degeneration. Depressing levels of NMN confers axon protection even in the face of lower NAD+ levels. However, elevating NMN was not sufficient to cause axon degeneration, as neurons treated with NAR and NMNsyn displayed an elevation in NMN but did not degenerate. It is possible that the augmented level of NAD+ in this setting was protective (17). In addition, how NMN relates mechanistically to axon degeneration, and presumably SARM1 activation, remains unresolved. There is no evidence supporting the notion that NMN regulates SARM1 directly, raising the possibility that some other NMN-binding protein is required in SARM1 regulation.
Finally, the viability of NAR-addicted neurons has implications for how mitochondrial NAD+ levels are maintained. In these neurons, there is no cytoplasmic NMN, and all NAD+ is derived through the deamidated pathway. The final step in this pathway depends on NAD+ synthetase, an enzyme that is excluded from mitochondria (27). Thus, all NAD+ in the NMN-depleted cells must be produced in the cytoplasm, and as a consequence, mitochondrial NAD+ must be transported from the cytoplasm rather than generated in the matrix via NMNAT3. This finding supports our observation that knockdown of NMNAT2 in other cell types reduced mitochondrial NAD+ levels (28). Mitochondrial NAD+ transporters have been identified in yeast and bacteria, but have not been identified in mammals.
Materials and Methods
Animal use followed NIH guidelines and complied with Oregon Health and Science University Institutional Animal Care and Use Committee (IACUC) under an approved protocol. Methods of cell culture, lentiviral packaging, NAD+ metabolite extraction, LC-MS/MS analysis, axon degeneration assay, and statistical analyses are provided in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by NIH Grants 1R01AG055431-01 (to R.H.G.) and DP2GM126897 (to X.A.C.), Roy J. Carver Trust (C.B.), and Pew Biomedical Scholar (M.S.C.).
Footnotes
Conflict of interest statement: C.B. owns stock in and consults for ChromaDex, Inc., and consults for Cytokinetics, Inc. M.E.M. has consulted for ChromaDex, Inc.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1809392115/-/DCSupplemental.
References
- 1.Grisold W, Cavaletti G, Windebank AJ. Peripheral neuropathies from chemotherapeutics and targeted agents: Diagnosis, treatment, and prevention. Neuro-oncol. 2012;14:iv45–iv54. doi: 10.1093/neuonc/nos203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park SB, et al. Chemotherapy-induced peripheral neurotoxicity: A critical analysis. CA Cancer J Clin. 2013;63:419–437. doi: 10.3322/caac.21204. [DOI] [PubMed] [Google Scholar]
- 3.Conforti L, Gilley J, Coleman MP. Wallerian degeneration: An emerging axon death pathway linking injury and disease. Nat Rev Neurosci. 2014;15:394–409. doi: 10.1038/nrn3680. [DOI] [PubMed] [Google Scholar]
- 4.Lunn ER, Perry VH, Brown MC, Rosen H, Gordon S. Absence of Wallerian degeneration does not hinder regeneration in peripheral nerve. Eur J Neurosci. 1989;1:27–33. doi: 10.1111/j.1460-9568.1989.tb00771.x. [DOI] [PubMed] [Google Scholar]
- 5.Coleman MP, et al. An 85-kb tandem triplication in the slow Wallerian degeneration (Wlds) mouse. Proc Natl Acad Sci USA. 1998;95:9985–9990. doi: 10.1073/pnas.95.17.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mack TG, et al. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat Neurosci. 2001;4:1199–1206. doi: 10.1038/nn770. [DOI] [PubMed] [Google Scholar]
- 7.Gilley J, Coleman MP. Endogenous Nmnat2 is an essential survival factor for maintenance of healthy axons. PLoS Biol. 2010;8:e1000300. doi: 10.1371/journal.pbio.1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, et al. A local mechanism mediates NAD-dependent protection of axon degeneration. J Cell Biol. 2005;170:349–355. doi: 10.1083/jcb.200504028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sasaki Y, Araki T, Milbrandt J. Stimulation of nicotinamide adenine dinucleotide biosynthetic pathways delays axonal degeneration after axotomy. J Neurosci. 2006;26:8484–8491. doi: 10.1523/JNEUROSCI.2320-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osterloh JM, et al. dSarm/Sarm1 is required for activation of an injury-induced axon death pathway. Science. 2012;337:481–484. doi: 10.1126/science.1223899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerdts J, Summers DW, Sasaki Y, DiAntonio A, Milbrandt J. Sarm1-mediated axon degeneration requires both SAM and TIR interactions. J Neurosci. 2013;33:13569–13580. doi: 10.1523/JNEUROSCI.1197-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henninger N, et al. Attenuated traumatic axonal injury and improved functional outcome after traumatic brain injury in mice lacking Sarm1. Brain. 2016;139:1094–1105. doi: 10.1093/brain/aww001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerdts J, Brace EJ, Sasaki Y, DiAntonio A, Milbrandt J. SARM1 activation triggers axon degeneration locally via NAD+ destruction. Science. 2015;348:453–457. doi: 10.1126/science.1258366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Essuman K, et al. The SARM1 Toll/Interleukin-1 receptor domain possesses intrinsic NAD+ cleavage activity that promotes pathological axonal degeneration. Neuron. 2017;93:1334–1343. doi: 10.1016/j.neuron.2017.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cantó C, Menzies KJ, Auwerx J. NAD(+) metabolism and the control of energy homeostasis: A balancing act between mitochondria and the nucleus. Cell Metab. 2015;22:31–53. doi: 10.1016/j.cmet.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Stefano M, et al. A rise in NAD precursor nicotinamide mononucleotide (NMN) after injury promotes axon degeneration. Cell Death Differ. 2015;22:731–742. doi: 10.1038/cdd.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Stefano M, et al. NMN deamidase delays Wallerian degeneration and rescues axonal defects caused by NMNAT2 deficiency in vivo. Curr Biol. 2017;27:784–794. doi: 10.1016/j.cub.2017.01.070. [DOI] [PubMed] [Google Scholar]
- 18.Bieganowski P, Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell. 2004;117:495–502. doi: 10.1016/s0092-8674(04)00416-7. [DOI] [PubMed] [Google Scholar]
- 19.Tempel W, et al. Nicotinamide riboside kinase structures reveal new pathways to NAD+ PLoS Biol. 2007;5:e263. doi: 10.1371/journal.pbio.0050263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaur P, et al. Nicotinamide riboside, a form of vitamin B3, protects against excitotoxicity-induced axonal degeneration. FASEB J. 2017;31:5440–5452. doi: 10.1096/fj.201700221RR. [DOI] [PubMed] [Google Scholar]
- 21.Trammell SA, Brenner C. Targeted, LCMS-based metabolomics for quantitative measurement of NAD(+) metabolites. Comput Struct Biotechnol J. 2013;4:e201301012. doi: 10.5936/csbj.201301012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kornberg A. Reversible enzymatic synthesis of diphosphopyridine nucleotide and inorganic pyrophosphate. J Biol Chem. 1950;182:779–793. [PubMed] [Google Scholar]
- 23.Sasaki Y, Nakagawa T, Mao X, DiAntonio A, Milbrandt J. NMNAT1 inhibits axon degeneration via blockade of SARM1-mediated NAD+ depletion. eLife. 2016;5:e19749. doi: 10.7554/eLife.19749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bieganowski P, Pace HC, Brenner C. Eukaryotic NAD+ synthetase Qns1 contains an essential, obligate intramolecular thiol glutamine amidotransferase domain related to nitrilase. J Biol Chem. 2003;278:33049–33055. doi: 10.1074/jbc.M302257200. [DOI] [PubMed] [Google Scholar]
- 25.Trammell SA, et al. Nicotinamide riboside opposes type 2 diabetes and neuropathy in mice. Sci Rep. 2016;6:26933. doi: 10.1038/srep26933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diguet N, et al. Nicotinamide riboside preserves cardiac function in a mouse model of dilated cardiomyopathy. Circulation. 2018;137:2256–2273. doi: 10.1161/CIRCULATIONAHA.116.026099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nikiforov A, Dölle C, Niere M, Ziegler M. Pathways and subcellular compartmentation of NAD biosynthesis in human cells: From entry of extracellular precursors to mitochondrial NAD generation. J Biol Chem. 2011;286:21767–21778. doi: 10.1074/jbc.M110.213298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cambronne XA, et al. Biosensor reveals multiple sources for mitochondrial NAD+ Science. 2016;352:1474–1477. doi: 10.1126/science.aad5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.