Significance
People’s emotions are heavily influenced by others around them. While previous studies have increased our understanding of the neuroscience of social influence in various contexts, such as music ratings and facial attraction, less is known about the neural basis of social influence on emotion processing and whether the human brain is able to track information from two different sources, such as ingroup and outgroup members. We address these two gaps in the literature by showing that the brain is able to differentially track, on a trial-by-trail basis, social influence of emotions deriving from ingroup and outgroup members. Results contribute to our understanding of the neural representations subserving everyday behaviors, such as people’s influence on others’ emotions.
Keywords: intergroup social influence, social neuroscience, emotion processing
Abstract
Emotions usually occur in a social context; yet little is known about how similar and dissimilar others influence our emotions. In the current study, we examined whether ingroup and outgroup members have differential influence on emotion processing at the behavioral and neural levels. To this end, we recruited 45 participants to rate a series of images displaying people engaged in different emotional contexts. Participants then underwent an fMRI scan where they viewed the same images along with information on how ingroup and outgroup members rated them, and they were asked to rate the images again. We found that participants shifted their emotions to be more in alignment with the ingroup over the outgroup, and that neural regions implicated in positive valuation [ventral striatum (VS) and ventromedial prefrontal cortex (vmPFC)], mentalizing [dorsomedial prefrontal cortex (dmPFC), medial prefrontal cortex (mPFC), posterior superior temporal sulcus (pSTS), and temporal pole], as well as emotion processing and salience detection (amygdala and insula), linearly tracked this behavior such that the extent of neural activity in these regions paralleled changes in participants’ emotions. Results illustrate the powerful impact that ingroup members have on our emotions.
On an everyday basis, people’s emotional experiences are heavily influenced by the emotions of others. Seeing other people unhappy can prompt individuals to become sad. This tendency for emotions to be contagious can be adaptive because emotional convergence serves as a building block for human interactions, as it promotes behavioral synchrony, facilitates rapport, and fosters effective interpersonal processes and social communication (1). However, not everyone influences us in the same way; those more similar to us (ingroup members) are more influential than those who are different (outgroup members). In this study, we aimed to examine the behavioral and neural mechanisms underlying conformity to emotions in an intergroup context.
Social Influence in an Intergroup Context
People’s motivations to conform, or change their behavior to match those of others, are shaped by their desire to form accurate perceptions of reality, engage in social affiliation, and maintain positive self-concepts (2). These drivers of conformity are particularly important in intergroup contexts where influence can come from two sources: ingroup and outgroup members (3). People have a greater tendency to change their beliefs, behaviors, and attitudes to be in accordance with the ingroup over the outgroup (2–4).
Theoretically based accounts on the advantages of ingroup conformity are grounded in evolutionary explanations, as conforming to and being more similar to ingroup members serves a more adaptive role compared with outgroup members by increasing chances of survival and reproduction through protection and provision of material resources (5). In addition, self-categorization theory (6) posits that conforming to ingroup (vs. outgroup) members provides individuals with multiple benefits, such as a sense of belonging and self-esteem (7, 8). These intergroup biases result in people perceiving, evaluating, and behaving more favorably toward ingroup than outgroup members (9, 10). However, although much research has focused on the impact of ingroup and outgroup members on attitudes and behaviors, less is known about how intergroup social influence can have an impact on emotion-related experiences.
Neural Basis of Social Influence and Intergroup Biases
Neuroimaging techniques have begun to provide insights into psychological processes that may be hard to capture through behavioral measures alone. This approach is especially useful when studying intergroup processes, as it allows researchers to investigate the underlying neural representation of biases in behavior and attitudes, which are difficult to capture through self-reports. Neuroimaging techniques are also beneficial when examining social influence, as it allows experimenters to disentangle between different types of conformity, such as public compliance and private acceptance (see refs. 2 and 11). In conjunction with observed behavioral outcomes, neuroscience can shed light on the mechanisms involved in intergroup social influence.
A growing body of research has begun to unpack the neural processes underlying conformity and social influence (for reviews, see refs. 12–16). People’s sensitivity to social reward (when conforming) and punishment (when violating a group’s norm) shapes their susceptibility to normative influences, and such conformity is mediated by the reward and social pain neural networks (13). The same neural systems that track reward signals, integrate value information, and motivate people to act [e.g., ventral striatum (VS) and ventromedial prefrontal cortex (vmPFC)] (17–19), are also implicated when people update their behavior to be in alignment with that of a group in social conformity contexts (11, 20, 21). Thus, activity in these neural systems might reflect the brain’s response to social reward and social bonding that results from conforming with others.
Similarly, social pain and rejection, powerful motivators of people’s desire to avoid exclusion and thus conform with others, tend to activate the dorsal anterior cingulate cortex (dACC) and anterior insula, common regions involved in conflict detection (22, 23). For example, increased neural activation in the dACC and anterior insula is present when updating one’s behavior if it differs from that of the group (20, 24). Thus, these regions might potentially track the cost of nonconformity to motivate people to change their behavior to be in line with the group.
Finally, regions associated with mentalizing [e.g., dorsomedial prefrontal cortex (dmPFC), medial prefrontal cortex (mPFC), temporoparietal junction (TPJ), posterior superior temporal sulcus (pSTS), and temporal poles] (25, 26), are likely to be involved in an intergroup social influence context. Previous research on the neuroscience of social influence has found that neural regions implicated in mentalizing are involved when conforming to others’ feedback (27, 28). Being influenced by others often entails taking the perspective of and making inferences about others’ mental states, both of which are critical for empathy and cooperation (29). The degree to which individuals engage in mentalizing and emotional understanding can depend on the source of the targets. For example, previous studies have shown that individuals engage in more individuated judgments about ingroup members compared with more superficial judgment about outgroup members (9, 29, 30). These differences are also reflected in the brain, where individuals recruit mentalizing regions to different extents, depending on the group membership of the target (29, 31, 32). Thus, it would be reasonable to hypothesize that mentalizing regions would be recruited to a greater extent for ingroup than outgroup members in an intergroup social influence context.
Social influence in an emotional context might be particularly strong when negative emotions are involved, as individuals are more likely to attend to and be more influenced by negative than positive information (33), a term referred to as a negativity bias. In emotional contexts, the insula is recruited to a greater extent for negative than positive stimuli (34), and in a social influence context, is associated with discomfort that might result from the mismatch between one’s ratings and those of others (24). Moreover, in intergroup contexts, the amygdala tends to be activated for biologically salient and motivationally relevant stimuli, such as ingroup members (35). Therefore, the insula and amygdala are candidate regions for the representation of the negativity bias effect as well as motivationally relevant groups, respectively. Given the importance of studying emotion processing in intergroup contexts, we sought to examine the behavioral and neural correlates of intergroup social influence in negative emotional contexts.
Current Study
In the current study, we designed a social influence task (Fig. 1) to examine how ingroup and outgroup members differentially influence people’s emotions. In line with previous evidence suggesting the advantage of ingroup conformity, we hypothesized that participants would be more likely to conform with the ingroup over the outgroup and that this behavior would be represented at the neural level by increased activation when conforming to ingroups over outgroups in areas previously associated with reward, positive valuation, and value integration (VS and vmPFC), mentalizing (dmPFC, mPFC, TPJ, pSTS, and temporal poles), and emotion and salience processing of biologically relevant stimuli (amygdala and insula).
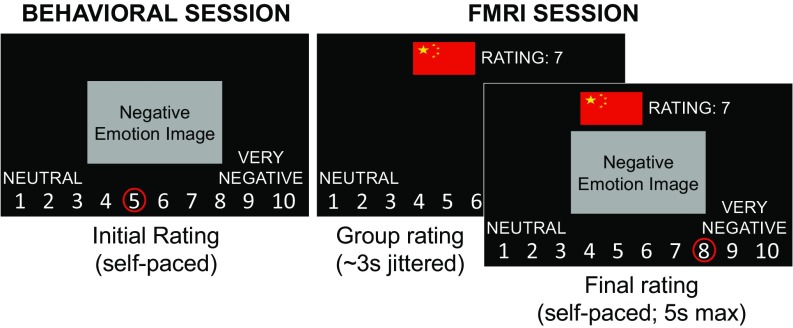
Fig. 1.
Overview of experimental design. Participants (American and Chinese) first rated a series of negatively valenced images during a behavioral session. Approximately 1 wk later, they completed the social influence task in the fMRI scanner. For each trial of the social influence task, participants first saw an American or Chinese flag (or no flag) indicating which group the feedback would be coming from along with a score indicating how students from a collaborating university in those countries rated an image (no ratings displayed for neutral trials). Around 3 s later, the image appeared on the screen and participants had a maximum of 5 s to give a final rating for the emotional image using the same scale as in the behavioral session. The images presented during the fMRI session were a subset of the ones participants rated during the behavioral session. We calculated influence scores based on whether participants changed their emotion ratings between the behavioral and scan sessions to conform (or not) with the group feedback.
We were also interested in examining whether culture modulates intergroup conformity. On the one hand, the cultural environment may influence the extent to which people display an ingroup bias in a social influence context. For example, East Asian culture emphasizes interdependence and group harmony, which could make individuals from these cultures more likely to conform to their ingroup compared with Western individuals who are more individualistic (36–38). On the other hand, given the well-established phenomenon that ingroup biases exist across cultures (39), intergroup conformity of emotional processes may exist similarly across cultures. Including culture as a factor in our study of intergroup social influence is important to understand the universality and boundaries of these processes.
Results
Behavioral Conformity to Ingroup over Outgroup Members.
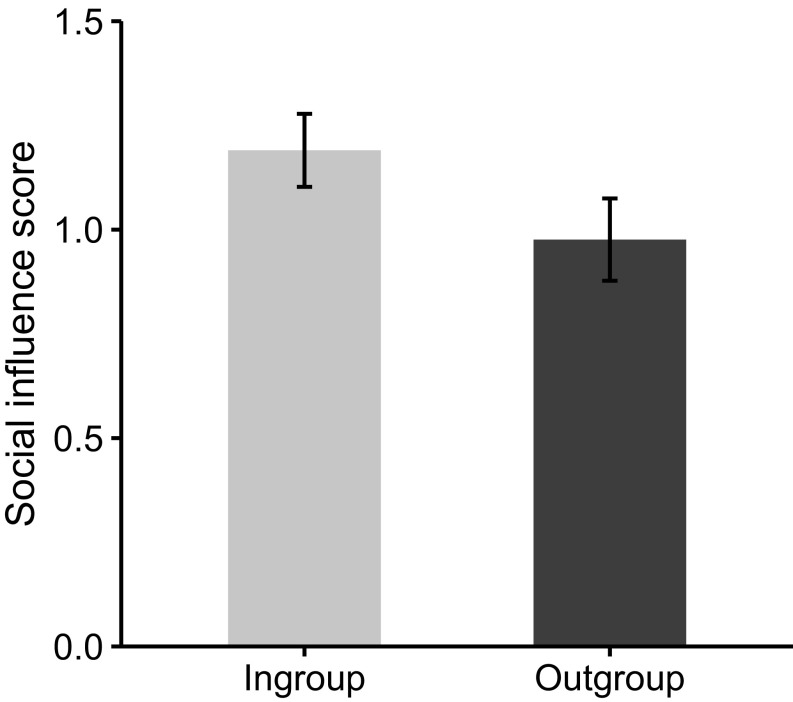
We first conducted a manipulation check for our social influence task and found that participants’ emotion ratings changed more in trials in which they were presented with group feedback compared with trials in which they were not presented with any feedback (SI Appendix). We then examined conformity to ingroup relative to outgroup ratings on trials where their initial ratings were moderate, as extreme scores are less likely to change (Methods). On these trials, participants were significantly more likely to be influenced by the ingroup (vs. outgroup), t (44) = 2.25, P = 0.03 (Fig. 2). Participants shifted their ratings on average by about 1.19 points in the direction of the ingroup and less than 1 point in the direction of the outgroup. Repeated measures ANOVA revealed no differences in ingroup and outgroup influence between American and Chinese participants, F(1,43) < 0.001, P = 0.99. These results suggest that ingroup conformity to negative emotions may be similar across these two cultural groups, which was further supported by post hoc analyses conducted within each cultural group separately (SI Appendix).
Fig. 2.
Behavioral conformity to ingroup over outgroup members. Participants aligned their emotion ratings to go along with ingroup more than outgroup members. Error bars represent SEM.
Increased Neural Tracking of Social Influence to Ingroup over Outgroup Members.
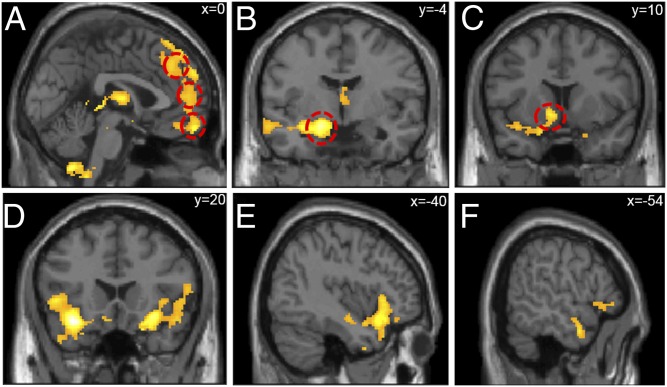
We next investigated whether participants showed different patterns of neural activation when shifting their behavior to be closer in alignment to the ingroup (vs. outgroup). To this end, we conducted a whole-brain one-sample (combining American and Chinese participants) t test, examining the contrast (ingroup − outgroup). Results revealed greater recruitment of the dmPFC, mPFC, vmPFC, left amygdala, left VS, bilateral insula, left temporal pole, right pSTS, and bilateral ventrolateral prefrontal cortex (vlPFC) when shifting emotional ratings toward the ingroup compared with the outgroup (Fig. 3 and SI Appendix, Fig. S1 and Table S1). In other words, these brain regions linearly tracked the amount of social influence to ingroups relative to outgroups. No neural regions were engaged more when aligning emotions with the outgroup over the ingroup. A whole brain two-sample (American vs. Chinese participants) t test revealed no differences between cultures. Post hoc one-sample t tests within each cultural group also revealed similar patterns of neural activation for American and Chinese participants when aligning their emotions with the ingroup over the outgroup (SI Appendix).
Fig. 3.
Neural regions that tracked social influence from ingroup more than outgroup members. Across both cultures, participants showed more recruitment of the (A) dmPFC, mPFC, and vmPFC; (B) left amygdala; (C) left VS; (D) insula; (E) vlPFC; and (F) left temporal pole, when aligning their emotional ratings to go with the ingroup compared with the outgroup.
Discussion
In the current study, we investigated the behavioral and neural mechanisms involved in tracking intergroup conformity in negative emotional contexts. We found that participants aligned their emotional responses with the ingroup over the outgroup, and that shifting their emotions toward the ingroup was associated with increased activation in neural regions implicated in reward, positive valuation, and value integration (VS and vmPFC), mentalizing (dmPFC, mPFC, pSTS, and temporal pole), and emotion and salience detection of biologically relevant stimuli (amygdala and insula). We did not find evidence that culture modulates these results, and our post hoc results show evidence of similar intergroup processes in behavior and brain across both of our cultural groups, suggesting that the behavioral and neural ingroup biases observed in our study may be present across different cultures.
Putative mechanisms underlying people’s shift in emotions to move in the direction of the ingroup (over the outgroup) were related to differences in the engagement of neural networks involved in reward, valuation, value integration, mentalizing, and emotion and salience processing, though we acknowledge our analyses cannot make the distinction between the possibility that participants engaged different neural regions when conforming to ingroup (vs. outgroup) members or whether they engaged similar neural regions but to different extents. Several hypotheses have been proposed regarding the role of these brain regions in social conformity, each not exclusive of one another. For example, one model proposes that agreement and disagreement with others results in error prediction signals that guide conformist behavior through reinforcement learning (15, 20). Another model posits that cognitive inconsistency between an individual and others creates an imbalance that ultimately results in behavioral adjustment (40). Finally, some researchers theorize that social influence can result in an actual modification of the neural representations of the objects being evaluated (their value or their perceptual features) that can ultimately lead to alignment with others’ behaviors (11, 15, 41, 42). Ultimately, all of these mechanisms might play a role in the neural signals that guide the adjustment of behavior to align with the group.
Our findings expand the rich literature on the neural basis of social influence that has been studied in the domains of food preferences, music ratings, and facial attraction (11, 20, 21, 24, 43) by extending the investigation to the area of intergroup influences on emotion processing. They complement previous studies that have demonstrated the powerful impact of intergroup social influence on attitudes and behaviors both at the behavioral (3) and neural levels (40, 44) by demonstrating that differential social influence from ingroup and outgroup members is just as strong in emotional contexts.
Neural Regions Implicated in Social Conformity.
vmPFC and VS.
Consistent with previous studies that have implicated the VS–vmPFC circuit in response to a mismatch between individual and group judgments, and updating one’s response to be in alignment to those of others (11, 20, 43), results from our study provide support for the idea that social conformity is associated with changes in the neural representation of value in response to social feedback and increased reward-related activity when conforming with others. We expand on previous findings by demonstrating that these changes are seen not only when one is in line with others, but more specifically, when one is in line with ingroup over outgroup members. Findings of the vmPFC converge with those examining group norms and food preferences, where vmPFC activity tracked social influence and VS activity increased when agreeing (vs. disagreeing) with others (21). Here, we extend these findings by demonstrating that agreement with similar vs. dissimilar others recruit vmPFC to different extents, with increased neural tracking in the VS and vmPFC for ingroup relative to outgroup social influence, potentially reflecting the rewarding nature of agreeing with groups that are more relevant to the self.
The VS’s role in positive valuation and reward in previous studies (17, 19) might also suggest a difference in valuation occurring when viewing the feedback from ingroups and outgroups, and is consistent with previous evidence suggesting that the subjective value placed on stimuli is sensitive to social feedback (11, 15, 21, 43, 45). VS activity suggests that social influence may have altered the neural representation of value placed on the different stimuli, indicating that participants in our study may have genuinely accepted others’ ratings of the emotional images when aligning their own ratings to be in accordance with the ingroup over the outgroup, rather than publicly conforming with ingroups over outgroups but privately holding the same views (11, 46).
dmPFC, mPFC, pSTS, and temporal pole.
As hypothesized, we observed increased dmPFC, mPFC, right pSTS, and left temporal pole activation when participants aligned their emotions to go along with ingroup over outgroup members. These regions have been implicated in theory of mind (25, 26), and their pattern of activation in our study suggests that when making the decision on the emotional content of the images, participants were processing the feedback provided by others to a greater extent when conforming to ingroups than outgroups. This is consistent with previous studies demonstrating more mentalizing and individuated (subordinate) levels of processing for ingroup members compared with more deindividuated (superordinate) processing for outgroup members (9, 29–31). In addition, previous studies have found overlapping neural regions between self-related and social-identity processing that involve medial prefrontal and medial parietal areas, including mPFC and dmPFC, and that activation in these regions correlate with ingroup biases (47), which are consistent with findings from our study.
In the context of social conformity, the dmPFC has been found to track cognitive inconsistency, and the larger this activation when viewing the preferences of others, the more likely individuals are to align their behavior to go along with (liked) others (40). The results from our study are in line with this hypothesis, as increased dmPFC activity when conforming to ingroup over outgroup members could represent a neural error signal that indicates deviation from others, a signal that is stronger for ingroup than outgroup members and motivates individuals to distinctively conform with different groups. dmPFC activation in our study, therefore, likely serves both mentalizing and tracking of cognitive imbalance functions, both processes likely involved in reinforcing behavioral adjustment to align with ingroup over outgroup members.
Insula and amygdala.
Results also indicated more insula and left amygdala activation when aligning emotions to go along with ingroup over outgroup members. Previous studies on the neural bases of social influence have interpreted insula activation as tracking an error occurring from a mismatch between an individual’s preference and a group’s behavior (15, 48, 49) that can result in arousal and negative affect (24) and ultimately facilitates behavioral alignment with the group (42). The current study suggests that these signals tracking deviations from others’ ratings and the ensuing negative affect might be stronger for ingroup than outgroup members. In addition, amygdala findings in our study could indicate higher depth of processing for motivationally relevant ingroup over outgroup members (35), with one implication being that emotion processing might occur at a deeper level when it is influenced by ingroup than outgroup members.
vlPFC.
We also observed increased activation in the vlPFC when participants adjusted their emotion ratings to go along with ingroup over outgroup members. The vlPFC has been implicated in cognitive control and emotion regulation (50). While this neural activation was not predicted a priori, previous studies have found increased vlPFC activation in relation to susceptibility to social influence (28). Our findings could suggest that participants directed their attention and engaged in more self-control to a greater extent when conforming to the emotions of ingroup over outgroup members.
Limitations and Future Directions.
The design of our study has several methodological strengths compared with previous studies examining the neural basis of social conformity (e.g., refs. 11, 20, 21, 24, 40, and 41). Mainly, participants made their initial and postsocial influence final ratings in two separate sessions days apart and were not reminded of their initial ratings during the scan session. In conjunction with the nature of our task that focused on emotions, these differences in experimental design may be why the main effect of influence (collapsing across ingroup and outgroup trials) vs. no-influence trials did not reveal divergent engagement of neural regions (SI Appendix), which suggests that our results might be more specific to how people respond to ingroup (vs. outgroup) influence rather than how they respond to social influence (vs. no influence) more generally. This paradigm minimizes demand characteristics that might result from completing initial and postgroup feedback ratings in the same experimental session by lowering the pressure to superficially comply with the ratings of others. It strengthens the claim that ingroup and outgroup members had divergent impacts on emotion processing both behaviorally and at the neural level, though our results suggest that at least at the behavioral level, this might be specific to situations where people’s initial ratings are moderate and not extreme (SI Appendix).
Our results on social influence in negative emotional contexts are consistent with previous research that found that people tend to have a negativity bias. Focusing on negative aspects of their environment might be particularly relevant in intergroup contexts, wherein strong and salient emotions might heighten the relevance of the ingroup. However, it would be interesting to explore whether the results from our study would hold for other emotions, such as positive, neutral, or even different kinds of negative emotions, such as anger, fear, and sadness.
The lack of cultural differences suggests that intergroup influences on emotion processing might be present both in Chinese and American cultures. Previous studies examining intergroup cultural influences on neural processing in other domains, such as empathy and prosociality, have found differential neural responses for ingroups and outgroups across cultures (51, 52). However, given that social influence is a powerful determinant of people’s behaviors and emotions, the results from our study suggest that social influence in an intergroup context might be just as powerful in individualistic and interdependent cultures when emotions are involved. Previous neuroimaging studies have examined the psychological and neural mechanisms involved in social influence, but research examining intercultural variation in these processes has lagged behind. Thus, while we did not find cultural differences, we highlight the importance of including culture as a factor for determining the universality of research findings.
Similar studies with participants from other sociocultural environments and from different age groups could also give us a more complete picture of the behavioral and neural substrates of social influence in intergroup contexts. Intra- and interindividual variability in the samples that are studied will help in addressing some of the lingering questions regarding the specific role of the neural systems involved in social conformity, such as whether value-related activation tracks conformity, valuation, or an interaction between the two, depending on the context (see ref. 12).
Conclusion
We explored the behavioral and neural mechanisms underlying intergroup social influence in an emotional context. We show that those similar to us are more likely to be influential than those different from us and we provide evidence that the neural representations subserving behavioral conformity in emotional contexts are also different, depending on the group membership of the influencers. This research improves our understanding of social influence on intergroup emotion processing by demonstrating that the brain is able to track social influence from different sources in emotional contexts, and that this might be a shared phenomenon across different cultures. In an increasingly globalized world, examining intergroup influence on emotion processing is a step forward for improving intergroup emotional understanding.
Methods
Participants.
Forty-five individuals participated in the study, including 22 American and 23 Chinese participants (mean age = 19.42 y, SD = 0.63, 24 female). All American participants were White/Caucasian and all Chinese participants were born in China and had been in the United States for less than 1 y. Participants were paid for their participation or given course credit, whichever the participant preferred. Subjects provided written consent and all procedures were approved by the University of Illinois’s Institutional Review Board.
Participants came for two separate sessions (a behavioral and an fMRI session), each about 1 wk apart. Instructions and materials for the study were translated and back translated to Chinese by bilingual speakers (53). American participants completed the English version of the tasks and questionnaires, and Chinese participants completed the Chinese version of the tasks and questionnaires.
Procedures.
Behavioral session.
In the first session, participants were presented with 100 images of people in negative contexts, such as an earthquake aftermath, at a funeral, or in the hospital. They were asked to imagine that they were the person in the picture and indicate, on a scale from 1 (neutral) to 10 (very negative), how they feel. Half of the targets in the pictures were Asian and half White. The task was self-paced.
fMRI session.
Approximately 1 wk after the behavioral session, participants underwent an fMRI scan while completing a social influence task (Fig. 1). In this task, participants viewed how ingroup and outgroup members (as defined by culture) rated a subset of the images they had rated during the behavioral session and were asked to rate the images again. In reality, group ratings were experimentally manipulated based on the participants’ initial ratings during the behavioral session. In total, each participant saw 24 ingroup, 24 outgroup ratings, and 12 no-rating trials. See SI Appendix for more details about the task and for information about the baseline ratings of the images that were used in the scan session (SI Appendix, Fig. S2).
fMRI Data Acquisition, Preprocessing, and Analysis.
All imaging data were acquired using a 3 Tesla Siemens Trio MRI scanner. T2*-weighted echoplanar images (EPIs) [slice thickness = 3 mm; 38 slices; repetition time (TR) = 2 s; echo time (TE) = 25 ms; matrix = 92 × 92; field of view (FOV) = 230 mm; voxel size 2.5 × 2.5 × 3 mm3] were acquired during the fMRI task. For the structural scans, a T2*-weighted, matched bandwidth (MBW), high-resolution, anatomical scan (TR = 4 s; TE = 64 ms; matrix = 192 × 192; FOV = 230 mm; slice thickness = 3 mm; 38 slices) and a T1* magnetization-prepared rapid-acquisition gradient echo (MPRAGE) (TR = 1.9 s; TE = 2.3 ms; matrix = 256 × 256; FOV = 230 mm; sagittal plane; slice thickness = 1 mm; 192 slices) were acquired for each participant. The MBW and EPI scans were oriented at an oblique axial angle to maximize brain coverage and reduce signal dropout.
Neuroimaging data were preprocessed using the FSL FMRIBs Software Library (FSL v6.0; https://fsl.fmrib.ox.ac.uk/fsl/fslwiki). For each participant, motion correction was performed using MCFLIRT (54). Spatial smoothing was applied using a Gaussian kernel of full width at half maximum (FWHM) of 6 mm. High-pass temporal filtering was then conducted with a filter width of 128 s (Gaussian-weighted least-squares straight line fitting, with sigma = 64.0 s) and images were skull stripped using FSL’s Brain Extraction Tool (55) based on the parameters suggested by ref. 56. Each functional image was registered to standard Montreal Neurological Institute (MNI) 2 mm brain through T2- and T1-weighted structural images using FLIRT (54, 57). An individual-level independent component analysis denoising procedure was also conducted using MELODIC (58) in conjunction with an automated signal classification toolbox (classifier Neyman–Pearson threshold = 0.3; see ref. 59 for more details) to remove motion- and physiological-related artifacts.
Statistical analyses were performed using SPM8 (Wellcome Department of Cognitive Neurology, London, UK). At the first level, each trial was modeled by convolving it with the canonical hemodynamic response function. In each participant’s fixed-effects analysis, a general linear model (GLM) was set up with the regressors of interest to separate different events, which included social influence from ingroup members, outgroup members, and no influence. The task was modeled as an event-related design and the duration of each trial was modeled from the initial presentation of the group rating (presentation of group rating or no rating) to the participant’s response. The jittered intertrial intervals were not explicitly modeled and thus constituted an implicit baseline.
For each trial, we also included a parametric modulator (PM) to track the participants’ change in initial to final rating in relation to the group rating. The PM was computed as follows: for those trials where participants saw a group rating, a 2 indicated if they moved fully in the direction of the group rating (changed their rating to be the same or more in the direction of the group rating), a 1 indicated partial movement toward the group rating (changed their rating to be in the direction of the group rating but still not as high or as low as the group rating), a 0 indicated they did not move at all, and a −1 indicated they moved in the opposite direction from the group rating. We chose to include 2 as the maximum score, as many of the scores were constricted to 2 points of movement. We stopped the parametric modulator at −1, given that there were not as many trials in which participants moved in the opposite direction of the influence group, as there were trials in which participants moved in the direction of the influence group, and such trials where they moved in the opposite direction had a smaller relative change. As an example of how trials were coded, if a participant initially rated an image as a 5 in the behavioral session, then provided a rating of an 8 following a group rating presentation of 8 during the scan, then that trial was coded as a 2. If the participant had provided a final rating of 7, then that trial was coded as 1. If they rated it a 5, then the trial was coded as 0, and if the participant rated it a 3, then it was coded as −1. For trials where participants did not see ratings from others (no group rating present), the PM was calculated based on the change from final to initial rating (SI Appendix). By including the PM representing change in ratings, we were able to examine how brain regions tracked the amount of social influence to in- and outgroups.
Linear contrast images comparing each of the conditions of interest were created based on the parameter estimates resulting from the GLM. Random effect analyses were then conducted by submitting the individual subject contrasts to the second level for group analyses. At the group level, we ran whole-brain, one-sample (combining American and Chinese participants) and two-sample t tests (American vs. Chinese participants) to examine similarities and cross-cultural differences in the neural correlates underlying intergroup social influence using the contrast (ingroup − outgroup).
We conducted a Monte Carlo stimulation to compute a cluster threshold to correct for multiple comparisons using the updated (April 2016) 3dFWHMx (-acf option) and 3dClustSim implemented in the AFNI software package (60), and the group-level brain mask for analyses of interest. The simulations indicated a voxel-wise threshold of P < 0.005 in combination with a minimum cluster size of 231 voxels for the one-sample t tests and 226 voxels for the two-sample t tests, corresponding to P < 0.05, family-wise error corrected. All results are available on Neurovault (61); see https://neurovault.org/collections/RHJBYKGH/.
Behavioral Data Analysis.
At the behavioral level, we computed an influence score on a trial-by-trial basis to calculate how much, for a given stimulus image, the participant was influenced by the group rating. This social influence score was calculated based on the difference in participants’ initial rating during the behavioral session and final rating after viewing the group rating during the fMRI scan. If the participant modified their rating to be away from the group feedback, then the corresponding influence score was negative. If they shifted their rating to be toward the group feedback, then the corresponding influence score was positive. Influence scores could not be calculated for no-influence trials, since no group feedback was provided. Because people are more likely to be influenced by others when their initial ratings are moderate and fall in the middle of the scale rather than at the extremes (2, 62), we only computed influence scores looking at trials where their initial ratings were moderate (e.g., between 3 and 8), as extreme scores (e.g., 1, 2, 9, and 10) are less likely to change (see SI Appendix for moderate vs. extreme trial comparisons in our data and SI Appendix, Fig. S3 for distributions of raw influence scores for ingroup and outgroup feedback trials).
Supplementary Material
Acknowledgments
We thank the Biomedical Imaging Center at the Beckman Institute for Advanced Science and Technology at the University of Illinois at Urbana–Champaign. This work was supported by National Science Foundation Grant SES 1459719 and generous funds from the Department of Psychology, University of Illinois at Urbana–Champaign.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1802111115/-/DCSupplemental.
References
- 1.Hatfield E, Rapson RL, Le Y-CL. Emotional contagion and empathy. In: Decety J, Ickes W, editors. The Social Neuroscience of Empathy. The MIT Press; Cambridge, MA: 2009. pp. 19–30. [Google Scholar]
- 2.Cialdini RB, Goldstein NJ. Social influence: Compliance and conformity. Annu Rev Psychol. 2004;55:591–621. doi: 10.1146/annurev.psych.55.090902.142015. [DOI] [PubMed] [Google Scholar]
- 3.Mackie DM, Wright CL. Social influence in an intergroup context. In: Brown R, Gaertner SL, editors. Blackwell Handbook of Social Psychology: Intergroup Processes. Wiley-Blackwell; Oxford: 2003. pp. 281–300. [Google Scholar]
- 4.MacKie DM, Gastardo-Conaco MC, Skelly JJ. Knowledge of the advocated position and the processing of in-group and out-group persuasive messages. Pers Soc Psychol Bull. 1992;18:145–151. [Google Scholar]
- 5.Correll J, Park B. A model of the ingroup as a social resource. Pers Soc Psychol Rev. 2005;9:341–359. doi: 10.1207/s15327957pspr0904_4. [DOI] [PubMed] [Google Scholar]
- 6.Turner JC. Social categorization and self-concept: A social cognitive theory of group behavior. In: Lawler EJ, editor. Advances in Group Process: Theory and Research. JAI Press; Greenwich, CT: 1985. pp. 77–121. [Google Scholar]
- 7.Brewer MB. The social self: On being the same and different at the same time. Pers Soc Psychol Bull. 1991;17:475–482. [Google Scholar]
- 8.Tajfel H, Turner J. An integrative theory of intergroup conflict. In: Austin W, Worchel S, editors. The Social Psychology of Intergroup Relations. Brooks/Cole; Monterey, CA: 1979. pp. 33–47. [Google Scholar]
- 9.Cikara M, Van Bavel JJ. The neuroscience of intergroup relations: An integrative review. Perspect Psychol Sci. 2014;9:245–274. doi: 10.1177/1745691614527464. [DOI] [PubMed] [Google Scholar]
- 10.Hewstone M, Rubin M, Willis H. Intergroup bias. Annu Rev Psychol. 2002;53:575–604. doi: 10.1146/annurev.psych.53.100901.135109. [DOI] [PubMed] [Google Scholar]
- 11.Zaki J, Schirmer J, Mitchell JP. Social influence modulates the neural computation of value. Psychol Sci. 2011;22:894–900. doi: 10.1177/0956797611411057. [DOI] [PubMed] [Google Scholar]
- 12.Falk E, Scholz C. Persuasion, influence, and value: Perspectives from communication and social neuroscience. Annu Rev Psychol. 2018;69:329–356. doi: 10.1146/annurev-psych-122216-011821. [DOI] [PubMed] [Google Scholar]
- 13.Falk EB, Way BM, Jasinska AJ. An imaging genetics approach to understanding social influence. Front Hum Neurosci. 2012;6:168. doi: 10.3389/fnhum.2012.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Izuma K. The neural basis of social influence and attitude change. Curr Opin Neurobiol. 2013;23:456–462. doi: 10.1016/j.conb.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Schnuerch R, Gibbons H. A Review of neurocognitive mechanisms of social conformity. Soc Psychol. 2014;45:466–478. [Google Scholar]
- 16.Cascio CN, Scholz C, Falk EB. Social influence and the brain: Persuasion, susceptibility to influence and retransmission. Curr Opin Behav Sci. 2015;3:51–57. [Google Scholar]
- 17.Bartra O, McGuire JT, Kable JW. The valuation system: A coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage. 2013;76:412–427. doi: 10.1016/j.neuroimage.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McClure SM, York MK, Montague PR. The neural substrates of reward processing in humans: The modern role of FMRI. Neuroscientist. 2004;10:260–268. doi: 10.1177/1073858404263526. [DOI] [PubMed] [Google Scholar]
- 20.Klucharev V, Hytönen K, Rijpkema M, Smidts A, Fernández G. Reinforcement learning signal predicts social conformity. Neuron. 2009;61:140–151. doi: 10.1016/j.neuron.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 21.Nook EC, Zaki J. Social norms shift behavioral and neural responses to foods. J Cogn Neurosci. 2015;27:1412–1426. doi: 10.1162/jocn_a_00795. [DOI] [PubMed] [Google Scholar]
- 22.Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- 23.Kross E, Berman MG, Mischel W, Smith EE, Wager TD. Social rejection shares somatosensory representations with physical pain. Proc Natl Acad Sci USA. 2011;108:6270–6275. doi: 10.1073/pnas.1102693108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berns GS, Capra CM, Moore S, Noussair C. Neural mechanisms of the influence of popularity on adolescent ratings of music. Neuroimage. 2010;49:2687–2696. doi: 10.1016/j.neuroimage.2009.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frith U, Frith CD. Development and neurophysiology of mentalizing. Philos Trans R Soc Lond B Biol Sci. 2003;358:459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frith CD, Frith U. The neural basis of mentalizing. Neuron. 2006;50:531–534. doi: 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Cascio CN, O’Donnell MB, Bayer J, Tinney FJ, Falk EB. Neural correlates of susceptibility to group opinions in online word-of-mouth recommendations. J Mark Res. 2015;52:559–575. [Google Scholar]
- 28.Welborn BL, et al. Neural mechanisms of social influence in adolescence. Soc Cogn Affect Neurosci. 2016;11:100–109. doi: 10.1093/scan/nsv095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heatherton TF. Neuroscience of self and self-regulation. Annu Rev Psychol. 2011;62:363–390. doi: 10.1146/annurev.psych.121208.131616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hugenberg K, Young SG, Bernstein MJ, Sacco DF. The categorization-individuation model: An integrative account of the other-race recognition deficit. Psychol Rev. 2010;117:1168–1187. doi: 10.1037/a0020463. [DOI] [PubMed] [Google Scholar]
- 31.Freeman JB, Schiller D, Rule NO, Ambady N. The neural origins of superficial and individuated judgments about ingroup and outgroup members. Hum Brain Mapp. 2010;31:150–159. doi: 10.1002/hbm.20852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris LT, Fiske ST. Dehumanizing the lowest of the low: Neuroimaging responses to extreme out-groups. Psychol Sci. 2006;17:847–853. doi: 10.1111/j.1467-9280.2006.01793.x. [DOI] [PubMed] [Google Scholar]
- 33.Baumeister RF, Bratslavsky E, Finkenauer C, Vohs KD. Bad is stronger than good. Rev Gen Psychol. 2001;5:323–370. [Google Scholar]
- 34.Cunningham WA, Raye CL, Johnson MK. Implicit and explicit evaluation: FMRI correlates of valence, emotional intensity, and control in the processing of attitudes. J Cogn Neurosci. 2004;16:1717–1729. doi: 10.1162/0898929042947919. [DOI] [PubMed] [Google Scholar]
- 35.Van Bavel JJ, Packer DJ, Cunningham WA. The neural substrates of in-group bias: A functional magnetic resonance imaging investigation. Psychol Sci. 2008;19:1131–1139. doi: 10.1111/j.1467-9280.2008.02214.x. [DOI] [PubMed] [Google Scholar]
- 36.Bond R, Smith PB. Culture and conformity: A meta-analysis of studies using Asch’s (1952b, 1956) line judgment task. Psychol Bull. 1996;119:111–137. [Google Scholar]
- 37.Markus HR, Kitayama S. Culture and the self: Implications for cognition, emotion, and motivation. Psychol Rev. 1991;98:224–253. [Google Scholar]
- 38.Triandis HC, Trafimow D. Culture and its implications for intergroup behavior. In: Brown R, Gaertner SL, editors. Blackwell Handbook of Social Psychology: Intergroup Processes. Wiley-Blackwell; Oxford: 2003. pp. 367–385. [Google Scholar]
- 39.Brown D. Human Universals. McGraw-Hill Humanities/Social Sciences/Languages; New York: 1991. [Google Scholar]
- 40.Izuma K, Adolphs R. Social manipulation of preference in the human brain. Neuron. 2013;78:563–573. doi: 10.1016/j.neuron.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berns GS, et al. Neurobiological correlates of social conformity and independence during mental rotation. Biol Psychiatry. 2005;58:245–253. doi: 10.1016/j.biopsych.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 42.Wu H, Luo Y, Feng C. Neural signatures of social conformity: A coordinate-based activation likelihood estimation meta-analysis of functional brain imaging studies. Neurosci Biobehav Rev. 2016;71:101–111. doi: 10.1016/j.neubiorev.2016.08.038. [DOI] [PubMed] [Google Scholar]
- 43.Campbell-Meiklejohn DK, Bach DR, Roepstorff A, Dolan RJ, Frith CD. How the opinion of others affects our valuation of objects. Curr Biol. 2010;20:1165–1170. doi: 10.1016/j.cub.2010.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stallen M, Smidts A, Sanfey AG. Peer influence: Neural mechanisms underlying in-group conformity. Front Hum Neurosci. 2013;7:50. doi: 10.3389/fnhum.2013.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mason MF, Dyer R, Norton MI. Neural mechanisms of social influence. Organ Behav Hum Decis Process. 2009;110:152–159. [Google Scholar]
- 46.Stallen M, Sanfey AG. The neuroscience of social conformity: Implications for fundamental and applied research. Front Neurosci. 2015;9:337. doi: 10.3389/fnins.2015.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Volz KG, Kessler T, von Cramon DY. In-group as part of the self: In-group favoritism is mediated by medial prefrontal cortex activation. Soc Neurosci. 2009;4:244–260. doi: 10.1080/17470910802553565. [DOI] [PubMed] [Google Scholar]
- 48.Chung D, Christopoulos GI, King-Casas B, Ball SB, Chiu PH. Social signals of safety and risk confer utility and have asymmetric effects on observers’ choices. Nat Neurosci. 2015;18:912–916. doi: 10.1038/nn.4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tomlin D, Nedic A, Prentice DA, Holmes P, Cohen JD. The neural substrates of social influence on decision making. PLoS One. 2013;8:e52630. doi: 10.1371/journal.pone.0052630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 51.Cheon BK, et al. Cultural influences on neural basis of intergroup empathy. Neuroimage. 2011;57:642–650. doi: 10.1016/j.neuroimage.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 52.Telzer EH, Ichien N, Qu Y. The ties that bind: Group membership shapes the neural correlates of in-group favoritism. Neuroimage. 2015;115:42–51. doi: 10.1016/j.neuroimage.2015.04.035. [DOI] [PubMed] [Google Scholar]
- 53.Brislin RW. Translation and content analysis of oral and written materials. In: Triandis HC, Berry JW, editors. Handbook of Cross-Cultural Psychology. Allyn & Bacon; Boston: 1980. pp. 389–444. [Google Scholar]
- 54.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 55.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Popescu V, et al. MAGNIMS Study Group Optimizing parameter choice for FSL-Brain Extraction Tool (BET) on 3D T1 images in multiple sclerosis. Neuroimage. 2012;61:1484–1494. doi: 10.1016/j.neuroimage.2012.03.074. [DOI] [PubMed] [Google Scholar]
- 57.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 58.Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- 59.Tohka J, et al. Automatic independent component labeling for artifact removal in fMRI. Neuroimage. 2008;39:1227–1245. doi: 10.1016/j.neuroimage.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ward BD. 2000 Simultaneous inference for fMRI data. Available at https://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf. Accessed July 3, 2018.
- 61.Gorgolewski KJ, et al. NeuroVault.org: A web-based repository for collecting and sharing unthresholded statistical maps of the human brain. Front Neuroinform. 2015;9:8. doi: 10.3389/fninf.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Erb H-P, Bohner G, Rank S, Einwiller S. Processing minority and majority communications: The role of conflict with prior attitudes. Pers Soc Psychol Bull. 2002;28:1172–1182. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.