Reading is a uniquely human skill, learned through extensive experience during childhood, with literacy becoming widespread only in the past few hundred years. Consequently, the neural circuitry underlying language could not have evolved to have circuitry genetically predefined for reading; this is unlike other expert skills such as face recognition, which can be seen in some of our evolutionary ancestors. Therefore, understanding the nature of the reading circuit strikes at the heart of the nature versus nurture debate about how expert skills shape, and are shaped by, brain circuitry. The PNAS article by Lerma-Usabiaga et al. (1) focuses on understanding the specialization of a particular brain territory, the left ventral occipitotemporal cortex (vOTC), known to be critical for visual word recognition. The study uses a combination of magnetic resonance imaging (MRI) and behavioral methods to carefully dissect two functionally and structurally distinct regions located within the vOTC that contribute to reading.
Lerma-Usabiaga et al. (1) were motivated by previous studies that have used functional MRI to localize the “visual word form area” (VWFA). Conceptually, the VWFA is a brain region within the left vOTC that responds preferentially to printed words and word-like stimuli. This orthographic selectivity emerges with the acquisition of literacy, indicating that reading experience tunes this region for skilled reading (2). Indeed, individuals with poorer reading ability exhibit reduced selectivity in the vOTC (3), and damage in adulthood causes acquired alexia, in which printed words can no longer be recognized automatically (4). While researchers agree on these broad facts about the VWFA, they disagree on the precise location of the VWFA and its specific role in reading (4–6).
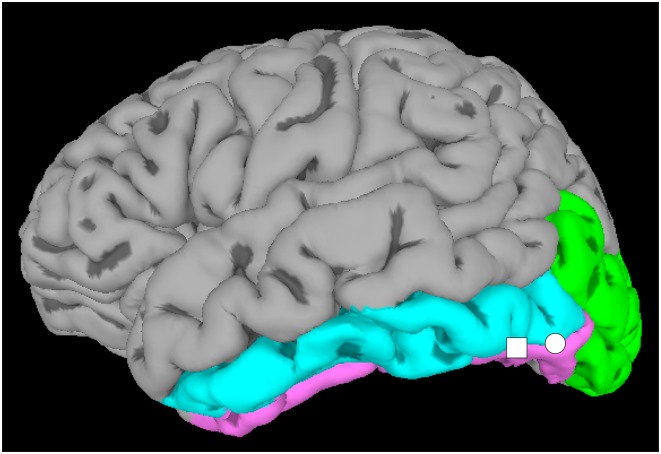
These disagreements could arise because different types of stimulus comparisons weight differentially for perceptual versus linguistic aspects of visual word recognition. Thus, like the proverbial men discussing different parts of the elephant, different groups may be localizing functionally different pieces of the vOTC and interpreting each as the VWFA. Instead, it might be more appropriate to regard the VWFA as an extended territory with distinct subregions. To test this idea, Lerma-Usabiaga et al. (1) employed two different types of stimulus comparisons: (i) perceptual comparisons localized vOTC tissue that responded more to printed words compared with meaningless stimuli without word-like visual structure, and (ii) lexical comparisons were finer grained and localized vOTC tissue that responded more to printed words compared with meaningless but visually word-like strings of letters and characters. Both the perceptual and lexical contrasts identified locations within a particular portion of the vOTC, the occipitotemporal sulcus (OTS) (Fig. 1). However, the perceptual contrasts yielded a maximal location within the posterior OTS (pOTS) region, while the lexical contrasts yielded a maximal location within the middle OTS (mOTS) region. These results suggest that word-responsive tissue within the vOTC can be functionally segregated, with the perceptual processing of visual word forms supported by the pOTS and the lexical processing supported by the mOTS.
Fig. 1.
View of the ventral brain surface. Lerma-Usabiago et al. (1) found two word-selective regions located along the OTS, which lies between the fusiform (pink) and the inferior temporal (blue) gyri, and abuts the inferior occipital (green) gyrus. A pOTS region (circle) showed functional specialization for the perceptual aspects of visual word recognition, while an mOTS region (square) showed specialization for the lexical aspects.
To examine the functional relevance of the pOTS and mOTS regions, Lerma-Usabiaga et al. (1) investigated the relationship between the neural response to orthographic stimuli and behavioral measures from a simple word-recognition task. Individuals with stronger functional specialization of the pOTS and mOTS, as indicated by larger signal differences in the perceptual contrasts, were able to distinguish between words (in the mOTS) and consonant letter strings (in both the pOTS and mOTS) more quickly than individuals with smaller signal differences. In other words, more-skilled readers putatively specialized the vOTC more strongly for reading.
Having differentiated the two vOTC regions on the basis of their functional characteristics, Lerma-Usabiaga et al. (1) considered whether these regions are also structurally distinct from each other. First, they evaluated the connections of the vOTC to other brain regions. Using diffusion imaging, a form of MRI that can visualize the anatomical connections of the brain, they found that connections from regions involved in visual processing terminated in portions of the vOTC that included the pOTS, but not the mOTS, region, whereas the opposite was true for the connections from regions involved in language processing. With the caveat that recent studies suggest diffusion imaging may be limited in its ability to make fine-grained connectivity comparisons (7), these results indicate that the two vOTC regions communicate with different processing networks within the brain, as appropriate for the different roles they play in word recognition.
Lastly, Lerma-Usabiaga et al. (1) evaluated the cellular structure of the vOTC. Building on previous research that defined vOTC subregions based on differences in cellular organization (8), they examined a component of the MRI signal that is influenced by the cellular structure of the tissue from which the signal arises. They observed differences in cellular structure across the vOTC and, importantly, found that their mOTS and pOTS regions fell on opposite sides of a vOTC border delineating portions of the vOTC with differing cellular structure. These results are provocative because differences in cellular structure are thought to endow brain regions with differing capacities for information processing (9).
In summary, the study by Lerma-Usabiaga et al. (1) provides compelling evidence for an extended VWFA territory that falls along the OTS. A pOTS region is more sensitive to perceptual information, and an mOTS region is more sensitive to linguistic information. Furthermore, these two regions show distinct cellular structures and anatomical connectivity, with the pOTS region possessing greater connectivity to visual areas, and the mOTS region to language areas.
Lerma-Usabiaga et al. (1) make a substantial contribution to our understanding of localized regions within the vOTC that support reading, but important questions remain. A recent study showed that a single word-sensitive location within the vOTC can participate in multiple stages of word processing. Activity shortly after seeing a word showed coarse representational structure sufficient to distinguish between visually dissimilar words. Activity about 100 ms later was sufficient to distinguish visually similar words from each other, suggesting a shift to lexical representation (10). Future studies are required to determine how interactions among the pOTS, mOTS, and other visual and language processing regions give rise to the dynamic representation of words within and across word-selective regions.
Perhaps the biggest questions regarding the influence of nature versus nurture in shaping the brain for expertise are how and why do regions modified by reading experience end up in the same vOTC locations across individuals? The structure–function relationships found by Lerma-Usabiaga et al. (1) provide an important clue. The major connections and cellular structure of the brain are under genetic control and substantially in place early in life (9, 11), and prereading morphological and connectivity differences in the vOTC predict the ultimate location of word-sensitive
The study by Lerma-Usabiaga et al. provides compelling evidence for an extended VWFA territory that falls along the OTS.
tissue and reading skill (12, 13). Such findings indicate that vOTC regions likely have predetermined structural characteristics that make them especially suited for reading (14). At the same time, the countless hours of reading practice needed to become a skilled reader may sculpt the cellular structure and anatomical connections of the mOTS and pOTS so that the parts of the brain that need to “work together” end up being more strongly “wired together”. This can explain why literacy drives changes in cellular structure and connectivity of the vOTC, even when it is acquired in adulthood (2). Thus, nature and nurture both seem to be at play in shaping the brain for reading.
A remaining question is why there should be a region in the visual processing stream that has connectivity to the language system before the onset of reading. Visual stimuli such as objects and faces are named, so some connectivity between the visual and language system is necessary (15); historically, many writing systems evolved from the use of pictograms (16). While learning to read, visual words may appropriate a portion of the visual system that is connected to the language system because this is precisely the infrastructure needed for visual word recognition. Another possibility is that the left vOTC is part of a circuit for lipreading (17). Even nonhuman primates have the capacity for a form of lipreading (18), face- and word-sensitive regions are interdigitated in the vOTC (19), and acquired alexia has been associated with lipreading deficits (20). Thus, a genetically and evolutionarily predetermined circuit for lipreading is feasible, and a portion of this circuit may be appropriated for word recognition because both of these processes involve visual forms being associated with spoken sounds. Testing these various hypotheses would provide an important contribution to our understanding of how cultural innovations, such as reading, capitalize on the innate potential of the brain for adaptation.
Acknowledgments
We gratefully acknowledge the support of the National Institute on Deafness and Other Communication Disorders under Grant K18DC014577 (to J.A.F.), the National Institute of Mental Health under Grant R01MH107797 (to A.S.G.), and the National Science Foundation under Grants 1734907 (to A.S.G.) and 1734735 (to J.A.F.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Science Foundation.
Footnotes
The authors declare no conflict of interest.
See companion article on page E9981.
References
- 1.Lerma-Usabiaga G, Carreiras M, Paz-Alonso PM. Converging evidence for functional and structural segregation within the left ventral occipitotemporal cortex in reading. Proc Natl Acad Sci USA. 2018;115:E9981–E9990. doi: 10.1073/pnas.1803003115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dehaene S, Cohen L, Morais J, Kolinsky R. Illiterate to literate: Behavioural and cerebral changes induced by reading acquisition. Nat Rev Neurosci. 2015;16:234–244. doi: 10.1038/nrn3924. [DOI] [PubMed] [Google Scholar]
- 3.Schlaggar BL, McCandliss BD. Development of neural systems for reading. Annu Rev Neurosci. 2007;30:475–503. doi: 10.1146/annurev.neuro.28.061604.135645. [DOI] [PubMed] [Google Scholar]
- 4.Cohen L, Dehaene S. Specialization within the ventral stream: The case for the visual word form area. Neuroimage. 2004;22:466–476. doi: 10.1016/j.neuroimage.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 5.Price CJ, Devlin JT. The myth of the visual word form area. Neuroimage. 2003;19:473–481. doi: 10.1016/s1053-8119(03)00084-3. [DOI] [PubMed] [Google Scholar]
- 6.Vogel AC, Petersen SE, Schlaggar BL. The VWFA: It’s not just for words anymore. Front Hum Neurosci. 2014;8:88. doi: 10.3389/fnhum.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas C, et al. Anatomical accuracy of brain connections derived from diffusion MRI tractography is inherently limited. Proc Natl Acad Sci USA. 2014;111:16574–16579. doi: 10.1073/pnas.1405672111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caspers J, et al. Cytoarchitectonical analysis and probabilistic mapping of two extrastriate areas of the human posterior fusiform gyrus. Brain Struct Funct. 2013;218:511–526. doi: 10.1007/s00429-012-0411-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amunts K, Zilles K. Architectonic mapping of the human brain beyond Brodmann. Neuron. 2015;88:1086–1107. doi: 10.1016/j.neuron.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Hirshorn EA, et al. Decoding and disrupting left midfusiform gyrus activity during word reading. Proc Natl Acad Sci USA. 2016;113:8162–8167. doi: 10.1073/pnas.1604126113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mangin JF, Jouvent E, Cachia A. In-vivo measurement of cortical morphology: Means and meanings. Curr Opin Neurol. 2010;23:359–367. doi: 10.1097/WCO.0b013e32833a0afc. [DOI] [PubMed] [Google Scholar]
- 12.Cachia A, et al. How interindividual differences in brain anatomy shape reading accuracy. Brain Struct Funct. 2018;223:701–712. doi: 10.1007/s00429-017-1516-x. [DOI] [PubMed] [Google Scholar]
- 13.Saygin ZM, et al. Connectivity precedes function in the development of the visual word form area. Nat Neurosci. 2016;19:1250–1255. doi: 10.1038/nn.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin A. Shades of Déjerine—Forging a causal link between the visual word form area and reading. Neuron. 2006;50:173–175. doi: 10.1016/j.neuron.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Price CJ, Devlin JT. The interactive account of ventral occipitotemporal contributions to reading. Trends Cogn Sci. 2011;15:246–253. doi: 10.1016/j.tics.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huey EB. History and Pedagogy of Reading. MacMillan; New York: 1918. [Google Scholar]
- 17.Hannagan T, Amedi A, Cohen L, Dehaene-Lambertz G, Dehaene S. Origins of the specialization for letters and numbers in ventral occipitotemporal cortex. Trends Cogn Sci. 2015;19:374–382. doi: 10.1016/j.tics.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Ghazanfar AA, Takahashi DY. The evolution of speech: Vision, rhythm, cooperation. Trends Cogn Sci. 2014;18:543–553. doi: 10.1016/j.tics.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuo T, et al. Alternating zones selective to faces and written words in the human ventral occipitotemporal cortex. Cereb Cortex. 2015;25:1265–1277. doi: 10.1093/cercor/bht319. [DOI] [PubMed] [Google Scholar]
- 20.Campbell R, Landis T, Regard M. Face recognition and lipreading. A neurological dissociation. Brain. 1986;109:509–521. doi: 10.1093/brain/109.3.509. [DOI] [PubMed] [Google Scholar]