Significance
FtsZ is the founding member of the prokaryotic tubulin family. It assembles into single-stranded filaments that coalesce to form the Z ring that drives bacterial cell division in most bacteria. FtsZ filaments undergo cooperative assembly and display treadmilling behavior. Here we investigate the kinetic polarity of FtsZ filaments by studying the toxicity of longitudinal interface mutants of FtsZ and find that bottom-face mutants are extremely toxic. This toxicity combined with a conformational switch induced during assembly reveals the kinetic polarity of FtsZ filaments, the basis for cooperative assembly, and explains the basis for other inhibitors of FtsZ assembly. It is likely that all members of the FtsZ/tubulin superfamily employ an assembly-induced conformational switch to generate kinetic polarity, which could be revealed by the same approach used here.
Keywords: FtsZ, capper, Z ring, treadmilling, tubulin
Abstract
FtsZ is the ancestral homolog of tubulin and assembles into the Z ring that organizes the division machinery to drive cell division in most bacteria. In contrast to tubulin that assembles into 13 stranded microtubules that undergo dynamic instability, FtsZ assembles into single-stranded filaments that treadmill to distribute the peptidoglycan synthetic machinery at the septum. Here, using longitudinal interface mutants of FtsZ, we demonstrate that the kinetic polarity of FtsZ filaments is opposite to that of microtubules. A conformational switch accompanying the assembly of FtsZ generates the kinetic polarity of FtsZ filaments, which explains the toxicity of interface mutants that function as a capper and reveals the mechanism of cooperative assembly. This approach can also be employed to determine the kinetic polarity of other filament-forming proteins.
Prokaryotic cytoskeletons play essential roles in many fundamental aspects of prokaryotic cell biology, including cytokinesis, cell-shape maintenance, chromosome segregation, and other aspects of cellular organization (1). FtsZ, a prokaryotic tubulin homolog, polymerizes into filaments that coalesce into the Z ring at midcell (2–4). The ring functions as a scaffold for recruitment of the machinery necessary to synthesize septal peptidoglycan (5, 6). Since formation of the Z ring is the first step in bacterial cytokinesis, antagonism of FtsZ polymerization is a prominent mechanism for regulating cell division in bacteria (7–11).
Like tubulin, FtsZ undergoes GTP-dependent, cooperative assembly with a critical concentration of about 1 µM (12, 13). However, FtsZ differs from tubulin in several ways. Tubulin is present as an αβ-heterodimer that assembles into microtubules containing 13 protofilaments (14), whereas FtsZ is a monomer that polymerizes into single-stranded filaments in vitro of about 120–200 nm in length (30–50 subunits) under conditions that best mimic in vivo conditions (5, 15). In vivo, FtsZ assembles into small clusters of filaments of unknown structure that together compose the Z ring (16, 17). Furthermore, microtubules undergo dynamic instability (18), whereas FtsZ filaments treadmill (16, 19). FtsZ filaments form the scaffold for the divisome, and the treadmilling distributes this peptidoglycan synthetic machinery around the septum (16, 20). FtsZ exists in two conformations: an open conformation observed in filaments and a closed conformation in the monomer (21). The basis of treadmilling is an assembly-associated conformational switch that promotes polymerization along with the assembly-associated GTP hydrolysis that weakens intersubunit interactions (21). However, the kinetic polarity of these FtsZ filaments, or how it is achieved, is not known (5).
Dynamic filaments have structural and kinetic polarity. The growing end is referred to as the “plus” end and the other end is referred to as the “minus” end. When tubulin assembles into microtubules, the bound GTP (top end) is exposed at the plus end, and incoming subunits interact with this end through their T7 loop (bottom end) (14). Like tubulin, FtsZ assembles in a head-to-tail manner, however, there are several indications that the polarity of FtsZ assembly is opposite to that of microtubules.
Since FtsZ filaments treadmill, longitudinal interface mutants of FtsZ should have different effects on assembly. Interface mutants that can add to the growing end but prevent further growth should be toxic to assembly and thus cell division at substoichiometric levels, whereas interface mutants that cannot add onto filaments should be much less toxic. Redick et al. (22) observed that some amino acid substitutions at the bottom end of FtsZ were toxic, whereas substitutions at the top end were not. Furthermore, expression of just the N-terminal domain (1–193, top end), but not the C-terminal domain of FtsZ, showed some toxicity (23). Also, the N-terminal domain of FtsZ has been shown to antagonize FtsZ polymerization in vitro (24). These observations suggest that FtsZ filaments have the opposite kinetic polarity of microtubules; however, previous measurements showed that neither the bottom-face mutants nor the N-terminal domain of FtsZ was toxic at substoichiometric levels but needed to be overexpressed three-to fivefold (22, 23), arguing against this suggestion. Since the mutations examined in the prior study were made before the structure of an FtsZ filament was known, and many of the mutants still assembled in vivo, we decided to reinvestigate the effect of interface mutants using known mutations. Our findings demonstrate that the kinetic polarity of FtsZ filaments is the opposite of tubulin assembly into microtubules. Furthermore, we propose how the conformational switch coupled to assembly promotes polymerization.
Results
Strategy for Determining Polarity of FtsZ Filaments.
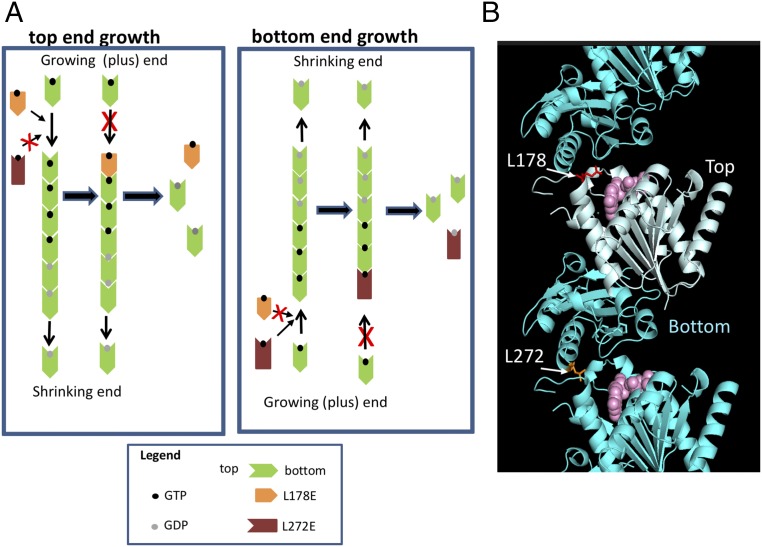
The expected effect of top and bottom interface mutants on treadmilling of FtsZ is outlined in Fig. 1A. In the first scenario, FtsZ polymerizes in the same direction as tubulin (top-end growth), and a top interface mutant would add to the growing or plus end blocking the growth of the filament, resulting in disassembly. Such a mutant would be expected to be dominant-negative and inhibit cell division, whereas a bottom interface mutant would not affect polymerization and thus would be nontoxic. In the second scenario, FtsZ polymerizes in the opposite direction of tubulin (bottom-end growth), and the effects of the top- and bottom-face mutants on polymerization and cell division would be reversed. Li et al. (25) described interface mutants that are unable to assemble including top [FtsZL178E(top)] and bottom-face mutants [FtsZL272E(bottom)]. These mutants do not assemble because charged residues would be forced into hydrophobic pockets formed between subunits in the filament (Fig. 1B).
Fig. 1.
FtsZ longitudinal interface mutants and the scheme used to determine FtsZ polymer polarity. (A) Two possible scenarios for the growth of FtsZ polymers. In the first case (top-end growth), FtsZ polymerizes in the same direction as tubulin with the bottom surface of an incoming subunit interacting with the top surface (GTP end) of the subunit at the growing end of a polymer, called the plus (+) end in tubulin. Subunits at the shrinking end dissociate from the filament following GTP hydrolysis. In this case, the top-surface mutant (FtsZL178E) adds to a treadmilling filament blocking further subunit addition resulting in disassembly from the shrinking end, while the bottom-surface mutant (FtsZL272E) does not have an effect. In the second situation, FtsZ polymerizes in the opposite direction of tubulin. In this case, the bottom-surface mutant FtsZL272E adds to the growing end of the filament and would block further growth, while the top-surface mutant does not have an effect. (B) FtsZ interface mutants used in this study. The FtsZL178E monomer was superimposed on the FtsZ filament from Staphylococcus aureus (PDB::3VOB). The GTP-binding domain (N-terminal) is the top interface (light cyan), while the domain containing the T7 synergy loop (C-terminal) is the bottom interface (dark cyan). GDP is pink.
The Bottom Interface Mutant Is Toxic at Substoichiometric Levels.
Two plasmid vectors were used to express the ftsZ alleles: one expressed the alleles under the control of an arabinose-inducible promoter and the other under the control of an isopropyl β-d-thiogalactopyranoside (IPTG)-inducible promoter. Initially, the two interface mutations, ftsZL178E and ftsZL272E, were introduced into pSEB135 (Para::ftsZ) and tested for their ability to complement a depletion strain [SD18 ftsZo recA56/repATs (PftsZ::ftsZ)] that has ftsZ provided by a temperature-sensitive plasmid. We also included ftsZ360, an allele deleted for the conserved tail of ftsZ that mediates interaction with multiple FtsZ partners (26), the expression of which is known to be toxic (27). We confirmed that these three alleles were unable to complement the depletion strain at the nonpermissive temperature of 42 °C (SI Appendix, Fig. S1). Furthermore, we observed that expression of ftsZL272E and ftsZ360 was toxic at 30 °C, whereas expression of ftsZ178E was not. By tagging the proteins with GFP, we confirmed that FtsZL178E and FtsZL272E were unable to polymerize in vivo (unable to localize to midcell) (SI Appendix, Fig. S2A). Furthermore, we confirmed that the purified proteins were unable to polymerize in vitro (SI Appendix, Fig. S2B).
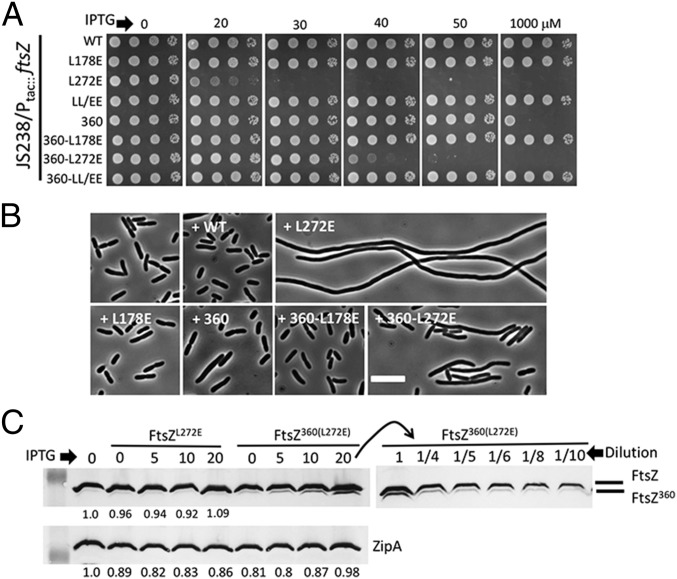
Although arabinose-inducible vectors offer some advantages (tighter off regulation), they have some disadvantages (variable cell-to-cell induction) (28). To better assess the toxicity of ftsZL272E, we attempted to clone it into an IPTG-inducible vector; however, we were unsuccessful. Since this was likely due to toxicity from leaky expression, we modified the ribosome binding site for ftsZ on the plasmid to reduce expression (from CGGAGA to CGCAGA). Following this change, we were able to clone all ftsZ alleles into the IPTG-inducible vector. Induction of ftsZL272E and ftsZ360 from this plasmid was toxic with ftsZL272E being much more toxic (Fig. 2A). Induction of ftsZL272E in a WT strain caused filamentation and blocked colony formation at 30 μM IPTG (Fig. 2 A and B). Since induction of ftsZ with 1 mM IPTG from this plasmid does not provide enough FtsZ to fully complement a ftsZ-depletion strain (SI Appendix, Fig. S3), we conclude that FtsZL272E is inhibitory at substoichiometric levels. If the toxicity of FtsZL272E is due to incorporation into filaments, then addition of the L178E substitution to disrupt the other longitudinal interface should prevent incorporation into the polymer and alleviate the toxicity. Indeed, expression of the double mutant (ftsZLL/EE) did not block division and was not toxic (Fig. 2 A and B). The toxicity of FtsZ360, which lacks the conserved C-terminal peptide that mediates interaction with multiple FtsZ partners (27), was also eliminated by addition of the L178E substitution, whereas addition of the L272E substitution increased the toxicity of FtsZ360 (Fig. 2 A and B). These results are consistent with L178E substitution preventing subunit addition to a filament whereas the L272E substitution likely allows subunit addition but prevents further growth of the filament.
Fig. 2.
The bottom-surface mutant (FtsZL272E) is dominant negative with substoichiometric toxicity. Plasmid pSD334 (Ptac::ftsZ) or derivatives containing different ftsZ alleles were transformed into strain JS238, and transformants were tested for toxicity. A 3-µL aliquot from each 10-fold serial dilution was spotted onto LB plates with chloramphenicol and IPTG and incubated at 37 °C overnight before imaging. (B) Cultures of some of the strains from A were induced with 30 μM IPTG for 2 h, and cells were photographed. (Scale bar: 5 µm.) (C) JS238-containing derivatives of pSD334 expressing ftsZL272E or ftsZ360(L272E) were induced with the indicated concentration of IPTG for 2 h, and a Western blot was performed to assess the level of the induced proteins. ZipA was blotted as a control. The sample from the 20-μM induction of ftsZ360(L272E) was serially diluted as indicated to assess the induced amount of FtsZ360(L272E) relative to the endogenous level of FtsZ. The level of FtsZ in the 1/10 dilution was about the level of FtsZ360(L272E) in the undiluted sample.
As shown in Fig. 2B and SI Appendix, Fig. S4, induction of ftsZL272E with as little as 5 μM ITPG caused cells to elongate, and they became extremely filamentous with 20 µM IPTG even though the level of FtsZ did not increase significantly (endogenous FtsZ + induced FtsZL272E) (Fig. 2C). To determine the level of FtsZL272E necessary to cause toxicity, we measured the level of FtsZ360(L272E), which can be distinguished from full-length FtsZ, and found that, with 20 µM IPTG, its level was only 10% of the level of the endogenous FtsZ (Fig. 2C). This result confirms that FtsZL272E is toxic at a substoichiometric level. This result is in contrast to previous observations that bottom-face mutants had to be overexpressed three- to fivefold to block colony formation using a plasmid with ftsZ under the control of an arabinose-inducible promoter (22). As shown in SI Appendix, Fig. S1, complementation with the arabinose-inducible promoter required 0.05–0.1% arabinose, but expression of ftsZL272E was already toxic to WT cells at 0.006% (SI Appendix, Fig. S5), consistent with FtsZL272E being toxic at substoichiometric levels.
FtsZL272E Incorporates into Z Rings to Cause Disruption.
To understand how the FtsZ mutants blocked cell division, we expressed the various alleles of ftsZ under the control of the arabinose promoter and examined Z-ring formation using ZipA-GFP as a proxy. Following induction of WT FtsZ with 0.05% arabinose for 2 h, 91% of the cells had ZipA-GFP rings, whereas induction of FtsZL272E reduced the number of cells with ZipA-GFP rings to 0.7% (Fig. 3A and SI Appendix, Fig. S6A). Induction of FtsZL178E or FtsZLL/EE had little effect (86 and 88% of cells with rings, respectively) even though they were induced to the same level (SI Appendix, Fig. S6 B and C). With induction of FtsZ360, the percentage of cells with rings was reduced to 32% as many rings had started to spiral away, likely due to heteropolymers containing FtsZ360 (unable to bind to FtsZ’s membrane tethers) poorly anchored to the membrane. Kinetic analysis revealed that Z rings started to disappear at 80 min after induction of FtsZL272E (SI Appendix, Fig. S7 A and B) before the level of FtsZ (FtsZ360 as a proxy) could be observed to increase (SI Appendix, Fig. S7C). These induction kinetics (rapid increase in the induced protein between 90 and 100 min) with the arabinose system are likely responsible for the previous underestimate of the toxicity (22).
Fig. 3.
FtsZL272E disrupts Z rings and antagonizes FtsZ polymerization. (A) FtsZL272E disrupts Z rings. Exponentially growing cultures of W3110 carrying derivatives of pSEB135 (pBAD18, Para::ftsZ) with various ftsZ alleles and pSEB206 (pEXT22, Ptac::zipA-gfp) growing in LB with 30 μM IPTG were induced for 2 h with 0.05% arabinose, and samples were visualized by fluorescence microscopy to assess ZipA-GFP localization (as a proxy for Z rings). (Scale bar: 5 µm.) (B) FtsZL272E localizes to Z rings. FtsZL272E-mNeon was observed in constricting cells whereas FtsZL178E-mNeon did not localize. Samples of transformants of JS238 with derivatives of pSD334 (Ptac::ftsZ) with various alleles of ftsZ were taken from the selection plates (with 0.2% glucose) while the colonies were still small and visualized by fluorescence microscopy. (Scale bar: 3 µm.) (C) Diagram of the constructs used. WT ftsZ is on the chromosome, and the FtsZ indicated in A and B was induced from the plasmid.
To see if FtsZL272E localizes to Z rings before they are disrupted, we used ftsZ-mNeonGreen fusions [which are brighter than GFP (29)] expressed from the IPTG-inducible vector. Whereas FtsZ-mNeonGreen was readily observed in rings at midcell, FtsZL272E-mNeonGreen was not as easy to detect; however, we observed localization in some constricting cells. In contrast, no localization of FtsZL178E-mNeonGreen to the midcell was detected (Fig. 3B). Thus, FtsZL272E is incorporated into FtsZ polymers at the Z ring before the ring is disrupted.
FtsZL272E Blocks Polymerization of FtsZ.
We next examined the effect of FtsZL178E and FtsZL272E on FtsZ polymerization in vitro using a sedimentation assay and electron microscopy. For the sedimentation test, we used FtsZ∆CL, a variant of FtsZ (deletion of the long carboxy linker) that polymerizes well and can be distinguished from FtsZL178E and FtsZL272E on SDS/PAGE gels (30). FtsZ∆CL sedimented in the presence of GTP, while FtsZL178E, FtsZL272E, and FtsZLL/EE did not (SI Appendix, Fig. S8). Addition of FtsZL178E to FtsZ∆CL did not affect the sedimentation of FtsZ∆CL, whereas FtsZL272E reduced the amount of FtsZ∆CL in the pellet in a dose-dependent manner (Fig. 4A). Adding the L178E substitution to FtsZL272E (FtsZLL/EE) eliminated its ability to antagonize FtsZ∆CL sedimentation. Electron microscopy revealed that FtsZL272E (1:1 ratio) dramatically reduced the number of FtsZ or FtsZ∆CL filaments (Fig. 4B and SI Appendix, Fig. S9, respectively) whereas FtsZL178E or FtsZLL/EE did not have an effect. Since the addition of FtsZL272E to FtsZ (1:1 ratio) increased the GTPase activity (Fig. 4C) while dramatically reducing the number of filaments (Fig. 4B), sequestration is ruled out and suggests that short filaments (of a few subunits) not detected in the EM are capable of GTPase activity. Importantly, the addition of L178E to FtsZL272E (FtsZLL/EE) reduced the ability of FtsZL272E to stimulate the GTPase activity. FtsZL178E also increased the GTPase activity but to a lesser extent than FtsZL272E did. FtsZL178E likely stimulates the GTPase because it can participate in nucleation (being the first subunit in a filament). However, it is possible that FtsZL178E and FtsZLL/EE retain weak interaction with FtsZ that is sufficient to stimulate the GTPase activity. Together, these results are consistent with the bottom-face mutant capping the end of the filament to block subunit addition leading to disassembly of the filament.
Fig. 4.
FtsZL272E antagonizes FtsZ assembly but enhances its GTPase activity. (A) The various FtsZ mutants were added to FtsZΔCL (kept at 5 μM) at the indicated ratios. GTP was added to 1 mM, and after 5 min of incubation the samples were centrifuged and the pellets and supernatants were analyzed by SDS/PAGE. (B) FtsZ (2 μM) was mixed with the indicated FtsZ mutants (2 μM) and polymerized by the addition of GTP and examined by negative-stain electron microscopy. (C) The various FtsZ mutants were mixed with FtsZ (2 μM) at a 1:1 ratio. GTP was added, and GTPase activity was determined as described in Materials and Methods.
Computation Model for FtsZ Treadmilling.
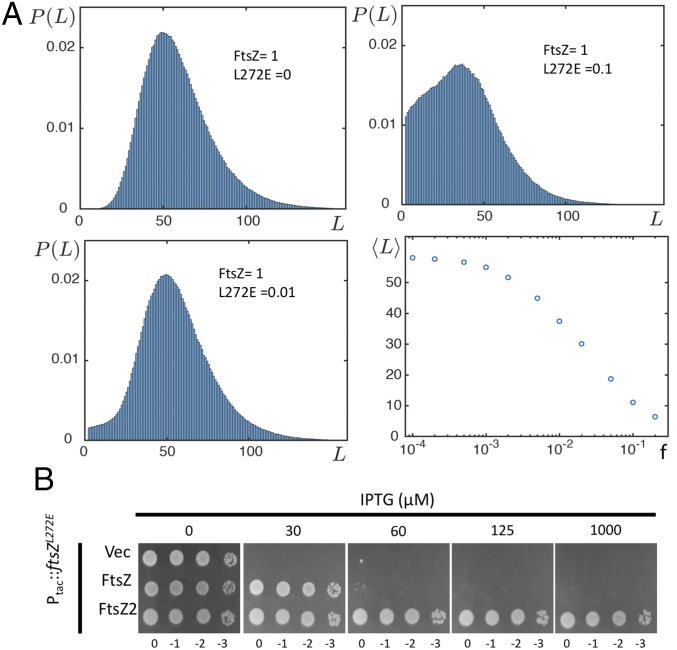
To further assess the impact of FtsZL272E on FtsZ assembly, we employed a computational model for treadmilling (31, 32) based upon what is known about FtsZ assembly (Materials and Methods). This model accounts for the following processes: (i) GTP subunit attachment at the bottom end and GDP subunit detachment at the top end, (ii) conversion of GTP to GDP subunits within the filament, and (iii) the attachment of mutant subunits that cap the filament and prevent further binding of subunits to the bottom end. Also, hydrolysis occurs at a constant rate, and there is no impact on the hydrolysis rate by the state of neighboring subunits in the filament. The parameters are kA (rate for GTP subunit attachment), kD (rate for GDP subunit detachment), kH for the GTPase, and f (ratio of FtsZ mutant to FtsZ). With the parameters used, the average length of FtsZ filaments is 55 subunits with a range from 25 to over 100 (Fig. 5A). As FtsZL272E is titrated in, the average length starts to decrease in a dose-dependent manner. At a ratio of 1:10 (FtsZL272E to WT), the average length of FtsZ polymers is reduced about 75%. This result would be consistent with FtsZL272E reducing polymer length in vivo leading to dissolution of the Z ring. If so, increasing the amount of FtsZ should provide some resistance to FtsZL272E due to an increase in the ratio of FtsZ to FtsZL272E. Indeed, adding a plasmid that increases the level of FtsZ about twofold (33) increased the concentration of IPTG (and therefore the amount of FtsZL272E) required to prevent colony formation from 30 to 60 μM (Fig. 5B).
Fig. 5.
Modeling the effect of FtsZL272E on FtsZ polymer length and the effect of increasing FtsZ and decreasing GTPase activity on suppressing FtsZL272E toxicity. (A) Treadmilling model for FtsZ was used to assess the effect of FtsZL272E on FtsZ filament length with FtsZ polymer-length distribution as a function of the ratio of FtsZL272E to FtsZ. The fourth panel plots the average polymer length versus the FtsZL272E-to-FtsZ ratio. (B) Increasing FtsZ and reducing GTPase activity suppress the toxicity of FtsZL272E. JS238/pSD334-L272E (pEXT22, Ptac::ftsZL272E) containing plasmids expressing ftsZ (pBEF0), ftsZD212G (pBEF2), or a vector control (pGB2) was subjected to a spot test on plates with increasing IPTG.
Effect of GTPase Mutant on Toxicity of FtsZL272E.
All known inhibitors of FtsZ polymerization require the GTPase activity of FtsZ to antagonize FtsZ polymerization, and a reduction in the GTPase activity (due to mutation) is a nonspecific mechanism resulting in resistance (9). We therefore tested whether FtsZL272E behaved similarly by testing its effect on FtsZD212N, which has been previously shown to be competent for polymerization but defective in GTPase activity (34). Importantly, FtsZL272E did not affect FtsZD212N sedimentation (SI Appendix, Fig. S10), indicating that FtsZL272E also requires the GTPase activity of FtsZ to antagonize polymerization. To further confirm this, we tested whether expression of a GTPase mutant of FtsZ would provide resistance to the toxicity of FtsZL272E in vivo. For this we used FtsZD212G (FtsZ2), which has very low GTPase activity and provides resistance to the well-characterized FtsZ inhibitors SulA and MinC/MinD (35, 36). As shown in Fig. 5B, the plasmid expressing ftsZ enabled cells to grow at 30 µM IPTG, but the presence of ftsZ2 allowed cells to grow at 1 mM IPTG. Thus, increasing the ratio of FtsZ to FtsZL272E suppresses FtsZL272E toxicity, but increasing the stability of FtsZ polymers by copolymerization of a mutant with decreased GTPase activity is dramatically more effective.
Discussion
FtsZ is an ancient filament-forming protein that undergoes cooperative assembly. FtsZ filaments assemble into the Z ring, which is used by most prokaryotic cells for division (2, 37, 38). FtsZ filaments display treadmilling behavior that is used by bacteria to distribute the septal biosynthetic machinery around the septum (16, 20). Here we show that a bottom-face mutant inhibits division in vivo by incorporating into the Z ring, leading to its dissolution, whereas in vitro this mutant antagonizes FtsZ assembly without inhibiting its GTPase activity. In contrast, a top-face mutant is not toxic and has no effect on FtsZ assembly. Furthermore, such a top-face mutation also eliminates the effects of the bottom-face mutant on cell division as well as FtsZ assembly. These observations are consistent with a bottom-face mutant functioning as a capper that blocks filament growth leading to filament disassembly. Thus, we conclude that FtsZ filaments have opposite kinetic polarity of microtubules (bottom-end growth, Figs. 1A and 6).
Fig. 6.
Model for FtsZ assembly that accounts for the toxicity of FtsZL272E. FtsZ with GTP bound exists in two conformations, open and closed, with the closed form preferred. Above the critical concentration, two subunits in the open form combine to generate a nucleus, and additional subunits in the closed form add to the bottom end with the energy of binding causing a conformational switch (snap into the open form) to produce a bottom end with high affinity for another GTP-bound subunit. GTP hydrolysis follows assembly resulting in subunits disassociating from the top end, leading to treadmilling. FtsZL272E adds to the bottom end and snaps into the open form, but subsequent subunit addition is prevented, leading to disassembly of the filament. As subunits disassemble, they return to the closed form and rapidly undergo exchange to the GTP form.
For a linear filament like FtsZ to undergo cooperative assembly, it was proposed that FtsZ undergoes a conformational switch upon assembly (9, 39, 40). In fact, two conformational forms of FtsZ have been observed: an open form seen when bound to the PC190723 inhibitor and in a filament and a closed form observed in an FtsZ monomer (21, 41). The GTP-bound form of FtsZ exists in these two conformations with the closed conformation the preferred form. Above the critical concentration, sufficient FtsZ exists in the open form to form nuclei consisting of at least a dimer of FtsZ with both subunits in the open form. Based upon our results we propose that the switch to the open form causes the bottom end (T7 end) to have high affinity for the GTP end of another FtsZ molecule, whether it is in the closed or open conformation. This allows an FtsZ monomer in the closed form to add on, which then snaps into the open form, and the process is repeated, resulting in cooperative assembly (Fig. 6). FtsZL272E can add to the end of a filament and snap to the open form, but subsequent addition is blocked by the substitution, causing it to act as a cap. This cap persists until the filament disassembles, making it possible to detect FtsZL272E at the Z ring despite its toxicity. In contrast, FtsZL178E can participate only in nucleation of a filament (the first subunit only), but due to treadmilling is also the first to disassemble, making it difficult to detect at the Z ring.
The above model is also consistent with the effect of the known FtsZ inhibitors MciZ and SulA. MciZ prevents or dismantles Z rings in the mother cell following asymmetric septation during sporulation in Bacillus subtilis by reducing the average length of FtsZ filaments by 50% (10, 42). From the crystal structure of the MciZ–FtsZ complex it is clear that MciZ binds to the closed form of FtsZ (10, 42). This suggests that the MciZ–FtsZ complex adds to the end of the filament, which prevents an additional FtsZ subunit from adding on. Thus, an MciZ–FtsZ complex is equivalent to FtsZL272E (Fig. 6). Previously, it was proposed that MciZ acts primarily by preventing filament annealing (10). Although observed in vitro with purified FtsZ (43, 44), it is not clear whether two membrane-tethered FtsZ filaments would have sufficient mobility to encounter each other in vivo to undergo annealling. SulA, an inhibitor of Z-ring assembly produced in response to DNA damage in E. coli (45), has been characterized as sequestering FtsZ since it blocks the GTPase activity (9, 11). However, it is likely that at lower concentrations it functions as a capper as it binds to the same end of FtsZ as MciZ (46). Thus, the FtsZ–SulA complex would also mimic FtsZL272E.
Both FtsZL272E and MciZ cause a collapse of the Z ring due to shortening of FtsZ filaments. The addition of FtsZ2 provides dramatic resistance to FtsZL272E, indicating that mixing in a GTPase-deficient mutant is sufficient to reduce treadmilling and produce longer filaments to rescue the Z ring. This reduced rate of treadmilling leading to an increased average length of filaments is presumably why reduction in the GTPase activity of FtsZ is a nonspecific mechanism to provide resistance to FtsZ inhibitors (47).
Our finding that FtsZ filaments have the opposite kinetic polarity of microtubules is somewhat surprising, considering that FtsZ and tubulin are related (48). Importantly, the conformational change associated with FtsZ assembly (closed to open form) makes it possible for another FtsZ (closed form) to add to the filament. A conformational change is likely also associated with microtubule assembly because the αβ-tubulin heterodimer has been shown to adopt curved and straight conformations (49). Similar to FtsZ, we propose that an unpolymerized GTP-bound αβ-tubulin heterodimer exists in both the curved and the straight forms with the curved form preferred. A curved αβ-tubulin heterodimer adds to the growing microtubule and snaps into the straight form, which has high affinity for a new incoming curved dimer. This is very similar to FtsZ except that the conformational change in αβ-tubulin is reversed, and assembly results in the generation of a GTP end (β-tubulin) with high affinity for the T7 end of an incoming subunit (α-tubulin). The employment of a polymerization-induced conformational switch to generate kinetic polarity is likely conserved in other members of the FtsZ/tubulin family, such as TubZ, PhuZ, and CetZ, as recently suggested (21). Examining the effect of interface mutants with FtsZ as we have done here should allow determination of the kinetic polarity of such filaments, and this approach can also be employed to determine the kinetic polarity of other filament-forming proteins.
Materials and Methods
SI Appendix, Materials and Methods, contains descriptions of bacterial strains, plasmids, strain and plasmid construction, growth conditions, and procedures. SI Appendix, Materials and Methods, also contains procedures for complementation assay, immunofluorescence microscopy, bacterial two-hybrid assays, visualization of GFP fusion proteins, GTPase assay, purification of FtsZ and mutants, sedimentation and electron microscopy assays for FtsZ polymerization, and Western blot. It also contains a section on the simulation of FtsZ treadmilling. The bacterial strains and plasmids used in this study are listed in SI Appendix, Table S1, and the oligonucleotide primers are listed in SI Appendix, Table S2.
Supplementary Material
Acknowledgments
We thank Scott Lovell for the structural overlays and Tom Bernhardt for plasmid pHC892. This work was supported by NIH Grant GM29764 (to J.L.)
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1811919115/-/DCSupplemental.
References
- 1.Wagstaff J, Löwe J. Prokaryotic cytoskeletons: Protein filaments organizing small cells. Nat Rev Microbiol. 2018;16:187–201. doi: 10.1038/nrmicro.2017.153. [DOI] [PubMed] [Google Scholar]
- 2.Bi EF, Lutkenhaus J. FtsZ ring structure associated with division in Escherichia coli. Nature. 1991;354:161–164. doi: 10.1038/354161a0. [DOI] [PubMed] [Google Scholar]
- 3.Mukherjee A, Lutkenhaus J. Guanine nucleotide-dependent assembly of FtsZ into filaments. J Bacteriol. 1994;176:2754–2758. doi: 10.1128/jb.176.9.2754-2758.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Löwe J, Amos LA. Crystal structure of the bacterial cell-division protein FtsZ. Nature. 1998;391:203–206. doi: 10.1038/34472. [DOI] [PubMed] [Google Scholar]
- 5.Erickson HP, Anderson DE, Osawa M. FtsZ in bacterial cytokinesis: Cytoskeleton and force generator all in one. Microbiol Mol Biol Rev. 2010;74:504–528. doi: 10.1128/MMBR.00021-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du S, Lutkenhaus J. Assembly and activation of the Escherichia coli divisome. Mol Microbiol. 2017;105:177–187. doi: 10.1111/mmi.13696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lutkenhaus J. Assembly dynamics of the bacterial MinCDE system and spatial regulation of the Z ring. Annu Rev Biochem. 2007;76:539–562. doi: 10.1146/annurev.biochem.75.103004.142652. [DOI] [PubMed] [Google Scholar]
- 8.Bi E, Lutkenhaus J. Cell division inhibitors SulA and MinCD prevent formation of the FtsZ ring. J Bacteriol. 1993;175:1118–1125. doi: 10.1128/jb.175.4.1118-1125.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dajkovic A, Mukherjee A, Lutkenhaus J. Investigation of regulation of FtsZ assembly by SulA and development of a model for FtsZ polymerization. J Bacteriol. 2008;190:2513–2526. doi: 10.1128/JB.01612-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bisson-Filho AW, et al. FtsZ filament capping by MciZ, a developmental regulator of bacterial division. Proc Natl Acad Sci USA. 2015;112:E2130–E2138. doi: 10.1073/pnas.1414242112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Milam SL, Erickson HP. SulA inhibits assembly of FtsZ by a simple sequestration mechanism. Biochemistry. 2012;51:3100–3109. doi: 10.1021/bi201669d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mukherjee A, Lutkenhaus J. Dynamic assembly of FtsZ regulated by GTP hydrolysis. EMBO J. 1998;17:462–469. doi: 10.1093/emboj/17.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Erickson HP. Rapid in vitro assembly dynamics and subunit turnover of FtsZ demonstrated by fluorescence resonance energy transfer. J Biol Chem. 2005;280:22549–22554. doi: 10.1074/jbc.M500895200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desai A, Mitchison TJ. Microtubule polymerization dynamics. Annu Rev Cell Dev Biol. 1997;13:83–117. doi: 10.1146/annurev.cellbio.13.1.83. [DOI] [PubMed] [Google Scholar]
- 15.Huecas S, et al. Energetics and geometry of FtsZ polymers: Nucleated self-assembly of single protofilaments. Biophys J. 2008;94:1796–1806. doi: 10.1529/biophysj.107.115493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X, et al. GTPase activity-coupled treadmilling of the bacterial tubulin FtsZ organizes septal cell wall synthesis. Science. 2017;355:744–747. doi: 10.1126/science.aak9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strauss MP, et al. 3D-SIM super resolution microscopy reveals a bead-like arrangement for FtsZ and the division machinery: Implications for triggering cytokinesis. PLoS Biol. 2012;10:e1001389. doi: 10.1371/journal.pbio.1001389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchison T, Kirschner M. Dynamic instability of microtubule growth. Nature. 1984;312:237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- 19.Loose M, Mitchison TJ. The bacterial cell division proteins FtsA and FtsZ self-organize into dynamic cytoskeletal patterns. Nat Cell Biol. 2014;16:38–46. doi: 10.1038/ncb2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bisson-Filho AW, et al. Treadmilling by FtsZ filaments drives peptidoglycan synthesis and bacterial cell division. Science. 2017;355:739–743. doi: 10.1126/science.aak9973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagstaff JM, et al. A polymerization-associated structural switch in FtsZ that enables treadmilling of model filaments. MBio. 2017;8:e00254-17. doi: 10.1128/mBio.00254-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Redick SD, Stricker J, Briscoe G, Erickson HP. Mutants of FtsZ targeting the protofilament interface: Effects on cell division and GTPase activity. J Bacteriol. 2005;187:2727–2736. doi: 10.1128/JB.187.8.2727-2736.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osawa M, Erickson HP. Probing the domain structure of FtsZ by random truncation and insertion of GFP. Microbiology. 2005;151:4033–4043. doi: 10.1099/mic.0.28219-0. [DOI] [PubMed] [Google Scholar]
- 24.Arumugam S, Petrašek Z, Schwille P. MinCDE exploits the dynamic nature of FtsZ filaments for its spatial regulation. Proc Natl Acad Sci USA. 2014;111:E1192–E1200. doi: 10.1073/pnas.1317764111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, et al. FtsZ protofilaments use a hinge-opening mechanism for constrictive force generation. Science. 2013;341:392–395. doi: 10.1126/science.1239248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ortiz C, Natale P, Cueto L, Vicente M. The keepers of the ring: Regulators of FtsZ assembly. FEMS Microbiol Rev. 2016;40:57–67. doi: 10.1093/femsre/fuv040. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Huang J, Mukherjee A, Cao C, Lutkenhaus J. Analysis of the interaction of FtsZ with itself, GTP, and FtsA. J Bacteriol. 1997;179:5551–5559. doi: 10.1128/jb.179.17.5551-5559.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guzman LM, Belin D, Carson MJ, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaner NC, et al. A bright monomeric green fluorescent protein derived from Branchiostoma lanceolatum. Nat Methods. 2013;10:407–409. doi: 10.1038/nmeth.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du S, Park KT, Lutkenhaus J. Oligomerization of FtsZ converts the FtsZ tail motif (conserved carboxy-terminal peptide) into a multivalent ligand with high avidity for partners ZipA and SlmA. Mol Microbiol. 2015;95:173–188. doi: 10.1111/mmi.12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erlenkämper C, Kruse K. Uncorrelated changes of subunit stability can generate length-dependent disassembly of treadmilling filaments. Phys Biol. 2009;6:046016. doi: 10.1088/1478-3975/6/4/046016. [DOI] [PubMed] [Google Scholar]
- 32.Erlenkämper C, Kruse K. Treadmilling and length distributions of active polar filaments. J Chem Phys. 2013;139:164907. doi: 10.1063/1.4825248. [DOI] [PubMed] [Google Scholar]
- 33.Bi E, Lutkenhaus J. FtsZ regulates frequency of cell division in Escherichia coli. J Bacteriol. 1990;172:2765–2768. doi: 10.1128/jb.172.5.2765-2768.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho H, McManus HR, Dove SL, Bernhardt TG. Nucleoid occlusion factor SlmA is a DNA-activated FtsZ polymerization antagonist. Proc Natl Acad Sci USA. 2011;108:3773–3778. doi: 10.1073/pnas.1018674108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bi E, Lutkenhaus J. Analysis of ftsZ mutations that confer resistance to the cell division inhibitor SulA (SfiA) J Bacteriol. 1990;172:5602–5609. doi: 10.1128/jb.172.10.5602-5609.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mukherjee A, Saez C, Lutkenhaus J. Assembly of an FtsZ mutant deficient in GTPase activity has implications for FtsZ assembly and the role of the Z ring in cell division. J Bacteriol. 2001;183:7190–7197. doi: 10.1128/JB.183.24.7190-7197.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Margolin W. Themes and variations in prokaryotic cell division. FEMS Microbiol Rev. 2000;24:531–548. doi: 10.1111/j.1574-6976.2000.tb00554.x. [DOI] [PubMed] [Google Scholar]
- 38.Margolin W. FtsZ and the division of prokaryotic cells and organelles. Nat Rev Mol Cell Biol. 2005;6:862–871. doi: 10.1038/nrm1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miraldi ER, Thomas PJ, Romberg L. Allosteric models for cooperative polymerization of linear polymers. Biophys J. 2008;95:2470–2486. doi: 10.1529/biophysj.107.126219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.González JM, et al. Essential cell division protein FtsZ assembles into one monomer-thick ribbons under conditions resembling the crowded intracellular environment. J Biol Chem. 2003;278:37664–37671. doi: 10.1074/jbc.M305230200. [DOI] [PubMed] [Google Scholar]
- 41.Matsui T, Han X, Yu J, Yao M, Tanaka I. Structural change in FtsZ induced by intermolecular interactions between bound GTP and the T7 loop. J Biol Chem. 2014;289:3501–3509. doi: 10.1074/jbc.M113.514901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Handler AA, Lim JE, Losick R. Peptide inhibitor of cytokinesis during sporulation in Bacillus subtilis. Mol Microbiol. 2008;68:588–599. doi: 10.1111/j.1365-2958.2008.06173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Y, Erickson HP. FtsZ filament dynamics at steady state: Subunit exchange with and without nucleotide hydrolysis. Biochemistry. 2009;48:6664–6673. doi: 10.1021/bi8022653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mingorance J, et al. Visualization of single Escherichia coli FtsZ filament dynamics with atomic force microscopy. J Biol Chem. 2005;280:20909–20914. doi: 10.1074/jbc.M503059200. [DOI] [PubMed] [Google Scholar]
- 45.Huisman O, D’Ari R. An inducible DNA replication-cell division coupling mechanism in E. coli. Nature. 1981;290:797–799. doi: 10.1038/290797a0. [DOI] [PubMed] [Google Scholar]
- 46.Cordell SC, Robinson EJ, Lowe J. Crystal structure of the SOS cell division inhibitor SulA and in complex with FtsZ. Proc Natl Acad Sci USA. 2003;100:7889–7894. doi: 10.1073/pnas.1330742100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dai K, Mukherjee A, Xu Y, Lutkenhaus J. Mutations in ftsZ that confer resistance to SulA affect the interaction of FtsZ with GTP. J Bacteriol. 1994;176:130–136. doi: 10.1128/jb.176.1.130-136.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nogales E, Downing KH, Amos LA, Löwe J. Tubulin and FtsZ form a distinct family of GTPases. Nat Struct Biol. 1998;5:451–458. doi: 10.1038/nsb0698-451. [DOI] [PubMed] [Google Scholar]
- 49.Brouhard GJ, Rice LM. The contribution of αβ-tubulin curvature to microtubule dynamics. J Cell Biol. 2014;207:323–334. doi: 10.1083/jcb.201407095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.