Abstract
The aim of the present study was to define the function of microRNA-424-5p (miR-424) in breast cancer cells. The present study investigated the level and the potential function of miR-424 in breast cancer by reverse transcription-quantitative polymerase chain reaction assays. miR-424 expression was decreased in the majority of human breast cancer specimens and cell lines used in the present study. The MTT assay, plate colony formation assay and flow cytometry analyses were used to characterize the function of miR-424 in two types of breast cancer cell lines. Upregulation of miR-424 inhibited cellular proliferation and regulated the cell cycle by arresting cells in the G2/M cell phase. The dual-luciferase reporter assay was used to confirm the direct association between miR-424 and cyclin-dependent kinase 1 (CDK1). Silencing of CDK1 expression by CDK1 short interfering RNA also significantly suppressed cell proliferation and arrested cells in the G2/M cell phase. The results of the present study indicated that miR-424 can suppress cell proliferation and arrest cells in G2/M cell phase by negatively regulating CDK1 mRNA in human breast cancer, possibly through the Hippo pathway and the extracellular signal-regulated kinase pathway. The results of the present study provided novel evidence for the role of miR-424 in breast cancer.
Keywords: microRNA-424, breast cancer, cyclin-dependent kinase 1, Hippo pathway, extracellular signal-regulated kinase pathway, cell cycle
Introduction
Breast cancer is the most common type of carcinoma and the second most common cause of cancer-associated mortality in females (1). MicroRNAs (miRNAs) are a group of small, single-stranded, non-coding RNAs that regulate gene expression by partial base pairing with the 3′-untranslated region (3′-UTR) or enhancer region of targeted genes (2–5). In addition to regulating particular mRNAs directly, miRNAs can also affect the association between effectors and target mRNAs (6). Each miRNA may regulate a variety proteins and serve an important role in more than one key cellular progress, including cell proliferation, cell survival and cell fate determination (7). Evidence has indicated that deregulation of miRNA expression may contribute toward the development of various types of human diseases, including cancer. The miRNAs are therefore becoming increasingly appreciated as potential biomarkers for the diagnosis, treatment and prognosis of diseases.
Recent studies have identified microRNA-424-5p (miR-424) as a crucial regulator in the development of several types of cancer, including bladder (8) and cervical cancer (9). In bladder cancer, the decreased expression of miR-424 was associated with invasive tumor growth, advanced clinical stage and a poor prognosis. Increased miR-424 levels inhibited tumor growth and invasive ability. In cervical cancer, miR-424 may act as an anti-oncogene by suppressing cell growth. However, the role of miR-424 in breast cancer remains poorly defined.
The aim of the present study was to define the function of miR-424 in breast cancer cells. The experiments performed indicated that miR-424 may suppress cell proliferation and arrest cells in the G2/M cell phase by negatively regulating cyclin-dependent kinase 1 (CDK1) mRNA in human breast cancer, and that this may occur through the Hippo pathway and the extracellular signal-regulated kinase (ERK) pathway. The results of the present study provided novel evidence for the role of miR-424 in breast cancer.
Methods and materials
Specimens, cell lines and culture conditions
The present study collected 17 pairs of breast cancer and matched adjacent normal control samples from the Department of Breast and Thyroid Surgery of Shanghai Tenth People's Hospital (Shanghai, China) between February and April 2017, which were histologically confirmed to be invasive ductal breast cancer. The patients were females aged between 34 and 74 years, with a mean age of 55 years. All these tissues were immediately snap-frozen to −196°C in liquid nitrogen. None of these patients had received any radiotherapy or chemotherapy prior to surgery.
Human triple-negative breast cancer MDA-MB-231 and HCC1937 cell lines, and the non-malignant breast epithelial MCF-10A cell line, were used. The cells were purchased from the Chinese Academy of Sciences. The HEK-293T cell line was a gift from the laboratory department of Shanghai Tenth People's Hospital. All cells were cultured in Dulbecco's modified Eagle's medium (DMEM; cat. no. C11995500BT; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), supplemented with 10% fetal bovine serum (FBS; cat. no. 900-108; Gemini Bio Products, West Sacramento, CA, USA), penicillin (100 U/ml) and streptomycin (100 µg/ml; PS; cat. no. 15140-163; Gibco; Thermo Fisher Scientific, Inc.). Cells were incubated at 37°C in a humidified atmosphere containing 5% CO2.
Transfection assays
The cells (1×106) were cultured in 6-well plates (Corning Incorporated, Corning, NY, USA). The cell density reached 30–50% confluency after <24 h. Next, the miR-424 mimics or miR-424 inhibitor or negative control (NC) or CDK1 small interfering RNA (siR-CDK1) or siR-NC were transfected separately into cells at a final concentration of 100 nmol/l using Lipofectamine® 2000 (cat. no. 11668-019; Invitrogen; Thermo Fisher Scientific, Inc.) in DMEM. The medium was replaced with complete DMEM after 4–6 h of incubation. The cells were incubated as described earlier and used for future analyses after 24 h.
miR-424 mimics (5′-CAGCAGCAAUUCAUGUUUUGAA-3′), miR-424 inhibitor (5′-GUCGUCGUUAAGUACAAAACUU-3′) and NC mimics (5′-UUUGUACUACACAAAAGUACUG-3′) were chemosynthesized by Guangzhou RiboBio Co., Ltd. (Guangzhou, China). siR-CDK1 (sense, 5′-GGCACUGAAUCAUCCAUAUTT-3′ and antisense, 5′-AUAUGGAUGAUUCAGUGCCTT-3′) and siR-NC (sense, 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense, 5′-ACGUGACACGUUCGGAGAATT-3′) were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China).
MTT assays
Cell proliferation ability was estimated by the MTT assay (cat. no. A100793-0001; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) every 24 h for a period of 120 h. Cells were plated into 96-well plates (Corning Incorporated) at a density of 500 cells/well. In brief, the MTT solution was added to each well and continuously incubated for 4 h. Subsequently, the medium was replaced by dimethylsulfoxide (DMSO; cat. no. A503039-0500; Sigma-Aldrich; Merck KGaA) to dissolve the purple formazan. The absorbance at optical density (OD) of 492 nm was measured by a microplate spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA).
Plate colony formation assays
Cells were plated in 6-well plates at a density of 500 cells/well and incubated for 7–14 days or until the colonies were visible to the eye. The colonies were fixed in 95% ethanol for 10 min, dried and stained with 0.1% crystal violet solution for 15 min at room temperature. Next, the plates were gently washed three times with water. Colonies with diameters of >1.5 mm were counted as live cells.
Protein extraction and western blot analyses
Following transfection for 48–72 h, the cells were resuspended in 80 µl/well radioimmunoprecipitation assay lysis buffer (cat. no. P0013C; Beyotime Institute of Biotechnology, Haimen, China) for 20 min. The protein concentrations were quantified using a bicinchoninic acid protein assay kit (P0010; Beyotime Institute of Biotechnology). Protein samples were denatured with 6× SDS sample loading buffer (P0015F; Beyotime Institute of Biotechnology) at 100°C for 10 min. Equal amounts of protein (30 µg) from each sample were separated by 10% SDS-PAGE and transferred onto 0.45-µm nitrocellulose membranes (Beyotime Institute of Biotechnology). The membranes were blocked with 5% skimmed milk for 60 min at room temperature and then probed with antibodies against CDK1 (dilution, 1:1,000; cat. no. BS6467; Bioworld Technology, Inc., Freemont, CA, USA), proliferating cell nuclear antigen [PCNA; dilution, 1:1,000; cat. no. 13110; Cell Signaling Technology, Inc., Danvers, MA, USA (CST)], Yes-associated protein (YAP; dilution, 1:1,000; cat. no. 14074; CST), ERK (dilution, 1:10,000; cat. no. ab184699; Abcam, Cambridge, UK), phosphorylated ERK (p-ERK; dilution, 1:10,000; cat. no. ab201015; Abcam) and β-actin (dilution, 1:1,000; cat. no. BS6498; Bioworld Technology, Inc.) overnight at 4°C. The membranes were incubated with IRDye® 800CW-conjugated anti-mouse (dilution, 1:1,000; cat. no. 926-32210; LI-COR Biosciences, Lincoln, NE, USA) or IRDye® 800CW-conjugated anti-rabbit secondary antibodies (dilution, 1:1,000; cat. no. 926-32211; LI-COR Biosciences) for 60 min. Finally, the bands were detected using an Odyssey Scanning system (LI-COR Biosciences).
Cell cycle assays
The cells were trypsinized and centrifuged at 1,000 rpm for 5 min at room temperature, and washed in cold PBS twice. Then cells were fixed in cold 70% ethanol at 4°C overnight. Each sample were resuspended in 300 µl 0.05 g/l propidium iodide (PI/RNase Staining Buffer Solution, 550825, BD Pharmingen) for 30 min in the dark at room temperature. Next, cell cycles were analyzed by flow cytometry (FACSCanto™ II; BD Biosciences, Franklin Lakes, NJ, USA) and the software used for analysis was ModFit LT 3.2 (Flow Cytometry DNA Modeling Software, Verity Software House, USA).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cells (following transfection for 48 h) or tissues using TRIzol® reagent (cat. no. 15596-026; Invitrogen; Thermo Fisher Scientific, Inc.). RNA was reverse transcribed using a PrimeScript™ RT-PCR kit (cat. no. RR037A; Takara Biotechnology Co., Ltd., Dalian, China) according to the manufacturer's protocol. The SYBRGreen PCR master mix (cat. no. KK4601; Kapa Biosystems, Inc., Wilmington, MA, USA) was used for RT-qPCR, which was followed by detection using a 7900HT fast RT-PCR instrument (Applied Biosystems; Thermo Fisher Scientific, Inc.).
miR-424 (miRQ0001341-1-1) and U6 (MQP-0201) primers, purchased from Guangzhou RiboBio Co., Ltd., were used for the detection of miR-424. The primer sequences were as follows: miR-424 forward, 5′-CAGCAGCAAUUCAUGUUUUGAA-3′ and reverse, 5′-CAGTGCGTGTCGTGGAGT-3′; and U6 (internal standard) forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′. For CDK1 mRNA analyses, β-actin was used as an internal standard. The primer sequences (Sangon Biotech Co., Ltd.) were as follows: CDK1 forward, 5′-GGATGTGCTTATGCAGGATTCC-3′ and reverse, 5′-CATGTACTGACCAGGAGGGATAG-3′; and β-actin forward, 5′-CAGAGCCTCGCCTTTGCC-3′ and reverse, 5′-GTCGCCCACATAGGAATC-3′. The amplification procedures were as follows: 3 min at 95°C, followed by 40 cycles of 3 sec at 95°C and 30 sec at 60°C.
The relative expression of miRNA or mRNA was assessed using the 2−ΔΔCq method (10).
Dual-luciferase reporter assay
The psiCHECK-2/CDK1 3′-UTR wild-type and mutant reporter plasmids (G0001) were purchased from Shanghai Integrated Biotech Solutions, China. HEK-293T cells were transiently co-transfected with 40 ng wild-type or mutant reporter plasmids with 100 nmol/l miR-424 or NC using Lipofectamine® 2000 reagent. Following transfection for 5 h, the complete medium was changed. Following transfection for 24 h, the cells were detected using a Dual Luciferase Assay (cat. no. E1910; Promega Corporation, Madison, WI, USA). The Renilla luciferase (RL) activity was normalized to firefly luciferase (FL) activity and the ratio of RL/FL was recorded. Each sample was tested with three replicates.
Statistical analysis
All statistical analyses were performed using GraphPad Prism version 6.0 (GraphPad Software, Inc., La Jolla, CA, USA). Data from >3 independent experiments are presented as the mean ± standard deviation. The differences between two groups were compared using Student's t-test. Differences between >2 groups were compared using one-way analysis of variance followed by Tukey's post hoc test. Kaplan-Meier, followed by the log-rank test, was used for survival analyses. P<0.05 were considered to indicate a statistically significant difference.
Results
miR-424 expression is decreased in breast cancer
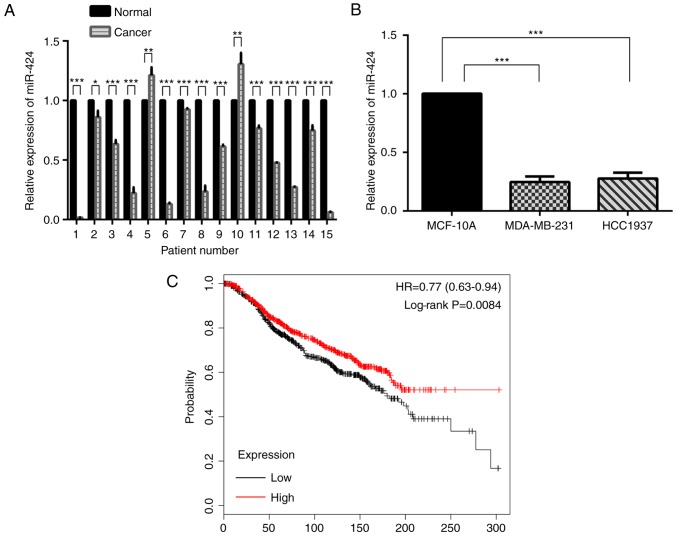
The expression of miR-424 was decreased in human breast cancer samples compared with that in matched normal breast tissue samples (Fig. 1A). In MDA-MB-231 and HCC-1937 cells, miR-424 expression was also decreased compared with that in MCF-10A cells (Fig. 1B). Kaplan-Meier survival analyses suggested that miR-424 acted as an anti-oncogene (Fig. 1C).
Figure 1.
Relative expression of miR-424. (A) The relative expression of miR-424 in 15 randomly selected paired breast cancer tissues and normal tissues. The graph represents the 2−ΔΔCq values ± standard deviation. *P<0.05; **P<0.01; ***P<0.001. (B) The relative expression of miR-424 in breast cancer MDA-MB-231 and HCC-1937 cell lines, compared with the normal breast epithelial MCF-10A cell line. ***P<0.001. (C) Kaplan-Meier survival analyses (from 1,262 cases of breast cancer, kmplot.com, ID in KMPLOT: has-mir-424) revealed that patients with high miR-424 expression exhibited longer overall survival times. P=0.0084. miR, microRNA; HR, hazard ratio.
miR-424 suppresses cell proliferation in breast cancer
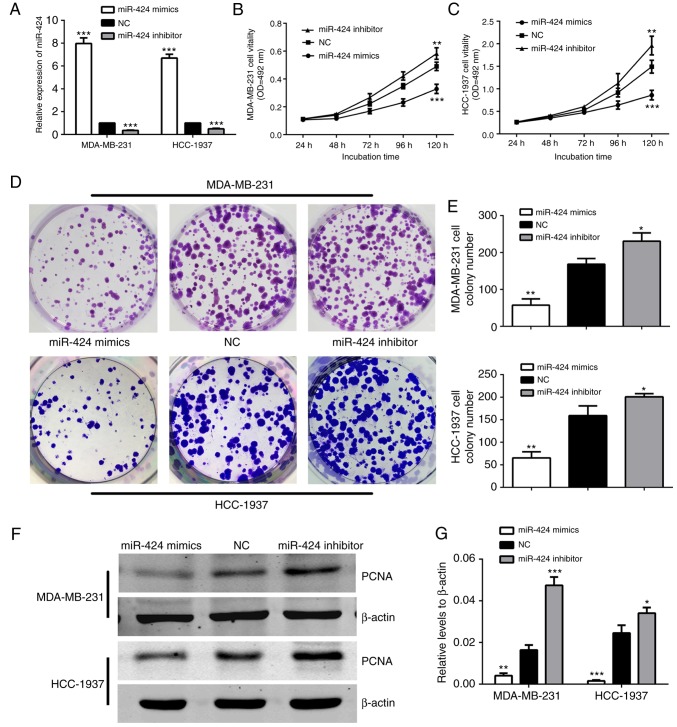
To begin with, RT-qPCR was conducted to examine the expression levels of miR-424 in MDA-MB-231 and HCC-1937 cells. As demonstrated in Fig. 2A, cells were successfully transfected with miR-424 mimics or inhibitors, leading to increased or decreased miR-424 expression, respectively. The MDA-MB-231 and HCC-1937 cell lines were used in the subsequent experiments. In order to determine the effects of miR-424 in breast cancer cells, the effect of miR-424 on cell proliferation was verified. The expression of miR-424 was upregulated and downregulated when compared with that in NC. The cells were respectively transfected with miR-424 mimics, inhibitor or NC, prior to MTT assays and colony formation assays being performed following transfection for 24 h. The MTT assay results demonstrated that cell proliferation was decreased in cells transfected with miR-424 mimics, compared with the NC cell group, while the cells transfected with inhibitors exhibited the opposite results (Fig. 2B and C). The colony formation assays also indicated that the number of colonies in cells with upregulated miR-424 was less, and in cells with downregulated miR-424, the colony number was greater than that in the NC group (Fig. 2D and E). In addition, western blot analysis was used to detect PCNA protein levels to represent the level of cell proliferation, which was also decreased by upregulation of miR-424 (Fig. 2F and G). All these data revealed that miR-424 suppresses cell proliferation in breast cancer.
Figure 2.
miR-424 inhibited MDA-MB-231 and HCC-1937 cell proliferation. (A) The relative expression of miR-424 was detected by reverse transcription-quantitative polymerase chain reaction in cells following transfection with miR-424 mimics, inhibitors or NC for 48 h. ***P<0.001. (B) The MTT assay was used to assess the proliferation level of MDA-MB-231 cells. The data represent the OD at 492 nm ± SD. **P<0.01, ***P<0.001. (C) The MTT assay was used to assess the proliferation level of HCC-1937 cells. The data represent the OD at 492 nm ± SD. **P<0.01, ***P<0.001. (D) The colony formation assays demonstrated the colony forming ability of the cells. MDA-MB-231 and HCC-1937 cells transfected with miR-424 mimics, inhibitors or NC are shown. (E) Cells transfected with miR-424 mimics exhibited fewer colonies than the NC group. *P<0.05, **P<0.01. (F) Western blots demonstrated the relative expression of PCNA protein. (G) The graph shows the mean ± SD of PCNA protein levels relative to β-actin, which was used as a control. All protein levels were quantified by measuring the IDV of each protein band. *P<0.05, **P<0.01, ***P<0.001. miR, microRNA; NC, negative control; SD, standard deviation; PCNA, proliferating cell nuclear antigen; IDV, integrated density value; OD, optical density.
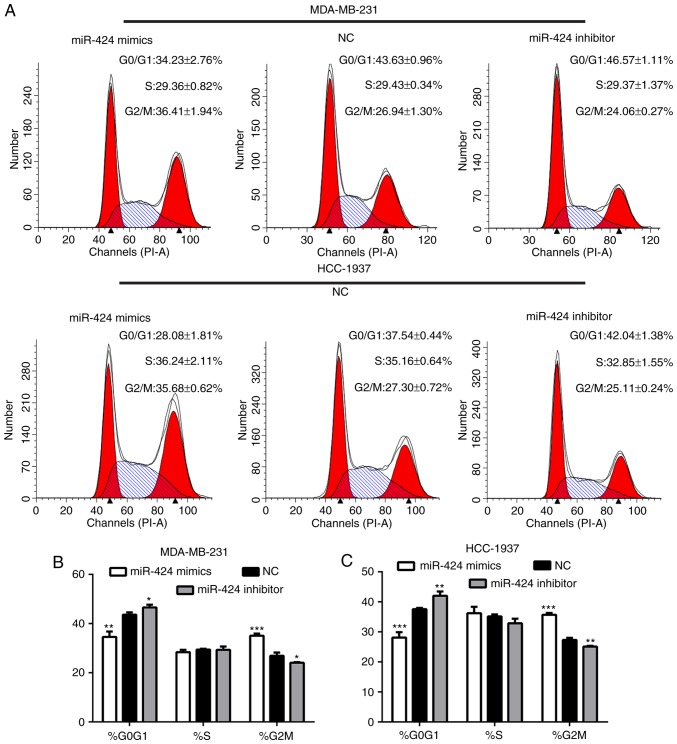
miR-424 regulates the cell cycle of breast cancer cells
Following transfection for 48 h, the cells were collected for flow cytometry analyses. This demonstrated that the proportion of G2/M phase cells in the miR-424 mimic group was significantly increased compared with that of the inhibitor and NC groups, and that the proportion of G0/G1 phase cells was decreased (Fig. 3). This result indicated that miR-424 regulated the cell cycle by arresting cells in the G2/M cell phase.
Figure 3.
Effects of miR-424 on the cell cycle in breast cancer cells. (A) Flow cytometry was used to analyze the cell cycle of MDA-MB-231 and HCC-1937 cells. (B) The proportions of MDA-MB-231 cells at each interface in every group are shown. *P<0.05, **P<0.01, ***P<0.001. (C) The proportions of HCC-1937 cells at each interface in every group are shown. **P<0.01, ***P<0.001. miR, microRNA; CDK, cyclin-dependent kinase; NC, negative control.
miR-424 suppresses CDK1 expression and certain key genes in the Hippo and ERK pathways
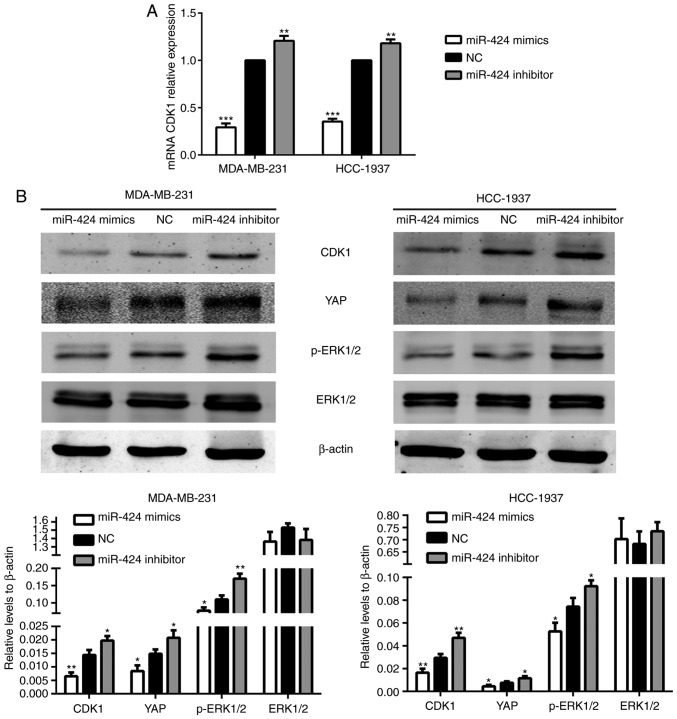
To further investigate the mechanism of action of miR-424 in breast cancer, TargetScan (http://www.targetscan.org/) was searched to identify target genes of miR-424. It suggested that CDK1 may be a potential target of miR-424. To determine whether miR-424 regulated endogenous CDK1, miR-424 mimics, inhibitors and NC were transfected into MDA-MB-231 and HCC-1937 cells, prior to the levels of CDK1 protein being detected by western blot analysis, and mRNA was monitored by RT-qPCR 48 h after transfection. It revealed that miR-424 mimics significantly suppressed the expression of endogenous CDK1 protein and mRNA compared with that of the NC group. By contrast, the miR-424 inhibitor enhanced the expression of CDK1 protein and mRNA (Fig. 4).
Figure 4.
miR-424 increased the levels of CDK1 and YAP, and the phosphorylation of ERK1/2. (A) The relative expression of CDK1 mRNA in transfected cells. **P<0.01, ***P<0.001. (B) The relative expression of CDK1, YAP, ERK1/2 and p-ERK1/2 proteins. *P<0.05, **P<0.01. miR, microRNA; CDK, cyclin-dependent kinase; YAP, yes-associated protein; ERK, extracellular signal-regulated kinase; p-ERK, phosphorylated ERK.
The Hippo and ERK pathways have been extensively studied in breast cancer in recent years. Therefore, the present study attempted to identify an association between these two pathways and miR-424. In the Hippo pathway, it was revealed that the protein expression level of YAP was decreased in the miR-424 mimic group and was increased in the miR-424 inhibitor group, compared with the NC group. In the ERK pathway, the expression of ERK1/2 did not differ between each group, while the protein expression level of p-ERK1/2 was decreased in the miR-424 mimic group (Fig. 4B). We hypothesized that these results may be due to variations in CDK1, and subsequent results verified this hypothesis.
CDK1 is a direct target of miR-424
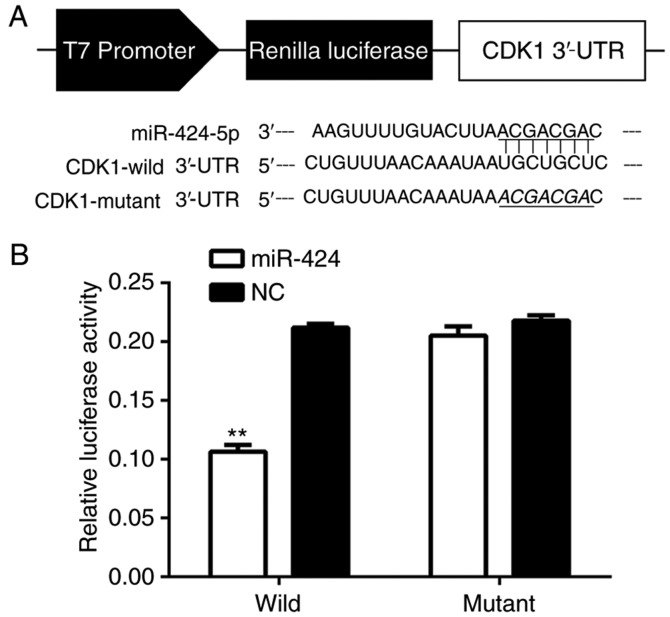
To determine if miR-424 binded to CDK1, the miR-424 and CDK1 mRNA sequences were analyzed and seven complementary nucleotides were identified (Fig. 5A). The luciferase reporter assay was used in the HEK-293T cell line, and the luciferase activity was suppressed in cells co-transfected with psi-CHECK-2/CDK1 3′-UTR and miR-424 mimics compared with NC, indicating that CDK1 was a direct target of miR-424 (Fig. 5B).
Figure 5.
CDK1 was a target gene of miR-424. (A) The binding site for miR-424 in CDK1 mRNA. (B) The relative luciferase activity of HEK-293T cells following co-transfection. **P<0.01. CDK, cyclin-dependent kinase; miR, microRNA; UTR, untranslated region; NC, negative control.
CDK1 promotes cell proliferation and regulates the cell cycle in breast cancer
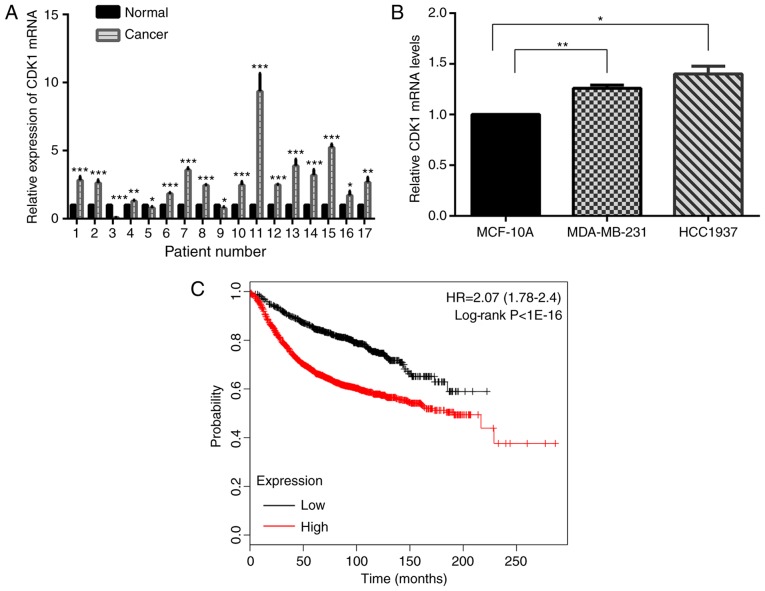
The expression level of CDK1 mRNA was tested in 17 pairs of breast cancer and matched normal specimens. It demonstrated that CDK1 was increased in human breast cancer specimens compared with normal breast specimens (Fig. 6A). Further study yielded the same results in MDA-MB-231 and HCC-1937 cells, compared with MCF-10A cells (Fig. 6B). Kaplan-Meier survival analyses suggested that CDK1 acted as an oncogene (Fig. 6C).
Figure 6.
Relative expression of CDK1. (A) The relative expression of CDK1 in 17 paired clinical breast cancer samples compared with matched normal tissues. *P<0.05; **P<0.01; ***P<0.001. (B) The relative expression of CDK1 in MDA-MB-231 and HCC-1937 cells compared with MCF-10A cells. *P<0.05; **P<0.01. (C) Kaplan-Meier survival analyses (from 3,951 cases of breast cancer, kmplot.com, ID in KMPLOT: 203213_at) revealed that patients with CDK1-overexpression exhibited shorter overall survival times. P<0.001. CDK, cyclin-dependent kinase; HR, hazard ratio.
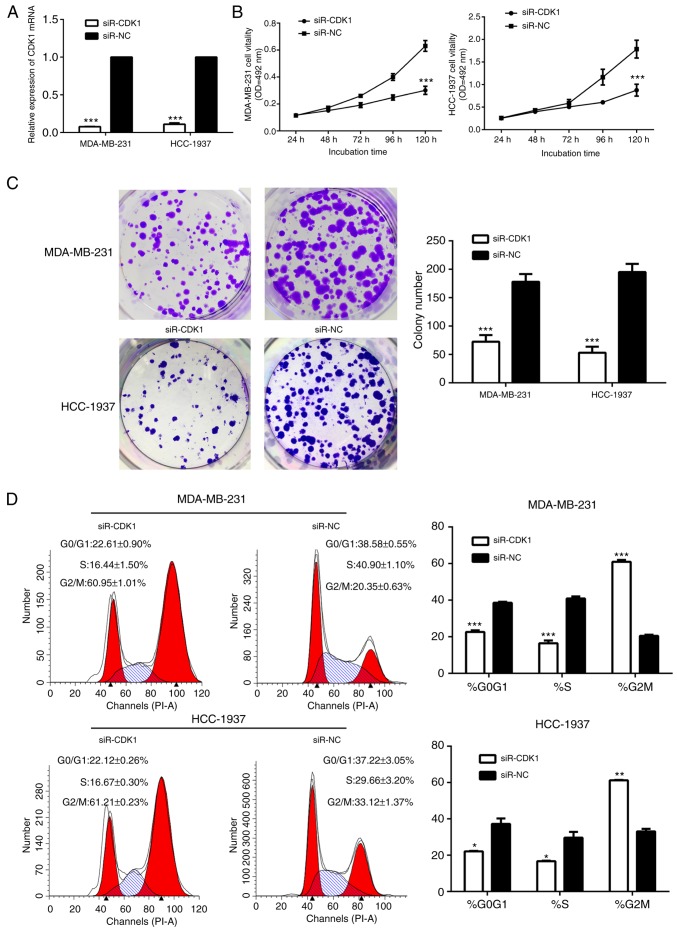
Therefore, the present study investigated the role of CDK1 in breast cancer. The cells in the siR-CDK1-treated group exhibited notably decreased CDK1 protein and mRNA expression, compared with the siR-NC-treated group, suggesting that CDK1 was successfully silenced by siR-CDK1 (Figs. 7A and 8). Silencing of CDK1 inhibited cell proliferation (Fig. 7B and C), and arrested the cells in the G2/M phase (Fig. 7D). In addition, the levels of associated proteins were decreased by silencing of CDK1, which further confirmed the results of the present study (Fig. 8).
Figure 7.
The effects of CDK1 on cell proliferation and the cell cycle. (A) The mRNA level of CDK1 was induced in the siRNA-CDK1-treated group compared with the siRNA-NC-treated group in MDA-MB-231 and HCC-1937 cells. ***P<0.001. (B) MTT assay colony forming assays showed that silencing of CDK1 suppressed cell proliferation. ***P<0.001. (C) Colony forming assays showed that silencing of CDK1 suppressed the colony forming ability of cells. ***P<0.001 (D) Silencing of CDK1 disrupted the cell cycle. *P<0.05, **P<0.01, ***P<0.001. siRNA, small interfering RNA; CDK, cyclin-dependent kinase; NC, negative control.
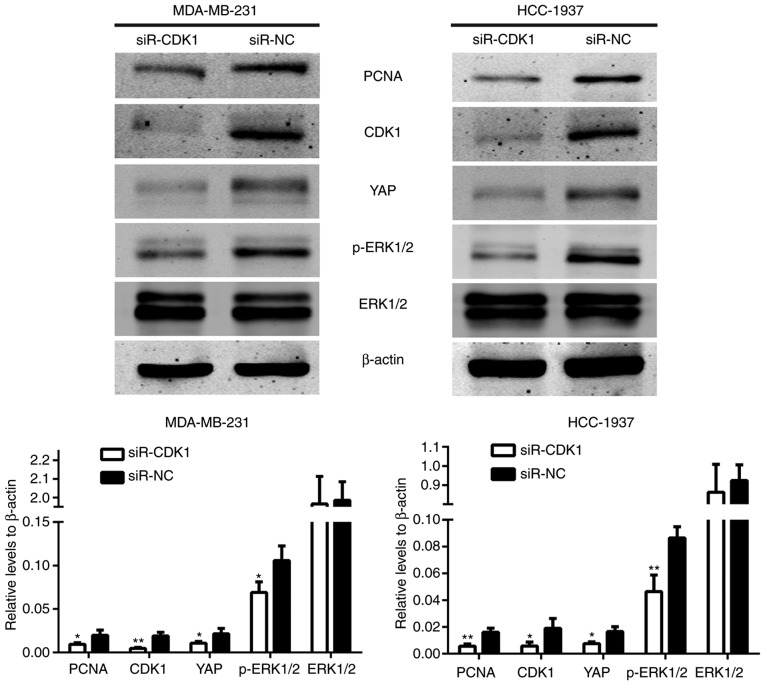
Figure 8.
siRNA-CDK1 significantly reduced the expression of CDK1. The protein expression levels of PCNA, cyclin B, YAP and p-ERK1/2 were decreased by silencing of CDK1. *P<0.05, **P<0.01. siRNA, small interfering RNA; CDK, cyclin-dependent kinase; PCNA, proliferating cell nuclear antigen; YAP, yes-associated protein; p-ERK1/2, phosphorylated extracellular signal-regulated kinase 1/2.
As demonstrated in Fig. 4B, the protein expression levels were regulated by miR-424. The protein expression levels of these genes in the siR-CDK1-treated group and the siR-NC-treated group were also assessed. The results demonstrated that the protein expression levels of YAP and p-ERK1/2 were also induced in the siR-CDK1-treated group compared with the siR-NC-treated group (Fig. 8). These data demonstrated that the effect of miR-424 on regulating the Hippo and ERK pathways were achieved through regulating CDK1.
Discussion
It has been reported that there are an estimated 1.7 million females expected to be diagnosed with breast cancer by 2020, and there has been a 26% increase from current recorded levels (11,12). Although the mortality rate in this decade has decreased, breast cancer remains a major life threatening disease with 1.3 million newly diagnosed cases each year among females worldwide (13). Therefore, it is crucial to investigate and develop more effective ways to diagnose and treat breast cancer at an early stage.
As a type of potential regulator, miRNAs serve a key role in tumorigenesis and the progression of breast cancer (14). They participate in numerous different biological processes, including cell proliferation, differentiation, invasion, migration and transcription. miRNAs may act as oncogenes or anti-oncogenes, such that their dysregulation is associated with the initiation and progression of breast cancer (15–17). miRNA-based therapies have already been used as vital strategies for breast cancer treatment (18). Additionally, it has been reported that several miRNAs participated in cell tumorigenesis and metastasis (19). The overexpression of anti-oncogenic miRNAs could therefore be a novel therapeutic approach for breast cancer treatment (20–22). The present study emphasized the role of miR-424 and its possible mechanism in breast cancer.
In recent years, several studies reported that miR-424 may act as an anti-oncogene in certain types of cancer. Therefore, the expression level of miR-424 was measured, and it was revealed to be decreased in human breast cancer tissues and cell lines, compared with normal tissues and cells, respectively. Similar observations have been reported in several different tumor types, including minimal deviation adenocarcinoma (23), cervical cancer (9) and bladder cancer (8). Although the number of clinical specimens was not large enough to confirm this conclusion, these results still have utility. Kaplan-Meier survival analyses indicated that overexpression of miR-424 resulted in longer overall survival times. We hypothesized that miR-424 served an anti-oncogene role in breast cancer. Therefore, miR-424 mimics and inhibitors were transfected into MDA-MB-231 and HCC-1937 cells to upregulate or downregulate the expression of miR-424, respectively, and miR-424 expression was determined by RT-qPCR. Next, MTT and colony formation assay results demonstrated that high miR-424 expression remarkably repressed the proliferation and colony formation ability of breast cancer cells. Flow cytometric analysis indicated that miR-424 significantly arrested cells in the G2/M phase. Furthermore, the expression levels of PCNA, which are associated with cell proliferation, were also inhibited by miR-424. To improve understanding regarding the functions of miR-424, it was critical to identify its target genes. TargetScan revealed that CDK1 may be a direct target of miR-424.
CDKs are a family of serine/threonine kinases that are critical regulatory enzymes for cell cycle transitions (24). Aberrant activation of CDKs may enhance tumor proliferation and chromosomal instability; therefore, they were focuses for the development of anticancer drug (25). In fact, numerous effective and selective inhibitors of CDKs have been identified in recent years, including inhibitors of CDK4/6 that have recently been approved by the FDA (26–29). CDK1 is the only CDK that is able to initiate mitosis. Its association with cyclin B is the primary impetus for entry into mitosis. CDK1 is required for mammalian cell proliferation, as confirmed by mouse knockout experiments (30). Additionally, there have been related reports regarding cell cycle and CDKs in recent years. CDK8 is able to promote cell proliferation in breast cancer, and is directly regulated by miRNA-26b and miRNA-107 (31–33). As an upstream component of CDK1, cyclin-dependent kinase regulatory subunit 2 (CKS2) is involved in the progression of thyroid papillary cancer cells, while CDK1 and cyclin B are regulated by the miR-7-CKS2 axis (34). Western blot analysis demonstrated that upregulation of miR-424 significantly inhibited CDK1 protein expression, while RT-qPCR confirmed that miR-424-overexpression significantly suppressed the level of CDK1 mRNA. Next, miR-424 directly binds to CDK1 as confirmed by luciferase reporter assays. Subsequently, it was revealed that the effect of CDK1 in the survival rate and relative expression levels in cancer tissues or cells was opposite to the effects of miR-424 in human breast cancer. Following decreasing the CDK1 levels by transfection with siR-CDK1, it was revealed that the effects on cell proliferation and the cell cycle were similar to those identified following upregulation of miR-424. Therefore, we hypothesized that the effect of miR-424 on these biological processes was exerted through targeting CDK1.
Furthermore, the present study measured the expression levels of certain Hippo and ERK pathway proteins in cells with miR-424 upregulation or downregulation. The expression alterations of miR-424 in MDA-MB-231 and HCC-1937 cells changed the protein expression levels of YAP and p-ERK1/2, but not those of ERK1/2. Similar results were also observed in CDK1-silenced cells. YAP is a core downstream molecule of the Hippo signaling pathway in mammals, which acts as an oncogene in the initiation and development of breast cancer. A previous study demonstrated that numerous proteins can promote the progression of breast cancer through the Hippo signaling pathway (35). The results of the present study suggested that miR-424 affected the Hippo pathway through targeting CDK1 and then regulating the expression level of YAP. The ERK pathway is a classic mitogen-activated protein kinase (MAPK) signaling cascade that regulates cell proliferation, malignant transformation, autophagy and differentiation, with core members, including Ras, Raf, MEK1/2 and ERK1/2 (36,37). ERK1/2 are serine-threonine kinases that are activated through phosphorylation by MEK1/2 (38). They served important roles in malignant cell transformation. Based on these results, we hypothesized that the miR-424-CDK1 axis altered the activation levels of ERK1/2 rather than their protein expression levels.
In conclusion, the present study demonstrated that miR-424 acted as a tumor suppressor in breast cancer cells. miR-424-overexpression suppressed cell growth and disrupted the cell cycle, likely by targeting CDK1 and further regulating the Hippo and ERK pathways; therefore, miR-424 may be a potential novel therapeutic target for human breast cancer.
Acknowledgements
The authors would like to thank all the teachers at the Central Laboratory of Shanghai Tenth People's Hospital (Shanghai, China) for providing support.
Funding
The present study was supported by the National Natural Sciences Foundation of China (grant no. 81272240), the Shanghai Municipal Health Bureau of Shanghai, China (grant no. 201640097) and the Shanghai Municipal Science and Technology Commission of Shanghai, China (grant no. 17411967200).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
DX and LF conceived and designed the study. DX, HS and TW performed the experiments. HX, BZ and CW analyzed the data. DX, KH and DL wrote the manuscript. KH and DL were also involved in the conception of this study. JH, CJ and YD acquired the reagents, the materials and the analysis tools. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
All procedures involving human participants in the present study were performed in accordance with the Ethical Standards of the Institutional and/or National Research Committee, and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bombonati A, Sgroi DC. The molecular pathology of breast cancer progression. J PATHOL. 2011;223:307–31. doi: 10.1002/path.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yates LA, Norbury CJ, Gilbert RJ. The long and short of microRNA. Cell. 2013;153:516–519. doi: 10.1016/j.cell.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 4.Xiao M, Li J, Li W, Wang Y, Wu F, Xi Y, Zhang L, Ding C, Luo H, Li Y, et al. MicroRNAs activate gene transcription epigenetically as an enhancer trigger. RNA Biol. 2017;14:1326–1334. doi: 10.1080/15476286.2015.1112487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dvinge H, Git A, Graf S, Salmon-Divon M, Curtis C, Sottoriva A, Zhao Y, Hirst M, Armisen J, Miska EA, et al. The shaping and functional consequences of the microRNA landscape in breast cancer. Nature. 2013;497:378–382. doi: 10.1038/nature12108. [DOI] [PubMed] [Google Scholar]
- 7.Cekaite L, Eide PW, Lind GE, Skotheim RI, Lothe RA. MicroRNAs as growth regulators, their function and biomarker status in colorectal cancer. Oncotarget. 2016;7:6476–6505. doi: 10.18632/oncotarget.6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu CT, Lin WY, Chang YH, Lin PY, Chen WC, Chen MF. DNMT1-dependent suppression of microRNA424 regulates tumor progression in human bladder cancer. Oncotarget. 2015;6:24119–24131. doi: 10.18632/oncotarget.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Y, An Q, Guo RX, Qiao YH, Li LX, Zhang XY, Zhao XL. miR424-5p functions as an anti-oncogene in cesrvical cancer cell growth by targeting KDM5B via the Notch signaling pathway. Life Sci. 2017;171:9–15. doi: 10.1016/j.lfs.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Kong X, Li J, Luo Q, Li X, Shen L, Chen L, Fang L. miR-96 promotes tumor proliferation and invasion by targeting RECK in breast cancer. Oncol Rep. 2014;31:1357–1363. doi: 10.3892/or.2013.2934. [DOI] [PubMed] [Google Scholar]
- 11.Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 12.Rahmani-Nezhad S, Safavi M, Pordeli M, Ardestani SK, Khosravani L, Pourshojaei Y, Mahdavi M, Emami S, Foroumadi A, Shafiee A. Synthesis, in vitro cytotoxicity and apoptosis inducing study of 2-aryl-3-nitro-2H-chromene derivatives as potent anti-breast cancer agents. Eur J Med Chem. 2014;86:562–569. doi: 10.1016/j.ejmech.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Tirona MT, Sehgal R, Ballester O. Prevention of breast cancer (part I): Epidemiology, risk factors, and risk assessment tools. Cancer Invest. 2010;28:743–750. doi: 10.3109/07357907.2010.512593. [DOI] [PubMed] [Google Scholar]
- 14.Jahagirdar D, Purohit S, Jain A, Sharma NK. Export of microRNAs: A bridge between breast carcinoma and their neighboring cells. Front Oncol. 2016;6:147. doi: 10.3389/fonc.2016.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo Q, Li X, Li J, Kong X, Zhang J, Chen L, Huang Y, Fang L. MiR-15a is underexpressed and inhibits the cell cycle by targeting CCNE1 in breast cancer. Int J Oncol. 2013;43:1212–128. doi: 10.3892/ijo.2013.2034. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Kong X, Zhang J, Luo Q, Li X, Fang L. Correction: MiRNA-26b inhibits proliferation by targeting PTGS2 in breast cancer. Cancer Cell Int. 2013;13:17. doi: 10.1186/1475-2867-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braza-Boils A, Mari-Alexandre J, Gilabert J, Sanchez-Izquierdo D, Espana F, Estelles A, Gilabert-Estellés J. MicroRNA expression profile in endometriosis: Its relation to angiogenesis and fibrinolytic factors. Hum Reprod. 2014;29:978–988. doi: 10.1093/humrep/deu019. [DOI] [PubMed] [Google Scholar]
- 18.Gambari R, Brognara E, Spandidos DA, Fabbri E. Targeting oncomiRNAs and mimicking tumor suppressor miRNAs: New trends in the development of miRNA therapeutic strategies in oncology (Review) Int J Oncol. 2016;49:5–32. doi: 10.3892/ijo.2016.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jahagirdar D, Purohit S, Jain A, Sharma NK. Export of microRNAs: A bridge between breast carcinoma and their neighboring cells. Front Oncol. 2016;6:147. doi: 10.3389/fonc.2016.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertoli G, Cava C, Castiglioni I. The potential of miRNAs for diagnosis, treatment and monitoring of breast cancer. Scand J Clin Lab Invest Suppl. 2016;245:S34–S39. doi: 10.1080/00365513.2016.1208444. [DOI] [PubMed] [Google Scholar]
- 21.Bertoli G, Cava C, Castiglioni I. MicroRNAs: New biomarkers for deiagnosis, prognosis, therapy prediction and therapeutic tools for breast cancer. Theranostics. 2015;5:1122–1143. doi: 10.7150/thno.11543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cava C, Bertoli G, Castiglioni I. Integrating genetics and epigenetics in breast cancer: Biological insights, experimental, computational methods and therapeutic potential. BMC Syst Biol. 2015;9:62. doi: 10.1186/s12918-015-0211-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee H, Kim KR, Cho NH, Hong SR, Jeong H, Kwon SY, Park KH, An HJ, Kim TH, Kim I, et al. MicroRNA expression profiling and Notch1 and Notch2 expression in minimal deviation adenocarcinoma of uterine cervix. World J Surg Oncol. 2014;12:334. doi: 10.1186/1477-7819-12-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nurse P, Masui Y, Hartwell L. Understanding the cell cycle. Nat Med. 1998;4:1103–1116. doi: 10.1038/2594. [DOI] [PubMed] [Google Scholar]
- 25.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: A changing paradigm. Nat Rev Cancer. 2009;9:153–1566. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 26.Lapenna S, Giordano A. Cell cycle kinases as therapeutic targets for cancer. Nat Rev Drug Discov. 2009;8:547–566. doi: 10.1038/nrd2907. [DOI] [PubMed] [Google Scholar]
- 27.Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov. 2015;14:130–146. doi: 10.1038/nrd4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, Ettl J, Patel R, Pinter T, Schmidt M, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole vs letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 29.Sherr CJ, Beach D, Shapiro GI. Targeting CDK4 and CDK6: From discovery to therapy. Cancer Discov. 2016;6:353–367. doi: 10.1158/2159-8290.CD-15-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santamaria D, Barriere C, Cerqueira A, Hunt S, Tardy C, Newton K, Cáceres JF, Dubus P, Malumbres M, Barbacid M. Cdk1 is sufficient to drive the mammalian cell cycle. Nature. 2007;448:811–815. doi: 10.1038/nature06046. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Li X, Kong X, Luo Q, Zhang J, Fang L. MiRNA-26b inhibits cellular proliferation by targeting CDK8 in breast cancer. Int J Clin Exp Med. 2014;7:558–565. [PMC free article] [PubMed] [Google Scholar]
- 32.Li XY, Luo QF, Wei CK, Li DF, Li J, Fang L. MiRNA-107 inhibits proliferation and migration by targeting CDK8 in breast cancer. Int J Clin Exp Med. 2014;7:32–40. [PMC free article] [PubMed] [Google Scholar]
- 33.Li XY, Luo QF, Wei CK, Li DF, Fang L. siRNA-mediated silencing of CDK8 inhibits proliferation and growth in breast cancer cells. Int J Clin Exp Pathol. 2013;7:92–100. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Hua K, Jin J, Zhang H, Zhao B, Wu C, Xu H, Fang L. MicroRNA-7 inhibits proliferation, migration and invasion of thyroid papillary cancer cells via targeting CKS2. Int J Oncol. 2016;49:1531–1540. doi: 10.3892/ijo.2016.3660. [DOI] [PubMed] [Google Scholar]
- 35.Shi P, Feng J, Chen C. Hippo pathway in mammary gland development and breast cancer. Acta Biochim Biophys Sin. 2015;47:53–59. doi: 10.1093/abbs/gmu114. [DOI] [PubMed] [Google Scholar]
- 36.McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011;75:50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang F, Steelman LS, Lee JT, Shelton JG, Navolanic PM, Blalock WL, Franklin RA, McCubrey JA. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: Potential targeting for therapeutic intervention. Leukemia. 2003;17:1263–1293. doi: 10.1038/sj.leu.2402945. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.