Abstract
Asthma and chronic obstructive pulmonary disease (COPD) are both highly prevalent conditions that can coexist in the same individual: the so-called ‘asthma -COPD overlap’ (ACO). Its prevalence and prognosis vary widely depending on how ACO is defined in each publication, the severity of bronchial obstruction of patients included and the treatment they are receiving. Although there is a lack of evidence about the biology of ACO, the overlap of both diseases should express a mixture of a Th1 inflammatory pattern (characteristic of COPD) and a Th2 signature (characteristic of asthma). In this review we support a novel algorithm for ACO diagnosis proposed by the Spanish Respiratory Society (SEPAR), based on a sequential evaluation that considers: (a) the presence of chronic airflow limitation in a smoker or ex-smoker patient ⩾35 years old; (b) a current diagnosis of asthma; and (c) the existence of a very positive bronchodilator test (PBT; ⩾15% and ⩾400 ml) or the presence of eosinophilia in blood (⩾300 eosinophils/μl). This algorithm can identify those patients who may benefit from a treatment with inhaled corticosteroids (ICSs) and maybe from biological drugs in a near future. In addition, it is easily applicable in clinical practice. The major disadvantage is that it groups patients with very different characteristics under the ACO’s umbrella. In view of this heterogeneity, we recommend a strategy of defining specific and measurable therapeutic objectives for every single patient and identifying the traits that can be treated to achieve those objectives.
Keywords: asthma, COPD, asthma-COPD overlap
Introduction
Asthma and chronic obstructive pulmonary disease (COPD) are two inflammatory diseases characterized by airflow obstruction that have different pathogenic mechanisms and different degrees of response to anti-inflammatory treatment. Given the fact that both are highly prevalent conditions, it is very likely that they overlap in some individuals. In the last decade there has been increasing interest in this entity that is now known as asthma–COPD overlap (ACO). However, this interest is not new, and was already addressed by Burrows and colleagues in 1987, describing a group of patients who had a clinical evolution and a prognosis that was between asthma and COPD,1 at that time labelled as ‘asthmatiform bronchitis’, supporting the view of a common origin of asthma and COPD, the so-called Dutch hypothesis.2 Recent studies of lung function trajectories in COPD also support the influence of early childhood asthma in lung development.3 The reason for this renewed interest has to do, on the one hand, with the proposal of identification of phenotypes with different prognosis and response to therapy in COPD and, on the other, with the warning provoked by the indiscriminate use of inhaled corticosteroids (ICSs) in patients with COPD that led to an increased signal of pneumonia in some clinical trials.
Taking apart the academic discussions and, in keeping with Burrows’ observations, the reality is that some patients frequently appear with clinical characteristics that overlap both diseases. These real-life patients, not clearly represented in clinical trials, might have a different evolution and prognosis, especially in the most severe forms, so their early identification is clinically relevant. The use of biomarkers such as periostin, eosinophilia in sputum or blood, bronchial hyperresponsiveness or nitric oxide in exhaled air4 has shown unequal results. There is some controversy regarding the clinical and prognostic repercussion of the ACO, not to mention that there are authors that question its own existence, or the criteria used to define it. Some studies5,6 conclude that it leads to more frequent and serious exacerbations as well as a worse quality of life, while others indicate the opposite.7 In this review we will try to clarify the repercussion that the presence of ACO may have, how to identify it in a simple way and propose a pragmatic therapeutic approach.
Definition of the asthma–COPD overlap and its prevalence
The identification or definition of ACO has different perspectives and, as a consequence, to estimate its prevalence is complex since it varies according the criteria used to define it.8–10 The COPDGene5 study used the coexistence of the diagnostic code of asthma (diagnosed before the age of 40 years) and COPD in the clinical history of the same patient and found a prevalence of 13% of this overlap that was associated with an increased risk of exacerbations and hospitalizations. These figures are similar to those reported in the PLATINO study6 that adopted a similar definition: 12% prevalence of ACO and more risk of exacerbations in these patients [odds ratio (OR) 3.01]. In Spain, an expert consensus11 proposed a series of major criteria (history of asthma, eosinophils in sputum >5% or bronchodilator test >400 ml and >15%) and minor criteria [immunoglobulin (Ig)E >100, allergy and two or more bronchodilator tests >200 ml and 12%] that were subsequently adopted by the Spanish COPD guidelines. Later on, a validation study in a cohort of patients with COPD found a very low prevalence of ACO using these criteria (0.5%) which led to propose including eosinophilia in peripheral blood (>5%) as a minor criterion, which raised the prevalence to 15%. Using these modified criteria, ACO patients seemed to show lower mortality rates after 1 year of follow up,7 the same that was shown by the de Hokkaido cohort (a COPD cohort that excluded patients with diagnosis of asthma) after 10 years of follow up.12 Such a discrepancy can be explained by how the entity is defined in each publication, by considering that the populations included in the different studies vary in the degree of bronchial obstruction (in patients with ACO and mild obstruction, the burden of asthma could be heavier than that of COPD, which would result in a better therapeutic response and a more favorable prognosis) and by the fact that the rate of patients with ACO who were receiving ICSs was far from being uniform (ACO patients are more likely to benefit from this therapy, likely leading to a better prognosis in comparison with the non-ACO groups). The prognostic significance of having a diagnosis of ACO must be further assessed in the light of a prospective cohort study, designed to compare the outcomes of COPD patients, asthmatics and individuals with both diseases.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) has incorporated a consensus document developed jointly with the Global Initiative for Asthma (GINA), which defines ACO and proposes a different approach in which at least three characteristics of asthma and three of COPD (taken from a given list of symptoms and clinical characteristics) should be fulfilled in the same patient.13 More recently, a task force of the Spanish Guidelines for COPD,14 and Spanish Guidelines for Asthma (GEMA) proposed a unified diagnostic algorithm.15 Both approaches will be discussed in detail further in this review.
Regardless of the suggested definition, there is a fundamental problem: in some way they incorporate COPD and asthma definitions, which are themselves imprecise and based on nonspecific clinical, inflammatory or physiological features. Furthermore, they do not take into account ACO’s specific inflammatory characteristics, which remain largely unknown (if they really do exist).
The biological basis
COPD and asthma share a series of clinical and biological similarities that often make their differentiation complex, especially in smokers with a history of atopy. However, both processes can have a pathogenic and pathophysiological basis easily differentiable in most cases.14 The clinical characteristics shared by both diseases are based on the inflammation and obstruction of the airway, the latter being poorly reversible and progressive in COPD and variable and reversible in asthma. Likewise, the location of the inflammatory response among these pathologies also has differences, being the predominant involvement of COPD in peripheral airways and lung parenchyma, in contrast with the lack of lung parenchyma damage and the panfocal involvement of the airway in asthma. Moreover, the key cells and mediators in both process also differ, being neutrophils, CD8 + T-lymphocytes and macrophages with interleukin (IL)-8 and tumor necrosis factor-alpha (TNF-α), among others, playing a predominant role in the case of COPD. In asthma, eosinophils, mast cells, CD4 + T-lymphocytes and a smaller number of macrophages are the representative cells14 with multiple inflammatory mediators involved in asthma15 such as histamine, leukotrienes, IL-4, IL-5 and IL-13. The presence of high bronchodilator responsiveness as a read out of bronchial hyperresponsiveness (BHR), is characteristic of asthma and is partially correlated with the severity of the disease and with markers of inflammation. In COPD, as we will discuss later, the presence of BHR is not considered a predominant finding. However, when analyzing the behavior of BHR in people older than 65 years, smokers and nonsmokers, an association with excessive loss of lung function, measured through the forced expiratory volume in one second (FEV1), was found.16 The consequence of the inflammatory cascade in both pathologies causes a progressive and scarcely reversible loss of lung function in COPD that is characterized by a bronchiolitis that evolves to fibrosis, where it is possible to observe areas of epithelial metaplasia of the mucus-producing cells. The remodeling of the airway present in asthma, due to the deposition of subepithelial collagen and the hypertrophy of the bronchial smooth muscle, may be responsible for the progression of the loss of lung function in persistent asthma. Conversely, in some patients the inflammatory profile of asthma and COPD could be similar, such as (1) neutrophilic asthma: a pattern of neutrophilic inflammation similar to that of COPD is found in smoking asthmatic patients, with predominance of neutrophils in the sputum, increased IL-8, TNF-α and oxidative stress, who also have a poor response to both inhaled and systemic corticosteroids;17 (2) COPD with reversibility to bronchodilators may have increased eosinophils in the induced sputum, increased levels of exhaled nitric oxide (NO)4 and better response to treatment with corticosteroids,17 all characteristic of asthma; and (3) COPD with eosinophilia may show an underlying Th2 signature, expressed by high blood eosinophil counts that has been associated to a higher reversibility, and better response to ICSs.18 Also, exacerbations of asthma and COPD can have common triggers (viruses, bacteria, environmental pollution, fumes). In both diseases exacerbations are associated with an increase in airway inflammation, an increase in the number of cells and higher concentrations of proinflammatory cytokines. Exacerbations of asthma show an increase in neutrophils and eosinophils, whereas exacerbations of COPD may present eosinophilia in the sputum.17
ACO is a disorder of adulthood that may begin early in life and several potential pathways might bring about its occurrence. One such pathway begins in early-onset allergic asthma. Smoking habits later in life might lead to the development of fixed airflow limitation and COPD in many of these patients. A second potential pathway recognizes patients with a lifetime smoking history, subsequent COPD and late-onset features of asthma (adult-onset eosinophilic asthma) or COPD with eosinophilic inflammation. Since both asthma and COPD are inflammatory diseases that affect the bronchial tree, the overlap of both diseases should show some evidence of a Th1 inflammatory pattern (characteristic of COPD) and some evidence of a Th2 (characteristic of asthma). However, there is a lack of evidence about the biology of ACO, with very few studies supporting this intuitive hypothesis.18,19
ACO is a heterogenous disorder
As we explained before, the problem with ACO is that clusters two entities with a different inflammatory substrate and clinical characteristics: smoking asthmatics and eosinophilic COPD (e-COPD), probably leading to a confused signal. Discrepant results when looking at clinical outcomes across the studies could be due to this uncertain clustering. An initial analysis of the CHACOS study population (a cohort comprising patients with all the different forms of chronic bronchial obstruction) showed that the clinical history of ACO patients did not differ significantly (in terms of previous exacerbations and symptoms measured by the asthma control test and COPD assessment test) from patients with COPD or asthma.20 However, when patients were reclassified according to their inflammatory pattern as ‘type 2-high’ (⩾300 eosinophils/l in blood or ⩾3% in sputum), or ‘type 2-low’, two groups of chronic airflow limitation patients emerged that did show different clinical characteristics.20 Consequently, this new categorization helped in selecting patients who were candidates for treatments aimed at specific inflammatory patterns, such as ICSs or biological agents. In a second study, we investigated the value and interactions of blood biomarkers of systemic inflammation (IL-6, IL-8, TNF-α, IL-17) and type 2 inflammation (periostin, IL-5, and IL-13) in patients with asthma, COPD, and ACO. A network analysis and a principal component analysis showed the inflammatory pattern of ACO to be a mixture of the patterns observed in asthma and COPD, but no single biomarkers nor any combination of biomarkers were accurate enough to differentiate ACO from asthma or COPD.18
We also compared the clinical characteristics and the inflammatory profile of e-COPD and smoking asthmatics. Patients classified as e-COPD were older and more often male and showed significantly impaired pulmonary function, likely explained by a heavier smoking habit. On the contrary, smoking asthmatics had more atopic features, more reversibility of airflow obstruction and higher IgE levels. The concentrations of IL-5, IL-13, IL-8, IL-6, TNF-α, and IL-17 in serum were similar between the two groups. However, type 2-related biomarkers [periostin, nitric oxide (FeNO) and blood eosinophils] showed higher median values in e-COPD patients.21 Our findings reinforce the notion that ACO is a heterogeneous disorder and, as a consequence, it might be unacceptable to offer the same treatment for two related but different conditions.22
Diagnosis of ACO
The interest in identifying patients with ACO over the last few years has led to the publication of several diagnostic algorithms, developed by groups of experts, organizations and national or international societies.23–25 Currently, there is no widely accepted classification criteria, so the diagnosis of ACO depends ultimately on the adopted definition and the population under study.26 This makes it extremely difficult to share knowledge and carry out clinical trials specifically designed to study this entity.
As we have already mentioned GOLD and GINA have published a joint document in which they suggest an approach to ACO diagnosis.24 They recommend collecting clinical, functional and radiological characteristics of asthma and COPD to identify a group of patients that share features of both diseases. However, in our opinion, diagnostic criteria of GINA/GOLD are imprecise and have limited practical value, because it has not been clearly established how many of the features are necessary or whether they have the same relevance for the diagnosis of ACO. For instance, significant exposure to tobacco (or biomass) smoke is considered as one of the features that point towards COPD diagnosis. However, smoking is an essential factor for the identification of ACO, because it is mandatory to diagnose COPD, one of the components of this entity; a never-smoking asthmatic with persistent airflow obstruction cannot be diagnosed with ACO.27 Moreover, this approach has completely ignored the nature of the underlying bronchial inflammation, denoted by the presence or absence of several biomarkers (mainly blood eosinophilia) that have been proven to predict therapeutic response to ICSs.28
The Spanish Respiratory Society (SEPAR) has proposed another diagnostic algorithm.23 According to this proposal, the diagnosis will be confirmed based on the following sequential evaluation (Figure 1):
Figure 1.
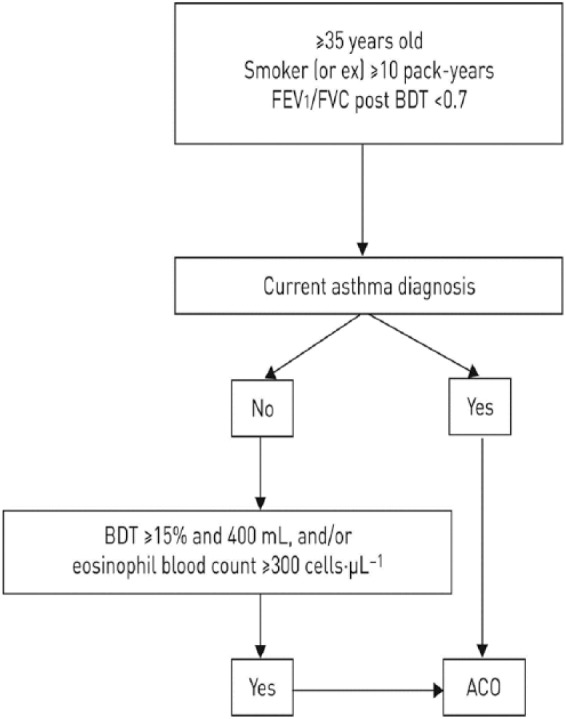
Spanish Respiratory Society’s algorithm for the identification of patients with ACO.
ACO, asthma–COPD overlap; BDT, bronchodilator test; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
(1) Presence of chronic airflow limitation [FEV1/forced vital capacity (FVC) post-bronchodilator <70%] in a patient ⩾35 years old, smoker or ex-smoker with a smoking history of at least 10 pack-years.
(2) Current diagnosis asthma, that should include: (a) history or symptoms of clinical suspicion: family history of asthma or personal history of asthma in childhood or personal history of atopy (sensitization to certain allergens), with respiratory symptoms (wheezing, cough, chest tightness) of variable course, sometimes in the form of dyspnea crisis of also variable intensity, or inflammation of the upper airway (rhinosinusitis with or without nasal polyposis); and (b) objective diagnostic confirmation of asthma with reversibility of airflow obstruction by spirometry or a PBT (⩾12% and ⩾200 ml), or a circadian variability of peak expiratory flow (PEF) ⩾ 20% or an exhaled fraction of FeNO ⩾ 50 ppb.
(3) In the event that the diagnosis of asthma cannot be established, the diagnosis of ACO will be confirmed in the presence of a very positive bronchodilator test (PBT ⩾15% and ⩾400 ml); or, in the presence of eosinophilia in blood (⩾300 eosinophils/μl) or both. These characteristics, although they are not diagnostic of asthma by themselves, point towards the existence of a high type 2 inflammatory pattern, which in a smoking patient with chronic airflow obstruction, allows it to be classified as ACO.
This approach was recently examined in a population of 292 patients with chronic bronchial obstruction (COPD, nonsmoking asthmatics, smoking asthmatics and e-COPD). This algorithm classifies as ACO all smoking asthmatics with nonfully reversible airway obstruction and a considerable proportion of e-COPD patients (48%). However, no patient in our study was classified as having ACO on the basis of a ‘very positive bronchodilator response’ as a single diagnostic feature, because all patients with this characteristic had either a current asthma diagnosis or high blood eosinophilia. Although the exact cut-off point for blood eosinophilia remains controversial, the authors of this algorithm have decided to choose 300 cells/µl. The main justifications for their position are the following: (a) Wagener and colleagues found that the best cut-off point for blood eosinophils was 270 cells/µl to detect sputum eosinophilia ⩾3% (AUROC 89%) in asthma patients;29 (b) patients with moderate-to-severe COPD and blood eosinophil counts of ⩾300 cells/μl have shown an increased risk exacerbations in the COPDGene study, which was prospectively validated in the ECLIPSE study;30 (c) it has been found that COPD patients with blood eosinophil levels >300 cells/µl achieved a greater reduction in exacerbations following ICS treatment compared with bronchodilators.31 Therefore, as a main advantage, the algorithm can identify those patients with bronchial chronic obstruction who may benefit from a treatment with ICSs and maybe from biological drugs in the near future. In addition, it is easily applicable in clinical practice, given the fact that it uses widely available data. The major disadvantage is that it groups patients with very different characteristics under the ACO umbrella, as we have stated above.
Treatment of ACO
Addressing the best therapeutic approach for patients with ACO remains a challenge, since they have been intentionally excluded from clinical trials: most clinical trials for asthma excluded patients with features of COPD, and COPD clinical trials have not usually included patients who might have an asthmatic component to their disease. Moreover, the aforementioned heterogeneity of ACO makes it impossible to recommend a simplistic, ‘one size fits all’ approach to the management of these patients. Thus, following the above arguments, it might be more appropriate to classify ACO patients into two different categories (or phenotypes): e-COPD and smoking asthmatics, separately reviewing the evidence relating to the treatment of each of them.
Eosinophilic COPD
It has been shown that ICSs reduce exacerbations in patients with moderate-to-severe COPD and a history of exacerbations;32–36 however, the overall treatment effect is modest, individual patient responses are variable and important potential adverse effects exist, including increased risk of pneumonia.37 To identify which COPD patients are most likely to benefit from ICSs has been a challenge for respiratory physicians and validation of a simple biomarker to allow targeted treatment has been long pursued. Several studies show that an increased induced sputum eosinophil count predicts response to oral steroids38–40 and ICSs.41,42 However, this technique is time consuming and a high degree of experimental rigor is needed, complicating the setting up of the test in routine clinical practice.
A post-hoc analysis of data from two replicate, randomized, double-blind trials comparing once a day vilanterol with three different doses of vilanterol-fluticasone furoate combination in patients with moderate-to-severe COPD showed that patients with a high blood eosinophil count gained greater benefit from treatment with ICSs to reduce exacerbation frequency than those with a low eosinophil count.31 The relationship between baseline blood eosinophils and the rate of exacerbations with indacaterol/glycopyrronium compared with salmeterol/fluticasone was examined through analysis of data from the FLAME study. The main conclusion is that ‘indacaterol/glycopyrronium provides superior or similar benefits over salmeterol/fluticasone regardless of blood eosinophil levels in patients with COPD’, which does not exactly mean that blood eosinophil counts make no difference in influencing the response.43 In fact, some authors advocate the use of a blood eosinophil threshold of 300 cells/µl to determine the likelihood of response to ICSs in asthma and COPD.44 However, this publication shows that the point estimate of hazard ratios increases and gets closer to 1 when the blood eosinophil count increases but, in patients with blood eosinophils ⩾300/µl, the point estimate is almost equal to but not greater than 1 suggesting a lack of difference between treatment arms. Therefore, the exact blood eosinophils cut-off point that best reflects an increased risk for exacerbation and a better response to ICSs remains controversial, and some authors argue that it should be used as a continuous variable, like serum cholesterol, considering other factors such as age, comorbidities, and cardiovascular risk in the decision-making process.45
TRINITY was a 52-week double-blind, double-dummy, randomized, multicenter, three-arm parallel-group, clinical trial to assess the superiority of extrafine beclometasone dipropionate (BDP), formoterol fumarate (FF), and glycopyrronium bromide, known as a fixed triple, versus tiotropium, and BDP/FF plus tiotropium (open triple). The fixed triple was superior to tiotropium and noninferior to the open triple and, interestingly, the effect of the two triple therapies on the exacerbation rate was greater in the subgroups with higher eosinophil concentrations (at least 2%).46
On the other hand, the recently published IMPACT study showed that once-daily triple therapy with fluticasone furoate, umeclidinium, and vilanterol resulted in a significantly lower rate of COPD exacerbations and better lung function and health-related quality of life than dual therapy with fluticasone furoate-vilanterol or the dual bronchodilator umeclidinium-vilanterol among patients with severe COPD and a history of exacerbations.47 A greater reduction in the exacerbation rate was observed in patients with eosinophil levels of at least 150 cells/µl. Altogether, these studies seem to indicate that clinical response to ICSs is related to a ‘type 2-high’ inflammatory bronchial response, clinically signaled by increased blood eosinophils levels.
Theoretically, blocking the pathway activated by IL-5 may have a beneficial impact in e-COPD patients. To date, only data on benralizumab and mepolizumab are available. A phase IIa clinical trial showed that numerical, albeit nonsignificant, improvement in COPD exacerbations, quality of life and FEV1 were greater in benralizumab-treated patients with high baseline blood eosinophil concentrations compared with placebo.48 In addition, mepolizumab at a dose of 100 mg was associated with a lower annual rate of exacerbations than placebo among patients with COPD and an eosinophilic phenotype.49 Further research is needed to clarify whether anti-IL-5 drugs should be employed in specific COPD subpopulations characterized by ‘type 2-high’ inflammation.
Smoking asthmatics
As previously noted, smoking asthmatics constitute a more heterogeneous group from the inflammatory point of view than eosinophilic COPD, probably encompassing ‘type 2-high’, neutrophilic and mixed endotypes. Previous studies have shown that only ‘type 2-high’ asthma patients respond to a course of ICSs50 and that smoking is associated with attenuated response to these drugs.51 However, International Guidelines recommend the use of ICSs in virtually all asthmatic patients and we do not yet have enough evidence to question this dogma at the current time.24
It has been demonstrated that tiotropium reduces exacerbations by 21% and improves pulmonary function and symptoms when given as an add-on therapy in asthma patients who remain uncontrolled despite been treated with a combination of ICSs and long-acting β adrenoceptor agonists (LABAs)52 and remarkably, an exploratory subgroup analysis of four large randomized trials suggests that the results are independent of a type 2 phenotype.53 Since tiotropium seems to be well tolerated for asthma patients, it would be almost mandatory to recommend triple inhaled therapy for severe asthma patients with persistent bronchial obstruction.
On the other hand, azithromycin has been shown to significantly reduce exacerbations and improve health-related quality of life in adults with uncontrolled asthma, currently symptomatic despite ICS and LABA use. The AMAZES study included patients with mild bronchial obstruction and with a median of one exacerbation during the prior year.54 Therefore, they are not the most severe patients we can find in our clinical practice. Patients with a hearing impairment or abnormally prolonged QTc interval were excluded and also those who were active smokers or ex-smokers with more than 10 pack-years if their diffusing capacity for carbon monoxide was less than 70% of the predicted value (this criterion rules out most ACO patients). The authors did not observe a reduction in inflammatory cell counts in the sputum to support a definite anti-inflammatory effect and azithromycin was effective in patients with and without potentially pathogenic microorganisms in sputum cultures at baseline. Azithromycin was effective irrespective of the number of eosinophils in blood. Thus, the exact mechanism of action remains poorly understood. Diarrhea (but no other potentially drug-related adverse effect) was more common in the active arm of the clinical trial; sputum cultures of azithromycin patients showed a nonsignificant increase in azithromycin-resistant bacteria. Since microbial resistance generates justified concern, add-on therapy with azithromycin in asthma should be restricted to those patients with the highest unmet medical need (e.g. frequent exacerbators) and, maybe, to winter months (periods with the greatest risk of exacerbations).
Limited clinical data exist on the efficacy of biological drugs in a population with overlapping asthma and COPD. Data from the Australian Omalizumab Registry were used to compare treatment responses in ACO patients with responses in patients with severe asthma alone. This study, although with clear limitations due to methodological bias and limited scope, suggests that omalizumab can improve symptoms and health-related quality of life in individuals with ACO.55
Proposal for the treatment of ACO
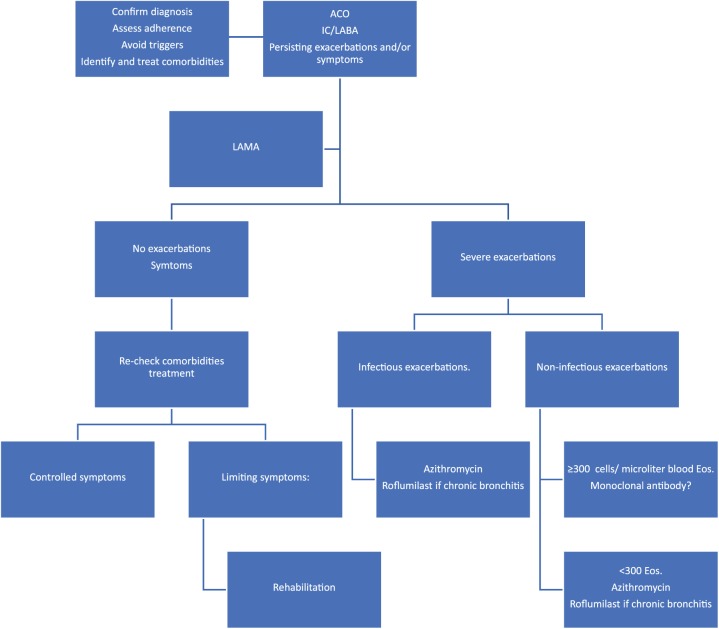
As mentioned above, ACO is a heterogeneous and somewhat inconsistent entity, characterized by wide clinical and biological variability. This fact reflects the need for personalized medicine to define each specific patient as the combination of distinct biological, clinical and social features. Maybe the widespread use of ‘omics’ in the near future will provide the physicians with the information they need to accurately categorize individuals with chronic obstructive bronchial disease. In the meantime, it seems reasonable to adopt a pragmatic and realistic course of action defining specific and measurable therapeutic objectives for every single patient and identifying the traits that can be treated to achieve those objectives. Figure 2 illustrates ACO treatment based on such an approach.
Figure 2.
An objectives/treatable traits approach to ACO treatment.
ACO, asthma–COPD overlap; COPD, chronic obstructive pulmonary disease; Eos, eosinophils; IC, Inhaled corticosteroid; LABA, long-acting β adrenoceptor agonist; LAMA, long-acting antimuscarinic agent.
Briefly, an important principle of ACO management is to avoid treatment with LABAs alone (LABA monotherapy) without ICSs in patients with asthma symptoms. ACO patients have irreversible bronchial obstruction that can be treated to improve severe exacerbations and symptoms with intensive bronchodilation. Therefore, the use of a LABA is mandatory and the addition of a long-acting antimuscarinic agent (LAMA) should be the first-line option if severe exacerbations or symptoms persist. If the patient continues having symptoms but no exacerbations, comorbidities should be identified and treated because they are very common in severe asthma and they can have a great impact in patient symptomatology. If severe exacerbations persist despite treatment with a LAMA, azithromycin is a good option (if not contraindicated), irrespectively of the inflammatory signature, but particularly if the etiology of the exacerbations is infectious. If this option fails, treatment with a monoclonal antibody could be considered in patients with a type 2 inflammatory pattern (peripheral eosinophilia or high IgE) and persistent severe exacerbations despite treatment with triple inhaled therapy, although their efficacy remains to be demonstrated in this setting. On the other hand, it has been found that almost a third of the patients with uncontrolled asthma have bronchiectasis56 and many of these patients can be chronically infected by a diversity of microbes. If that is the case, the role of inhaled antibiotic therapy needs to be determined. Finally, roflumilast, is a selective phosphodiesterase 4 inhibitor that has shown efficacy in severe COPD with frequent exacerbations and a chronic bronchitis phenotype and could also be effective in patients with asthma.57 This could also be an option in ACO, although further evidence is required.
Conclusion
ACO is the coexistence of two bronchial inflammatory disorders in the same individual. Given the fact that COPD and asthma can present with a variety of clinicopathological features, it should come as no surprise that ACO lacks a universally applicable definition and a precise set of diagnostic criteria. In this context, the Spanish Respiratory Society’s algorithm represents a practical and easy-to-implement solution, because it allows to identify those patients with bronchial chronic obstruction who can benefit from treatment with ICSs, and, in a hypothetical near future, from biological drugs. One pragmatic way to account for the heterogeneity of ACO is to adopt a strategy of defining specific and measurable therapeutic objectives for every single patient and identifying the traits that can be treated to achieve those objectives. Nevertheless, more studies are needed in order to clarify several important issues with regard to ACO, such as the molecular pathways and underlying mechanisms, the identification of possible specific biomarkers for diagnosis and targeted treatment, the prognosis and, finally, the optimal therapeutic interventions for this entity.
Footnotes
Funding: Dr Pérez de Llano reports personal fees and nonfinancial support from Novartis, grants and personal fees from Astra-Zeneca, personal fees and nonfinancial support from GSK, grants, personal fees and nonfinancial support from Teva, personal fees and nonfinancial support from Boehringer-Ingelheim, grants and personal fees from Chiesi, personal fees from Sanofi, nonfinancial support from Menarini, personal fees and nonfinancial support from Mundipharma, personal fees and nonfinancial support from Esteve, outside the submitted work.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Borja G. Cosío  https://orcid.org/0000-0002-6388-8209
https://orcid.org/0000-0002-6388-8209
Luis Pérez de Llano  https://orcid.org/0000-0003-2652-6847
https://orcid.org/0000-0003-2652-6847
Contributor Information
Borja G. Cosío, Department of Respiratory Medicine, Hospital Universitario Son Espases-IdISBa, Palma de Mallorca, Spain CIBER de Enfermedades Respiratorias (CIBERES), Instituto de Salud Carlos III, Madrid, Spain.
David Dacal, Department of Respiratory Medicine, Hospital Arquitecto Marcide, Ferrol, Spain.
Luis Pérez de Llano, Department of Respiratory Medicine, Hospital Lucus Augusti, C/ Dr Ulises Romero, nº 1, Lugo, 27004, Spain.
References
- 1. Burrows B, Bloom JW, Traver GA, et al. The course and prognosis of different forms of chronic airways obstruction in a sample from the general population. N Engl J Med 1987; 317: 1309–1314. [DOI] [PubMed] [Google Scholar]
- 2. Postma DS, Weiss ST, van den Berge M, et al. Revisiting the Dutch hypothesis. J Allergy Clin Immunol 2015; 136: 521–529. [DOI] [PubMed] [Google Scholar]
- 3. Bui DS, Lodge CJ, Burgess JA, et al. Childhood predictors of lung function trajectories and future COPD risk: a prospective cohort study from the first to the sixth decade of life. Lancet Respir Med 2018; 6: 535–544. [DOI] [PubMed] [Google Scholar]
- 4. Papi A, Romagnoli M, Baraldo S, et al. Partial reversibility of airflow limitation and increased exhaled NO and sputum eosinophilia in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000; 162: 1773–1777. [DOI] [PubMed] [Google Scholar]
- 5. Hardin M, Silverman EK, Barr RG, et al. The clinical features of the overlap between COPD and asthma. Respir Res 2011; 12: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Menezes AM, Montes de OM, Perez-Padilla R, et al. Increased risk of exacerbation and hospitalization in subjects with an overlap phenotype: COPD-asthma. Chest 2014; 145: 297–304. [DOI] [PubMed] [Google Scholar]
- 7. Cosio BG, Soriano JB, Lopez-Campos JL, et al. Defining the asthma-COPD overlap syndrome in a COPD cohort. Chest 2016; 149: 45–52. [DOI] [PubMed] [Google Scholar]
- 8. van Boven JF, Roman-Rodriguez M, Palmer JF, et al. Comorbidome, pattern and impact of asthma-COPD overlap syndrome (ACOS) in real-life. Chest 2016; 149: 1011–1020. [DOI] [PubMed] [Google Scholar]
- 9. Alshabanat A, Zafari Z, Albanyan O, et al. Asthma and COPD overlap syndrome (ACOS): a systematic review and meta analysis. PLoS One 2015; 10: e0136065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fernandez-Villar A, Lopez-Campos JL. Mixed COPD-asthma phenotype: ACOS or CAOS? A reflection on recent guidelines and recommendations. Arch Bronconeumol 2016; 52: 277–278. [DOI] [PubMed] [Google Scholar]
- 11. Soler-Cataluna JJ, Cosio B, Izquierdo JL, et al. Consensus document on the overlap phenotype COPD-asthma in COPD. Arch Bronconeumol 2012; 48: 331–337. [DOI] [PubMed] [Google Scholar]
- 12. Suzuki M, Makita H, Konno S, et al. Asthma-like features and clinical course of chronic obstructive pulmonary disease. An analysis from the Hokkaido COPD cohort study. Am J Respir Crit Care Med 2016; 194: 1358–1365. [DOI] [PubMed] [Google Scholar]
- 13. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Arch Bronconeumol 2017; 53: 128–149. [DOI] [PubMed] [Google Scholar]
- 14. Miravitlles M, Soler-Cataluna JJ, Calle M, et al. Spanish guidelines for management of chronic obstructive pulmonary disease (GesEPOC) 2017. Pharmacological treatment of stable phase. Arch Bronconeumol 2017; 53: 324–335. [DOI] [PubMed] [Google Scholar]
- 15. Miravitlles M, Alvarez-Gutierrez FJ, Calle M, et al. Algorithm for identification of asthma-COPD overlap: consensus between the Spanish COPD and asthma guidelines. Eur Respir J 2017; 49. [DOI] [PubMed] [Google Scholar]
- 16. Perez de Llano L, Cosio BG, Miravitlles M, et al. Accuracy of a new algorithm to identify asthma-COPD overlap (ACO) patients in a cohort of patients with chronic obstructive airway disease. Arch Bronconeumol 2018; 54: 198–204. [DOI] [PubMed] [Google Scholar]
- 17. Chanez P, Vignola AM, O’Shaugnessy T, et al. Corticosteroid reversibility in COPD is related to features of asthma. Am J Respir Crit Care Med 1997; 155: 1529–1534. [DOI] [PubMed] [Google Scholar]
- 18. Perez de Llano L, Cosio BG, Iglesias A, et al. Mixed Type 2 and non-Type 2 inflammatory pattern in the asthma-COPD overlap: a network approach. Int J Chron Obstruct Pulmon Dis 2018; 13: 591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Christenson SA, Steiling K, van den Berge M, et al. Asthma-COPD overlap. Clinical relevance of genomic signatures of type 2 inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015; 191: 758–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cosio BG, Perez de LL, Lopez VA, et al. Th-2 signature in chronic airway diseases: towards the extinction of asthma-COPD overlap syndrome? Eur Respir J 2017; 49. [DOI] [PubMed] [Google Scholar]
- 21. Perez-de-Llano L, Cosio BG. Asthma-COPD overlap is not a homogeneous disorder: further supporting data. Respir Res 2017; 18: 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Perez de Llano LA, Cosio BG. Asthma, chronic obstructive pulmonary disease and other combinations. Arch Bronconeumol 2016; 52: 499–500. [DOI] [PubMed] [Google Scholar]
- 23. Plaza V, Álvarez F, Calle M, et al. Consensus on the asthma-COPD overlap syndrome (ACOS) between the Spanish COPD guidelines (GesEPOC) and the Spanish guidelines on the management of asthma (GEMA). Arch Bronconeumol 2017; 53: 443–449. [DOI] [PubMed] [Google Scholar]
- 24. Global Initiative for Asthma (GINA). Diagnosis of diseases of chronic airflow limitation: asthma, COPD and asthma–COPD overlap syndrome (ACOS). Global strategy for asthma management and prevention, 2014. http://ginasthma.org/asthma-copd-and-asthma-copd-overlap-syndrome-acos/ (accessed 7 January 2017) [Google Scholar]
- 25. Sin DD, Miravitlles M, Mannino DM, et al. What is asthma-COPD overlap syndrome? Towards a consensus definition from a round table discussion. Eur Respir J 2016; 48: 664–673. [DOI] [PubMed] [Google Scholar]
- 26. Bonten TN, Kasteleyn MJ, de Mutsert R, et al. Defining asthma-COPD overlap syndrome: a population-based study. Eur Respir J 2017; 49. [DOI] [PubMed] [Google Scholar]
- 27. Miravitlles M. Diagnosis of asthma - COPD overlap: the five commandments. Eur Respir J 2017; 49. [DOI] [PubMed] [Google Scholar]
- 28. Bafadhel M, Pavord ID, Russell REK. Eosinophils in COPD: just another biomarker? Lancet Respir Med 2017; 5: 747–759. [DOI] [PubMed] [Google Scholar]
- 29. Wagener AH, de Nijs SB, Lutter R, et al. External validation of blood eosinophils, FE(NO) and serum periostin as surrogates for sputum eosinophils in asthma. Thorax 2015; 70: 115–120. [DOI] [PubMed] [Google Scholar]
- 30. Yun JH, Lamb A, Chase R, et al. Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol 141: 2037–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pascoe S, Locantore N, Dransfield MT, et al. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med 2015; 3: 435–442. [DOI] [PubMed] [Google Scholar]
- 32. Ferguson GT, Anzueto A, Fei R, Emmett A, et al. Effect of fl uticasone propionate/salmeterol (250/50 microg) or salmeterol (50 microg) on COPD exacerbations. Respir Med 2008; 102: 1099–1108. [DOI] [PubMed] [Google Scholar]
- 33. Dransfield MT, Bourbeau J, Jones PW, et al. Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. Lancet Respir Med 2013; 1: 210–223. [DOI] [PubMed] [Google Scholar]
- 34. Szafranski WW, Cukier A, Ramirez A, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J 2003; 21: 74–81. [DOI] [PubMed] [Google Scholar]
- 35. Sharafkhaneh A, Southard JG, Goldman M, et al. Effect of budesonide/formoterol pMDI on COPD exacerbations: a double-blind, randomized study. Respir Med 2012; 106: 257–268. [DOI] [PubMed] [Google Scholar]
- 36. Agarwal R, Aggarwal AN, Gupta D, et al. Inhaled corticosteroids vs placebo for preventing COPD exacerbations: a systematic review and metaregression of randomized controlled trials. Chest 2010; 137: 318–325. [DOI] [PubMed] [Google Scholar]
- 37. Suissa S. Number needed to treat in COPD: exacerbations versus pneumonias. Thorax 2013; 68: 540–543. [DOI] [PubMed] [Google Scholar]
- 38. Brightling CE, Monteiro W, Ward R, et al. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2000; 356: 1480–1485. [DOI] [PubMed] [Google Scholar]
- 39. Bafadhel M, McKenna S, Terry S, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med 2012; 186: 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pizzichini E, Pizzichini MM, Gibson P, et al. Sputum eosinophilia predicts benefit from prednisone in smokers with chronic obstructive bronchitis. Am J Respir Crit Care Med 1998; 158: 1511–1517. [DOI] [PubMed] [Google Scholar]
- 41. Brightling CE, McKenna S, Hargadon B, et al. Sputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary disease. Thorax 2005; 60: 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Leigh R, Pizzichini MM, Morris MM, et al. Stable COPD: predicting benefi t from high-dose inhaled corticosteroid treatment. Eur Respir J 2006; 27: 964–971. [DOI] [PubMed] [Google Scholar]
- 43. Roche N, Chapman KR, Vogelmeier CF, et al. Blood eosinophils and response to maintenance chronic obstructive pulmonary disease treatment. Data from the FLAME trial. Am J Respir Crit Care Med 2017; 195: 1189–1197. [DOI] [PubMed] [Google Scholar]
- 44. Yilmaz I, Turk M. What should be the cutoff value of blood eosinophilia as a predictor of inhaled corticosteroid responsiveness in patients with chronic obstructive pulmonary disease? Am J Respir Crit Care Med 2017; 196: 1229–1230. [DOI] [PubMed] [Google Scholar]
- 45. Pascoe S, Pavord I, Hinds D, et al. The association between blood eosinophils and risk and treatment outcome in COPD is not dichotomised. Lancet Respir Med 2018; 6: e18. [DOI] [PubMed] [Google Scholar]
- 46. Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet 2017; 389: 1919–1929. [DOI] [PubMed] [Google Scholar]
- 47. Lipson DA, Barnhart F, Brealey N, et al. IMPACT investigators. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med 2018; 378: 1671–1680. [DOI] [PubMed] [Google Scholar]
- 48. Brightling CE, Bleecker ER, Panettieri RA, Jr., et al. Benralizumab for chronic obstructive pulmonary disease and sputum eosinophilia: a randomised, double-blind, placebo-controlled, phase 2a study. Lancet Respir Med 2014; 2: 891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pavord ID, Chanez P, Criner GJ, et al. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N Engl J Med 2017; 377: 1613–1629. [DOI] [PubMed] [Google Scholar]
- 50. Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med 2009; 180: 388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zheng X, Guan W, Zheng J, et al. Smoking influences response to inhaled corticosteroids in patients with asthma: a meta-analysis. Curr Med Res Opin 2012; 28: 1791–1798. [DOI] [PubMed] [Google Scholar]
- 52. Kerstjens HA, Engel M, Dahl R, et al. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med 2012; 367: 1198–1207. [DOI] [PubMed] [Google Scholar]
- 53. Casale TB, Bateman ED, Vandewalker M, et al. Tiotropium respimat add-on is efficacious in symptomatic asthma, independent of T2 phenotype. J Allergy Clin Immunol Pract. Epub ahead of print 22 November 2017. DOI: 10.1016/j.jaip.2017.08.037. [DOI] [PubMed] [Google Scholar]
- 54. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet 2017; 390: 659–668. [DOI] [PubMed] [Google Scholar]
- 55. Maltby S, Gibson PG, Powell H, et al. Omalizumab treatment response in a population with severe allergic asthma and overlapping COPD. Chest 2017; 151: 78–89. [DOI] [PubMed] [Google Scholar]
- 56. Padilla-Galo A, Olveira C, Fernández de Rota-Garcia L, et al. Factors associated with bronchiectasis in patients with uncontrolled asthma; the NOPES score: a study in 398 patients. Respir Res 2018; 19: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bateman ED, Bousquet J, Aubier M, et al. Roflumilast for asthma: efficacy findings in non-placebo-controlled comparator and dosing studies. Pulm Pharmacol Ther 2015; 35(Suppl.): S11–S19. [DOI] [PubMed] [Google Scholar]