Short abstract
Background
Severe postoperative pain remains a clinical problem that impacts patient’s rehabilitation. The present work aims to investigate the role of Toll-like receptor-4 (TLR4) activation in wounded plantar tissue and dorsal root ganglion (DRG) in the genesis of postoperative pain and its underlying mechanisms.
Results
Postoperative pain was induced by plantar incision in rat hind paw. Plantar incision led to increased expression of TLR4 in ipsilateral lumbar 4–5 (L4/L5) DRGs, which occurred at 2 h and was persistent to the third day after surgery. Similar to the change in TLR4 expression, there was also significant increase in phosphorylated nuclear factor-kappa B p65 (p-p65) in DRGs after surgery. Immunofluorescence staining revealed that the increased expressions of TLR4 and p-p65 not only in neuronal cells but also in satellite glial cells in DRG. Furthermore, the enhanced expressions of TLR4 and p-p65 were also detected in plantar tissues around the incision, which was observed starting at 2 h and lasting until the third day after surgery. Prior intrathecal (i.t.) injections of TAK-242 (a TLR4-specific antagonist) or 4',6-diamidino-2-phenylindole-dihydrochloride (PDTC, a nuclear factor-kappa B activation inhibitor) dose dependently alleviated plantar incision-induced mechanical allodynia and thermal hyperalgesia and inhibited the increased expressions of p-p65, tumor necrosis factor-alpha, and interleukin-1 beta in DRG. Prior subcutaneous (s.c.) plantar injection of TAK-242 or PDTC also ameliorated pain-related hypersensitivity following plantar incision. Moreover, the plantar s.c. injection of TAK-242 or PDTC inhibited the increased expressions of p-p65, tumor necrosis factor-alpha, and interleukin-1 beta not only in local wounded plantar tissue but also dramatically in ipsilateral lumbar 4–5 DRGs.
Conclusion
TLR4/ nuclear factor-kappa B signaling activation in local injured tissue and DRG contribute to the development of postoperative pain via regulating pro-inflammatory cytokines release. Targeting TLR4/ nuclear factor-kappa B signaling in local tissue at early stage of surgery may be an effective strategy for the treatment of postoperative pain.
Keywords: Postoperative pain, Toll-like receptor-4/nuclear factor-kappa B signaling, dorsal root ganglion, plantar tissue, plantar incision
Background
More than 80% of patients who undergo surgery suffer acute postoperative pain, and less than half of patients report adequate pain relief. Researchers have estimated that only one in four surgical patients in the United States received adequate relief of acute pain.1 Inadequately controlled pain dramatically increases the risk of postsurgical complications and the risk of persistent postsurgical pain.2 Despite increased preclinical and clinical research have improved understanding of its pathological mechanisms, appropriate postoperative pain therapy still remains a challenge for physicians.3,4
It has been reported that central sensitization in the spinal cord is critical for the maintenance of postoperative pain.5–7 However, cellular and molecular reactions at the local tissue of surgical incision and the dorsal root ganglion (DRG) are also relevant3,8,9. It is well clarified that inflammatory mediators which are released locally after tissue injury directly stimulate and cause sensitization of pain-related nociceptors located at nerve fibers of primary afferent neurons in peripheral tissues.9–11 Thus, acute peripheral inflammation is intimately linked to the development of postsurgical acute pain.12 Although previous studies have shown that tissue and peripheral nerve injury following surgery leads to local inflammatory reaction, accompanied by elevated levels of biological mediators, including prostaglandins, bradykinins, substance P, calcitonin gene-related peptide, and the pro-inflammatory cytokines,10,13 the underlying mechanisms which regulate the release of these pain-related substances still remain unclear. Toll-like receptors (TLRs) are pattern recognition receptors that are the vital elements of innate immunity. TLR4 activation results in a pro-inflammatory cascade, which displays as regulating release of some cytokines, such as tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β).14 It has been well documented that TLR4 signaling activation is involved in the development of chronic pain.15 Either genetic deletion or pharmaceutical inhibition of TLR4 relieves the neuropathic, inflammatory, and cancer pain.16–20 Using skin and muscle incision and the retraction (SMIR) model, Chen et al. reported that the activation of p38 and IL-1β signaling pathway via TLR4 in DRG mediates mechanical allodynia after SMIR surgery.21 In the present study, we hypothesize that TLR4/nuclear factor-kappa B (NF-κB) signaling activation in plantar tissue and DRG via regulating pro-inflammatory cytokines release might be involved in the development of acute postoperative pain.
The animal model of postoperative pain consisting of incision at the plantar hind paw was developed in rats in 1996.22 This experimental model is characterized by spontaneous pain, allodynia and hyperalgesia, lasting for several days and corresponding with the time course of postoperative pain in patients.3 Thus, in the current study, the plantar incision (PI) model in the rat hind paw was used to investigate the role of TLR4/NF-κB signaling activation in plantar tissue and DRG in the development of postoperative pain. We first observed the role of TLR4/NF-κB signaling in the generation of incisional pain by injection (intrathecal or intraplantar) of TAK-242 or PDTC combined with pain-related behavioral tests. Then, we examined the expressions of TLR4 and NF-κB in the local tissue around the incision and lumbar 4–5 (L4/L5) DRGs following PI. Finally, we further explored whether the TLR4-induced postoperative pain was mediated by NF-κB activation and consequently increased the expressions of inflammatory cytokines TNF-α and IL-1β in DRG or local wounded plantar tissue.
Methods
Animal preparation
Male Sprague-Dawley rats weighing 250–300 g (purchased from the Laboratory Animal Center of Zhengzhou University, Zhengzhou, China) were housed in separate cages with free access to food and water. The room temperature was maintained at 23 ± 2°C under a natural light–dark cycle. All animal experimental procedures were approved by the Institutional Animal Care and Use Committee of Zhengzhou University and were performed according to the guidelines of the National Institutes of Health (NIH) on animal care.
Planter incision
The animal model of postoperative pain was performed by PI as our previously described.23 In brief, Male Sprague-Dawley rats were anesthetized with sevoflurane (2%–3%) vaporized through a nose cone. The plantar aspect of the left hind paw was scrubbed with 10% povidone-iodine three times. A 1-cm long incision, starting 0.5 cm from the heel and extending toward the toes, was made through the skin and fascia of the plantar aspect of the left hind paw including the underlying muscle. The plantaris muscle was isolated; elevated slightly, incised longitudinally, and then put it back to its original position. The exposed incision site of the plantaris muscle was desiccated and abraded with sterile gauze until hemorrhage was stopped. The wound was closed with two mattress sutures of 2-0 nylon. Rats were allowed to recover from the anesthesia before returning to their home cage. Sham animals were anesthetized and the left hind paw scrubbed with 10% povidone-iodine three times, but no incision was made. The incision was checked daily, and the rats that displayed wound infection or dehiscence were excluded from the study.
Intrathecal catheterization and drugs delivery
The TLR4-specific antagonist TAK-242 (Millipore, 508336) was dissolved in sterile saline containing 10% DMSO. Ammonium pyrrolidinedithiocarbamate (PDTC)
, a specific inhibitor of NF-κB activation, was purchased form Sigma (St. Louis, MO) and freshly dissolved daily in normal sterile saline. Drugs were delivered by intrathecal (i.t.) or subcutaneous (s.c.) plantar injection to rats. The intrathecal catheterization was performed as previously described.24,25 In brief, a polyethylene-10 (outside diameter, 0.61 mm; inside diameter, 0.28 mm) catheter was inserted into the rat’s subarachnoid space through the L5–L6 intervertebral space, and the tip of the catheter was located at the L5 spinal segmental level. The doses of TAK-242 (5, 20 µg/10 µl)26,27 and PDTC (0.2, 0.5 µg/10 µl)28,29 used in i.t. injection were based on the previous studies. The doses of s.c. injection of TAK-242 (20 µg/20 µl) or PDTC (0.5 µg/20 µl) were based on the doses of i.t. injection.
Behavioral tests
The behavioral tests were performed in accordance with our previous described methods.30,31 All rats were adapted to the testing environment for at least three days before baseline measurement. The paw withdrawal threshold (PWT) to assess mechanical sensitivity was determined by applying von Frey hairs to the plantar surface of the hind paw, and 50% PWT was determined using the up–down method.32 Heat hypersensitivity was evaluated by testing paw withdrawal latency (PWL) using a plantar analgesia tester (7370, Ugo Basile, Comeria, Italy) according to the method described by Hargreaves et al.33 A radiant heat source under the glass floor was aimed at the plantar surface of the hind paw. Three latency measurements were taken for each hind paw in each test session. The hind paws were tested alternately, and the intervals between consecutive tests were more than 5 min. The three latency measurements for the right paw and the left paw were averaged separately.
Western blotting
Western blotting was performed according to our previous published procedures.31,34 Briefly, the animals were sacrificed by decapitation at a designed time point. The ipsilateral plantar skin samples with deep fascia and muscle and L4/L5 DRGs were harvested and placed temporarily in liquid nitrogen. Next, the samples were homogenized with ice-cold lysis buffer (10 mM Tris, 5 mM EGTA, 0.5% Triton X-100, 2 mM benzamidine, 0.1 mM PMSF, 40 mM leupeptin, 150 mM NaCl, 1% phosphatase inhibitor cocktail IIand III). The crude homogenate was centrifuged at 4°C for 15 min at 3000 r/min, and the supernatants were collected. After the protein concentrations were measured, the samples were heated for 5 min at 99°C, and 30–60 µg protein was loaded onto 10% sodium dodecyl sulfate-polyacrylamide gels. The proteins were electrophoretically transferred onto polyvinylidene difluoride membranes. The blotting membranes were blocked with 3% nonfat milk for 1 h and incubated overnight at 4°C with the primary antibody. The following primary antibodies were used: rabbit anti-TLR4 (ABclonal, 1:1000), rabbit anti-NF-κB p65, rabbit anti-NF-κB p-p65 (Cell Signaling, 1:1000) , rabbit anti-TNF-α, rabbit anti-IL-1β (ABclonal, 1:1000), and mouse anti-β-actin (Sigma, 1:10, 000). The proteins were detected with horseradish peroxidase-conjugated antimouse or antirabbit secondary antibodies (BIORad, 1:3000), visualized using the chemiluminescence reagents provided with the ECL kit (BIORad) and detected by a machine of ProteinSimple (FluorChem E, San Jose, CA). The intensities of the blots were quantified by computer-assisted imaging analysis system (Image J; NIH, Bethesda, MD). The blot density of the control rats was set as 100%. The relative density values of the other groups were determined by dividing their optical density values by that of the control rats.
Immunohistochemistry
Immunohistochemistry was done following our previous methods.35 Briefly, one day after PI, rats were deeply anesthetized with sevoflurane and perfused from the ascending aorta with normal saline, followed by 4% paraformaldehyde in 0.1M phosphate buffer. After perfusion, ipsilateral plantar skin samples with deep fascia muscle and L4/L5 DRGs were removed and postfixed in same fixative for 2 h, which was then replaced by 30% of sucrose phosphate-buffered saline over two nights. Transverse paw tissue sections (15 µm) and DRG sections (15 µm) were cut on a cryostat (Leica, CM1950) and prepared for immunofluorescence staining. Sections were randomly selected and put into different wells of a 24-well plate. After washing with phosphate-buffered saline, the sections were blocked with 5% goat serum in 0.3% Triton X-100 for 1 h at 37°C and incubated with primary antibody overnight at 4°C. For double immunofluorescence staining, the sections (except for isolectin B4 (IB4)-treated DRG sections, which were only incubated with Cy3-conjugated secondary antibody) were incubated with a mixture of goat antimouse fluorescein isothiocyanate (FITC)-conjugated secondary antibody (1: 200, Jackson ImmunoResearch, Amish, PA) and goat antirabbit Cy3-conjugated secondary antibody (1:400, Jackson ImmunoResearch) for 2 h at 37°C. The sections were incubated in 4′,6-diamidino-2-phenylindole(dihydrochloride, DAPI) for 10 min at room temperature. The stained sections were mounted onto slides and examined with an Olympus BX53 (Olympus Optical, Tokyo, Japan) fluorescence microscope, and images were captured with a CCD spot camera. The primary antibodies used were as follows: rabbit anti-TLR4 (1:100, ABclonal) and rabbit anti-NF-κB p-p65 (1:100, Cell Signaling). The following cell-specific markers were used: neurofilament-200 (NF-200, a marker for medium/large cells and myelinated Aβ fibers, 1:200, Chemicon, Billerica, MA), FITC-conjugated IB4 (a marker for small nonpeptidergic neurons, 20 µg/mL, Sigma), glial fibrillary acidic protein (GFAP, a marker for satellite glia cells, 1:200, Chemicon). The quantification of TLR4 and NF-κB p-p65 positive staining area in paw tissue was performed using a computerized image analysis system (Image J; NIH).
The specificity of anti-TLR4 antibody was tested by a proincubation method.35 Briefly, the DRG sections that had been blocked in 5% goat serum were preincubated with lipopolysaccharide (LPS), a classical ligand of TLR4, for 1 h at room temperature. The anti-TLR4 antibody was then directly loaded onto the sections, and immunohistochemistry was used to assess TLR4-immunoreactivity (TLR4-IR). The results showed that the TLR4-IR in sections which preincubated with LPS was lower than that in the sections without preincubation (data not shown). The specificity of anti-NF-κB p-p65 antibody was tested by a method that using NF-κB p65 siRNA-treated cultured PC12 cells. Then, the anti-p-p65 antibody was used to detect the change in phosphorylation level of the NF-κB p65 in p65 siRNA-treated cells by Western blot. The results showed the level of NF-κB p65 phosphorylation displayed a significant decrease in NF-κB p65 siRNA-treated cells (data not shown).
Statistical analysis
Statistical tests were performed with SPSS 10.0 (SPSS Inc., Chicago, IL) and SigmaStat (Systat, San Jose, CA). All data were presented as mean ± standard error. For behavioral analysis, two-way analysis of variance (ANOVA) with repeated measures followed by Tukey’s post hoc test was used for difference over time. The one-way ANOVA followed by individual post hoc comparisons (Tukey’s post hoc tests) were carried out for the data between groups at the same time points. For Western blot and immunohistochemistry data, the differences were tested using one-way ANOVA followed by individual post hoc comparisons (Tukey’s post hoc tests) or using Student’s t test if only two groups were applied. A value of P < 0.05 was considered significant.
Results
PT-induced pain-related hypersensitivity and activation of TLR4/NF-κB signaling in DRG
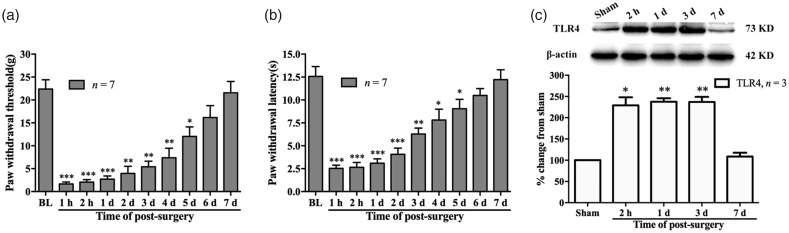
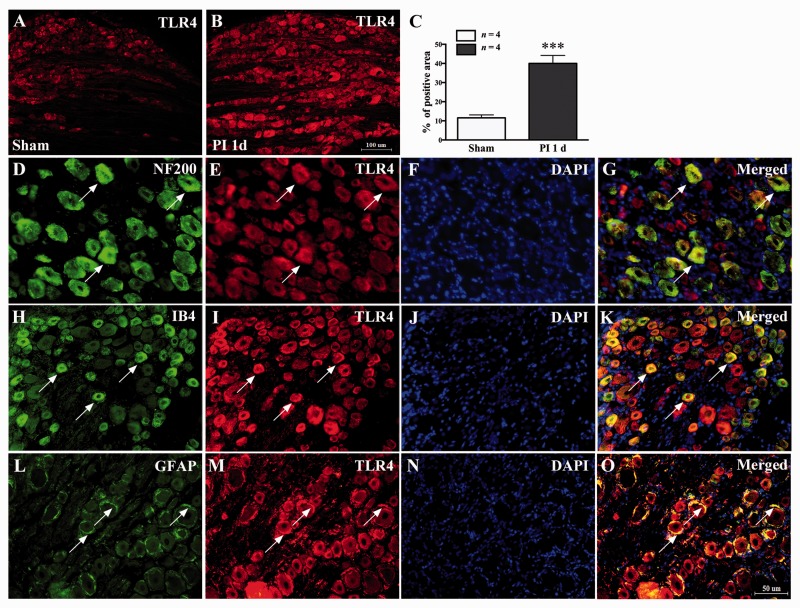
Consistent with the previous studies, PI induced an acute mechanical allodynia and thermal hyperalgesia for several days. Behavioral tests showed a clear reduction in PWT (compared with baseline value, 1 h, P < 0.001; 2 h, P < 0.001; one day, P < 0.001; two days, P < 0.01; three days, P < 0.01; four days, P < 0.01; five days, P < 0.05, two-way ANOVA, Figure 1(a)) and PWL (compared with baseline value, 1 h, P < 0.001; 2 h, P < 0.001; one day, P < 0.001; two days, P < 0.001; three days, P < 0.01; four days, P < 0.05; five days, P < 0.05, two-way ANOVA, Figure 1(b)), which started at 1 h and lasted to the fifth day after PI. Considering the potential role of TLR4/NF-κB signaling activation in postoperative pain, the expression of TLR4 was examined in DRG after surgery. The Western blot data showed that the protein expression of TLR4 in L4/L5 DRGs increased following PI. Compared with sham group, the significant increased expression of TLR4 occurred at 2 h and lasted to the third day (2 h, P < 0.05; one day, P < 0.01; three days, P < 0.01; one-way ANOVA, Figure 1(c) and (d)) after PI. Results of immunofluorescence staining also showed significant increased positive staining cells in ipsilateral L4 and L5 DRGs (***P < 0.01 vs. sham group; Student’s t test, Figure 2(a) to (c)). To identify the cell types that expressed TLR4 in DRG after PI, we performed triple immunofluorescence staining of TLR4 with three cell-specific markers: NF-200 (A-type neuron), IB4 (C-type neuron), and GFAP (satellite glial cells) and nuclear marker: DAPI. The results showed that the increase in TLR4 was colocalized with neurons (A-type and C-type) and satellite glial cells (Figure 2(g), (k), and (o)).
Figure 1.
Plantar incision (PI)-induced mechanical allodynia, thermal hyperalgesia, and upregulation of TLR4 in L4/L5 DRGs in rats. Behavioral data showing reduction in paw withdrawal threshold (a) and paw withdrawal latency (b) following PI. *P < 0.05; **P < 0.01; ***P< 0.001 versus baseline value, two-way ANOVA. BL: baseline. (c) Western blotting data showing increased expression of TLR4 in ipsilateral L4/L5 DRGs following PI. *P < 0.05; **P < 0.01 versus sham group, one-way ANOVA. TLR4: Toll-like receptor-4.
Figure 2.
Distribution and cell-type of TLR4 expression in ipsilateral L5 DRG following PI. (a–c) PI led to significant increased positive staining cells in DRG (PI 1 d vs. sham, ***P < 0.001, Student’s t test). (d–g) Representative pictures showing that the TLR4 colocalized with A-type neurons marker NF-200. (h–k) TLR4 colocalized with C-type nonpeptidergic neurons marker IB4. (l–o) TLR4 colocalized with satellite glial cells marker GFAP. (f), (j), and (n) showing DAPI marked the nucleus. Scale bar: (a) and (b) = 100 µm; (d) to (o) = 50 µm. TLR4: Toll-like receptor-4; PI: plantar incision; NF-200: neurofilament-200; DAPI: 4′,6-diamidino-2-phenylindole; IB4: isolectin B4; GFAP: glial fibrillary acidic protein.
It has been demonstrated that TLR4-triggered-NF-κB signaling activation mediates inflammatory reactions of cells.18 Our immunofluorescence staining data showing clearly increased p-p65 positive staining cells in ipsilateral L4/L5 DRGs one day after PI (** P < 0.01 vs. sham group; Student’s t test, Figure 3(a) to (c)). Double immunofluorescence staining revealed that the increased expression of p-p65 colocalized with both neuronal cell marker (NF-200 and IB4) and satellite glial marker GFAP (Figure 3(h), (l), and (p)). We further observed the PI-induced expression and activation of NF-κB in DRG by Western blot. Consistent with the change in TLR4, the enhanced expression of NF-κB p-p65 also started at 2 h and was lasted to the third day (compared with sham group, 2 h, P < 0.01; one day, P < 0.01; three days, P < 0.05; one-way ANOVA, Figure 3(d)) in PI rats. However, the total NF-κB p65 protein in DRGs was not changed following surgery (Figure 3(d)).
Figure 3.
Distribution and cell type of NF-κB p-p65 expressed in ipsilateral L5 DRG following PI. (a–c) The immunofluorescence staining pictures showing increased NF-κB p-p65-positive staining cells in DRG after PI (PI 1 d vs. sham, **P < 0.01, Student’s t test). (d) Western blotting data showing an increased level of phosphorylated NF-κB p65 (p-p65), but not total p65, in DRGs following PI (*P < 0.05; **P < 0.01 vs. sham group, one-way ANOVA). (e–p) Representative pictures showing that the p-p65 colocalized with A-type neurons marker NF-200 (h), C-type nonpeptide neurons marker IB4 (l), and satellite glial cells marker GFAP (p). DAPI marked the nucleus at (g), (k), and (o). Scale bar: (a) and (b) = 100 µm; (e) to (p) = 20 µm. PI: plantar incision; NF-200: neurofilament-200; DAPI: 4′,6-diamidino-2-phenylindole; IB4: isolectin B4; GFAP: glial fibrillary acidic protein.
TLR4/NF-κB signaling inhibition impaired the development of postoperative pain following PI in rats
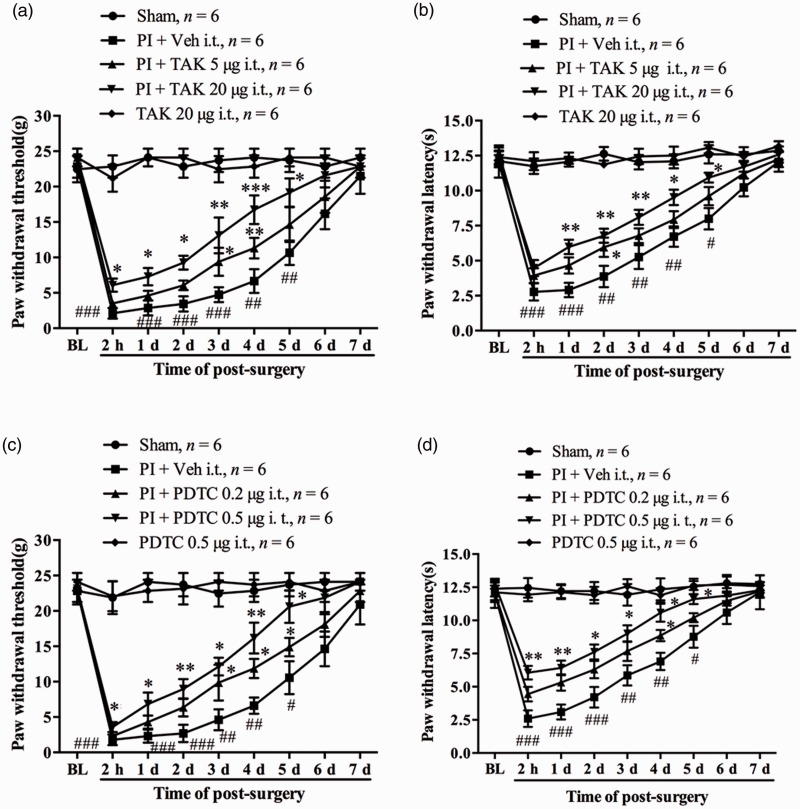
Because the spinal dural membrane in rats extends into the capsule of the DRG, the proximal face of the DRG is in direct continuity with the subarachnoid space.36 Thus, the intrathecal injection (i.t.) of TAK-242, a specific antagonist of TLR4, and PDTC, an inhibitor of NF-κB activation, were performed in the following experiments in view of the potential role of TLR4/NF-κB signaling activation in DRG in the development of postsurgical pain. The rats were injected with different doses of TAK-242 (5, 20 µg/10 µl) intrathecally 30 min before PI and daily for five days. Compared with the vehicle group, TAK-242 treatment dose dependently attenuated PI-induced mechanical allodynia and thermal hyperalgesia. The statistical difference in PWT in high-dose group occurred at 2 h and lasted to the fifth day after PI (compared with PI + vehicle group, 2 h, P < 0.05; one day, P < 0.05; two days, P < 0.05; three days, P < 0.01; four days, P < 0.001; five days, P < 0.05, one-way ANOVA, Figure 4(a)). TAK-242 treatment in the high-dose group also significantly increased PWL which occurred at first day and was maintained to the fifth day (compared with PI + vehicle group, one day, P < 0.01; two days, P < 0.01; three days, P < 0.01; four days, P < 0.05; five days, P < 0.05, one-way ANOVA, Figure 4(b)). However, for the low-dose TAK-242 group, the significant increased PWT occurred on day 3 and day 4 (Figure 4(a)) and PWL occurred on day 2 after surgery (Figure 4(b)). The basal PWT and PWL in naive rats were not changed by i.t. TAK-242 (20 µg) alone daily for five days (Figure 4(a) and (b)). Next, we examined the role of NF-κB activation in the genesis of postsurgical pain after PI. Behavior tests showed that prior i.t. injection of PDTC (0.2, 0.5 µg/10 µl, 30 min before surgery and daily for five days) dose dependently prevented the PI-induced reduction in PWT and PWL. The significant difference in PWT (compared with PI + vehicle group, 2 h, P < 0.05; one day, P < 0.05; two days, P < 0.01; three days, P < 0.05; four days, P < 0.01; five days, P < 0.05, one-way ANOVA, Figure 4(c)) and PWL (compared with PI + vehicle group, 2 h, P < 0.01; one day, P < 0.01; two days, P < 0.05; three days, P < 0.05; four days, P < 0.05; five days, P < 0.05, one-way ANOVA, Figure 4(d)) started at 2 h after surgery and lasted to the fifth day in the high-dose group. Whereas the statistic difference just occurred at day 3, day 4, and day5 for PWT (Figure 4(c)) and at day 4 for PWL (Figure 4(d)) in low-dose PDTC group. The basal PWT and PWL in naive rats were not changed by i.t. PDTC (0.5 µg) alone daily for five days. These results imply that TLR4/NF-κB signaling activation contributes to the development of PI-induced postoperative pain.
Figure 4.
Inhibition of TLR4/NF-κB signaling activation attenuated postoperative pain following PI in rats. Prior i.t. injection of TAK-242 partially prevented the reductions in paw withdrawal threshold (PWT) (a) and paw withdrawal latency (PWL) (b) following PI. Prior i.t. administration of PDTC partially attenuated PI-induced decrease in PWT (c) and PWL (d). *P < 0.05; **P < 0.01; ***P < 0.001 versus PI + vehicle group at same time points between different groups, one-way ANOVA. #P < 0.05; ##P < 0.01; ###P < 0.001 versus baseline value, two-way ANOVA. PI: plantar incision; BL: baseline; PDTC: ▪; TAK: ▪.
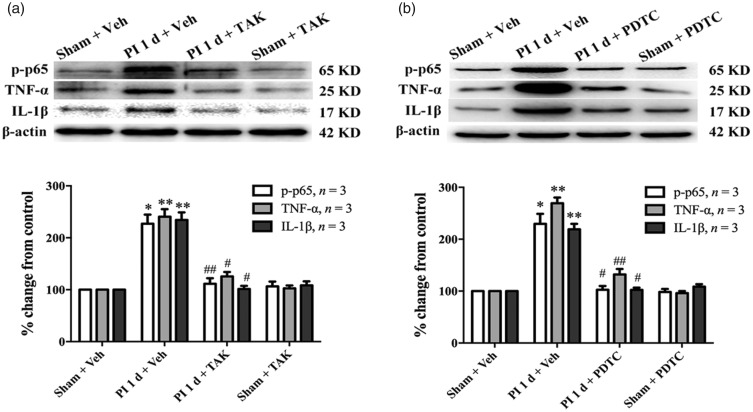
To further confirm the above results, we performed Western blot to observe the effect of i.t. administration of TAK-242 on NF-κB activation in DRG. Our results showed that prior i.t. administration of TAK-242 significantly reduced the phosphorylation level of NF-κB p65 in ipsilateral L4/L5 DRGs (PI + vehicle vs. sham + vehicle, P < 0.05; PI + TAK vs. PI + vehicle, P < 0.01, one-way ANOVA, Figure 5(a)). Furthermore, the increased expressions of pro-inflammatory cytokines TNF-α and IL-1β were also inhibited by the TAK-242 injection. Compared with sham group, rats that received PI showed significant increased expressions of TNF-α and IL-1β in L4/L5 DRGs (PI + vehicle vs. sham + vehicle, P < 0.01, one-way ANOVA, Figure 5(a)). However, in the PI + TAK-242 i.t. group, the increased expressions of TNF-α and IL-1β were clearly inhibited when compared with the PI + vehicle i.t. group (P < 0.05, one-way ANOVA, Figure 5(a)). Moreover, prior i.t. injections of PDTC also suppressed the increased expressions of p-p65, TNF-α, and IL-1β in ipsilateral L4/L5 DRGs one day after surgery (*P < 0.05, **P < 0.01 vs. sham group; #P < 0.05, ##P < 0.01 vs. PI + vehicle group, one-way ANOVA, Figure 5(b)). The expressions of p-p65, TNF-α, and IL-1β were not changed in contralateral L4/L5 DRGs (data not shown).
Figure 5.
Prior i.t. administration of TAK-242 and PDTC inhibited activation of NF-κB and reduced pro-inflammatory cytokines (TNF-α and IL-1β) expression in DRGs following PI. The Western blot data showing reduced expressions of p-p65, TNF-α, and IL-1β in ipsilateral L4/L5 DRGs following repeated i.t. administration of TAK-242 (a) or PDTC (b). *P < 0.05; **P < 0.01 versus sham + vehicle (veh) group; #P < 0.05; ##P < 0.01 versus PI + veh group, one-way ANOVA. TNF-α: tumor necrosis factor-alpha; PI: plantar incision; PDTC: ▪; TAK: ▪; IL-1β: interleukin-1 beta.
TLR4/NF-κB signaling activation in plantar tissue following PI
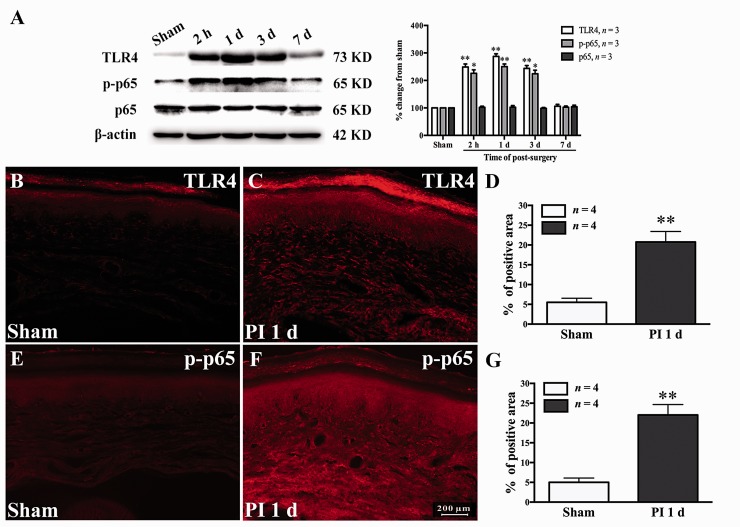
Previous studies have shown that TLR4/NF-κB signaling activation in the DRG and spinal cord contributes to neuropathic, inflammatory18 and muscle incision and retraction induced-chronic pain.37 However, the expression and activation of TLR4/NF-κB signaling in local plantar tissue after PI still remain unclear. In the current study, the expressions of TLR4 and NF-κB p-p65 in plantar tissue were examined at different time points after PI. Our Western blot results showed that the PI led to a clear expression of TLR4 in local plantar tissue. Compared with the sham group, the PI group showed significant increased expression of TLR4, which started at 2 h and persistent to the third day after surgery (2 h, P < 0.01; day 1, P < 0.01; day 3, P < 0.01; one-way ANOVA, Figure 6(a)). Correlated with the change in TLR4, PI also caused markedly increased expression of NF-κB p-p65 in the wounded plantar tissue. Compared with sham group, the statistical difference in NF-κB p-p65 occurred at 2 h and persistent to the third day following PI (2 h, P < 0.05; day 1, P < 0.01; day 3, P < 0.05; one-way ANOVA, Figure 6(a)). The total NF-κB p65 protein was not changed after surgery (Figure 6(a)). To further confirm the above results, immunohistochemistry was performed to examine the IR of TLR4 and NF-κB p-p65 in the wounded plantar tissue (skin, fascia, and muscle) one day after PI. Compared with sham group, the rats that received PI showed significant increased TLR4-IR (P < 0.01, Student’s t test, Figure 6(b) to (d)) and NF-κB p-p65-IR (P < 0.01, Student’s t test, Figure 6(e) to (g)). These data indicate that PI results in significant increased expressions of TLR4 and NF-κB p-p65 in local wounded plantar tissue.
Figure 6.
Expressions of TLR4 and NF-κB p-p65 in the injured plantar tissue (skin, fascia, and muscle) after PI. (a) The Western blotting data showing increased expressions of TLR4 and p-p65 in the wound plantar tissue following PI. Total NF-κB p65 was not changed after PI. *P < 0.05; **P < 0.01 versus sham group, one-way ANOVA. (b–d) The immunofluorescence staining pictures showing the increased expression of TLR4 in local injured plantar tissue one day after PI. **P < 0.01 versus sham group, Student’s t test. (e–g) The immunofluorescence staining pictures showing increased expression of p-p65 in local injured plantar tissue one day after PI. **P < 0.01 versus sham group, Student’s t test. PI: plantar incision; TLR4: Toll-like receptor-4.
Role of TLR4/NF-κB signaling activation in local wounded tissue in PI induced-postoperative pain
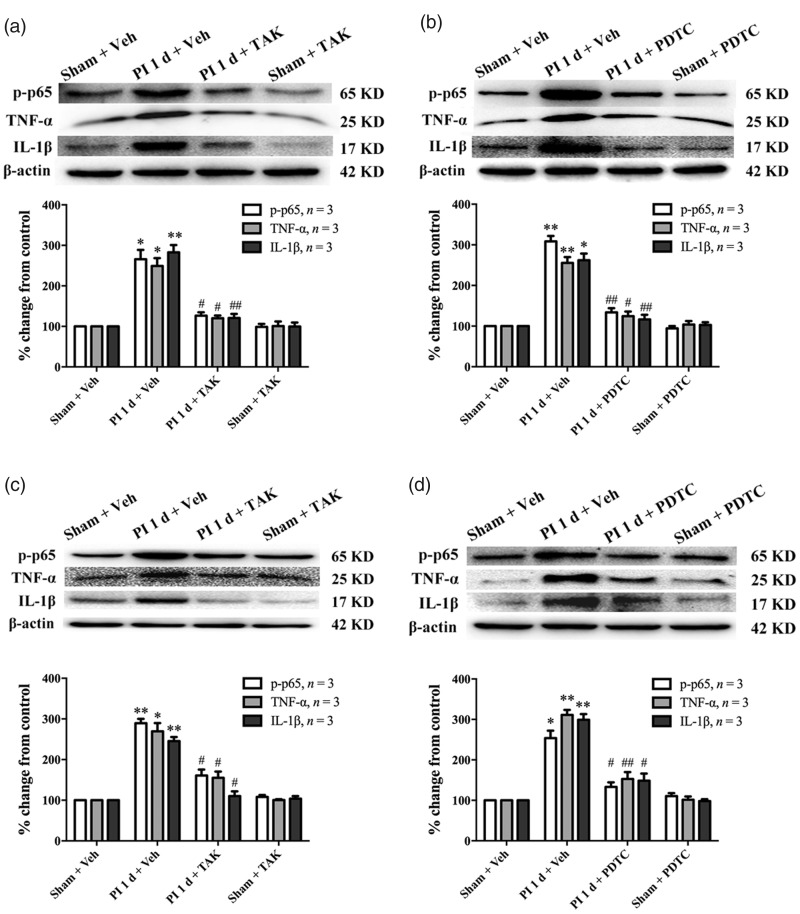
Based on the above results, the effect of local inhibition of TLR4/NF-κB signaling on PI-induced pain hypersensitivity was observed by s.c. injection of TAK-242 or PDTC in plantar tissue. Western blot data showed that the PI-induced increased expressions of NF-κB p-p65, TNF-α, and IL-1β in local wounded plantar tissue were significantly inhibited by plantar injection of TAK-242 (*P < 0.05, **P < 0.01 vs. sham + vehicle group; #P < 0.05, ##P < 0.01 vs. PI + vehicle group, one-way ANOVA, Figure 7(a)) or PDTC (*P < 0.05, **P < 0.01 vs. sham + vehicle group; #P < 0.05, ##P < 0.01 vs. PI + vehicle group, one-way ANOVA, Figure 7(b)). In addition, s.c. injections of TAK-242 or PDTC around wounded plantar tissue also dramatically reduced the expressions of NF-κB p-p65, TNF-α, and IL-1β at ipsilateral L4/L5 DRGs (Figure 7(c) and (d)).
Figure 7.
Intraplantar injection of TAK-242 or PDTC inhibited the expressions of NF-κB p-p65, TNF-α, and IL-1β in injured plantar tissue and ipsilateral L4/L5 DRGs following PI. The PI-induced increased expressions of p-p65, TNF-α, and IL-1β in injured plantar tissue were inhibited by prior to s.c. injection of TAK-242 (a) or PDTC (b) around injured plantar tissue. The expressions of p-p65, TNF-α, and IL-1β in ipsilateral L4/L5 DRGs were reduced after PI by prior to s.c. injection of TAK-242 (c) or PDTC (d) around injured plantar tissue. *P < 0.05; **P < 0.01 versus sham + vehicle (veh) group; #P < 0.05; ##P < 0.01 versus PI + veh group, one-way ANOVA. TNF-α: tumor necrosis factor-alpha; IL-1β: interleukin-1 beta; PI: plantar incision; PDTC: ▪; TAK: ▪.
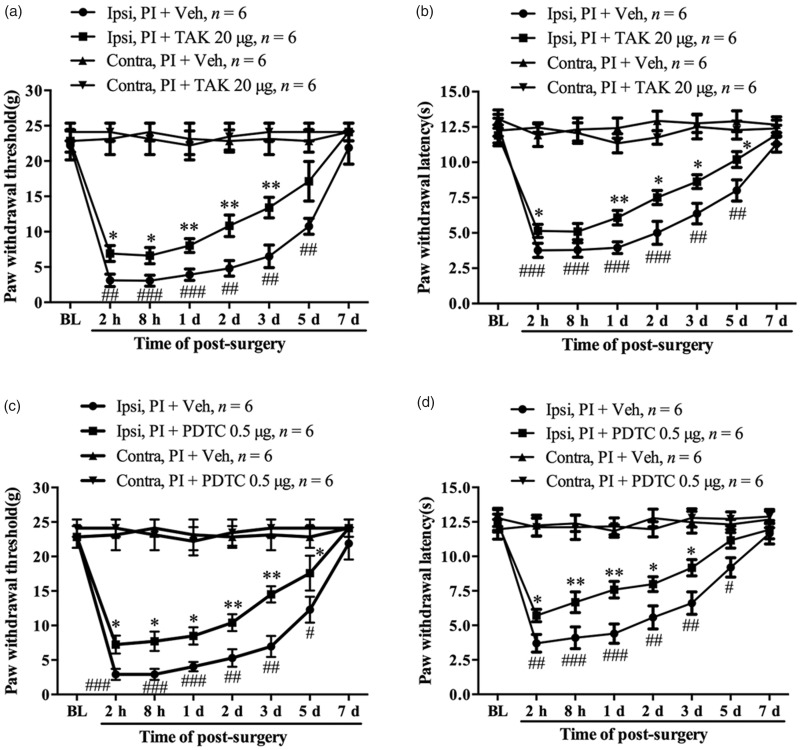
Behavioral data showed that the TAK-242-treated group (20 µg/20 µl s.c., 30 min before surgery and daily for five days) displayed significantly increased PWT (PI + TAK vs. PI + vehicle, 2 h, P < 0.05; 8 h, P < 0.05; one day, P < 0.01; two days, P < 0.01; three days, P < 0.01, one-way ANOVA, Figure 8(a)) and PWL (PI + TAK vs. PI + vehicle, 2 h, P < 0.05; one day, P < 0.01; two days, P < 0.05; three days, P < 0.05; five days, P < 0.05, one-way ANOVA, Figure 8(b)) in the ipsilateral hind paw. Subcutaneous injection of PDTC (0.5 µg/20 µl, 30 min before surgery and daily for five days) in plantar was then performed in another group of rats. Behavioral data showed that prior injection of PDTC significantly prevented the PI-induced reductions in PWT (PI + PDTC vs. PI + vehicle, 2 h, P < 0.05; 8 h, P < 0.05; one day, P < 0.05; two days, P < 0.01; three days, P < 0.01, five days, P < 0.05, one-way ANOVA, Figure 8(c)) and PWL (PI + PDTC vs. PI + vehicle, 2 h, P < 0.05; 8 h, P < 0.01; one day, P < 0.01; two days, P < 0.05; three days, P < 0.05, one-way ANOVA, Figure 8(d)) in the ipsilateral hind paw. The PWT and PWL in the contralateral hind paw were not changed by the local treatment of TAK-242 or PDTC. These results suggest that activation of TLR4/NF-κB signaling in local wounded tissue might play a critical role in the development of postsurgical pain.
Figure 8.
The role of TLR4/NF-κB signaling activation in injured plantar tissue in the development of postoperative pain. Prior intraplantar injection of TAK-242 partially prevented the reduction in paw withdrawal threshold (a) and paw withdrawal latency (b) following PI. Prior intraplantar injection of PDTC partially attenuated PI-induced mechanical allodynia (c) and thermal hyperalgesia (d). *P < 0.05; **P < 0.01 versus PI + vehicle group at same time points, Student’s t test. #P < 0.05; ##P < 0.01; ###P < 0.001 versus baseline value, two-way ANOVA. PI: plantar incision; PDTC: ▪; TAK: ▪.
Discussion
Previous studies have shown that TLR4/NF-κB signaling activation contributes to the development of several types of chronic pain.10,18,38 Here, we demonstrated that TLR4/NF-κB signaling activation in wounded plantar tissue and DRG is involved in postoperative pain. Our results revealed that PI resulted in increased expression of TLR4 both in wounded plantar tissue and ipsilateral L4/L5 DRGs. Correlated with the change in TLR4, PI also led to significant enhanced expression of NF-κB p-p65 in the same tissue. Prior i.t. administration of TAK-242 or PDTC reduced the expressions of NF-κB p-p65, TNF-α, and IL-1β in DRG and attenuated the mechanical allodynia and thermal hyperalgesia induced by PI. Moreover, prior s.c. injection of TAK-242 or PDTC in plantar tissue also alleviated the pain-related hypersensitivity and suppressed the expressions of NF-κB p-p65, TNF-α, and IL-1β in the wounded plantar tissue after surgery. The NF-κB p-p65, TNF-α, and IL-1β expressions in ipsilateral L4/L5 DRGs were also dramatically reduced by the prior to s.c. injection of TAK-242 in plantar tissue. Taken together, these results indicate that TLR4/NF-κB signaling activation in wounded tissue and DRG contribute to the development of postoperative pain by regulating the expressions of TNF-α and IL-1β at local plantar tissue and DRGs.
Compelling evidence shows that TLR4/NF-κB signaling activation in DRG and spinal cord is critical to the development of chronic pain.39–41 Postoperative pain characterized by acute pain in the area of incision is also determined by inflammatory response in local injured tissue and spinal cord.22 In the present study, our data revealed that PI led to significant increased expressions of TLR4 and NF-κB p-p65 not only in DRG but also in injured plantar tissues. Compared with sham group, the statistical difference in TLR4 and NF-κB p-p65 occurred as early as 2 h after surgery and persistent more than three days. Recently, using the SMIR model, Sun et al. reported a significant increased expression of TLR4 in the spinal cord and L3/4 DRGs, but the time course is somewhat different from our current study.37 The SMIR model results in persistent postsurgical pain and sustained expression of TLR4, which last more than 20 days after surgery. However, the PI model used in the present study induces acute postoperative pain which last for several days. Therefore, we observed a transient (about three days) increased expressions of TLR4 and NF-κB p-p65 in DRG and in wounded plantar tissues. As a vital element of regulating innate immunity, although the direct target of TLR4 is MyD88, NF-κB is one of its important downstream signaling pathways, which mediates inflammatory reactions.10,14,40,42 The p50/p65 complex is the most common functional heterodimer of NF-κB in cells.43 It has been demonstrated that NF-κB activation was evidenced by the increased expression of p-p65 because NF-κB p65 requires phosphorylation prior to its binding to a specific target gene in the nucleus.44,45 However, the site of p65 phosphorylation is in cytoplasm which depended on PI3K activation.46 Our results showed that most of positive-staining p-p65 located in cytoplasm. I think that may be the content of p-p65 which was translocated to nucleus is too small to be stained by our immunohistochemistry method. Therefore, the upregulation of TLR4 accompanied by the increased phosphorylation of NF-κB p65 might represent TLR4/NF-κB signaling activation in DRG and in injured plantar tissues after PI.
In view of the potential role of TLR4/NF-κB signaling activation in injured plantar tissue and DRG in the development of postsurgical pain, the i.t. injection of TAK-242, a specific TLR4 antagonist, and PDTC, an inhibitor of NF-κB activation, were performed to examine the effect of TLR4/NF-κB signaling inhibition on pain-related behaviors, firstly. The data showed that not only TAK-242 but also PDTC i.t. administration clearly alleviated the mechanical allodynia and thermal hyperalgesia following PI. Although it has been demonstrated that the spinal dural membrane in rats extends into the capsule of the DRG and that the proximal face of the DRG is in direct continuity with the subarachnoid space,36 we still cannot exclude that partial effects of TAK-242 and PDTC in the above study came from the direct action in the spinal cord. Obviously, direct microinjection of TAK-242 or PDTC in DRG is a better method to the present study. But, the severe injury to local tissue which caused by DRG injection itself will affect the performance of PI-induced pain behaviors at hind paws. To provide evidence of intrathecal TAK-242 inhibiting the inflammatory response in DRG, we performed Western blot to examine the expressions of NF-κB p-p65, TNF-α, and IL-β in L4/L5 DRGs after rats received TAK-242 treatment intrathecally. We found the planter incision-induced increased expressions of NF-κB p-p65, TNF-α, and IL-β in ipsilateral L4/L5 DRGs were clearly inhibited by prior to i.t. administration of TAK-242 or PDTC. Our double immunofluorescence data showing the upregulated TLR4 and NF-κB p-p65 not only colocalized with DRG neurons but also satellite glial cells. Although we cannot dissect which one is more important in postsurgical pain by the present data, the role of spinal glial cells, may be also include DRG satellite glial cells, in postsurgical pain has been demonstrated by studies in which the gial inhibitor minocycline or fluorocitrate were administered intrathecally in PI rats.47,48 Because there are no direct connections with spinal sensory neuron, the contribution of DRG satellite glial cells to postsurgical pain may essentially depend on the activity of DRG neurons.
To further confirm the role of peripheral TLR4/NF-κB signaling activation in the development of postoperative pain, the expressions of TLR4 and NF-κB p-p65 were examined in injured plantar tissue after surgery. We found that PI also led to an enhanced expression of TLR4, which was accompanied by an increased phosphorylation of NF-κB p65, in the local wounded plantar tissue. Then, we performed intraplantar (subcutaneous) injection of TAK-242 or PDTC around the incision. Behavioral data revealed that both TAK-242 and PDTC treatment clearly attenuated the pain-related hypersensitivity induced by PI. To rule out the possible systemic effects of subcutaneous injection of TAK-242 and PDTC, the doses of the two drugs which are used in current experiment are lowered in i.t. administration. The effects of s.c. injection of TAK-242 and PDTC in plantar on the expressions of NF-κB p-p65, TNF-α, and IL-1β in injured plantar tissue and ipsilateral L4/L5 DRGs were verified by Western blot assay. The results showed that TAK-242 and PDTC treatment not only prevented TLR4/NF-κB activation in local plantar tissue but also dramatically reduced the expressions of NF-κB p-p65, TNF-α, and IL-1β in ipsilateral L4/L5 DRGs. Previous studies have shown that tissue and peripheral nerve injury leads to local inflammatory reaction, accompanied by elevated levels of biological mediators, including IL-1β, IL-6, and TNF-α.1,9,49 The nociceptive activity of these cytokines are also verified by experiments in which intraplantar injections of IL-1β, IL-6, or TNF-α in naive rats result in acute mechanical allodynia and thermal hyperalgesia.10,50–53 However, the roles of TLR4/NF-κB signaling in the releases of these cytokines in local injured tissue and in the development of postoperative pain have no yet been determined. Especially, the more interesting founding of the current study is that s.c. injection of TAK-242 in plantar reduced the TLR4/NF-κB signaling activation in ipsilateral L4/L5 DRGs and alleviate pain-related hypersensitivity after surgery. It implies that inflammatory response at wounded tissue may act as initial step during the development of pain-related sensitization at DRG and spinal cord. The local injection of TLR4/NF-κB activation inhibitor or blocking related-nerve before surgery might be effective methods to relieve acute postsurgical pain or even prevent the translation of acute pain to chronic pain after surgery.
Conclusions
Our results reveal that TLR4/NF-κB signaling activation in local injured tissue and DRG contributes to the development of postoperative pain via regulating pro-inflammatory cytokines, TNF-α and IL-1β, release. Targeting TLR4/NF-κB signaling in local tissue at early stage of surgery may be an effective strategy for the treatment of postoperative pain.
Authors' Note
Yuan-Xiang Tao is now affiliated with Department of Anesthesiology, Rutgers, The State University of New Jersey, New Jersey Medical School, Newark, NJ, USA.
Authors’ Contributions
WZ, FX, and JTX conceived of the project, designed the experiments. FX, JW, JZ, and HG carried out all experiments. FX, LB, and ZL analyzed the data. YXT, WZ, and JTX supervised the overall experiment. YXT and JTX revised the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Natural Science Foundation of China (81571079, 81571082, and 81501070).
References
- 1.Blichfeldt-Eckhardt MR. From acute to chronic postsurgical pain: the significance of the acute pain response. Dan Med J 2018; 65: B5326. [PubMed] [Google Scholar]
- 2.Pogatzki-Zahn EM, Segelcke D, Schug SA. Postoperative pain-from mechanisms to treatment. Pain Rep 2017; 2: e588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brennan TJ, Zahn PK, Pogatzki-Zahn EM. Mechanisms of incisional pain. Anesthesiol Clin North America 2005; 23: 1–20. [DOI] [PubMed] [Google Scholar]
- 4.Richebe P, Capdevila X, Rivat C. Persistent postsurgical pain: pathophysiology and preventative pharmacologic considerations. Anesthesiology 2018; 7: 10. [DOI] [PubMed] [Google Scholar]
- 5.Nishimura W, Muratani T, Tatsumi S, Sakimura K, Mishina M, Minami T, Ito S. Characterization of N-methyl-D-aspartate receptor subunits responsible for postoperative pain. Eur J Pharmacol 2004; 503: 71–75. [DOI] [PubMed] [Google Scholar]
- 6.Xu B, Guan X-H, Yu J-X, Lv J, Zhang H-X, Fu Q-C, Xiang H-B, Bu H-L, Shi D, Shu B, Qin L-S, Manyande A, Tian Y-K. Activation of spinal phosphatidylinositol 3-kinase/protein kinase B mediates pain behavior induced by plantar incision in mice. Exp Neurol 2014; 255: 71–82. [DOI] [PubMed] [Google Scholar]
- 7.Zahn PK, Pogatzki-Zahn EM, Brennan TJ. Spinal administration of MK-801 and NBQX demonstrates NMDA-independent dorsal horn sensitization in incisional pain. Pain 2005; 114: 499–510. [DOI] [PubMed] [Google Scholar]
- 8.Lavand'homme P. Perioperative pain. Curr Opin Anaesthesiol 2006; 19: 556–561. [DOI] [PubMed] [Google Scholar]
- 9.Amaya F, Izumi Y, Matsuda M, Sasaki M. Tissue injury and related mediators of pain exacerbation. Curr Neuropharmacol 2013; 11: 592–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMahon SB, Cafferty WB, Marchand F. Immune and glial cell factors as pain mediators and modulators. Exp Neurol 2005; 192: 444–462. [DOI] [PubMed] [Google Scholar]
- 11.Miura M, Sasaki M, Mizukoshi K, Shibasaki M, Izumi Y, Shimosato G, Amaya F. Peripheral sensitization caused by insulin-like growth factor 1 contributes to pain hypersensitivity after tissue injury. Pain 2011; 152: 888–895. [DOI] [PubMed] [Google Scholar]
- 12.Wooden SR. Chronic postsurgical pain. Annu Rev Nurs Res 2017; 35: 91–115. [DOI] [PubMed] [Google Scholar]
- 13.Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov 2014; 13: 533–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peri F, Piazza M. Therapeutic targeting of innate immunity with Toll-like receptor 4 (TLR4) antagonists. Biotechnol Adv 2012; 30: 251–260. [DOI] [PubMed] [Google Scholar]
- 15.Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci U S A 2005; 102: 5856–5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou D-m, Zhuang Y, Chen W-j, Li W, Miao B. Effects of duloxetine on the Toll-like receptor 4 signaling pathway in spinal dorsal horn in a rat model of diabetic neuropathic pain. Pain Med 2018; 19: 580–588. [DOI] [PubMed] [Google Scholar]
- 17.Sorge RE, LaCroix-Fralish ML, Tuttle AH, Sotocinal SG, Austin J-S, Ritchie J, Chanda ML, Graham AC, Topham L, Beggs S, Salter MW, Mogil JS. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J Neurosci 2011; 31: 15450–15454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu F-x, Bian J-j, Miao X-r, Huang S-d, Xu X-w, Gong D-j, Sun Y-m, Lu Z-j, Yu W-f. Intrathecal siRNA against Toll-like receptor 4 reduces nociception in a rat model of neuropathic pain. Int J Med Sci 2010; 7: 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Z, Wang S, Wu I, Mata M, Fink DJ. Activation of TLR-4 to produce tumour necrosis factor-alpha in neuropathic pain caused by paclitaxel. Eur J Pain 2015; 19: 889–898. [DOI] [PubMed] [Google Scholar]
- 20.Liu T, Gao YJ, Ji RR. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci Bull 2012; 28: 131–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen H, Jiang Y-s, Sun Y, Xiong Y-c. p38 and interleukin-1 beta pathway via Toll-like receptor 4 contributed to the skin and muscle incision and retraction-induced allodynia. J Surg Res 2015; 197: 339–347. [DOI] [PubMed] [Google Scholar]
- 22.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain 1996; 64: 493–501. [DOI] [PubMed] [Google Scholar]
- 23.Xing F, Kong C, Bai L, Qian J, Yuan J, Li Z, Zhang W, Xu J-T. CXCL12/CXCR4 signaling mediated ERK1/2 activation in spinal cord contributes to the pathogenesis of postsurgical pain in rats. Mol Pain 2017; 13: 1744806917718753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bai L, Wang X, Li Z, Kong C, Zhao Y, Qian J-L, Kan Q, Zhang W, Xu J-T. Upregulation of chemokine CXCL12 in the dorsal root ganglia and spinal cord contributes to the development and maintenance of neuropathic pain following spared nerve injury in rats. Neurosci Bull 2016; 32: 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu J-T, Tu H-Y, Xin W-J, Liu X-G, Zhang G-H, Zhai C-H. Activation of phosphatidylinositol 3-kinase and protein kinase B/Akt in dorsal root ganglia and spinal cord contributes to the neuropathic pain induced by spinal nerve ligation in rats. Exp Neurol 2007; 206: 269–279. [DOI] [PubMed] [Google Scholar]
- 26.Su M, Ran Y, He Z, Zhang M, Hu G, Tang W, Zhao D, Yu S. Inhibition of Toll-like receptor 4 alleviates hyperalgesia induced by acute dural inflammation in experimental migraine. Mol Pain 2018; 14: 174480691875461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Y, Xin Y, Gao J, Teng R-Y, Chu H-C. Analgesic effect of TAK-242 on neuropathic pain in rats. Int J Clin Exp Med 2015; 8: 11202–11207. [PMC free article] [PubMed] [Google Scholar]
- 28.Bai L, Zhai C, Han K, Li Z, Qian J, Jing Y, Zhang W, Xu J-T. Toll-like receptor 4-mediated nuclear factor-kappaB activation in spinal cord contributes to chronic morphine-induced analgesic tolerance and hyperalgesia in rats. Neurosci Bull 2014; 30: 936–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ledeboer A, Gamanos M, Lai W, Martin D, Maier S F, Watkins L R, Quan N. Involvement of spinal cord nuclear factor kappaB activation in rat models of proinflammatory cytokine-mediated pain facilitation. Eur J Neurosci 2005; 22: 1977–1986. [DOI] [PubMed] [Google Scholar]
- 30.Xu J-T, Xin W-J, Wei X-H, Wu C-Y, Ge Y-X, Liu Y-L, Zang Y, Zhang T, Li Y-Y, Liu X-G. p38 activation in uninjured primary afferent neurons and in spinal microglia contributes to the development of neuropathic pain induced by selective motor fiber injury. Exp Neurol 2007; 204: 355–365. [DOI] [PubMed] [Google Scholar]
- 31.Xu J-T, Zhao J-Y, Zhao X, Ligons D, Tiwari V, Atianjoh F E, Lee C-Y, Liang L, Zang W, Njoku D, Raja SN, Yaster M, Tao Y-X. Opioid receptor-triggered spinal mTORC1 activation contributes to morphine tolerance and hyperalgesia. J Clin Invest 2014; 124: 592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 33.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988; 32: 77–88. [DOI] [PubMed] [Google Scholar]
- 34.Xu J-T, Zhao X, Yaster M, Tao Y-X. Expression and distribution of mTOR, p70S6K, 4E-BP1, and their phosphorylated counterparts in rat dorsal root ganglion and spinal cord dorsal horn. Brain Res 2010; 1336: 46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu J-T, Xin W-J, Zang Y, Wu C-Y, Liu X-G. The role of tumor necrosis factor-alpha in the neuropathic pain induced by Lumbar 5 ventral root transection in rat. Pain 2006; 123: 306–321. [DOI] [PubMed] [Google Scholar]
- 36.Ji R-R, Samad TA, Jin S-X, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 2002; 36: 57–68. [DOI] [PubMed] [Google Scholar]
- 37.Sun Y, Yang M, Tang H, Ma Z, Liang Y, Li Z. The over-production of TNF-alpha via Toll-like receptor 4 in spinal dorsal horn contributes to the chronic postsurgical pain in rat. J Anesth 2015; 29: 734–740. [DOI] [PubMed] [Google Scholar]
- 38.Zhao L-X, Jiang B-C, Wu X-B, Cao D-L, Gao Y-J. Ligustilide attenuates inflammatory pain via inhibition of NFkappaB-mediated chemokines production in spinal astrocytes. Eur J Neurosci 2014; 39: 1391–1402. [DOI] [PubMed] [Google Scholar]
- 39.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol 2002; 2: 725–734. [DOI] [PubMed] [Google Scholar]
- 40.Tse K-H, Chow KBS, Leung WK, Wong YH, Wise H. Lipopolysaccharide differentially modulates expression of cytokines and cyclooxygenases in dorsal root ganglion cells via Toll-like receptor-4 dependent pathways. Neuroscience 2014; 267: 241–251. [DOI] [PubMed] [Google Scholar]
- 41.Liu T, Han Q, Chen G, Huang Y, Zhao L-X, Berta T, Gao Y-J, Ji R-R. Toll-like receptor 4 contributes to chronic itch, alloknesis, and spinal astrocyte activation in male mice. Pain 2016; 157: 806–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Neill LA. Primer: Toll-like receptor signaling pathways—what do rheumatologists need to know? Nat Clin Pract Rheumatol 2008; 4: 319–327. [DOI] [PubMed] [Google Scholar]
- 43.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell 2002; 109: S81–S96. [DOI] [PubMed] [Google Scholar]
- 44.Vermeulen L, De Wilde G, Notebaert S, Vanden Berghe W, Haegeman G. Regulation of the transcriptional activity of the nuclear factor-kappaB p65 subunit. Biochem Pharmacol 2002; 64: 963–970. [DOI] [PubMed] [Google Scholar]
- 45.Viatour P, Merville M-P, Bours V, Chariot A. Phosphorylation of NF-kappaB and IkappaB proteins: implications in cancer and inflammation. Trends Biochem Sci 2005; 30: 43–52. [DOI] [PubMed] [Google Scholar]
- 46.Sizemore N, Lerner N, Dombrowski N, Sakurai H, Stark GR. Distinct roles of the Ikappa B kinase alpha and beta subunits in liberating nuclear factor kappa B (NF-kappa B) from Ikappa B and in phosphorylating the p65 subunit of NF-kappa B. J Biol Chem 2002; 277: 3863–3869. [DOI] [PubMed] [Google Scholar]
- 47.Li K, Fu K-Y, Light AR, Mao J. Systemic minocycline differentially influences changes in spinal microglial markers following formalin-induced nociception. J Neuroimmunol 2010; 221: 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Obata H, Eisenach JC, Hussain H, Bynum T, Vincler M. Spinal glial activation contributes to postoperative mechanical hypersensitivity in the rat. J Pain 2006; 7: 816–822. [DOI] [PubMed] [Google Scholar]
- 49.Grace PM, Rolan PE, Hutchinson MR. Peripheral immune contributions to the maintenance of central glial activation underlying neuropathic pain. Brain Behav Immun 2011; 25: 1322–1332. [DOI] [PubMed] [Google Scholar]
- 50.Dahl E, Cohen SP. Perineural injection of etanercept as a treatment for postamputation pain. Clin J Pain 2008; 24: 172–175. [DOI] [PubMed] [Google Scholar]
- 51.Wolf G, Livshits D, Beilin B, Yirmiya R, Shavit Y. Interleukin-1 signaling is required for induction and maintenance of postoperative incisional pain: genetic and pharmacological studies in mice. Brain Behav Immun 2008; 22: 1072–1077. [DOI] [PubMed] [Google Scholar]
- 52.Fukuoka H, Kawatani M, Hisamitsu T, Takeshige C. Cutaneous hyperalgesia induced by peripheral injection of interleukin-1 beta in the rat. Brain Res 1994; 657: 133–140. [DOI] [PubMed] [Google Scholar]
- 53.Wei X-H, Zang Y, Wu C-Y, Xu J-T, Xin W-J, Liu X-G. Peri-sciatic administration of recombinant rat TNF-alpha induces mechanical allodynia via upregulation of TNF-alpha in dorsal root ganglia and in spinal dorsal horn: the role of NF-kappa B pathway. Exp Neurol 2007; 205: 471–484. [DOI] [PubMed] [Google Scholar]