Abstract
Background:
To evaluate the benefits of teduglutide in a real-life setting, we analyzed the data of 14 patients with short bowel syndrome treated with teduglutide. Additionally, we studied glucagon-like peptide 2 (GLP-2) receptor expression in samples of small intestinal and colonic tissue to provide explanations for clinical observations.
Methods:
Stool frequency and consistency, sensation of thirst, parental calorie or fluid uptake and the number of days on parenteral support per week were collected for up to 2 years. Quantitative real-time polymerase chain reaction of the GLP-2 receptor in healthy controls was performed to better understand clinical response in different patient subgroups.
Results:
There was a significant reduction in parenteral support after 24 and 48 weeks (by 11.0 and 36.6%, respectively; p < 0.05). Further major improvements were made in several patients after over 1 year (reduction by 79.3%, p < 0.05). The proportion of patients who reduced parenteral support by at least 20% was 33.3%, 54.5% and 71.3% after 24 weeks, 48 weeks and beyond 1 year, respectively. Patients on daily parenteral support showed late but strong amelioration. The reduction of thirst was the earliest marker for response. While stool consistency increased (p < 0.01), stool frequency decreased (p < 0.05) significantly after 12 weeks. This reduction was even more pronounced in patients with colon in continuity. Supporting these clinical observations, we found a stronger physiological expression of the GLP-2 receptor in the colon than in the small intestine.
Conclusions:
Patients benefit from teduglutide in a real-life setting, but in contrast to randomized, controlled studies reduction of parenteral support took longer. We identified early clinical markers of response, such as stool consistency and frequency as well as sensation of thirst. Clinical and molecular observations support the role of the colon as an important target organ of teduglutide.
Keywords: glucagon-like peptide 2 expression, intestinal failure, short bowel syndrome, teduglutide
Introduction
Short bowel syndrome is usually a postsurgical condition with a multifactorial etiology defined by a significant insufficiency of the intestine in the absorption of macro- and micronutrients that leads to the individual’s inability to maintain a stable nutrient and hydration status.1,2 With a prevalence of 10–34 per 1,000,000, short bowel syndrome is considered an orphan disease.3,4 Nevertheless the burden for affected patients is high.5 While advances in therapy were made in recent decades, patients continue to be affected by a high degree of morbidity and mortality and treatment creates a high economic burden.6–10 Established therapy so far has been largely based on symptomatic treatment such as opioids, somatostatin analogues and parenteral substitution of fluids and nutrients.11–14 Nutritional options for stimulation of physiological adaptation in intestinal failure are available but limited.15 Intestinal transplantation has gained importance since the 1990s and important advances have been made since.16 But it is costly, related to a high post-transplantation morbidity and mortality rate and the available long-term data are not yet satisfactory.17–19
In 2014, teduglutide was approved for the treatment of short bowel syndrome. It is an analogue to the physiological glucagon-like peptide 2 (GLP-2). In contrast to the physiological peptide, the altered-drug peptide is more resistant to cleavage by the protease dipeptidylpeptidase IV (DPP-IV), resulting in an extended half life and allowing daily dosing.
Teduglutide causes an enlargement of the remaining intestinal surface by growth of the intestinal villi and crypts as well as a reduction in motility and augmentation of intestinal blood flow, all of which lead to a better adsorption of fluids, micro- and macronutrients.20–25 In randomized, controlled clinical trials teduglutide was shown to significantly reduce demand for parenteral support in patients with short bowel syndrome.26,27 But there is limited information on the benefits of teduglutide in a real-world setting. While studies request a strict regimen and controlled setting concerning changes in parenteral support, treatment success in a real-life setting is influenced by different factors including patients’ subjective perception of their state of health, their cautiousness concerning changes in treatment and physicians’ experience with a new drug.
Besides collecting data on changes in parenteral support we aimed to assess some aspects not previously reported but which we believe are important for patients with short bowel syndrome such as sensation of thirst, stool frequency and consistency. Another objective of our investigation was to find additional early markers for clinical response.
Using endoscopic samples of the small intestine and colon of healthy individuals we acquired data on the physiological expression of the teduglutide target, the GLP-2 receptor, in different parts of the bowel to interpret clinical findings.
Materials and methods
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in the prior approval by the Ethical Committee of University Hospital Tübingen on 23 May 2015 (reference number 687/ 2012B01). Written and informed consent for data acquisition was obtained from all patients included in this study prior to treatment initiation. Written and informed consent for biopsy sampling and analysis was obtained from all healthy controls beforehand.
Patient biopsies
Biopsies from healthy control patients from the small intestine (that is, the terminal ileum) and colon were sampled during routine colonoscopy at the Robert Bosch Hospital, Stuttgart, Germany.
Clinical data
At the University Hospital Tübingen, 14 patients with short bowel syndrome were treated with teduglutide. Clinical data were collected systematically by a specialized homecare service provider (Healthcare at Home, Weinheim, Germany). Parameters recorded were age, weight, stool consistency and frequency within 24 h, sensation of thirst, oral fluid uptake, intravenous calorie uptake per week, total intravenous fluid uptake per week, number of days on parenteral support per week and urinary output. For ostomy patients stool frequency represents the number of ostomy bag emptyings per day. We opted for this instead of measuring the output volume for several reasons. First, we expected compliance in providing data in this delicate area to be higher when patients did not have to undergo the trouble of measuring the ostomy output at all times. Second, we felt that in order to assess the benefit from teduglutide, the number of times patients with ostomy have to use the bathroom to empty the bag is more relevant than the ostomy output volume. Ultimately, this approach allows easy comparison to patients without the condition. Among our 14 patients, 5 were started on teduglutide less than a year after initiation of parenteral support because of their general state of health, severity of malnutrition and the high strain demanded an earlier intervention.
Comparisons of values were made between the start of treatment (n = 14), 12 (n = 14), 24 (n = 13) and 48 (n = 11) weeks after treatment initiation. Additional patient data from a treatment period of more than 12 months were also included (n = 7). Teduglutide treatment was not initiated simultaneously. Therefore, at the time of data assessment, treatment length and therefore group numbers varied. Two subgroups of patients were formed depending on postsurgical situs [patients with colon in continuity (n = 9) and patients with a jejuno- or ileostomy (n = 5)].
Quantitative polymerase chain reaction
For analysis of GLP-2 receptor expression, we used biopsies of the colon (n = 25) and small intestine (n = 24) acquired from healthy individuals during routine screening colonoscopy since 2001.
Quantitative polymerase chain reaction was performed as previously described by Wehkamp and colleagues28 using Roche equipment (LightCycler 480, Roche Diagnostics, Indianapolis, IN, USA). Single-stranded complementary DNA corresponding to 10 ng of RNA was used as a template with specific oligonucleotide primer pairs (Table 1). To assess copy numbers, we used specific plasmid standards for each product which were designed using a TOPO TA Cloning Kit (Invitrogen, Carlsbad, CA, USA) and confirmed by sequencing analysis. β-actin expression was assessed for normalization of GLP-2 receptor expression.
Table 1.
Primer for real-time PCR.
| Product | Primer forward | Primer reverse |
|---|---|---|
| β-actin | GCC AAC CGC GAG AAG ATG A | CAT CAC GAT GCC AGT GGT A |
| GLP-2 receptor | ACC TTG GTG GAG TGA AGA GAG | CAT TCG GAG TCA TCC TGC CA |
Immunohistochemistry
Immunohistochemical staining for GLP-2 receptor was based on the EnVision technique by Dako (Glostrup, Denmark) and conducted according to their protocol. The primary anti-GLP-2-receptor (Anti-GLP-2 rabbit, Sigma, St Louis, MO, USA) was used in a dilution of 1:50 in Antibody Diluent (Dako) at 4°C overnight. The horseradish peroxidase-labeled secondary antibody (detection kit by Dako) was applied for 30 min at room temperature. This procedure was visualized by a Dako DAB chromogen. An additional 15 s hematoxylin counterstaining was performed to visualize cell nuclei.
Statistics
Graphs are presented as means ± standard error of the mean (SEM) and were generated using GraphPad Prism V7 (GraphPad Software Inc., La Jolla, CA, USA). For clinical data, mean and SEM were calculated using SPSS 24 (IBM SPSS Statistics, IBM Corporation, Somers, NY, USA) and statistical relevance was assessed performing the paired t test. For statistical analysis of GLP-2 receptor expression, the Wilcoxon Mann–Whitney test for non-normally distributed groups was performed using GraphPad Prism V7.
For all values, p up to 0.05 was considered to be statistically significant (*p < 0.05, **p < 0.01, ***p < 0.001).
Results
Baseline patient characteristics are shown in Table 2. Treatment with antimotility agents such as loperamide had failed in our patients and these drugs were not taken routinely before or after treatment initiation. No patient took proton pump inhibitors regularly.
Table 2.
Patient characteristics.
| Patient | Age | Sex | Etiology of SBS | Initial body weight (kg) | Anatomical situation | Remaining length of small intestine | Last GI tract operation | Begin parental support | Begin Teduglutide (Shire Pharmaceuticals Ireland Limited, Dublin) | Weight start (kg) | Weight at assessment (kg) | Months of observation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 30 | F | Small intestine volvulus | 49.1 | Colon in continuity | 120 cm | 02/2013 | 03/2010 | 11/2014 | 49.6 | 59.8 | 27 |
| 2 | 82 | F | Acute mesenteric arterial occlusion | 55.7 | Colon in continuity | 20 cm | 12/2003 | 01/2004 | 11/2014 | 55.7 | 55 | 27 |
| 3 | 26 | F | Acute mesenteric arterial occlusion | 95 | Colon in continuity | 45 cm | 11/2014 | 11/2014 | 07/2015 | 95 | 88 | 20 |
| 4 | 31 | F | Crohn’s disease | 54 | Ileostomy | 40 cm | 08/2010 | 08/2010 | 03/2015 | 54 | 60 | 23 |
| 5 | 57 | M | Crohn’s disease | 71.8 | Ileostomy | Unknown | 11/2003 | 11/2014 | 06/2015 | 71.8 | 66 | 19 |
| 6 | 57 | F | Acute mesenteric arterial occlusion | 49.6 | Colon in continuity | 15 cm | 10/2009 | 11/2009 | 07/2015 | 49.6 | 51.3 | 18 |
| 7 | 69 | F | Crohn’s disease | 54.5 | Colon in continuity | 60 cm | 07/2006 | 07/2006 | 10/2015 | 54.5 | 55.9 | 15 |
| 8 | 68 | M | Crohn’s disease | 89 | Colon in continuity | 80 cm | 11/2008 | 09/2009 | 01/2016 | 89 | 98 | 13 |
| 9 | 68 | F | Crohn’s disease | 40 | Colon in continuity | 100 cm | 01/2007 | 12/2015 | 12/2015 | 40 | 48.2 | 14 |
| 10 | 50 | F | Uncontrolled bleeding from angiodysplasias | 42 | Colon in continuity | 150 cm | Uncertain, but before 2010 | 12/2015 | 01/2016 | 43.5 | 45 | 13 |
| 11 | 29 | F | Mesenteric venous thrombosis | 65 | Ileostomy | Unknown | 06/2009 | 07/2009 | 02/2016 | 65 | 64.5 | 12 |
| 12 | 43 | M | Crohn’s disease | 89.3 | Ileostomy | 50 cm | 12/2013 | 01/2014 | 08/2016 | 89.3 | 86.1 | 7 |
| 13 | 51 | M | Acute mesenteric arterial occlusion | 74 | Colon in continuity | 30 cm | 03/2016 | 04/2016 | 10/2016 | 74 | 77 | 4 |
| 14 | 26 | M | Crohn’s disease | 48.6 | Ileostomy | unknown | 06/2005 | 08/2015 | 07/2016 | 48.6 | 49.3 | 8 |
GI, gastrointestinal; SBS, short bowel syndrome.
All patients reported a general improvement in their situation and wish to continue treatment with teduglutide. So far, no patient has discontinued treatment with teduglutide. Reported side effects such as nausea, abdominal distention or bloating, or inflammation of the site of injection were generally mild in nature. One patient encountered a significant growth of the ostomy nipple and the baseplate had to be readapted. Another patient had an ostomy prolapse that needed no surgical intervention and could be reversed manually. No new polyps were found on routine endoscopy performed 6–12 months after treatment initiation.
Changes in parenteral support
We measured uptake of intravenous calories (kcal) per week, total fluid uptake per week (ml) and the days in a week a patient received parenteral support. Measures were compared with baseline after 12, 24 and 48 weeks and when looking at treatment length of over 12 months. The results are shown in Figure 1.
Figure 1.
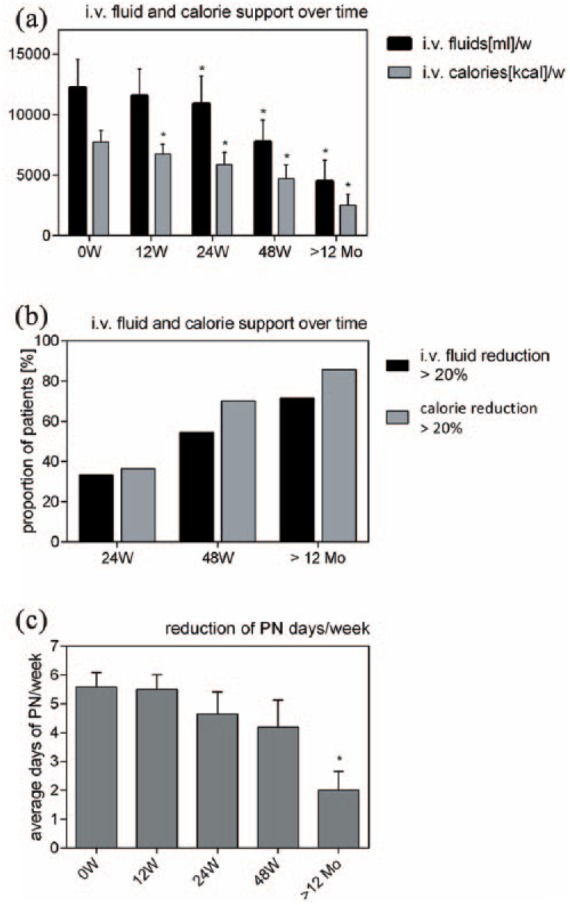
Changes in parenteral nutrition. Considering all patients, reduction of intravenous calories was significant after 12, 24 and 48 weeks and beyond 12 months of teduglutide treatment. Reduction of total intravenous fluid support was significant after 24 and 48 weeks as well as after treatment of over 12 months (a). The proportion of patients that reduced intravenous fluids by 20% or more was 33.3% after 24 weeks, 54.5% after 48 weeks and 71.3% in treatment of over 12 months. For intravenous calories the proportion was 36.4%, 70% and 85.7% after 24, 48 and over 12 months, respectively (b). The average number of days of parenteral support per week decreased from 5.6 days at baseline to 4.2 days after 48 weeks of treatment and to 2.0 days beyond 12 months of treatment. The latter showed statistical significance (c) (*p < 0.05). Mo, months; PN, parenteral nutrition; W, weeks.
Intravenous calories were significantly reduced compared with baseline (µ0W = 7731 ± 942 kcal; µ12W = 6733 ±792 kcal; µ24W = 5863 ± 1011 kcal; µ48W = 4689 ± 1161 kcal; µ>12Mo 4491 ± 1735 kcal; p < 0.05 for each value). Total amount of intravenous fluids diminished strongly with changes being statistically significant after 24, 48 weeks and beyond 12 months of treatment compared with baseline (µ0W = 12,229 ± 2318 ml; µ12W = 11,537 ± 2213 ml; µ24W = 10,882 ± 2290 ml; µ48W = 7751 ± 1786 ml; µ>12Mo 2531 ± 864 ml; p = 0.06 for µ12W and p < 0.05 for µ24W, µ48W and µ>12Mo) [Figure 1(a)].
After 24 weeks, the proportion of patients who had reduced total parenteral fluid support by at least 20% was 33.3% (4 out of 12). At 48 weeks, the proportion was 54.5% (6 out of 11) and 71.3% when treated over 12 months (5 out of 7). Concerning calories, 36.4% (4 out of 11) reduced their uptake by at least 20% after 24 weeks; it was 70.0% (7 out of 10) and 85.7% (6 out of 7) after 48 weeks and beyond 12 months, respectively (Figure 1b).
Baseline value for days on parenteral support per week was 5.6 days per week. After 48 weeks (n = 10) the average reduction was 1.4 days of parenteral support per week. When looking at a treatment of over 12 months (n = 7), average reduction was 3.6 days [Figure 1(c)]. Two patients were weaned off parenteral support completely after 48 weeks of treatment, one initially with four and the other initially on 7 days of parenteral support per week (not shown).
Role of initial need for parenteral support
Figure 2 shows that in contrast to these expectations, patients on daily parenteral support (n = 6) particularly benefitted from teduglutide and that the most dramatic improvements were made after treatment length of 1 year or more. Compared with baseline (µ0W = 17,430 ± 3763, n = 6), total intravenous fluid support per week in patients initially on daily parenteral support was reduced by 5.5% after 12 weeks (µ12W = 16,466 ± 3591 ml, n = 6, p > 0.05), 13.6 % after 24 weeks (µ24W = 15,067 ± 3481 ml, n = 6, p < 0.05), 38.3% after 48 weeks (µ48W = 10,755 ± 2502 ml, n = 6, p > 0.05) and 81.0% after treatment of over 1 year (µ>12Mo = 3317 ± 1658 ml, n = 3, p < 0.05), respectively. By contrast, in the subgroup of patients receiving 1–4 days of parenteral support per week, reduction of total intravenous fluid support per week was 5.9% after 12 weeks (µ0W = 7771 ± 1634 ml, n = 7; µ12W = 7311 ± 1589 ml, n = 7), 13.8% after 24 weeks (µ24W = 6696 ± 1985 ml, n = 6), 46.7% after 48 weeks (µ48W = 4146 ± 1482 ml, n = 5) and 30.9% (after treatment of over 1 year, µ0W = 5273 ± 2926 ml, n = 4) (p > 0.05 for all values) [Figure 2(a)].
Figure 2.
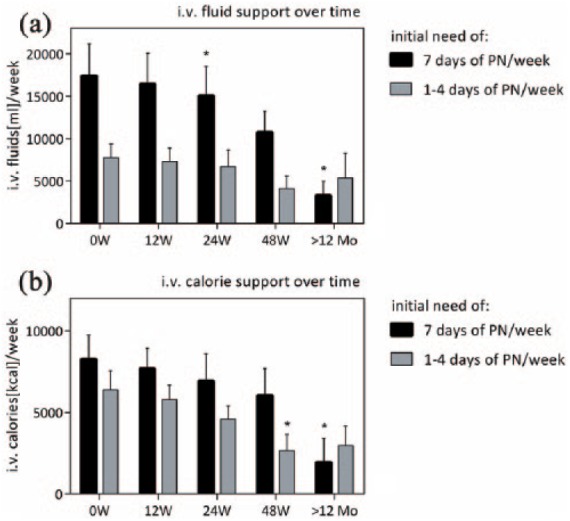
Impact of initial need for parenteral nutrition. For patients initially on daily parenteral support, reduction of intravenous fluids and calories was statistically significant after treatment over 12 months. In patients initially needing 1–4 days of parenteral support per week, reduction of intravenous calories was significant after 48 weeks but not after over a year of treatment. No significant change was seen in total intravenous fluids in this group (*p < 0.05). Mo, months; PN, parenteral nutrition; W, weeks.
For intravenous calories, there was no significant reduction in patients initially receiving daily parenteral support after 12 weeks (µ12W = 7680 ± 1261 kcal, n = 6), 24 weeks (µ24W = 6930 ± 1673 kcal, n = 6) and 48 weeks (µ48W = 6048 ± 1648 kcal, n = 6) compared with baseline (µ0W = 8275 ± 1474 kcal, n = 6). However, changes were statistically significant after treatment of over 1 year (µ>12Mo = 1940 ± 1460 kcal, n = 3, p < 0.05). In the subgroup of patients receiving 1–4 days of parenteral support per week, reduction of intravenous calories was not significant after 12 weeks (µ12W = 5787 ± 901 kcal, n = 6) or 24 weeks (µ24W = 4584 ± 821 kcal, n = 5) but after 48 weeks of treatment (µ48W = 2650 ± 1008 kcal, n = 4, p < 0.05) compared with baseline (µ0W = 6387 ± 1171 kcal, n = 6). However, there was no more significance beyond 12 months of treatment (µ>12Mo = 2975 ± 1181 kcal, n = 4) [Figure 2(b)].
Changes in stool frequency and consistency
Figure 3 shows that there was a significant reduction in bowel movement frequency after 12, 24 and 48 weeks of treatment compared with baseline (µ0W = 9.18 ± 1.22; µ12W = 5.86 ± 0.97; µ24W = 6.0 ± 0.73, µ48W = 5.95 ± 0.71, p < 0.05 for each value). There was no further significant change in stool frequency after 12 weeks [Figure 3(a)].
Figure 3.
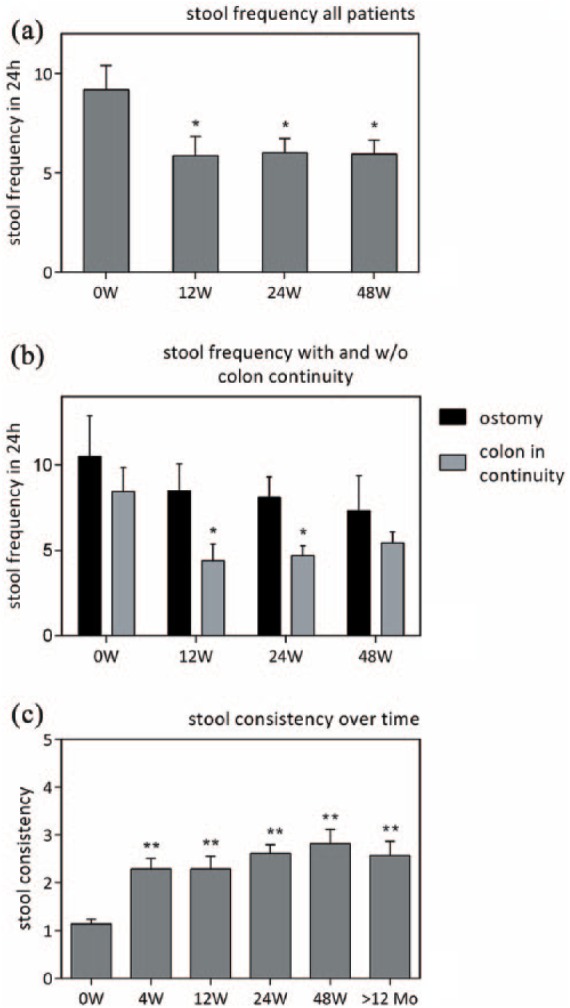
Changes in stool frequency and consistency. Considering all patients, there was a significant reduction in stool frequency after 12, 24 and 48 weeks (a). Dividing patients into those with a jejuno- or ileostomy and those with a colon in continuity (b), changes in stool frequency are only statistically significant for patients with a colon in continuity after 12 and 24 weeks but not 48 weeks. Patients with an ostomy do not show a significant reduction in stool frequency at any time. (c) Visualizes improvement in stool consistency in all patients on a scale from 1 to 5 (1 = watery, 2 = loose/mushy, 3 = soft, 4 = formed, 5 = hard) (*p < 0.05, **p < 0.01). Mo, months; W, weeks.
When dividing patients into those with a colon in continuity and those with an ileo- or jejunostomy it becomes clear that there was a stronger reduction in bowel movement frequency in the patients with colon in continuity (labeled µcc) after 12, 24 and 48 weeks (µcc0W = 8.44 ± 1.41; µcc12W = 4.23 ± 0.97; µcc24W = 4.69 ± 0.59; µcc48W = 5.44 ± 0.64; p < 0.05 for all values compared with baseline) compared with those with an ostomy (labeled µs) (µs0W = 10.50 ± 2.38; µs12W = 8.50 ± 1.58; µs24W = 8.10 ± 1.20; µs48W = 7.33 ± 3.02, p > 0.05 for all values compared with baseline). The relative reduction in relation to baseline value (colon in continuity versus ostomy) was 47.99% versus 19.05% after 12 weeks, 44.43% versus 22.86 % after 24 weeks, and 35.55% versus 30.19 % after 48 weeks. The effect in the group with colon in continuity was statistically significant after 12 and 24 weeks (p < 0.05) but not after 48 weeks compared with baseline value. There was no statistical difference in the ostomy group between any of the values [Figure 3(b)].
We categorized stool consistency as watery, loose/mushy, soft, formed or hard and attributed numbers from 1 to 5 (1 being watery and 5 being hard) for better visualization. An average was calculated for all patients after 4, 12, 24 and 48 weeks as well as with values beyond 12 months of treatment. As shown here, a major amelioration was achieved within 4 weeks with only moderate change afterwards [Figure 3(c)]. There was a significant improvement for all values compared with baseline (p < 0.05).
Sensation of thirst
Information on thirst (classified as none, low, moderate and severe) was available for 10 out of 14 patients. Figure 4 shows that within 2 weeks, four out of five patients with severe sensation of thirst lost this feeling completely. While 50% of patients reported none or only mild thirst on treatment initiation, it was 90% after 4 weeks. There was no further change in these values after 4 weeks.
Figure 4.
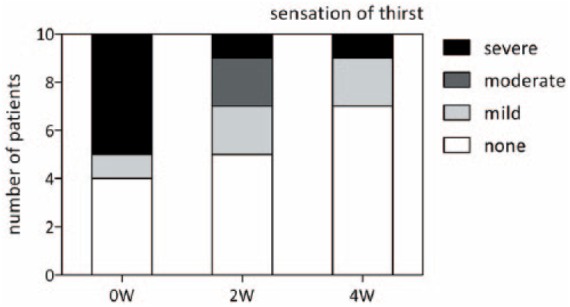
Sensation of thirst. Data on sensation of thirst was available for 10 patients. Five had a strong sensation of thirst before the start of treatment. Of these, four had improvement in thirst after 2 weeks. After 4 weeks, seven patients felt no thirst, two felt mild thirst and one patient continued to have a strong sensation of thirst. W, weeks.
GLP-2 receptor expression in the small and large intestine
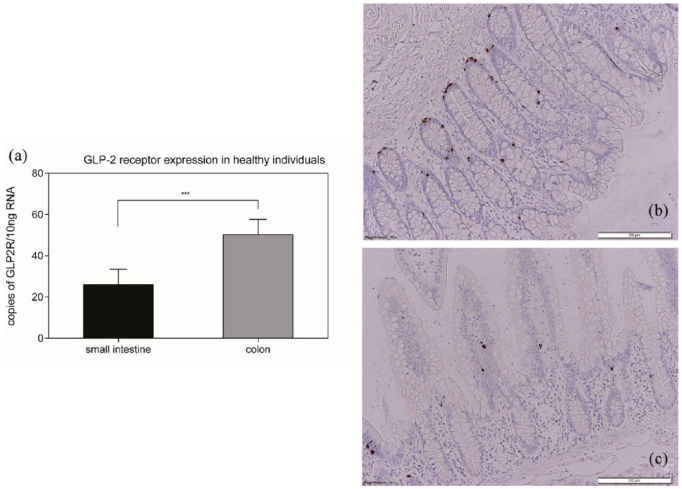
Figure 5(a) shows that copy numbers were significantly higher (50.33 ± 7.36 versus 26.08 ± 7.47) in the colon (n = 25) than in the small intestine (p < 0.001) (n = 24). The difference was still statistically significant (p < 0.05) when GLP-2 receptor expression was normalized to ß-actin expression (not shown). To illustrate these findings and to confirm these results, immunohistochemistry staining on samples of sections of the colon [Figure 5(b)] and the small intestine [Figure 5(c)] was performed.
Figure 5.
GLP-2 receptor expression. Analyzing the expression of the glucagon-like peptide 2 (GLP-2) receptor in the colon and small intestine of healthy individuals we found a significantly higher expression in the colon than in the small intestine (that is, the terminal ileum) (a) (***p < 0.001). Immunohistochemistry showed more GLP-2-positive cells in the colon (b) than in the small intestine (c).
Discussion
Here we report the single center experience of a cohort of patients with short bowel syndrome treated with teduglutide in a prospective, uncontrolled study. To our knowledge, this is the largest set of real-life data on teduglutide efficacy so far.
The main aim of our work was to investigate the benefits of the novel agent teduglutide in the treatment of short bowel syndrome in a real-life clinical setting outside a controlled study. Overall the treatment was well tolerated. All patients reported a general amelioration of their situation and wished to continue therapy.
The extent of clinically measurable effects and time until parenteral nutrients and fluids were reduced varied among the different patients. We found reduction of thirst as the earliest clinical marker for response. We show that improvement in stool frequency and consistency as well as loss of thirst can be considered as additional early markers of success. Of note, patients with colon in continuity were characterized by additional improvements, suggesting the colon to be an important, additional target organ for teduglutide. This finding is consistent with recently published real-life data29 as well as the post hoc analysis of the phase III placebo-controlled trials.30 This observation is supported by the high physiological expression of the GLP-2 receptor in the colon.
All observed adverse events were, if present at all, mild in nature and we can generally confirm recently published data on the real-life safety and efficacy of teduglutide.31
In our cohort, 36.4% of the patients had reduced their amount of weekly intravenous calorie uptake by at least 20% after 24 weeks and 70% of patients after 48 weeks. The success rate was even higher (85.7%) when patients had been treated for over 1 year. Rates for reduction of total intravenous fluid uptake were similar. Total amount of intravenous fluid and calorie uptake per week was significantly reduced over time, but it took longer than previously reported in a randomized and controlled clinical trial.26 Interestingly, as reported previously,32 baseline characteristics for amount of parenteral support before the start of treatment did not strictly determine the effect of teduglutide. Patients initially on daily parenteral support showed a strong improvement after treatment over 1 year.
Our approach to reduction of parenteral support, in contrast to algorithms used in clinical trials, was not strictly guided by changes in urinary output but based on several subjective markers besides urinary output, such as a patient’s general condition, weight, thirst, stool frequency and consistency. With regard to patients’ individual fear of negative effects of reduction of parenteral support, we left patients some degree of freedom to decide when to reduce parenteral support. Also, due to the lack of experience with this novel treatment approach and because of patient safety reasons, parenteral support was intentionally reduced slowly. In summary, our data are in line with previously published real-life data on the efficacy of teduglutide29,31 and we can confirm the benefits of, sometimes dramatic, reductions in parenteral support. However, these changes did take longer in a real-world setting than in the clinical studies.
While we did not systematically assess quality of life using a disease-specific scale as suggested in the past,33 all our patients reported good tolerance of the drug and reported a subjective improvement in their general situation, hence their quality of life. We believe this to be triggered not only by a reduction in parenteral support. The positive effect on outcome by including patients with short bowel syndrome in social activity and education programs has been demonstrated in the past.34 But high stool frequency in short bowel syndrome leads to impairment in social activity and therefore reduction in quality of life. The positive effect on quality of life of teduglutide has been shown,35 but the role of improvement of stool frequency was not clearly assessed. We believe a marked reduction in stool frequency is a major contributor to a better quality of life. The quick and continuing relief from thirst within the first 4 weeks of therapy clearly contributed to this effect.
Based on experimental and clinical data, the small intestine has so far largely been regarded as the only clinically relevant target organ of teduglutide therapy.20,36 When subdividing our patients into those with an ostomy and those without (hence a colon in continuity), a marked difference in the effect of teduglutide becomes obvious. When we analyzed endoscopic biopsies from healthy individuals we could show that the molecular target of teduglutide, the GLP-2 receptor, was expressed more than twice as much in the colon compared with the small intestine. Studies on GLP-2 receptor expression in the small and large intestine as well as a proliferative effect of GLP-2 on both sites were previously reported in rodents.37–39 To our knowledge, our data are the first quantification of GLP-2 receptor expression in the human small and large intestine. To date, there are no similar data on GLP-2 expression in patients with short bowel syndrome and one has to be careful in translating this finding to affected patients. Still, we believe that these results are interesting since, in contrast to prior descriptions, when no change in colonic crypt depth was observed,36 our data confirm prior clinical observations of a beneficial effect of teduglutide on colon in continuity response.32 We therefore conclude that the colon is, beside the small intestine, a clinically relevant target for teduglutide and might play a key role in mediating the observed clinical effects, such as the reduction in stool frequency and thirst, both of which we believe to be crucial for improvement of quality of life under therapy with teduglutide.
We acknowledge that our results should be interpreted within the context of several limitations. The small number of patients may lead to an overestimation of our observations due to individual patient differences and random fluctuation of the collected values. Additionally, exact information on the length of the remaining small intestine and colon was scarce, and we could not make further evaluations on the importance of the remaining length of intestine for the clinical effects of teduglutide. Also, the etiology of short bowel syndrome in our different patients is multifactorial and group sizes were too small to identify its impact on clinical response. Assessment of stool consistency and sensation of thirst are susceptible to interindividual differences in perception and therefore to bias. Ultimately, with a small number of patients and even smaller subgroup sizes, the statistical power of our analysis is low and the possibility to generalize our results is very limited. The assessed parameters varied strongly between the different patients under treatment with teduglutide. Concerning measurement of GLP-2 levels in the small and large intestine, values for patients with short bowel syndrome are lacking and extrapolating control data to affected patients must remain speculation for now.
Considering the annual cost of treatment with teduglutide and the resulting economic burden the establishment of predictive markers for a patient’s clinical response is crucial. Our data suggest that the clinical effects of teduglutide can in part be anticipated when considering the anatomical situation of the remaining gastrointestinal tract. Further evaluation in larger patient cohorts, such as analysis of GLP-2 receptor expression in treated patients, could help to create patient selection criteria in the future.
Acknowledgments
We thank Jutta Bader and Marion Strauß for excellent technical assistance. We thank Health Care at Home (Weinheim, Germany) for help in assessment of patient data. We thank Dr Thomas Welsh (Shire, Germany) for critical comments and discussion. Guarantor of article: Jan Wehkamp.
Author contributions: study concept and design (1); acquisition of data (2); analysis and interpretation of data (3); drafting of the manuscript (4); critical revision of the manuscript for important intellectual content (5); statistical analysis (6); obtained funding (7); administrative, technical, or material support (8); study supervision (9). Marc Schoeler: 1–5; Thomas Klag: 1, 3–5; Judith Wendler: 3–5; Simon Bernhard: 2, 5; Michael Adolph: 4, 5; Andreas Kirschniak: 4, 5; Martin Goetz: 4, 5; Nisar Malek: 4, 5, 7; Jan Wehkamp: 1–9. All authors approved the final version of the article, including the authorship list
Footnotes
Funding: This work was supported by the Deutsche Forschungsgemeinschaft (Germany, Heisenberg Program).
Conflict of interest statement: Jan Wehkamp served on national and international advisory boards for Shire. All other authors declare that they have no competing interests.
Contributor Information
Marc Schoeler, Department of Gastroenterology, Hepatology and Infectious Diseases, University Hospital Tübingen, Tübingen, Germany.
Thomas Klag, Department of Gastroenterology, Hepatology and Infectious Diseases, University Hospital Tübingen, Tübingen, Germany.
Judith Wendler, Department of Gastroenterology, Hepatology and Infectious Diseases, University Hospital Tübingen, Tübingen, Germany.
Simon Bernhard, Department of Gastroenterology, Hepatology and Infectious Diseases, University Hospital Tübingen, Tübingen, Germany.
Michael Adolph, Department of Anesthesiology, University Hospital Tübingen, Tübingen, Germany.
Andreas Kirschniak, Department of Surgery, University Hospital Tübingen, Tübingen, Germany.
Martin Goetz, Department of Gastroenterology, Hepatology and Infectious Diseases, University Hospital Tübingen, Tübingen, Germany.
Nisar Malek, Department of Gastroenterology, Hepatology and Infectious Diseases, University Hospital Tübingen, Tübingen, Germany.
Jan Wehkamp, Department of Gastroenterology, Hepatology and Infectious Diseases, University Hospital Tübingen, Tübingen, Otfried-Müller-Str. 10, 72076 Germany.
References
- 1. O’Keefe SJ, Buchman AL, Fishbein TM, et al. Short bowel syndrome and intestinal failure: consensus definitions and overview. Clin Gastroenterol Hepatol 2006; 4: 6–10. [DOI] [PubMed] [Google Scholar]
- 2. Jeppesen PB, Mortensen PB. Intestinal failure defined by measurements of intestinal energy and wet weight absorption. Gut 2000; 46: 701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Howard L, Ament M, Fleming CR, et al. Current use and clinical outcome of home parenteral and enteral nutrition therapies in the United States. Gastroenterology 1995; 109: 355–365. [DOI] [PubMed] [Google Scholar]
- 4. von Websky MW, Liermann U, Buchholz BM, et al. [Short bowel syndrome in Germany. Estimated prevalence and standard of care]. Chirurg 2014; 85: 433–439. [DOI] [PubMed] [Google Scholar]
- 5. Jeppesen PB, Langholz E, Mortensen PB. Quality of life in patients receiving home parenteral nutrition. Gut 1999; 44: 844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hofstetter S, Stern L, Willet J. Key issues in addressing the clinical and humanistic burden of short bowel syndrome in the US. Curr Med Res Opin 2013; 29: 495–504. [DOI] [PubMed] [Google Scholar]
- 7. Howard L. Home parenteral nutrition: survival, cost, and quality of life. Gastroenterology 2006; 130: S52–S59. [DOI] [PubMed] [Google Scholar]
- 8. Messing B, Crenn P, Beau P, et al. Long-term survival and parenteral nutrition-dependency of adult patients with nonmalignant short bowel. Transplant Proc 1998; 30: 2548. [DOI] [PubMed] [Google Scholar]
- 9. Schalamon J, Mayr JM, Hollwarth ME. Mortality and economics in short bowel syndrome. Best Pract Res Clin Gastroenterol 2003; 17: 931–942. [DOI] [PubMed] [Google Scholar]
- 10. Dibb M, Soop M, Teubner A, et al. Survival and nutritional dependence on home parenteral nutrition: Three decades of experience from a single referral centre. Clin Nutr 2017; 36: 570–576. [DOI] [PubMed] [Google Scholar]
- 11. Nightingale JM. Management of patients with a short bowel. Nutrition 1999; 15: 633–637. [DOI] [PubMed] [Google Scholar]
- 12. O’Keefe SJ, Haymond MW, Bennet WM, et al. Long-acting somatostatin analogue therapy and protein metabolism in patients with jejunostomies. Gastroenterology 1994; 107: 379–388. [DOI] [PubMed] [Google Scholar]
- 13. Lamprecht G, Pape U-F, Witte M, et al. S3-Guideline of the German Society for Nutritional Medicine (DGEM) in cooperation with the GESKES, the AKE and the DGVS Clinical Nutrition in the Gastroenterology (Part 3) – Chronic Intestinal Failure, https://www.awmf.org/uploads/tx_szleitlinien/073-026l_S3_Klin_Ern_Gastro_Chronisches_Darmversagen_2014-07.pdf
- 14. Pironi L, Arends J, Bozzetti F, et al. ESPEN guidelines on chronic intestinal failure in adults. Clin Nutr 2016; 35: 247–307. [DOI] [PubMed] [Google Scholar]
- 15. Lamprecht G, Bodammer P. Nutritional strategies to enhance adaptation in intestinal failure. Curr Opin Organ Transplant 2016; 21: 140–146. [DOI] [PubMed] [Google Scholar]
- 16. Harrison E, Allan P, Ramu A, et al. Management of intestinal failure in inflammatory bowel disease: small intestinal transplantation or home parenteral nutrition? World J Gastroenterol 2014; 20: 3153–3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buchman AL, Scolapio J, Fryer J. AGA technical review on short bowel syndrome and intestinal transplantation. Gastroenterology 2003; 124: 1111–1134. [DOI] [PubMed] [Google Scholar]
- 18. Grant D, Abu-Elmagd K, Mazariegos G, et al. Intestinal transplant registry report: global activity and trends. Am J Transplant 2015; 15: 210–219. [DOI] [PubMed] [Google Scholar]
- 19. Pironi L, Joly F, Forbes A, et al. Long-term follow-up of patients on home parenteral nutrition in Europe: implications for intestinal transplantation. Gut 2011; 60: 17–25. [DOI] [PubMed] [Google Scholar]
- 20. Drucker DJ, Erlich P, Asa SL, et al. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci USA 1996; 93: 7911–7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jeppesen PB, Hartmann B, Thulesen J, et al. Glucagon-like peptide 2 improves nutrient absorption and nutritional status in short-bowel patients with no colon. Gastroenterology 2001; 120: 806–815. [DOI] [PubMed] [Google Scholar]
- 22. Wojdemann M, Wettergren A, Hartmann B, et al. Glucagon-like peptide-2 inhibits centrally induced antral motility in pigs. Scand J Gastroenterol 1998; 33: 828–832. [DOI] [PubMed] [Google Scholar]
- 23. Brubaker PL, Izzo A, Hill M, et al. Intestinal function in mice with small bowel growth induced by glucagon-like peptide-2. Am J Physiol 1997; 272: E1050–E1058. [DOI] [PubMed] [Google Scholar]
- 24. Hoyerup P, Hellstrom PM, Schmidt PT, et al. Glucagon-like peptide-2 stimulates mucosal microcirculation measured by laser Doppler flowmetry in end-jejunostomy short bowel syndrome patients. Regul Pept 2013; 180: 12–16. [DOI] [PubMed] [Google Scholar]
- 25. Bremholm L, Hornum M, Henriksen BM, et al. Glucagon-like peptide-2 increases mesenteric blood flow in humans. Scand J Gastroenterol 2009; 44: 314–319. [DOI] [PubMed] [Google Scholar]
- 26. Jeppesen PB, Pertkiewicz M, Messing B, et al. Teduglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology 2012; 143: 1473–1481.e1473. [DOI] [PubMed] [Google Scholar]
- 27. Schwartz LK, O’Keefe SJ, Fujioka K, et al. Long-term Teduglutide for the treatment of patients with intestinal failure associated with short bowel syndrome. Clin Transl Gastroenterol 2016; 7: e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wehkamp J, Wang G, Kubler I, et al. The Paneth cell alpha-defensin deficiency of ileal Crohn’s disease is linked to Wnt/Tcf-4. J Immunol 2007; 179: 3109–3118. [DOI] [PubMed] [Google Scholar]
- 29. Lam K, Schwartz L, Batisti J, et al. Single-center experience with the use of Teduglutide in adult patients with short bowel syndrome. JPEN J Parenter Enteral Nutr 2018; 42: 225–230. [DOI] [PubMed] [Google Scholar]
- 30. Iyer KR, Kunecki M, Boullata JI, et al. Independence from parenteral nutrition and intravenous fluid support during treatment with Teduglutide among patients with intestinal failure associated with short bowel syndrome. JPEN J Parenter Enteral Nutr 2017; 41: 946–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kochar B, Long MD, Shelton E, et al. Safety and efficacy of Teduglutide (Gattex) in patients with Crohn’s disease and need for parenteral support due to short bowel syndrome-associated intestinal failure. J Clin Gastroenterol 2017; 51: 508–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Iyer KR, Kunecki M, Boullata JI, et al. Independence from parenteral nutrition and intravenous fluid support during treatment with Teduglutide among patients with intestinal failure associated with short bowel syndrome. JPEN J Parenter Enteral Nutr 2017; 41: 946–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Berghofer P, Fragkos KC, Baxter JP, et al. Development and validation of the disease-specific Short Bowel Syndrome-Quality of Life (SBS-QoL) scale. Clin Nutr 2013; 32: 789–796. [DOI] [PubMed] [Google Scholar]
- 34. Smith CE, Curtas S, Werkowitch M, et al. Home parenteral nutrition: does affiliation with a national support and educational organization improve patient outcomes? JPEN J Parenter Enteral Nutr 2002; 26: 159–163. [DOI] [PubMed] [Google Scholar]
- 35. Jeppesen PB, Pertkiewicz M, Forbes A, et al. Quality of life in patients with short bowel syndrome treated with the new glucagon-like peptide-2 analogue Teduglutide–analyses from a randomised, placebo-controlled study. Clin Nutr 2013; 32: 713–721. [DOI] [PubMed] [Google Scholar]
- 36. Jeppesen PB, Sanguinetti EL, Buchman A, et al. Teduglutide (ALX-0600), a dipeptidyl peptidase IV resistant glucagon-like peptide 2 analogue, improves intestinal function in short bowel syndrome patients. Gut 2005; 54: 1224–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yusta B, Huang L, Munroe D, et al. Enteroendocrine localization of GLP-2 receptor expression in humans and rodents. Gastroenterology 2000; 119: 744–755. [DOI] [PubMed] [Google Scholar]
- 38. Litvak DA, Hellmich MR, Evers BM, et al. Glucagon-like peptide 2 is a potent growth factor for small intestine and colon. J Gastrointest Surg 1998; 2: 146–150. [DOI] [PubMed] [Google Scholar]
- 39. Ghatei MA, Goodlad RA, Taheri S, et al. Proglucagon-derived peptides in intestinal epithelial proliferation: glucagon-like peptide-2 is a major mediator of intestinal epithelial proliferation in rats. Dig Dis Sci 2001; 46: 1255–1263. [DOI] [PubMed] [Google Scholar]