Abstract
Purpose:
Regional inductive moderate hyperthermia in combination with chemotherapy can improve the therapeutic efficacy in patients with breast cancer with multiple liver metastases.
Methods:
The study included 103 patients with breast cancer with multiple liver metastases: 53 patients (main group) who received a combined chemotherapy (TC drug combination) and regional inductive moderate hyperthermia treatment and 50 patients (control group) who received chemotherapy (TC drug combination) alone. Regional inductive moderate hyperthermia exploited electromagnetic fields with an operating frequency of 27.17 ± 0.16 MHz and output power of 75 W. Treatment results were assessed by computed tomography and ultrasound imaging.
Results:
Partial regression was defined as a 30% decrease in the sum of the maximum diameters of investigated tumors. In the current study, partial regression was described in 8 (15.1%) patients assigned to the main group and 2 (4%) patients in the control (P < .05). The process stabilization was reported in 32 (60.4%) patients receiving the combined treatment and 19 (38%) in the control (P < .05). Equally important, tumor progression was observed in 13 (24.5%) patients representing the main group and 29 (58%) in the control. During a 30-minute treatment session, a temperature increase overlaying greater than 90% of the liver projection exposed to electromagnetic irradiation was not exceeding 40°C.
Conclusion:
The combined regional inductive moderate hyperthermia and chemotherapy treatment increased the overall therapeutic efficacy by 33.9% (χ2 = 12.182; P < .01).
Keywords: breast cancer, liver metastases, chemotherapy, inductive hyperthermia, combined treatment planning
Introduction
Breast carcinoma is the most common cancer in women worldwide. The 5-year survival rate for all stages of breast cancer (BC) is close to 89.7%, based on data from the Surveillance, Epidemiology, and End Results program of the US National Cancer Institute SEER 18 2008 to 2014. The better outcome is expected in patients with localized BC who has a 5-year survival rate of 98.7%. As for 6% of patients with BC diagnosed at metastatic stage, the 5-year survival rate is about 27%.1
Palliative treatment for patients with metastatic BC frequently includes hyperthermia.2 Hyperthermia is a treatment in which a part of the body (eg, a body cavity, organ, or limb) is exposed to heat. It is usually given with other forms of cancer therapy, such as radiation therapy and chemotherapy.3–5 One of the approaches to noninvasive deep hyperthermia is to exploit the heat originated from eddy currents induced within conductors by a variable magnetic field. Frequency of induction heating can be used as a tool to adjust temperature delivered to the tissue at various depths, leading to different effects of cancer treatment.6–8
However, previous animal studies have shown that when hyperthermia is applied at a temperature range between 43°C and 45°C, blood flow in normal tissues tends to significantly increase even compared to a tumor.9 Storm et al have found out that a combination of regional inductive moderate hyperthermia (RIMH) and chemotherapy improves treatment outcomes in patients with cancer with advanced liver metastases at moderate temperatures ∼40°C in the liver. The combined treatment resulted in disease regression (30% of patients) and disease stabilization (50% of patients).10
A number of recent studies has conclusively shown that tumor response to moderate increase in temperature11 and electromagnetic irradiation12,13 can be mediated by oxidative stress. High levels of oxidative stress can inhibit endogenous antioxidant systems, denature proteins, modulate cellular functions, and further lead to cell death.
TC chemotherapy is a combination of drugs, paclitaxel and carboplatin, used to treat BC, which also induces high oxidative stress in the tumor.14,15 Previously reported research results have raised the key question whether RIMH can enhance the antitumor effect of chemotherapeutic drugs with fewer side effects. This article examines the influence of combined RIMH and chemotherapy on treatment efficacy of patients with BC with multiple liver metastases.
Materials and Methods
Patients
Patients (n = 103) included in this study had BC with multiple liver metastases and were inpatient at the Department of Solid Tumor Chemotherapy, National Cancer Institute of Ukraine during 2009 to 2013. The study was approved by the Regional Committee for Medical Research Ethics of National Cancer Institute (protocol 05.2012) and was performed under the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Written informed consents were obtained from patients prior to initiation of study procedures.
Clinical characteristics for patients in each group are listed in Table 1. The average age at first diagnosis was 57.4 ± 1.3 years. Seventy-four (71.8%) patients aged less than 60 years and 29 (28.2%) patients aged 60 years or older. Most patients across all groups were diagnosed with invasive lobular carcinoma: 38 (72.2% ± 6.3%) patients (main group) and 35 (70% ± 6.2%) patients (control group), P > .05. For both groups, there were no significant differences in age, tumor histology, tumor differentiation grade, tumor subtypes, distant metastases, concomitant diseases, and Eastern Cooperative Oncology Group (ECOG).
Table 1.
Clinical Characteristics of Patients.
| Main Group, n = 53 (%) | Control Group, n = 50 (%) | P | |
|---|---|---|---|
| Age | |||
| Young (28-29 years) | 1 (1.9%) | 0 | >.05 |
| Mature (30-44 years) | 12 (22.6%) | 9 (18%) | |
| Middle-aged (45-59 years) | 27 (50.9%) | 25 (50%) | |
| Elderly (60-74 years) | 13 (24.5%) | 16 (32%) | |
| Tumor histology | |||
| Infiltrative lobular carcinoma | 38 (71.7%) | 35 (70%) | >.05 |
| Infiltrative ductal carcinoma | 10 (18.9%) | 9 (18%) | |
| Mucinous carcinoma | 3 (5.7%) | 4 (8%) | |
| Medullary carcinoma | 2 (3.8%) | 2 (4%) | |
| Grade | |||
| 1 | 0 | 0 | >.05 |
| 2 | 36 (67.9%) | 34 (68%) | |
| 3 | 17 (32.1%) | 16 (32%) | |
| Tumor subtypes | |||
| Luminal A | 22 (41.5%) | 15 (30%) | >.05 |
| Luminal B Her 2 − | 24 (45.3%) | 28 (56%) | |
| Luminal B Her 2 + | 2 (3.8%) | 4 (8%) | |
| Her 2 | 1 (1.9%) | 1 (2%) | |
| Triple negative | 4 (7.5%) | 2 (4%) | |
| Distant metastases | |||
| Bones | 9 (9.6%) | 12 (13.2%) | >.05 |
| Lungs | 14 (14.9%) | 18 (19.8%) | |
| Liver | 53 (56.4%) | 51 (56%) | |
| Other | 18 (19.1%) | 10 (11%) | |
| Concomitant diseases | |||
| Cardiovascular system | 22 (53.7%) | 27 (55.1%) | >.05 |
| Gastrointestinal tract | 10 (24.4%) | 9 (18.4%) | |
| Genitourinary system | 1 (2.4%) | 3 (6.1%) | |
| Endocrine system | 8 (19.5%) | 10 (20.4%) | |
| ECOG | |||
| 0-1 | 53 (100%) | 50 (100%) | >.05 |
| 2-3 | 0 | 0 | |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; Her-2, human epidermal growth factor receptor-2.
Evaluation of estrogen receptor (ER), progesterone receptor (PgR), and human epidermal growth factor receptor-2 (Her-2/neu) expression was performed in biopsy specimens by immunohistochemistry following the standard methods.16 Monoclonal antibodies: ER α (Clone 1D5, ready-to-use; Dako, Santa Clara, California), PgR β (Clone PgR 636, ready-to-use; Dako), and a polyclonal antibody to detect tyrosine kinase receptor c-erbB-2 oncoprotein (Her-2/neu; in a 1:250 dilution; Dako) were used as the primary antibodies. The study utilized 7300/7500 Real-Time PCR Systems (Applied Bio Systems Inc, Foster City, California) and NanoDrop1000 (Thermo Fisher Scientific, Waltham, Massachusetts) to conduct immunohistochemistry assay.
Immunohistochemistry test and tumor differentiation grade results were analyzed in all patients. G3 was determined in 33 patients and G2 in 70 patients. The hormone receptor assay revealed ER- and PgR-positive tumors in 95 patients (“+” in 15 patients, “++” and “+++” in 80 patients, respectively). Six patients had ER- and PgR-negative tumors. Human epidermal growth factor receptor 2 was detected in 68 patients (“+” in 51 patients, “++” in 9 patients, and “+++” in 8 patients), reflecting the very small possibility of a favorable outcome. Thirty-five patients demonstrated HER-2/neu-negative tumors. The majority of patients had multiple metastases to other organs besides the liver: 39 patients (main group) and 40 patients (control group). Of the 103 patients, 74 had concomitant diseases: the cardiovascular (49 patients), genitourinary (4 patients), endocrine (18 patients) systems, and gastrointestinal tract (19 patients). Common concomitant diseases included the ischemic heart disease, hypertensive heart disease, chronic cholecystitis, and pancreatitis.
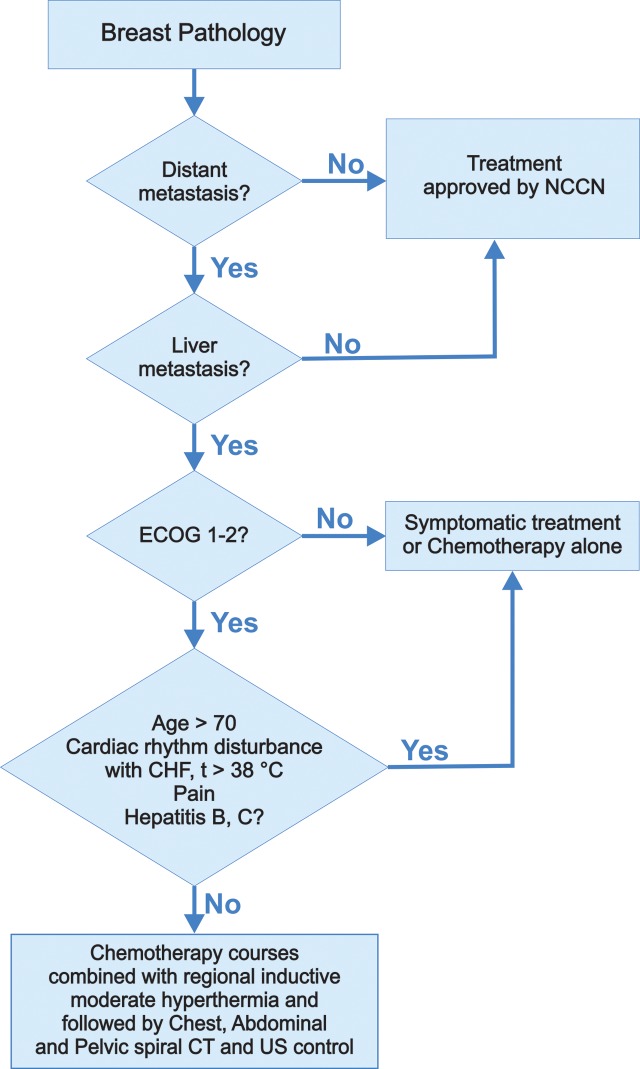
Patients were dichotomized into 2 groups: main (53 patients) and control (50 patients). The main group received the combined treatment with 6 cycles of paclitaxel 175 mg/m2 + AUC 6 carboplatin once every 3 weeks (TC drug combination) and RIMH, whereas the control received TC drug combination alone. The treatment algorithm for patients with BC with liver metastases can be seen from Figure 1. Once patients were diagnosed with BC, they underwent a complete examination. Instrumental study included a computed tomography (CT) of the chest with intravenous contrast enhancement for the abdomen and pelvis, esophagogastroscopy, electrocardiogram, and echocardiography. A full blood count and blood chemistry tests (measuring the level of glucose, general and direct bilirubin, creatinine, urea, total protein, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase) were routinely performed. For patients discovered to have a solitary liver metastasis, the surgeon assessed the patient’s fitness for intervention. If deemed medically unfit for surgery, patients received chemotherapy and RIMH. Regional inductive moderate hyperthermia was delivered 30 minutes after the complete drug infusion. A course of the combined treatment was given once every 3 weeks. For patients completed 3 cycles of the combined treatment, a CT of the chest, abdominal cavity, and pelvic organs was performed.
Figure 1.
Treatment algorithm for patients with BC with liver metastases. BC indicates breast cancer.
Regional Inductive Moderate Hyperthermia
Regional inductive moderate hyperthermia was used to treat patients with BC suitable for receiving chemotherapy with an isolated and inoperable liver metastasis, multiple liver metastases, and ECOG 0 to 2. The main contraindications to RIMH by MagTherm (Radmir, Kharkiv, Ukraine) included a condition of a fever; acute inflammatory process; systemic blood disease; active tuberculosis; severe ischemic heart disease; cardiac aneurysm; disturbance of the heart rhythm; insufficiency of blood circulation; metallic foreign bodies in tissues; pregnancy; epilepsy; complicated ulcer; exacerbation of mental illness; decompensated liver, kidney, pancreas disorders; respiratory failure; polyserositis; and leucopenia. As it was previously mentioned, RIMH was only to use in combination with chemotherapy and under no circumstances alone as monotherapy.17
MagTherm (Radmir, Kharkiv, Ukraine) apparatus generated electromagnetic fields with an operating frequency of 27.17 ± 0.16 MHz and output power of 75 W. The applicator of MagTherm (Radmir, Kharkiv, Ukraine) was set in the area of metastasis to generate the maximum intensity of electromagnetic irradiation that was calculated by the specific absorption rate (SAR) and temperature. Fiber optical thermometers TM-4 (Radmir, Kharkiv, Ukraine) were used to control temperatures. During the 30-minute treatment session, a temperature increase overlaying greater than 90% of the liver projection exposed to electromagnetic irradiation was not exceeding 40°C.
Imaging Techniques
Patients with BC underwent CT on a Brilliance Big Bore scanner (Philips Medical Systems, Best, the Netherlands) at 120 kV and 350 mA. Data acquisition was performed during a single breath-hold following the standard algorithm for all image displays.
NEMIO XG (Toshiba, Tustin, California) ultrasound (US) system with a microconvex transducer 3.5 to 5.0 MHz was used to measure the blood flow velocity and evaluate the efficacy of the combined chemotherapy and RIMH treatment, following the standard method for liver US imaging. Patients with BC underwent a comprehensive US examination of the liver in several modes: real-time mode, B-mode, tissue harmonic imaging, and Doppler mode (Doppler color and energy imaging of the hepatic artery and portal vein).
Image Analysis
The obtained CT and US scans were independently analyzed in a blinded fashion by 2 radiologists per modality. Radiologists were unaware of clinical findings or any imaging studies related to the patients, although they were informed that the patients had BC. The criteria used with CT imaging to diagnose liver metastases were described as defined heterogeneous nodules with higher attenuation than that of bile with some degree of enhancement.
Doppler US imaging allowed to measure blood flow characteristics of the hepatic artery, portal vein, and metastatic tumor vessels such as the peak systolic velocity, end-diastolic velocity, and resistive index, commonly known as the Pourcelot index RI = (S-D)/S.
Equation 1 reveals changes in blood flow rates:
| 1 |
where S 0 is the systolic amplitude before RIMH; S is the systolic amplitude after RIMH; D 0 is the diastolic amplitude before RIMH; D is the diastolic amplitude after RIMH; RI0 is the resistive index before RIMH; RI is the resistive index after RIMH.
Patients received 2 US scans: before combined chemotherapy and RIMH and 30 minutes after the combined treatment session.
Regional Inductive Moderate Hyperthermia Planning for Liver Metastases
The SolidWorks 2015 x64 Edition software tool was used to build a 3-D model of the liver with multiple metastases based on CT scans from patients with BC. The mean liver volume measured by CT was 1548 ± 233 cm3 before the combined treatment, whereas the mean volume of liver metastasis was equal to 56 ± 24 cm3. Generally, the liver in women had a volume of about 1300 ± 100 cm3.
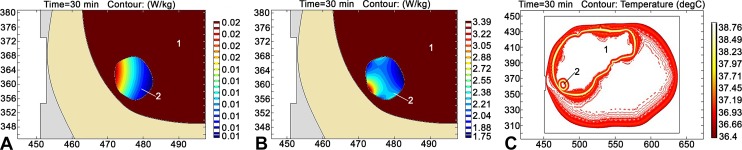
It was decided to adopt the COMSOL Multiphysics 5.2 software for modeling the optimal distribution of electromagnetic and temperature fields in liver metastasis for each patient during RIMH. Figure 2 illustrates the distribution of SAR calculated from the electric, magnetic, and temperature fields within the liver (1) and metastasis (2). The calculations were made based on a CT axial scan when modeling the impact of MagTherm (Radmir, Kharkiv, Ukraine) with an output power of 75 W. In particular, the maximum values of SAR calculated from the electric and magnetic fields were respectively equal to 0.02 and 3.39 W/kg. Such a difference between the maximum values can be explained due to absorption properties of the magnetic and electric components in the body tissues.18 The maximum value of the temperature field was 38.8°C. In regard to the abovementioned, the impact of electromagnetic irradiation could have only initiated moderate hyperthermia. We consider RIMH to be a more promising approach, since temperatures higher than 42°C lead to the production of heat shock proteins in a tumor cell, which may cause chemotherapy resistance and hypoperfusion of the tumor and its microenvironment.19,20
Figure 2.
Distribution of SAR calculated from the electric field (A), SAR calculated from the magnetic field (B), and temperature contour (C) in the liver metastasis based on a CT axial scan (axes in mm) for MagTherm (Radmir, Kharkiv, Ukraine) utilized with an output power of 75 W. 1 indicates liver; 2, metastasis; CT, computed tomography; SAR, specific absorption rate.
Statistical Analysis
Data comparisons were performed with Statistica 13.0 (StatSoft, Inc, 2015) software by the Student t test when the data complied with the conditions of normality. The Kolmogorov-Smirnov method was applied to test for normality. The significance criterion of P < .05, determined from 2-sided test, was used. The χ2 test was used for a nonparametric correlation analysis.
Results and Discussion
Patients with BC experienced mild (grade 1) adverse events commonly associated with chemotherapy (TC-drug combination) in both groups. No patient had a serious adverse event that was related to the treatment.21
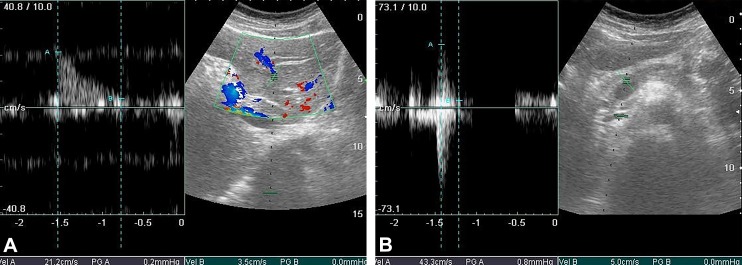
As is widely known, the liver vessels affected by metastasis show a decrease in blood flow which results in low Doppler signal.22 Targeting drugs to the liver metastases under conditions of impaired blood flow may be restricted. The results obtained from the preliminary analysis of the RIMH influence on blood flow are presented in Figure 3. The investigated metastasis located in the liver segment VI with the size of 16.2 × 15.0 mm is indicated by a dashed line. Blood vessels surrounding metastasis are also indicated by the dashed line.
Figure 3.
Liver sonogram, B-mode vessels imaging combined with Doppler flow measurement (color Doppler mapping, Pulsed wave [PW]Doppler), before RIMH (A) after RIMH (B). RIMH indicates regional inductive moderate hyperthermia.
The blood flow rate in the hepatic artery was 43.3 cm/s after RIMH. Thus, RIMH provided an increase in the hepatic artery flow by 22.1 cm/s (Figure 3) that could have improved drug delivery to liver metastases.
Three patients experienced local hyperemia in a specific area of the skin exposed to electromagnetic irradiation that disappeared within 30 to 40 minutes. The main notable side effects caused by the combined treatment were nausea, vomiting, alopecia, and leucopenia. The body weight of patients with BC during treatment varied from 71.17 ± 17.49 kg to 74.17 ± 14.39 kg.
Radiographic response was determined by the spiral CT appearance following definitions: complete regression (CR), a decrease in the tumor size with depiction of normal zonal anatomy; partial regression (PR), a 30% decrease in the sum of the maximum diameters of investigated tumors. The results of this study did not show any case of CR (Table 2). Partial regression was described in 8 (15.1%) patients assigned to the main group and 2 (4%) patients in the control (P < .05). The process stabilization was reported in 32 (60.4%) patients receiving the combined treatment and 19 (38%) in the control (P < .05). Equally important, tumor progression was observed in 13 (24.5%) patients representing the main group and 29 (58%) in the control. Consequently, the overall efficacy of treatment for patients with BC assigned to the main group was 75.9%, while in the control 42%. In other words, combined RIMH and chemotherapy increased the overall efficacy of treatment by 33.9% (χ2 = 12.182; P < .01).
Table 2.
The Main Group BC Treatment Results According to RECIST Criteria.
| Patient Group, n (%) | P | ||
|---|---|---|---|
| Main, n = 53 | Control, n = 50 | ||
| Complete regression | 0 (0) | 0 (0) | – |
| Partial regression | 8 (15.1) | 2 (4) | <.05 |
| Process stabilization | 32 (60.4) | 19 (38) | <.05 |
| Process progression | 13 (24.5) | 29 (58) | >.05 |
| Total | 53 (100) | 50 (100) | – |
Abbreviations: BC, breast cancer; RECIST, Response Evaluation Criteria in Solid Tumors.
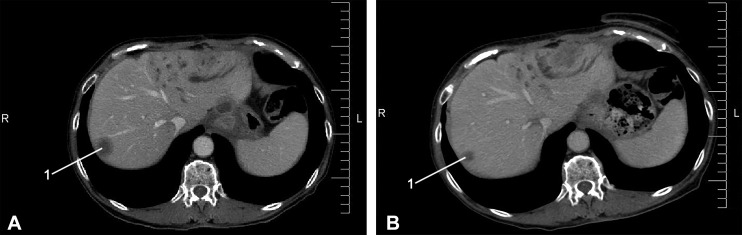
A typical example of axial CT scans for a 73-year-old female patient with BC with liver metastases before and after treatment showed PR (Figure 4). Computed tomography image analysis revealed that there was a ∼ 30% reduction in liver metastasis volume after completing the combined treatment.
Figure 4.
Axial CT scan for a 73-year-old female patient with BC with liver metastasis (1): (A) metastasis volume before chemotherapy and RIMH treatment 27 cm3 and (B) metastasis volume after the combined treatment 20 cm3. BC indicates breast cancer; CT, computed tomography; RIMH, regional inductive moderate hyperthermia.
Furthermore, liver metastases developed in patients assigned to the main group from 5 ± 0.01 months to 154 ± 0.08 months and on average of 50.72 months after BC diagnosis. Whereas in the control, the minimum and maximum time prior to liver metastases development was equal to 2.90 ± 0.01 months and 88.30 ± 0.04 months, respectively, with an average of 33.06 months after BC diagnosis. Thus, the time between BC diagnosis and liver metastases development was 8.51 ± 0.42 months in patients receiving combined RIMH and chemotherapy compared to 4.32 ± 0.31 months in the control group (P < .05).
The obtained results can be interpreted as clinical support for the concept that modulation of oxidative stress has already been exploited for therapeutic benefits.23 Magnetic fields generated during RIMH are able to significantly accelerate the chemical interactions between molecular oxygen and cell metabolites. Based on a triplet–singlet spin state interconversion in radical pairs, these interactions are used to modulate the oxidation rates of polyunsaturated acids and lipids.24 Moreover, the increase of oxidative stress in the tumor and its microenvironment significantly enhances hyperthermia-induced apoptosis.25 Oxidative stress also plays a key role in cell signaling pathways, including immune pathways in anticancer therapy. Phagocytes produce reactive oxygen species that could regulate cancer cell metabolism.26
The results of this investigation suggested that combined RIMH and chemotherapy improved the treatment efficacy of antitumor drugs and quality of life in patients with BC with multiple liver metastases. We believe that future research in the field of RIMH should be focused on developing adjuvant strategies for oxidative stress modulation in tumors.
Conclusion
Therefore, the current study has shown that the combined use of RIMH and chemotherapy in patients with BC with liver metastases does not cause significant adverse changes in blood count and blood chemistry tests. Likewise, the combined treatment was not associated with complications that could deteriorate the patient medical state and extend duration of hospital stay.
It is important to note that RIMH markedly increased liver blood flow in patients with BC. The combined use of RIMH and chemotherapy significantly improved the treatment results (χ2 = 12.182; P < .01) and immediate outcomes (disease stabilization 21.3%, PR 12.6%).
Quantitative assessment of the health status in patients following the questionnaire EQ-5D demonstrated an increase of 5.3% in the total score for the main group after the combined treatment, whereas the control showed a decrease of 12.1%. The quality of life for patients with BC with liver metastases who received combined RIMH and chemotherapy improved by 17.4% compared to the control. Finally, the median time prior to disease progression was greater in patients receiving the combined treatment: (8.51 ± 0.42) months compared to the control (4.32 ± 0.31) months (P < .05).
Abbreviations
- BC
breast cancer
- CR
complete regression
- CT
computed tomography
- ECOG
Eastern Cooperative Oncology Group
- ER
estrogen receptor
- Her-2/neu
human epidermal growth factor receptor-2
- PgR
progesterone receptor
- PR
partial regression
- RECIST
Response Evaluation Criteria in Solid Tumors
- RIMH
regional inductive moderate hyperthermia
- SAR
specific absorption rate
- US
ultrasound.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Research Programs of National Cancer Institute, Ukraine: Chemotherapy development for patients with malignant tumors of the thorax based on clinical and laboratory toxicity prognosis factors (code: BH. 14.01.07.131-11); Possible optimization methods in radiology applied to diagnose hepatobiliary malignant tumors (code BH.14.01.23.134-08); Optimal treatment tactics in metastatic breast cancer patients with prognostically unfavorable molecular subtypes (code: BH. 14.01.07.152-14).
ORCID iD: Valerii E. Orel, Prof, DSc  http://orcid.org/0000-0002-6319-4215
http://orcid.org/0000-0002-6319-4215
References
- 1. Surveillance, Epidemiology, and End Results (SEER) program of the US National Cancer Institute web site. https://seer.cancer.gov/statfacts/html/breast.html. Published 2008-2014. Accessed October 3, 2018.
- 2. Takeda T, Takeda T, Etani M, Kobayashi S, Takeda H. The effect of immunotherapy and hyperthermia on patients with advanced or recurrent breast cancer. Gan to Kagaku Ryoho. 2013;40(12):1596–1599. [PubMed] [Google Scholar]
- 3. van der Zee J. Heating the patient: a promising approach? Ann Oncol. 2002;13:1173–1184. 10.1093/annonc/mdf280. [DOI] [PubMed] [Google Scholar]
- 4. Issels RD. Regional hyperthermia in high-risk soft tissue sarcomas. Curr Opin Oncol. 2008;20(4):438–443. doi:10.1097/CCO.0b013e3283025e50. [DOI] [PubMed] [Google Scholar]
- 5. Yu JI, Park HC, Choi DH, et al. Prospective phase II trial of regional hyperthermia and whole liver irradiation for numerous chemorefractory liver metastases from colorectal cancer. Rad Oncol J. 2016;34(1):34–44. 10.3857/roj.2016.34.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Storm FK, Harrison WH, Elliott RS, Morton DL. Normal tissue and solid tumour effects of hyperthermia in animal models and clinical trials. Cancer Res. 1979;39(6 pt 1):2245–2251. [PubMed] [Google Scholar]
- 7. Storm FK, Morton DL. Localized hyperthermia in the treatment of cancer. CA Cancer J Clin. 1983;33(1):44–56. 10.3322/canjclin.33.1.44. [DOI] [PubMed] [Google Scholar]
- 8. Oleson JR. A review of magnetic induction methods for hyperthermia treatment of cancer. IEEE Trans Biomed Eng. 1984;31(1):91–97. doi:10.1109/TBME.1984.325374. [DOI] [PubMed] [Google Scholar]
- 9. Emami B, Song CW. Physiological mechanisms in hyperthermia: a review. Int J Radiat Oncol Biol Phys. 1984;10(2):289–295. 10.1016/0360-3016(84)90015-4. [DOI] [PubMed] [Google Scholar]
- 10. Storm FK, Kaiser LR, Goodnight JE, et al. Thermochemotherapy for melanoma metastases in liver. Cancer. 1982;49(6):1243–1248. . [DOI] [PubMed] [Google Scholar]
- 11. Dewhirst MW, Lee CT, Ashcraft KA. The future of biology in driving the field of hyperthermia. Int J Hyperthermia. 2016;32(1):4–13. doi:10.3109/02656736.2015.1091093. [DOI] [PubMed] [Google Scholar]
- 12. Barnes F, Greenebaum B. Role of radical pairs and feedback in weak radio frequency field effects on biological systems. Environ Res. 2018;163:165–170. doi:10.1016/j.envres.2018.01.038. [DOI] [PubMed] [Google Scholar]
- 13. Orel VE, Tselepi M, Mitrelias T, et al. Nanomagnetic modulation of tumor redox state. Nanomedicine. 2018;14(4):1249–1256. 10.1016/j.nano.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 14. Duggett NA, Griffiths LA, McKenna OE, et al. Oxidative stress in the development, maintenance and resolution of paclitaxel-induced painful neuropathy. Neuroscience. 2016;333:13–26. doi:10.1016/j.neuroscience.2016.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fath MA, Ahmad IM, Smith CJ, Spence J, Spitz DR. Enhancement of carboplatin-mediated lung cancer cell killing by simultaneous disruption of glutathione and thioredoxin metabolism. Clin Cancer Res. 2011;17(19):6206–6217. doi:10.1158/1078-0432.CCR-11-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beenken SW, Bland KI. Biomarkers for breast cancer. Minerva Chir. 2002;57(4):437–448. [PubMed] [Google Scholar]
- 17. Orel VE, Dzyatkovskaya NN, Romanov AV, Kozarenko TM. The effect of electromagnetic field and local inductive hyperthermia on nonlinear dynamics of the growth of transplanted animal tumours. Exp Oncol. 2007;29(2):156–158. [PubMed] [Google Scholar]
- 18. Vincze G, Szasz O, Szasz A. Generalization of the thermal dose of hyperthermia in oncology. Open J Biophys. 2015;5:97–114. doi:10.4236/ojbiphy.2015.54009. [Google Scholar]
- 19. Thiesen B, Jordan A. Clinical applications of magnetic nanoparticles for hyperthermia. Int J Hyperthermia. 2008;24(6):467–474. doi:10.1080/02656730802104757. [DOI] [PubMed] [Google Scholar]
- 20. Song CW. Effect of local hyperthermia on blood flow and microenvironment: a review. Cancer Res. 1984;44(10 suppl):4721s–4730s. [PubMed] [Google Scholar]
- 21. US Department of Health and Human Services, National Institutes of Health National Cancer Institute; Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. 2009 (v 4.03: June 14, 2010). [Google Scholar]
- 22. De Gaetano AM, Barbaro B, Chiarla C, De Franco A, Maresca G, Marano P. The tissue characterization of focal liver lesions by color Doppler echography. Radiol Med. 1995;89(4):453–463. [PubMed] [Google Scholar]
- 23. Gurer-Orhan H, Ince E, Konyar D, Saso L, Suzen S. The role of oxidative stress modulators in breast cancer. Curr Med Chem. 2017. doi:10.2174/0929867324666170711114336. [DOI] [PubMed] [Google Scholar]
- 24. Pliss EM, Grobov AM, Kuzaev AK, Buchachenko AL. Magnetic field effect on the oxidation of hydrocarbons by molecular oxygen. Mendeleev Commun. 2017;3:246–247. 10.1016/j.mencom.2017.05.009. [DOI] [Google Scholar]
- 25. Tabuchi Y, Ahmed K, Kondo T. Induction of oxidative stress by hyperthermia and enhancement of hyperthermia-induced apoptosis by oxidative stress modification In: Kokura S, Yoshikawa T, Ohnishi T, eds. Hyperthermic Oncology from Bench to Bedside. Singapore, Singapore: Springer; 2016:7–15. 10.1007/978-981-10-0719-4_2. [DOI] [Google Scholar]
- 26. Nathan C, Cunningham-Bussel A. Beyond oxidative stress: an immunologist’s guide to reactive oxygen species. Nat Rev Immunol. 2013;13(5):349–361, doi:10.1038/nri3423. [DOI] [PMC free article] [PubMed] [Google Scholar]