Abstract
Background:
Fixed- and adjustable-loop femoral cortical suspension devices are commonly used for femoral graft fixation during anterior cruciate ligament reconstruction (ACLR).
Purpose:
To compare the biomechanical results of fixed- versus adjustable-loop femoral cortical suspension devices in studies simulating ACLR with an isolated device and/or specimen setup using porcine femora and bovine flexor tendons.
Study Design:
Systematic review.
Methods:
Two independent reviewers searched PubMed, Embase, and the Cochrane Library databases to find studies comparing the biomechanical strength of fixed- and adjustable-loop cortical suspension devices for ACLR with isolated device and/or specimen setups using porcine femora and bovine flexor tendons. Studies that compared both devices with similar biomechanical methods were included. Data extracted included displacement during cyclic loading, ultimate load to failure, and mode of failure of the different cortical suspension devices for ACLR.
Results:
Six studies were identified that met the inclusion criteria, including a total of 76 fixed-loop devices and 120 adjustable-loop devices. Load to failure was significantly different (P < .0001), with the strongest fixation device being the ToggleLoc with ZipLoop adjustable-loop device (1443.9 ± 512.3 N), compared with the Endobutton CL fixed-loop device (1312.9 ± 258.1 N; P = .04) and the TightRope RT adjustable-loop device (863.8 ± 64.7 N; P = .01). Cyclic displacement was significantly different, with Endobutton CL (3.7 ± 3.9 mm) showing the least displacement, followed by ToggleLoc with ZipLoop (4.9 ± 2.3 mm) and TightRope RT (7.7 ± 11.1 mm) (P < .0001). Mode of failure was statistically different between the 3 groups (P = .01), with suture failure accounting for 83.8% of TightRope RT devices, 69.4% of ToggleLoc with ZipLoop devices, and 60.3% of Endobutton CL devices.
Conclusion:
Current biomechanical data suggest that the ToggleLoc with ZipLoop device is the strongest fixation device at “time zero” in terms of ultimate load to mechanical failure. However, the Endobutton CL device demonstrated the least cyclic displacement, which may be a more clinically applicable measure of device superiority.
Keywords: anterior cruciate ligament reconstruction, adjustable loop, fixed loop, cortical suspension, suspensory fixation device
The most frequent technical error leading to recurrent instability after anterior cruciate ligament (ACL) reconstruction (ACLR) is failure to replicate the native anatomic femoral and tibial footprints during ACL graft placement, although poor graft quality and inadequate graft tensioning are also causes of failure.11,15,17 Therefore, during primary ACLR, attention should be focused on attaining adequate graft fixation and placement so that patients are not subject to the complications inherent to revision surgical reconstruction.11,17 The most common methods of femoral graft fixation for ACLR include interference screws, cortical suspension devices, and cross-pins.1,7,10 Currently, there are 2 common types of cortical suspension devices: fixed loop and adjustable loop. With the fixed-loop cortical suspension device, the graft is attached to a continuous suture loop connected to a button, which is flipped and then fixed at the distal femoral cortex, and the tunnel is filled with the graft without any implant. In contrast, an adjustable-loop cortical suspension device has a button attached to the graft through the adjustable loop, and the loop is tightened to pull the graft through to the top of the femoral tunnel, which eliminates the additional tunnel length required to flip the button.4
Fixed-loop devices provide good graft fixation in terms of limiting graft slippage and providing sufficient graft strength, but the requirement of drilling the femoral socket to a specific tunnel depth to flip a button raises some concerns in terms of bone preservation, stability of the tendon graft, and tendon-bone healing as a result of insufficient graft length.1,7 Adjustable-loop devices allow the surgeon to adapt to different tunnel lengths intraoperatively, thereby avoiding the necessity for drilling a longer tunnel and maximizing the amount of graft within the tunnel by not leaving excess space in the bone tunnel.1,7,10 Additional advantages of the adjustable-loop devices include the ability to retension the graft on the femoral side after tibial fixation. However, the flexibility of the loop length of the adjustable-loop devices is concerning, as it may allow for increased graft slippage postoperatively.1,7,10 To date, there is no consensus on which cortical suspension device type is superior during ACLR. The purpose of this systematic review and meta-analysis was to compare the biomechanical outcomes of fixed- versus adjustable-loop femoral cortical suspension devices in studies simulating ACLR with an isolated device and/or specimen setup using porcine femora and bovine flexor tendons.
Methods
Literature Search
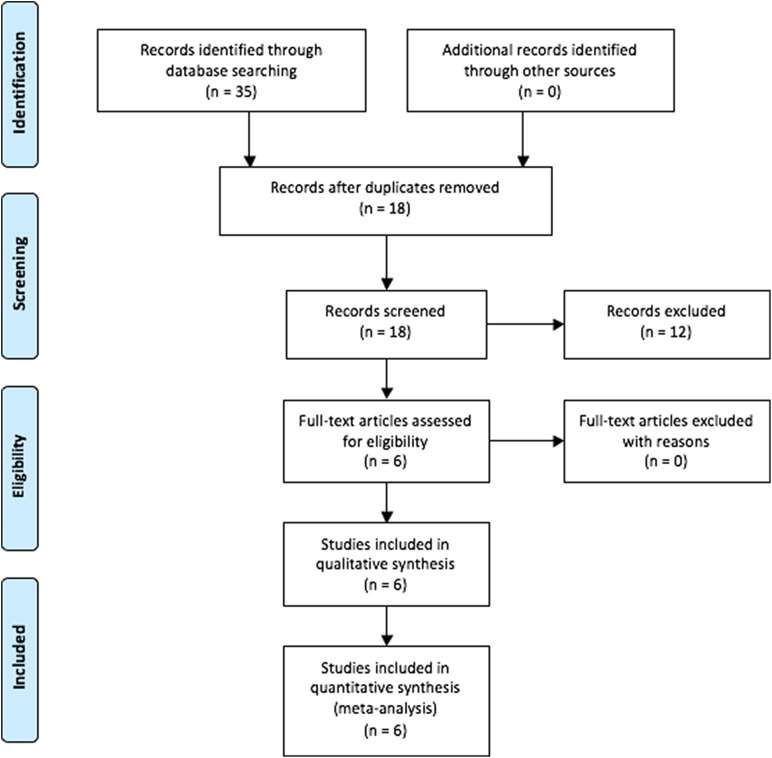
A systematic review of multiple databases was performed. Two independent reviewers (D.A.H., M.J.K.) searched PubMed, Embase, and the Cochrane Library up to November 20, 2017. The electronic search strategy used was the following: (fixed OR closed) AND (adjustable OR open) AND loop AND “anterior cruciate.” A total of 35 studies were reviewed by title and/or abstract to determine study eligibility. Inclusion criteria included studies that compared biomechanical outcomes of fixed-loop devices and at least 1 of 2 adjustable-loop cortical suspension devices for ACLR. Exclusion criteria included clinical studies, studies that did not have similar devices (same manufacturer), biomechanical testing protocols and surgical techniques, and editorial commentaries. Overall, 6 studies1,3,7,10,19,21 were determined to meet the inclusion and exclusion criteria by the 2 reviewers (Figure 1).
Figure 1.
Search strategy. The authors’ electronic search strategy is outlined using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.
Experimental Setup
The Endobutton CL (ECL; Smith & Nephew) fixed-loop femoral cortical device was used in all 6 studies (Table 1).1,3,7,10,19,21 The TightRope RT (TRT; Arthrex) adjustable-loop femoral cortical device was also used in all 6 studies,1,3,7,10,19,21 while the ToggleLoc with ZipLoop (TLZ; Biomet) adjustable-loop femoral cortical device was used in 4 studies.1,10,19,21 Two studies10,19 used TLZ Inline, which is a newer version compared with prior studies1,21 and is designed to be tensioned through the exit of the lateral femoral cortex rather than the anteromedial portal.
TABLE 1.
Summary of Included Studiesa
| Study | Journal | No. of Isolated Devices | No. of Specimens | ||||
|---|---|---|---|---|---|---|---|
| ECL | TRT | TLZ | ECL | TRT | TLZ | ||
| Barrow et al1 (2014) | AJSM | 6 | 6 | 6 | 0 | 0 | 0 |
| Chang et al3 (2018) | Arthroscopy | 6 | 6 | 0 | 6 | 6 | 0 |
| Eguchi et al7 (2014) | The Knee | 10 | 10 | 0 | 10 | 10 | 0 |
| Johnson et al10 (2015) | AJSM | 8 | 8 | 8 | 0 | 0 | 0 |
| Nye et al19 (2017) | Arthroscopy | 0 | 0 | 0 | 10 | 10 | 10 |
| Petre et al21 (2013) | AJSM | 10 | 10 | 10 | 10 | 10 | 10 |
aAJSM, The American Journal of Sports Medicine; ECL, Endobutton CL; TLZ, ToggleLoc with ZipLoop; TRT, TightRope RT.
To isolate the properties of the fixation devices without being influenced by the properties of the bone and tendon, 3 studies3,7,21 performed isolated device testing before specimen testing using fresh-frozen porcine femora and bovine flexor tendons as grafts. Two studies1,10 only performed isolated device testing, while 1 study19 solely performed specimen testing using fresh-frozen porcine femora and bovine flexor tendons as grafts. All studies followed the device insertion techniques in accordance with the manufacturers’ descriptions.
Mechanical Isolated Device Testing
To replicate an in vivo study, 1 study1 tested each device by drilling a 4.5 mm–diameter bone tunnel into a 5 mm–thick steel baseplate. Similarly, 2 studies10,21 drilled a 4 mm– and 4.5 mm–diameter bone tunnel into a 5-mm steel baseplate to test the TRT and TLZ devices, respectively. Additionally, Chang et al3 drilled a 4 mm–diameter hole into a 5-mm steel baseplate to test the TRT device. To test each ECL device, Petre et al21 drilled a 4 mm–diameter bone tunnel, while 3 studies3,7,10 drilled a 4.5 mm–diameter bone tunnel into a steel baseplate. Finally, Eguchi et al7 drilled a 3.6 mm–diameter bone tunnel into a steel baseplate to test the mechanical properties of the TRT device. After drilling a bone tunnel into the steel baseplate, the button was pulled through the tunnel and secured against the steel plate, which acted as the lateral femoral cortex. The adjustable-loop devices were then tightened to a length that corresponded to the inner diameter of the ECL loops.1,3,7,10,21 The loops were then placed around a steel rod with a diameter of 4.5 mm,10,21 5 mm,1 or 9 mm3 or a steel hook.7 The steel rod and hook were then attached and secured to the machine actuator.1,3,7,10,21
After securing the isolated devices to the testing apparatuses in the controlled laboratory setting, each isolated device underwent cyclic preconditioning.1,3,7,10,21 Barrow et al1 cyclically preconditioned each device at a force of 10 to 50 N for 10 cycles at 1 Hz. Three studies3,10,21 cyclically preconditioned each TRT,3,10,21 TLZ,10,21 and ECL3,10,21 device at a force of 10 to 50 N21 or 10 to 75 N3,10 for 10 cycles at 0.1 Hz. Eguchi et al7 preloaded each TRT and ECL device at a constant load of 50 N for 30 seconds. After preconditioning, each adjustable-loop device was retensioned,1,7,10,21 with the exception of that in the study of Chang et al.3 Two studies10,21 performed cyclic loading on each device at a force of 50 to 250 N21 or 100 to 400 N10 for 1000 cycles at 0.5 Hz. Chang et al3 performed cyclic loading on each TRT and ECL device at a force of 100 to 400 N for 4500 cycles at 0.5 Hz. Barrow et al1 cyclically loaded each device at a force of 10 to 250 N for 4500 cycles at 1 Hz, while Eguchi et al7 cyclically loaded each TRT and ECL device at a force of 50 to 250 N for 2000 cycles at 2 Hz. Each sample then underwent load to failure testing at a rate of 20 mm/min,1 50 mm/min,3,10,21 or 60 mm/min.7 Clinical failure was determined by 3 mm of device lengthening measured by mechanical testing apparatuses,1,3,10,21 based on KT-1000 arthrometer testing showing that a side-to-side difference in anterior tibial translation is a sensitive determinant of clinical ACL failure.5
Biomechanical Specimen Testing
Bovine flexor tendon grafts measuring 180 mm7,21 or 200 mm3,19 in length were doubled over to a diameter of 8 mm7,19 or 9 mm.3,21 After looping the graft around the device, the free ends were whipstitched together.3,7,19 Each study3,7,19,21 placed and drilled the femoral tunnels in the center of the porcine ACL footprint exiting on the anterolateral femoral cortex but did not specify specific drilling techniques.
Each TRT3,7,19,21 and TLZ19,21 device was inserted by drilling a guide pin through the lateral femoral cortex. The investigators then used a reamer to drill the femoral tunnel. Using passing sutures, the graft loop was passed through the femoral tunnel and then flipped on the lateral femoral cortex. The tensioning sutures were slowly and proximally pulled to fix the graft within the tunnel.3,7,19,21
Investigators of the 4 studies3,7,19,21 performing specimen testing inserted each ECL device by using a drill reamed over a drill pin. A reamer was used to drill the femoral tunnel to a depth that would allow the button to be flipped properly. The investigators used an eyelet pin loaded with the lead suture to insert the graft into the femoral tunnel. Tension was then applied to pull the insert through the tunnel. After being pulled through the lateral femoral cortex, the ECL device was then flipped over by applying tension on the trailing suture. Distal traction was applied to the graft as the device was fixed with the button perpendicular to the outer femoral cortex.3,7,19,21
Three studies3,7,21 used the same loading profile for both isolated device testing and specimen testing using porcine femora and bovine flexor tendons. Once the devices were secured, Nye et al19 cyclically preconditioned each device at a constant load of 25 N for 30 seconds. Then, each adjustable-loop device was retensioned. After preloading, the constructs were cycled at a force of 50 to 250 N for 1000 cycles at 100 mm/min. The constructs then underwent load to failure testing at a rate of 50 mm/min. This study also defined biomechanical failure as 3 mm of device lengthening.19
Data Extraction
Data extracted for this review included displacement during cyclic loading, ultimate load to failure, and mode of failure of the different cortical suspension devices for ACLR. Mode of failure was categorized as suture, incomplete testing, button, bone, and tendon.
Statistical Analysis
A weighted mean and composite SD were calculated for each group, as previously described.14 Data were then analyzed using 1-way analysis of variance (www.openepi.com). A chi-square test was used to analyze the data on mode of failure. Tukey post hoc analysis was used in cases in which P < .05 (http://astatsa.com/OneWay_Anova_with_TukeyHSD).
The included studies were arranged according to biomechanical test data (displacement during cyclic loading, ultimate load to failure) and device comparisons (ECL vs TRT, ECL vs TLZ). Because there were limited studies, we chose not to further stratify based on model (isolated device vs specimen). A standardized mean difference (SMD) was determined for continuous outcome data, and 95% CIs were determined for effect measures. The degree of study heterogeneity was estimated through the I 2 statistical test, and the Cochran chi-square test was used to test for heterogeneity. An I 2 value of 25%, 50%, and 75% represents a low, moderate, and high degree of heterogeneity according to guidelines from the Cochrane Collaboration.9
Summary measures were estimated for each biomechanical test datum and device comparison using random-effects models, and these were included in multiple forest plots. A random-effects model was used because there was some degree of anticipated heterogeneity among the eligible studies, and this model takes into account between-study variation. A meta-regression approach was not used because of the variability of biomechanical test data and device comparisons reported within these studies.24
Meta-analyses including tests for heterogeneity, the random-effects model, and generation of forest plots were performed using the metafor package.31
Results
Among the 6 studies reviewed, there were 76 fixed-loop models (40 isolated device, 36 specimens) and 120 adjustable-loop models (64 isolated device, 56 specimens) (Table 2). There was an overall significant difference in ultimate load to failure between the ECL, TRT, and TLZ devices (P < .0001), with the strongest fixation device being the TLZ adjustable-loop device (1443.9 ± 512.3 N), followed by the ECL fixed-loop device (1312.9 ± 258.1 N; P = .04) and the TRT adjustable-loop device (863.8 ± 64.7 N; P = .01). There was also an overall significant difference in cyclic displacement (P < .0001), with the ECL fixed-loop device (3.7 ± 3.9 mm) demonstrating the least displacement, followed by the TLZ adjustable-loop device (4.9 ± 2.3 mm) and the TRT adjustable-loop device (7.7 ± 11.1 mm).
TABLE 2.
Load to Failure and Cyclic Displacement Outcomesa
| Study | Model | n | Ultimate Load, N | Displacement, mm | ||||
|---|---|---|---|---|---|---|---|---|
| Fixed (n = 64) | Adjustable (n = 108) | Fixed (n = 76) | Adjustable (n = 120) | |||||
| ECL (n = 64) | TRT (n = 64) | TLZ (n = 44) | ECL (n = 76) | TRT (n = 76) | TLZ (n = 44) | |||
| Barrow et al1 (2014) | Isolated device | 18 | 1529.38 ± 26.07 | 809.11 ± 52.94 | 1652.13 ± 45.11 | 1.34 ± 0.03 | 42.45 ± 7.01 | 5.76 ± 0.35 |
| Chang et al3 (2018) | Isolated device | 12 | 1410 ± NRb | 925 ± 50b | NR | 0.79 ± 0.06 | 1.99 ± 0.53 | NR |
| Eguchi et al7 (2014) | Isolated device | 20 | 1430 ± 148 | 866 ± 53 | NR | 2.03 ± 0.31 | 4.05 ± 1.16 | NR |
| Johnson et al10 (2015) | Isolated device | 24 | 1530 ± 180 | 1020 ± 421 | 2231 ± 511 | 1.05 ± 0.05 | 1.81 ± 0.51 | 3.22 ± 1.41 |
| Petre et al21 (2013) | Isolated device | 30 | 1456 ± 130 | 841 ± 55 | 1561 ± 112 | 0.42 ± 0.08 | 1.10 ± 0.20 | 2.18 ± 0.31 |
| Chang et al3 (2018) | Specimen | 12 | 843 ± 146.09b | 888 ± 118.25b | NR | 14.88 ± 2.34 | 15.65 ± 3.19 | NR |
| Eguchi et al7 (2014) | Specimen | 20 | 1115 ± 274 | 860 ± 70 | NR | 5.88 ± 1.06 | 6.39 ± 2.32 | NR |
| Nye et al19 (2017) | Specimen | 30 | 803.9 ± 92.2 | 801.1 ± 56.3 | 682.1 ± 182.4 | 5.07 ± 0.56 | 5.09 ± 0.87 | 7.44 ± 1.63 |
| Petre et al21 (2013) | Specimen | 30 | 1456 ± 101 | 859 ± 43 | 1334 ± 81 | 3.37 ± 0.27 | 4.47 ± 0.65 | 6.02 ± 1.90 |
| Mean | 1312.9 ± 258.1 | 863.8 ± 64.7 | 1443.9 ± 512.3 | 3.7 ± 3.9 | 7.7 ± 11.1 | 4.9 ± 2.3 | ||
| P value (ECL vs TRT vs TLZ) | <.0001c | <.0001 | ||||||
aData are shown as mean ± SD. ECL, Endobutton CL; NR, not reported; TLZ, ToggleLoc with ZipLoop; TRT, TightRope RT.
bValues were not included in total.
cTukey post hoc analysis revealed that TLZ had a significantly higher ultimate load to failure compared with both ECL and TRT (P < .05).
Mode of failure was significantly different between the 3 groups (P = .01), with suture failure accounting for 83.8% of TRT devices, 69.4% of TLZ devices, and 60.3% of ECL devices (Table 3). One study10 did not report mode of failure, while 2 studies3,7 only reported mode of failure for the ECL and TRT devices.
TABLE 3.
Modes of Failurea
| Device | Suture | Incomplete Testing | Button | Bone | Tendon | Total |
|---|---|---|---|---|---|---|
| Fixed | ||||||
| ECL | 41 (60.3) | 1 (1.5) | 3 (4.4) | 15 (22.0) | 8 (11.8) | 68 (100.0) |
| Adjustable | ||||||
| TRT | 57 (83.8) | 2 (2.9) | 1 (1.5) | 5 (7.4) | 3 (4.4) | 68 (100.0) |
| TLZ | 25 (69.4) | 0 (0.0) | 0 (0.0) | 11 (30.6) | 0 (0.0) | 36 (100.0) |
| Total | 123 (71.5) | 3 (1.7) | 4 (2.3) | 31 (18.1) | 11 (6.4) | 172 (100.0) |
| P value | <.01b | |||||
aData are shown as n (%). ECL, Endobutton CL; TLZ, ToggleLoc with ZipLoop; TRT, TightRope RT.
bTukey post hoc analysis revealed that suture failure was significantly higher than incomplete testing failure, button failure, bone failure, and tendon failure (P < .05).
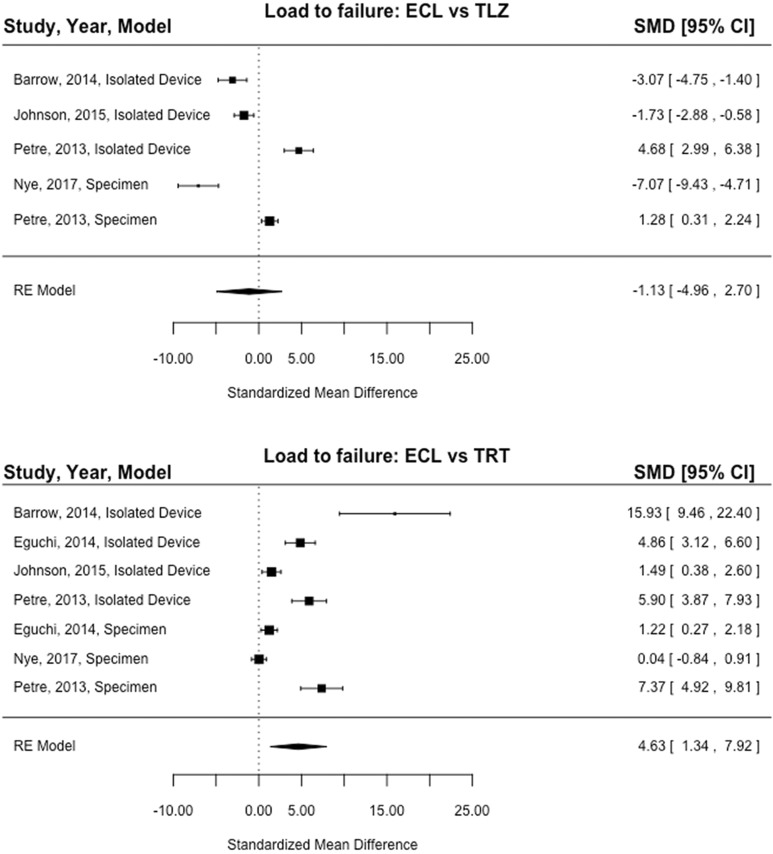
Of the 5 studies (4 isolated device, 3 specimen) included in the ultimate load-to-failure analysis, load to failure of ECL was significantly higher compared with TRT (SMD, 4.63; 95% CI, 1.34-7.92; P = .006) (Figure 2). The results of the 4 studies (3 isolated device, 2 specimen) reporting load to failure of the ECL versus TLZ devices showed no significant difference between devices (SMD, –1.13; 95% CI, –4.96 to 2.70; P = .56) (Figure 2). The results of the 6 studies (5 isolated device, 4 specimen) reporting displacement of ECL versus TRT showed that the TRT device had significantly higher displacement compared with the ECL device (SMD, –2.21; 95% CI, –3.60 to –0.82; P = .002) (Figure 3). The results of the 4 studies (3 isolated device, 2 specimen) reporting displacement of ECL versus TLZ demonstrated that TLZ had significantly higher displacement compared with ECL (SMD, –5.25; 95% CI, –9.95 to –0.55; P = .03) (Figure 3).
Figure 2.
Forest plot showing the standardized mean difference (SMD) in load to failure between fixed-loop and adjustable-loop devices. ECL, Endobutton CL; RE model, random-effects model; TLZ, ToggleLoc with ZipLoop; TRT, TightRope RT. Line of effect: left = favors higher load to failure of TLZ/TRT, right = favors higher load to failure of ECL.
Figure 3.
Forest plot showing the standardized mean difference (SMD) in displacement between fixed-loop and adjustable-loop devices. ECL, Endobutton CL; RE model, random-effects model; TLZ, ToggleLoc with ZipLoop; TRT, TightRope RT. Line of effect: left = favors higher displacement of TLZ/TRT, right = favors higher displacement of ECL.
The statistical assessment of heterogeneity conducted for load to failure of the ECL versus TRT devices found I 2 = 97.23% (95% CI, 92.74%-99.62%; P < .0001), for load to failure of the ECL versus TLZ devices found I 2 = 97.23% (95% CI, 92.05%-99.68%; P < .0001), for displacement of the ECL versus TRT devices found I 2 = 91.32% (95% CI, 81.50%-98.67%; P < .0001), and for displacement of the ECL versus TLZ devices found I 2 = 97.85% (95% CI, 92.93%-99.79%; P < .0001). This indicates that high heterogeneity of an effect may be present in the load to failure and displacement analyses of the ECL versus TRT and ECL versus TLZ group comparisons. These statistics are greatly underpowered and therefore limit a confident conclusion.
Discussion
This is the first systematic review and meta-analysis to compare the biomechanical outcomes of fixed- versus adjustable-loop femoral cortical suspension devices in studies simulating ACLR with an isolated device and/or specimen setup using porcine femora and bovine flexor tendons. The most important finding from this study is that the ECL fixed-loop device, on average, displaced significantly less than both the TRT and TLZ adjustable-loop devices. However, the TLZ adjustable-loop device demonstrated the highest ultimate load to failure. It is important to note that all devices had failure loads much higher than would be expected on the ACL graft during the early rehabilitation period. The mode of failure was significantly different between the 3 groups, with suture failure occurring among a significantly higher proportion of adjustable-loop devices than fixed-loop devices.
Positive clinical outcomes have been reported with all types of graft fixation devices, although interference screw fixation has been associated with graft slippage and lower ultimate failure loads,23,30 and cross-pins have been associated with several complications.12,13,20 A randomized experimental study by Kousa et al13 demonstrated that interference screws and cross-pins have low ultimate failure loads (interference screws, 546-794 N; cross-pins, 868 N) and high cyclic displacement (interference screws, 3.2-4.0 mm; cross-pins, 3.7 mm), providing further evidence that the design of interference screws influences the slippage of soft tissue fixation under cyclic loading conditions. To avoid complications and maintain graft tension before the graft is incorporated after primary ACLR, sufficient graft fixation and placement need to be obtained.1,10,11,13,17,29 Bone-tendon healing may require anywhere from 6 to 12 weeks for autografts, whereas allografts may require up to 6 months.1,10,22 During this initial healing stage, the graft is dependent on tibial and femoral fixation devices to prevent migration of the graft during early rehabilitation, which could result in laxity, instability, and functional failure.1,8,10,13 Furthermore, increases in residual displacement of the graft fixation device during the early postoperative period may lead to micromotion, resulting in tunnel widening and impaired healing of graft ingrowth.1,7,22
The advantage of avoiding overdrilling into the bone tunnel when using an adjustable-loop device may seem desirable, but the flexibility of being able to alter the loop length comes with the drawback of loop slippage and graft displacement on cyclic loading.1,7,10 Additionally, all 6 studies1,3,7,10,19,21 included in this meta-analysis suggested that the adjustable-loop devices lengthen under cyclic loading, which may result in graft fixation lengthening during the early postoperative period. It has been reported that ≥3 mm of side-to-side difference in anterior tibial translation is a sensitive determinant of clinical failure based on KT-1000 arthrometer testing, although this may not result in functional failure.5,21 Thus, a KT-1000 arthrometer measurement of 5 mm is commonly used to define failure in clinical settings.2,14 Cyclic displacement greatly affects the graft’s ability to heal properly, and if the graft displaces but still heals, patients may experience instability when returning to normal activity levels even without experiencing graft ruptures.16,21 Consequently, cyclic loading may be a more clinically applicable test than ultimate load to failure. Furthermore, the included studies demonstrated controversial findings when comparing load to failure outcomes of ECL versus TLZ. Thus, the ECL fixed-loop device may be best.
Interestingly, Nye et al19 observed a higher variability of measured outcomes in the TLZ device compared with the TRT and ECL devices, which may indicate that the sutures are sliding through the locking mechanism and thus allowing the loops to lengthen. Furthermore, Petre et al21 suggested that these adjustable-loop devices may need to be retensioned after cycling the knee and that the fixed-loop device may be superior because it allows less cyclic and initial displacement, thus providing better graft fixation in terms of limiting graft slippage and providing sufficient graft strength. A previous study by Kousa et al13 highlighted the importance of preconditioning and repetitive loading of grafts, which eliminates the natural elastic creep in the graft before testing the devices. While the high amount of displacement seen in the TRT adjustable-loop device could be explained by the lack of retensioning in the study by Chang et al,3 an almost equivalent amount of displacement was demonstrated in the ECL fixed-loop device, and it is unclear whether this was attributed to lengthening of the graft or lengthening of the device. However, Chang et al3 did cyclically load the devices at the highest force (100-400 N) of the included studies. Forces in the ACL during various movements range from 20 N to almost 600 N,18,19,25–28,32 with a native ACL yield strength of 2160 N.15 This review demonstrated that any of these constructs have stronger ultimate loads (863-1443 N) than the 303 to 590 N needed in the early rehabilitation phase after ACLR.18,21,26–28
Few clinical studies have compared adjustable- and fixed-loop femoral cortical suspension devices with respect to knee stability after ACLR. Boyle et al2 found no significant differences in postoperative knee stability and the graft failure rate between adjustable- and fixed-loop femoral cortical suspension devices in a consecutive series of 188 patients who underwent primary ACLR using a hamstring autograft. However, Choi et al4 demonstrated that femoral fixation by the use of the ECL fixed-loop device resulted in significantly better stability on the pivot-shift test than the TRT adjustable-loop device after ACLR with a hamstring graft. Interestingly, the patients in the adjustable-loop group who had grade 2 pivot-shift test findings had excellent Lysholm scores, and there was a trend toward better Lachman and KT-1000 arthrometer results in the adjustable-loop group compared with patients in the fixed-loop group.4
There are limitations to this study that should be noted. To net a large sample size for analysis, the results were not distinguished based on isolated device versus specimen-testing setups. Additionally, the specimen model introduces confounding interspecimen variability, as the porcine femora and bovine flexor tendons may differ in strength and elasticity, respectively, from those of humans.7,10 The high degree of heterogeneity of the data represents an appreciable limitation of this study. However, these statistics were extremely underpowered because of the limited number of studies for each summary estimate, and an inference about publication bias should be made with caution. Two studies3,7 did not examine the TLZ device. Chang et al3 did not retension the TRT device after preconditioning, which may have altered the displacement values. Two studies10,19 used TLZ Inline, and although TLZ and TLZ Inline are similar devices, the differences could be clinically relevant. Two studies1,10 performed a device-only model, which may represent an oversimplification of the true ACLR procedure. Additionally, the variable cyclic loading protocols used in vitro may not exactly reproduce the physiological loads experienced by the ACL. Furthermore, the accuracy of the force vectors experienced by the suspension devices was also not very easily reproduced with mechanical loading in line with the bone tunnel. These biomechanical studies may have been affected by technical errors due to a learning curve of deploying each device. DeBerardino et al6 supported this by repeating the testing protocol used by Barrow et al1 in 2 independent laboratories and found a displacement of less than 3 mm compared with the displacement previously reported of 42.45 mm. Finally, although biomechanical data are useful, recent studies2,4 have suggested that fixed- and adjustable-loop devices result in similar clinical outcomes. This conclusion may be more important when deciding which fixation device is superior. Despite the high level of heterogeneity of the included studies, which limits the ability to draw strong conclusions from these data, this study does provide some insight into the advantages and disadvantages of these different devices and encourages further clinical studies on this topic.
Conclusion
Current biomechanical data suggest that the TLZ device is the strongest fixation device at “time zero” in terms of ultimate load to mechanical failure. However, the ECL device demonstrated the least cyclic displacement, which may be a more clinically applicable measure of device superiority. Future clinical studies should aim to compare the clinical outcomes of fixed- versus adjustable-loop femoral cortical suspension devices for ACLR.
Acknowledgment
The authors thank Balaji Sridhar, PhD, for his assistance with the statistical analysis.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: E.C.M. receives royalties from Biomet and Elsevier, is a consultant for Biomet and DePuy, and receives research support from Biomet, Mitek, Smith & Nephew, and Stryker. J.T.B. is a consultant for DJ Orthopaedics, Shukla Medical, Encore Medical, and Smith & Nephew; receives royalties from Shukla Medical; receives research support from Stryker; has received hospitality payments from Encore Medical and Smith & Nephew; and has received fellowship funding from Smith & Nephew, Mitek, and Stryker. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Barrow AE, Pilia M, Guda T, Kadrmas WR, Burns TC. Femoral suspension devices for anterior cruciate ligament reconstruction: do adjustable loops lengthen? Am J Sports Med. 2014;42(2):343–349. [DOI] [PubMed] [Google Scholar]
- 2. Boyle MJ, Vovos TJ, Walker CG, Stabile KJ, Roth JM, Garrett WE., Jr Does adjustable-loop femoral cortical suspension loosen after anterior cruciate ligament reconstruction? A retrospective comparative study. Knee. 2015;22(4):304–308. [DOI] [PubMed] [Google Scholar]
- 3. Chang MJ, Bae TS, Moon YW, Ahn JH, Wang JH. A comparative biomechanical study of femoral cortical suspension devices for soft-tissue anterior cruciate ligament reconstruction: adjustable-length loop versus fixed-length loop. Arthroscopy. 2018;34(2):566–572. [DOI] [PubMed] [Google Scholar]
- 4. Choi NH, Yang BS, Victoroff BN. Clinical and radiological outcomes after hamstring anterior cruciate ligament reconstructions: comparison between fixed-loop and adjustable-loop cortical suspension devices. Am J Sports Med. 2017;45(4):826–831. [DOI] [PubMed] [Google Scholar]
- 5. Daniel DM, Stone ML, Sachs R, Malcom L. Instrumented measurement of anterior knee laxity in patients with acute anterior cruciate ligament disruption. Am J Sports Med. 1985;13(6):401–407. [DOI] [PubMed] [Google Scholar]
- 6. DeBerardino TM, Smith PA, Cook JL. Femoral suspension devices for anterior cruciate ligament reconstruction: letter to the editor. Am J Sports Med. 2014;42(2):NP15–NP16. [DOI] [PubMed] [Google Scholar]
- 7. Eguchi A, Ochi M, Adachi N, Deie M, Nakamae A, Usman MA. Mechanical properties of suspensory fixation devices for anterior cruciate ligament reconstruction: comparison of the fixed-length loop device versus the adjustable-length loop device. Knee. 2014;21(3):743–748. [DOI] [PubMed] [Google Scholar]
- 8. Heijne A, Fleming BC, Renstrom PA, Peura GD, Beynnon BD, Werner S. Strain on the anterior cruciate ligament during closed kinetic chain exercises. Med Sci Sports Exerc. 2004;36(6):935–941. [DOI] [PubMed] [Google Scholar]
- 9. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnson JS, Smith SD, LaPrade CM, Turnbull TL, LaPrade RF, Wijdicks CA. A biomechanical comparison of femoral cortical suspension devices for soft tissue anterior cruciate ligament reconstruction under high loads. Am J Sports Med. 2015;43(1):154–160. [DOI] [PubMed] [Google Scholar]
- 11. Kamath GV, Redfern JC, Greis PE, Burks RT. Revision anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39(1):199–217. [DOI] [PubMed] [Google Scholar]
- 12. Kokkinakis M, Ashmore A, El-Guindi M. Intraoperative complications using the Bio-Transfix femoral fixation implant in anterior cruciate ligament reconstruction. Arch Orthop Trauma Surg. 2010;130(3):375–379. [DOI] [PubMed] [Google Scholar]
- 13. Kousa P, Jarvinen TL, Vihavainen M, Kannus P, Jarvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction, part I: femoral site. Am J Sports Med. 2003;31(2):174–181. [DOI] [PubMed] [Google Scholar]
- 14. Kraeutler MJ, Bravman JT, McCarty EC. Bone-patellar tendon-bone autograft versus allograft in outcomes of anterior cruciate ligament reconstruction: a meta-analysis of 5182 patients. Am J Sports Med. 2013;41(10):2439–2448. [DOI] [PubMed] [Google Scholar]
- 15. Kraeutler MJ, Wolsky RM, Vidal AF, Bravman JT. Anatomy and biomechanics of the native and reconstructed anterior cruciate ligament: surgical implications. J Bone Joint Surg Am. 2017;99(5):438–445. [DOI] [PubMed] [Google Scholar]
- 16. Ma CB, Francis K, Towers J, Irrgang J, Fu FH, Harner CH. Hamstring anterior cruciate ligament reconstruction: a comparison of bioabsorbable interference screw and endobutton-post fixation. Arthroscopy. 2004;20(2):122–128. [DOI] [PubMed] [Google Scholar]
- 17. Marchant BG, Noyes FR, Barber-Westin SD, Fleckenstein C. Prevalence of nonanatomical graft placement in a series of failed anterior cruciate ligament reconstructions. Am J Sports Med. 2010;38(10):1987–1996. [DOI] [PubMed] [Google Scholar]
- 18. Markolf KL, Gorek JF, Kabo JM, Shapiro MS. Direct measurement of resultant forces in the anterior cruciate ligament: an in vitro study performed with a new experimental technique. J Bone Joint Surg Am. 1990;72(4):557–567. [PubMed] [Google Scholar]
- 19. Nye DD, Mitchell WR, Liu W, Ostrander RV. Biomechanical comparison of fixed-loop and adjustable-loop cortical suspensory devices for metaphyseal femoral-sided soft tissue graft fixation in anatomic anterior cruciate ligament reconstruction using a porcine model. Arthroscopy. 2017;33(6):1225–1232. [DOI] [PubMed] [Google Scholar]
- 20. Ozer H, Oznur A. Complications following hamstring anterior cruciate ligament reconstruction with femoral cross-pin fixation. Arthroscopy. 2005;21(11):1407–1408, author reply 1408. [DOI] [PubMed] [Google Scholar]
- 21. Petre BM, Smith SD, Jansson KS, et al. Femoral cortical suspension devices for soft tissue anterior cruciate ligament reconstruction: a comparative biomechanical study. Am J Sports Med. 2013;41(2):416–422. [DOI] [PubMed] [Google Scholar]
- 22. Rodeo SA, Kawamura S, Kim HJ, Dynybil C, Ying L. Tendon healing in a bone tunnel differs at the tunnel entrance versus the tunnel exit: an effect of graft-tunnel motion? Am J Sports Med. 2006;34(11):1790–1800. [DOI] [PubMed] [Google Scholar]
- 23. Scheffler SU, Sudkamp NP, Gockenjan A, Hoffmann RF, Weiler A. Biomechanical comparison of hamstring and patellar tendon graft anterior cruciate ligament reconstruction techniques: the impact of fixation level and fixation method under cyclic loading. Arthroscopy. 2002;18(3):304–315. [DOI] [PubMed] [Google Scholar]
- 24. Schrock JB, Kraeutler MJ, Houck DA, McQueen MB, McCarty EC. A cost-effectiveness analysis of surgical treatment modalities for chondral lesions of the knee: microfracture, osteochondral autograft transplantation, and autologous chondrocyte implantation. Orthop J Sports Med. 2017;5(5):2325967117704634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Serpas F, Yanagawa T, Pandy M. Forward-dynamics simulation of anterior cruciate ligament forces developed during isokinetic dynamometry. Comput Methods Biomech Biomed Engin. 2002;5(1):33–43. [DOI] [PubMed] [Google Scholar]
- 26. Shelburne KB, Pandy MG. Determinants of cruciate-ligament loading during rehabilitation exercise. Clin Biomech (Bristol, Avon). 1998;13(6):403–413. [DOI] [PubMed] [Google Scholar]
- 27. Shelburne KB, Pandy MG. A dynamic model of the knee and lower limb for simulating rising movements. Comput Methods Biomech Biomed Engin. 2002;5(2):149–159. [DOI] [PubMed] [Google Scholar]
- 28. Shelburne KB, Pandy MG, Anderson FC, Torry MR. Pattern of anterior cruciate ligament force in normal walking. J Biomech. 2004;37(6):797–805. [DOI] [PubMed] [Google Scholar]
- 29. Shelton WR, Fagan BC. Autografts commonly used in anterior cruciate ligament reconstruction. J Am Acad Orthop Surg. 2011;19(5):259–264. [DOI] [PubMed] [Google Scholar]
- 30. Stadelmaier DM, Lowe WR, Ilahi OA, Noble PC, Kohl HW., 3rd Cyclic pull-out strength of hamstring tendon graft fixation with soft tissue interference screws: influence of screw length. Am J Sports Med. 1999;27(6):778–783. [DOI] [PubMed] [Google Scholar]
- 31. Viechtbauer W. Conducting meta-analyses in R with the metafor package. Journal of Statistical Software. 2010;36(3):1–48. [Google Scholar]
- 32. Wascher DC, Markolf KL, Shapiro MS, Finerman GA. Direct in vitro measurement of forces in the cruciate ligaments, part I: the effect of multiplane loading in the intact knee. J Bone Joint Surg Am. 1993;75(3):377–386. [DOI] [PubMed] [Google Scholar]