Abstract
Sarcoidosis is a granulomatous inflammatory disease with unknown etiology. Epidemiological studies have contributed greatly to our knowledge about sarcoidosis, providing critical information on the determinants and distribution of the disease. In this review, we summarize recently published findings from epidemiological studies on sarcoidosis. We review the epidemiological tools used, the incidence and prevalence of disease, mortality and cancer risk after sarcoidosis and nongenetic risk factors for sarcoidosis. Genetics studies have not been included as they deserve a separate review. Leveraging existing epidemiological data to conduct etiological studies aimed towards understanding and preventing disease is critical for future sarcoidosis research.
Keywords: Epidemiology, Sarcoidosis, Incidence, Prevalence, Mortality
Introduction
Sarcoidosis is a systemic inflammatory disease which is characterized by granuloma formation and scarring which, in some cases, permanently impairs organ function. The etiology of sarcoidosis is unknown, but it is hypothesized that a combination of genetic and environmental factors contribute to its occurrence. Sarcoidosis can be challenging to study due to its relative rarity, lack of sensitive and specific diagnostic tests and its heterogeneous presentation which may reflect different etiologies.1,2 Epidemiological studies have contributed greatly to our knowledge about sarcoidosis through describing its distribution, examining long-term outcomes and identifying risk factors for the disease. Recently, the use of large datasets have helped us to better understand patterns in sarcoidosis occurrence. Yet important gaps remain. It is necessary to strategically build upon population-based data in order to enable in-depth, etiologic studies if we wish to move forward from gathering information to preventing disease.
In this review, we summarize findings from epidemiological studies on sarcoidosis published in the last 3 years and discuss future directions for sarcoidosis research. Genetics studies were excluded from this review because the large number would be sufficient for a separate review. We searched MEDLINE from January 2015 to December 2017 using the MeSH term as well as the free text term in all fields to identify epidemiological studies for inclusion.
Epidemiological tools to study sarcoidosis
Epidemiological studies can be used to describe the distribution of sarcoidosis in a population and investigate risk factors for the disease. The tools used in epidemiology can be descriptive or analytic and help to clarify patterns and associations on a population level.
Clinical studies
Although the first to identify new illnesses or adverse effects of new exposures, clinical science is mainly focused on the diagnosis, treatment, and prognosis of disease in individual patients. As a result, clinical studies often include a highly selected group of patients who receive care at a specialized hospital or clinic.3 While these studies provide excellent data on the clinical characteristics of those with the disease, the study sample is not often representative of the full spectrum of disease in the population at large because some sick (or otherwise undiagnosed) people never come to the attention of health care providers.3 Finally, the comparison groups are often composed of other cases with either more or less severe disease, or with differing levels of treatment. Thus, etiology or risk cannot be fully explored.
Case-control studies
The case-control study design is an efficient way to investigate risk factors for disease when the disease is rare.3 Researchers enroll ‘cases’ (those with disease) from hospitals, clinics, or disease registries, and a sample of nondiseased (‘controls’) from the source population which produced the cases. One such study was the ACCESS (A Case Controlled Etiologic Study of Sarcoidosis) study, which enrolled 736 incident cases of sarcoidosis and 706 control subjects from 10 clinical centers in the USA between 1997 and 1999.4,5 Case-control studies can be limited if data were collected retrospectively, increasing the chance of recall bias, and making it difficult to establish the temporal sequence between exposure and disease.3 The validity of case-control studies also depends on the appropriate selection of controls which in some circumstances can be challenging.
Cohort studies
Cohort studies assess the exposure status of healthy individuals and follow them over time to determine the incidence of disease or mortality. Cohort studies play an important role in understanding the etiology of sarcoidosis. Many such prospective studies [e.g. Black Women’s Health Study (BWHS), Nurses’ Health Study II (NHSII)] were originally initiated to study other outcomes (e.g. cancer, cardiovascular disease), but can be used to investigate prediagnosis factors not routinely captured in clinical databases such as dietary intake and lifestyle factors. It is not feasible in large observational cohorts to examine all participants for the presence of the disease of interest, a disadvantage associated with this study design. Thus, in contrast to smaller clinical studies, the BWHS and NHSII rely on self report of sarcoidosis followed by validation using supplemental questionnaires and medical record review in a subset.
Data from ACCESS, NHSII and BWHS have provided excellent information on risk factors for sarcoidosis. Newer and until recently untapped resources are population and administrative databases which can be used to conduct cohort and case-control studies. This is an efficient use of data that have already been collected, although not originally for sarcoidosis-specific research purposes. These databases often have large sample sizes and some are population-based, providing representative estimates in entire populations. However, because data were not collected for research purposes, they usually do not include detailed clinical data (e.g. sarcoidosis phenotype) and rely on International Classification of Disease (ICD) codes to identify sarcoidosis, which have not been validated. This leads to possible disease misclassification and mild and asymptomatic cases are likely to be underreported. Recent reports from Sweden, Taiwan and the USA have made efficient use of these already collected data to study sarcoidosis.
The Swedish National Patient Register is a nationwide database which has been used to identify sarcoidosis diagnoses from inpatient hospitalizations (since 1964) and outpatient care (since 2001) among all Swedish residents.6 These data were linked to other population-based registers containing information from before and after sarcoidosis diagnosis using each individual’s personal identification number. Another population-based and nationwide database is the Taiwan National Health Insurance Research Database (NHIRD), begun in 1995. The NHIRD captures demographics, clinical visits, prescriptions, medical specialty, and ICD diagnostic codes from insurance claim data for reimbursement on nearly 100% of the Taiwanese population.7 Such databases are not available in countries without a centralized healthcare system. The Optum healthcare database is an administrative database (not population-based) which includes approximately 15% of US residents who have commercial health insurance or are enrolled in Medicare parts C and D. (www.Medicare.gov) These data are powerful, including almost 30,000 patients with an insurance claim for sarcoidosis.8 However, generalizability may be limited as the population of patients captured in Optum is older than the general population of the USA and those without insurance coverage are underrepresented.
Incidence and prevalence
Race/geographical location
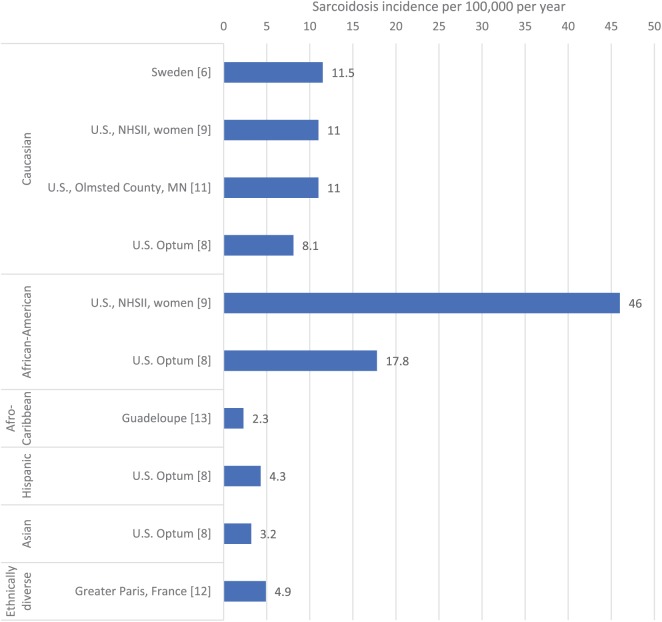
The incidence and prevalence of sarcoidosis has consistently been observed to be highest in Nordic countries and African Americans. Recent studies from Sweden6 and the USA8,9 have provided contemporary estimates of incidence and prevalence which lend further support that these populations experience the highest rates of sarcoidosis. Interestingly, several recent studies in other populations report a prevalence of sarcoidosis greater than 0.05%, indicating that the disease may not be as rare as previously thought. Studies reporting the incidence and prevalence of sarcoidosis in the last three years are presented in Table 1 and incidence estimates categorized by ethnic group are displayed in Figure 1.
Table 1.
Sarcoidosis incidence and prevalence estimates reported in the literature 2015–2017.
| Country, data source | Incidence per 100,000 per year | Prevalence per 100,000 | Age of onset, years | Female incident cases (%) | |
|---|---|---|---|---|---|
| Arkema 6 | Sweden, National Patient Register | 11.5 | 160 | Men: 45 Women: 55 | 45 |
| Baughman 8 | USA, Optum Health Care Database | African Americans: 17.8 White people: 8.1 Hispanics: 4.3 Asians: 3.2 |
African Americans: 14 White people: 50 Hispanics: 22 Asians: 19 |
NR | NR |
| Beghe 17 | Italy, University Hospital of Parma | NR | 49 | Men: 47 Women: 54 | 58 |
| Coquart 14 | Guadeloupe, hospital and laboratory databases | 2.3 | 21 | NR | 59 |
| Duchemann 13 | France, hospitals in Greater Paris, Seine-Saint-Denise County | 4.9 | 30 | NR | 55 |
| Dumas 9 | USA, Nurses’ Health Study (women only) | 11 African Americans: 46 White people: 11 |
100 African Americans: 519 White people: 92 |
Women: 48 | 100 |
| Ungprasert 11 | USA, Olmsted County, MN, healthcare providers | 11 | NR | Men: 43 Women: 48 | 50 |
| Wu 7 | Taiwan, National Health Insurance Research Database | NR | 2.17 | Men: 42Women: 51 | 63 |
NR, not reported.
Figure 1.
Incidence of sarcoidosis per 100,000 per year reported in the literature 2015–2017 categorized by ethnic group.
*Studies from Olmsted County, MN (USA) and Sweden include populations who are majority (>90%) white. NHSII, Nurses’ Health Study II.
A recent study from Sweden using nationwide population-based registers estimated an incidence of 11.5 per 100,000 per year and a prevalence of 0.16%.6 These estimates are lower than those reported in a previous Swedish study published in the 1980s, which observed an incidence of 19 per 100,000 per year.10 The reason for the discrepancy may be that different methods were used to identify sarcoidosis; the older study identified cases through mass radiographic survey of one county (Uppsala) and 10% of cases were discovered by chance. In contrast, the recent register-based Swedish study included individuals who received an ICD code in inpatient or outpatient care. Interestingly, a study from the Mayo Clinic (Olmsted County, MN, USA) using data from a cohort of mainly white people with a northern European ancestry observed an incidence rate of 11 per 100,000 per year, which is nearly identical to the recent results from Swedish data.11
NHSII, a prospective cohort of female nurses followed from 1989 which collected self-reported diagnoses of sarcoidosis via questionnaire, reported a similar incidence (11 per 100,000 per year) and prevalence (0.10%).9 The majority of the study population was white, but the 4% who were black had a higher incidence (43/100,000 per year) and prevalence (0.52%) compared with white women.26 These estimates are lower than what was previously reported in Black American women (Black Women’s Health Study, incidence 71/100,000 per year, prevalence 2.0%).12
In a study using the Optum health insurance medical claims database in the USA, the incidence rate ranged from 7.6 to 8.8 per 100,000 per year.8 African Americans had a higher incidence and prevalence compared with Caucasians, Hispanics and Asians. The prevalence in African Americans was 0.14% and the incidence was 17.8 per 100,000. Caucasians had a prevalence of 0.05% and the incidence was 8.1. Hispanics and Asians had the lowest rates, with a prevalence of 0.02% and an incidence between 3 and 4 per 100,000.8
In recent years, several studies have also described the occurrence of sarcoidosis in other populations not previously studied, providing a picture of the disease in other parts of the world. Data from the Taiwanese NHIRD reported an overall sarcoidosis prevalence of 2.17 per 100,000.7 In a study from Seine-Saint-Denise, a county of Greater Paris, France, the incidence was 4.9 per 100,000 per year and the prevalence was 0.03%.13 The population was quite diverse, allowing for an examination of the occurrence of sarcoidosis by geographical origin as a proxy for ethnicity. Afro Caribbeans had the highest incidence rates (16.9 per 100,000 per year) followed by North Africans (9.7), others (6.4) and Europeans (2.4).
One would think that Afro Caribbeans would have similar rates to Afro Caribbeans living in Europe if geographical origin is a suitable proxy for genetic risk through ethnicity. However, a study examining the occurrence of sarcoidosis in Afro Caribbeans in Guadeloupe reported an incidence of 2.3 per 100,000 and a prevalence of 0.02% which is much lower than the estimates seen in Afro Caribbeans in the French study.14 The difference could be due to more strict criteria for inclusion in this study, which required a positive biopsy. However, it may indicate that location matters as well as race and that ethnicity is not a perfect proxy for race. Another possible explanation for the increased rates observed among racial/ethnic minorities living outside of their respective geographical region of origin is what Hennessy and colleagues term ‘migratory bias’.15 A subtype of diagnostic access bias, migratory bias, posits that in the event of voluntary migration, immigrants may be more inclined to utilize healthcare in their new location than nonimmigrants. This differential utilization of healthcare provides greater access to diagnostic testing and can influence measures of disease incidence.16
Geographical location has been shown to be an important factor related to sarcoidosis occurrence and recent studies confirm variation by region. In the Swedish study, some northern counties had a much higher prevalence than others.6 In the NHSII, BWHS and in the Optum database, Western US states had the lowest rates.8,9,12 A recent study from the Italian province of Parma reported a prevalence of 0.05%, which varied by residential location from 0.03% to 0.09%.17
Age and sex
In addition to race and geographical location, sarcoidosis occurrence varies greatly by age and sex. What causes this variation is unknown, but it indicates that sex plays a role in the manifestation of disease. In some populations, there is a 10-year difference in age at diagnosis between men and women. In Sweden, the age at diagnosis in men was 45 compared with 54 in women.6 In Italy, the age at diagnosis was 47 in men and 54 in women.17 The difference in age is not so large in other populations such as Olmsted County, MN (men 43 versus women 48 years old)11 and in a Spanish urban tertiary teaching hospital report (men 44 versus women 49 years old).18 The overall average age of onset in most studies was between 47 and 51 years old, except for one report from Estonia (average age in men 34, women 43 years old).19 The statement repeated in the literature that sarcoidosis onset peaks between 20 and 45 years of age20 is not supported by the majority of recent studies, with the peak ages of onset closer to 30–55 years old.
The age at onset might have changed over time, as was shown in a study from Japan.21 Age increased over a 40-year period, and a previously high peak onset in men in their 20 s diminished. This may be due to an aging population or a change in detection over time (better diagnostics, less routine chest radiography done as part of workplace exams in men which were common in the 1980s, increased medicalization of women over the life course). It could, however, indicate that exposures to environmental factors which are related to sex have changed over time.
The difference in age at diagnosis between men and women suggests that there is something different in the disease pathogenesis between the sexes, which could be related to genetic factors or environmental exposures which are sex specific, such as reproductive factors. The clinical manifestations of sarcoidosis also appear to differ by sex, with women having more musculoskeletal symptoms than men in two studies.18,19 The observed patterns are not consistent across studies, however women have been reported to have more asymptomatic disease in one study,11 whereas another reported that men were more likely to have asymptomatic disease.18 Future investigations should take into account age when examining sex differences, since age and sex are closely correlated in sarcoidosis.
Mortality
For most patients, sarcoidosis has a benign clinical course, but for a subset of patients it is a chronic, life-threatening disease. A study from the French Epidemiologic Centre for the Medical Causes of Death identified death certificates listing sarcoidosis as the cause of death or a significant condition contributing to death.22 The age-standardized mortality rate was 3.6 per million and increased over time. Sarcoidosis-related deaths occurred at younger ages than the general population and the average age at death was similar for both men and women, but women died less frequently. Two studies from the USA reported pulmonary sarcoidosis-related age-standardized mortality rates using death certificate data from the National Center for Health Statistics.23,24 One study reported that the mortality rate from interstitial lung disease and pulmonary sarcoidosis increased from 2.7 deaths per 100,000 in 1980 to 5.5 deaths per 100,000 in 2014.23 Another study using the same data source but from 1999 through 2010 estimated a death rate of 2.8 per 1 million with sarcoidosis as the immediate cause.24 Mortality rates were higher in women compared with men (3.3 versus 2.3) and in African Americans versus white people (16 versus 1.3). There was a statistically significant increase in the sarcoidosis-related mortality rate in white individuals over the past decade.24
Mortality studies based on death certificates require that sarcoidosis be listed as a cause of death to be included in the study, which likely includes more severe cases of sarcoidosis. Results from these studies show how many people die with sarcoidosis listed, but do not provide an estimate of mortality among people who had sarcoidosis in the past. Individuals who die of a disease downstream of sarcoidosis may not be captured in these studies if sarcoidosis was not considered a contributing cause at the time of death. Furthermore, changes over time in sarcoidosis-related deaths captured by death certificates may reflect changes in practices of completing death certificates. Autopsies are performed less often. Therefore, changes over time should be interpreted with caution. Death certificate data are also more likely to report a higher rate of sarcoidosis-related death in women due to the fact that women are diagnosed later in life and therefore sarcoidosis is more likely to be present at the time of death at an older age.
Several recent studies have used cohort study designs which follow individuals from the time of their diagnosis until death. Using US Veterans Health Administration data (a racially mixed, predominantly male population; 50.8% black and 86.0% male), the 1-year all-cause mortality was estimated to be 2.0%.25 Male sex and black race were associated with an increase in mortality and ocular inflammation was associated with a decrease in mortality. The mortality rate was not compared with individuals without sarcoidosis. One study from the Mayo Clinic in Olmsted County, MN (USA) reported that mortality in patients with sarcoidosis was not higher than the general population [standardized mortality ratio 0.90; 95% confidence interval (CI) 0.74–1.08].26 The mortality rate in sarcoidosis patients was 13 per 1000 over the study period (1946–2013). The lack of an association between sarcoidosis and increased mortality is interesting considering that data from this very same study population found that sarcoidosis patients had increased morbidity,27 infection,28 and cardiovascular disease,29 but do not appear to die any younger than the general population.
Comorbidity has a significant impact on mortality in sarcoidosis. In a hospital-based study from Poland, the number of comorbidities and stage of sarcoidosis significantly predicted survival.30 Several recent investigations have elucidated the comorbidity burden on patients with sarcoidosis, and are summarized in Table 2. These studies have examined multimorbidity,27,31 infection,28,32 malignancy,33,34 cardiovascular disease,29 venous thromboembolism,35,36 fractures,37,38 and autoimmune diseases.7 Patients with sarcoidosis are hospitalized more often than individuals without sarcoidosis39 and in-hospital mortality is higher.40
Table 2.
Studies examining the association between sarcoidosis and subsequent comorbidity reported in the literature 2015-2017
| Author, year | Study population, country | No. patients with sarcoidosis | Average follow-up time | Outcome | Estimate of RR (95% CI) | Additional findings | Risk of outcome associated with sarcoidosis |
|---|---|---|---|---|---|---|---|
| Wu 7 | National Health Insurance Research Database, Taiwan | 1237 | NR | Autoimmune diseases | HR 1.66 (95% CI 1.37–2.00) | Highest risks observed for autoimmune thyroid disease, Sjögren’s syndrome, ankylosing spondylitis | ↑ |
| Ungprasert 29 | Olmsted County, MN, USA | 345 | 15.1 years | Cardiovascular disease | HR 1.65 (95% CI 1.08–2.53) | Highest risks observed for congestive heart failure, cerebrovascular accident | ↑ |
| Ungprasert 35 | Olmsted County, MN, USA | 345 | 15.1 years | Venus thromboembolism | HR 3.04 (95% CI 1.47–6.29) | ↑ | |
| Yaqoob 36 | Explores electronic medical records from 26 healthcare systems, USA | 53,680 | NA (cross-sectional study) | Venus thromboembolism | OR 3.35 (95% CI 3.25–3.46) | ↑ | |
| Bours 37 | Primary care medical records (Clinical Practice Research Datalink), UK | 5722 | 6.7 years | Vertebral and nonvertebral fractures | Any fracture RR 0.90 (95% CI 0.80–1.02) Vertebral RR 1.77 (95% CI 1.06–2.96) Nonvertebral 0.87 (95% CI 0.77–0.99) |
Glucocorticoid use associated with an increased risk of fractures | ↑ vertebral ↓ nonvertebral fractures |
| Ungprasert 38 | Olmsted County, MN, USA | 345 | 12.9 years | Fragility fracture | HR 2.18 (95% CI 1.23–3.88) | ↑ | |
| Sogaard 34 | Nationwide register study, Denmark | 12,890 | 10 years | Malignancy, excluding nonmelanoma skin cancers | SIR 1.3 (95% CI 1.3–1.4) | Risk of tonsil, colon and immune-related cancers remained increased >10 years after diagnosis | ↑ |
| Ungprasert 33 | Olmsted County, MN, USA | 345 | 12.9 years | Malignancy, excluding nonmelanoma skin cancers | HR 0.72 (95% CI 0.49–1.06) | Increased risk of hematologic malignancy among cases of systemic sarcoidosis compared with those without systemic disease | ↔ |
| Ungprasert 39 | Olmsted County, MN, USA | 332 | 14.8 years | Hospitalization | RR 1.37 (95% CI 1.24–1.52) | ↑ | |
| Ungprasert 28 | Olmsted County, MN, USA | 345 | 15.1 years | Hospitalized infection | HR 2.00 (95% CI 1.41–2.84) | Higher risk observed in patients who received immunosuppressive treatment | ↑ |
| Ungprasert 27 | Olmsted County, MN, USA | 345 | 12.9 years | Multimorbidity: at least two chronic conditions | HR 1.60 (95% CI 1.27. 2.01) | Higher risk of cardiovascular diseases, arthritis, depression and diabetes mellitus | ↑ |
| Brito-Zeron 31 | Hospital-based cohort of patients with sarcoidosis and controls from primary care, Spain | 218 | NA(case-control study) |
Comorbidities included in the Charlson Comorbidity Index | 51% of patients with sarcoidosis had at least one comorbidity compared with 29% of controls | Patients with sarcoidosis more likely to have chronic liver disease, autoimmune diseases, chronic pulmonary disease and malignancy | ↑ |
CI, confidence interval; HR, hazard ratio; NA, not applicable; NR, not reported; OR, odds ratio; RR, relative risk; SIR, standardized incidence ratio.
Overall, these data underscore the burden of sarcoidosis in terms of premature mortality and comorbidity. Because the disease has a heterogeneous onset and course, it is important in future studies to stratify patients by disease severity to provide estimates which take into account differences in patient characteristics.
Malignancy
The possible relationship between cancer and sarcoidosis has been investigated for decades with numerous reports of patients diagnosed with malignancies proximal to (immediately before or after) the diagnosis of sarcoidosis, yielding inconsistent results41–46 Hypotheses supporting a possible association include the chronic inflammation or immune system dysregulation of sarcoidosis promoting the biologic mechanism behind carcinogenesis.46,47 Additionally, immunosuppressive therapeutic regimen for sarcoidosis may increase the risk of cancer.48
A meta-analysis of studies from Japan, the UK, the USA, and Scandinavia found a pooled relative risk (RR) for all invasive cancers of 1.19 (95% CI 1.07–1.32).49 The observed risk was highest within 4 years of sarcoidosis diagnosis (RR 1.41; 95% CI 1.27–1.56), followed by those between 5 and 9 years post diagnosis (1.31; 95% CI 1.15–1.48); after 10 years, the risk dropped to 1.06 (95% CI 0.93–1.21). There was an approximately twofold increased risk of developing hematologic (lymphomas, Hodgkin’s lymphoma, leukemia), skin (melanoma, nonmelanoma), and organ (upper digestive tract, kidney, liver, colorectal, bladder) cancers.
Since the meta-analysis was published, two additional studies have reported estimates for the relative risk of cancer associated with sarcoidosis.33,34 A follow-up study of the Danish National Registry of Patients from 1980 to 2011 found increased short- and long-term cancer risk after sarcoidosis diagnosis.34 Specifically, patients with sarcoidosis had a 20% increased cancer risk 3–10 years post diagnosis, and a 10% increased risk more than 10 years post diagnosis. Patients were at increased risk of non-Hodgkins lymphoma, cancers of the tonsil, colon and biliary tract, and nonmelanoma skin cancer in the short term, while in the long term they were at increased risk of cancer of the biliary tract, basal cell carcinoma, and squamous cell skin.
In contrast, in the study from Olmsted County, MN (USA), from 1976 to 201333 there was a 28% reduced risk of any cancer among sarcoidosis cases compared with noncases [hazard ratio (HR) 0.72; 95% CI 0.49–1.06], and no increases (or decreases) of specific cancers among sarcoidosis cases. However, subgroup analyses found an increased risk of hematologic malignancy among cases of systemic (extra-thoracic) sarcoidosis compared with those with without extra-thoracic disease (HR 1.87; 95% CI 1.09–3.22), suggesting an elevated risk of cancer only among patients with extensive disease.
Taken together, these results continue to support the hypothesis of sarcoidosis as a risk factor for cancer, and that hematopoietic cancers may be the most likely manifestation. Additional cohorts of demographically and racially diverse study subjects are needed to further characterize this association.
Risk factors for sarcoidosis
The exact causes of sarcoidosis remain unknown, but a few factors have emerged which provide clues regarding disease etiology.
Obesity
The Black Women’s Health Study, a follow-up study of 59,000 African-American women, found that obesity [body mass index (BMI) ⩾30 kg/m2] at study baseline was associated with a 40% increased incidence of sarcoidosis over a 16-year period (1995–2011; HR 1.42; 95% CI 1.07–1.89).50 Investigators also found that obesity at age 18 and cumulative weight gain since age 18 of at least 30 kg were each associated with an increased incidence. The association between BMI and sarcoidosis was also investigated in the NHSII cohort, a follow-up study of over 116,000 predominantly white (>98%), female registered nurses (1989–2013).51 Current obesity (in the 2-year period prior to diagnosis) was associated with a 70% increased risk of developing sarcoidosis (HR 1.74; 95% CI 1.26–2.40). Associations with sarcoidosis incidence were also observed for BMI at age 18 and cumulative weight gain of at least 25 kg since age 18.51 Finally, a smaller, case-control study of Olmsted County, MN (USA) residents (313 sarcoidosis cases, 200 controls; >90% white) also found a positive association between BMI (within 1 year prior to 3 months post diagnosis) and sarcoidosis risk. The odds ratio of sarcoidosis among obese compared with normal or low BMI subjects was 2.54 (95% CI 1.58–4.06).52 These recent results from different US populations are consistent with those from an earlier study in Danish women in which prepregnancy overweight and obesity was associated with a two- to threefold increased risk of sarcoidosis.53 While the exact mechanism by which obesity influences sarcoidosis is not known, it is generally thought that the proinflammatory environment of obesity plays a primary role54–61 and has been observed with other conditions including asthma.62 Understanding this mechanism may provide insight into possible avenues of treatment development and prevention.
Smoking
One of the strongest associations to emerge from the ACCESS study was a 35% reduced odds of sarcoidosis among ever smokers compared with never smokers,63 a finding consistent with several earlier observations.64–67 Recently, a case-control study of Olmsted County, MN (USA) residents found a stronger negative association between smoking and sarcoidosis.52 Compared with never smokers, the odds ratios for current smokers and former smokers was 0.34 (95% CI 0.23–0.50) and 0.68 (95% CI 0.45–1.01), respectively.
Occupational risk factors
Crystalline silica is an occupational exposure released in mining, construction, and agricultural work. Silica has been shown to be associated with several autoimmune diseases such as systemic sclerosis, rheumatoid arthritis, systemic lupus erythematosus, and Antineutrophil cytoplasmic antibody (ANCA)-related vasculitis.68A recent cohort study conducted in silica-exposed workers in Sweden found that high exposure to silica dust (>0.048 mg/m3) was associated with an increased risk of sarcoidosis compared with those with less exposure (standardized incidence ratio 3.94; 95% CI 1.07–10.08).69 Although based on only seven cases, these results support previous findings of an association between silica and sarcoidosis.70 A better understanding of how silica triggers sarcoidosis and other autoimmune diseases may shed light on the pathogenesis of sarcoidosis. Firefighting has previously been suggested to increase the risk of sarcoidosis through the inhalation of unidentified toxins.71,72 After the World Trade Center disaster on September 11, 2001, several reports found that workers exposed to the debris, in particular firefighters, had an increased risk of sarcoidosis or “sarcoid-like” disease.73–75 A recent report examined this association further by comparing the rates observed in firefighters and emergency medical service workers who arrived as rescue/recovery workers soon after the disaster to age- and sex-specific sarcoidosis incidence rates from the Rochester Epidemiology Project.7676 The World Trade Center-exposed workers had a 2.8-fold increased risk of sarcoidosis and the rate was higher in those with increased exposure (defined by proximity in time to the event and work duration). This study was limited by the use of a comparison group which did not experience the same level of screening as the exposure group.
Future directions
Despite more than 100 years of inquiry and research, we still know very little about sarcoidosis. In particular, little is known about the occurrence of sarcoidosis in other regions, such as Africa, which to our knowledge is based on studies from 30–50 years ago.77–79 Because race has been shown to be an important factor in sarcoidosis occurrence, future studies should be conducted in diverse populations.
New ‘big data’ from population and administrative databases appear to have good validity and provide robust risk estimates among individuals seeking care for sarcoidosis. However, future work should focus on the validation of claims data and ICD codes used to diagnose sarcoidosis to better understand the data quality. The inability to stratify according to sarcoidosis phenotypes in these data is a limitation that should be addressed. Sarcoidosis is a heterogeneous disease and current estimates do not take into account the potentially wide variation in outcomes among different subsets of patients. Phenotyping of the disease is necessary for risk factor identification, as associations with specific subtypes may be missed if pooled together.
Disease registries may also play an important role in the study of sarcoidosis. The Worldwide Sarcoidosis Research Study (WISE)80 was designed to collect information about the characteristics and clinical course of sarcoidosis to increase our understanding and improve treatment of the disease. The future study of sarcoidosis must involve such voluntary enrollment registries, but also a larger network of mandatory registries akin to the US cancer registries (https://seer.cancer.gov/registries/) where diagnostic entities (e.g. hospitals, laboratories) are mandated to report to a central source. This system will best facilitate the identification and recruitment of patients for epidemiologic research studies, provide detailed data on disease phenotype, allow generation of population estimates and assessment of geographic and environmental influences, and identify and recruit patients for epidemiologic research studies.
In order to increase our understanding of long-suspected (e.g. smoking, infectious agents) and novel (e.g. obesity) risk factors for sarcoidosis, we will need the appropriate epidemiologic tools, specifically greater reliance on prospective cohort studies. For example, infectious factors including mycobacterial antigens such as mKatG and Mtb-hsp have long been considered to be potential causes of sarcoidosis,81–84 an association favored by pooled analyses of cross-sectional studies.85,86 However, the temporal sequence of the association has not been established, thus it remains unclear whether exposure to mycobacterial antigens preceded diagnosis of sarcoidosis. Cohort studies with prediagnostic banked biological samples have the potential to answer this question.
Ideally, epidemiological studies can be used to inform future clinical and preclinical investigations of the pathogenesis of disease. For example, one of the strongest and most consistent associations across several studies is a reduced risk of sarcoidosis among smokers compared with nonsmokers. To test the hypothesized mechanism that nicotine’s immunomodulatory properties suppress granulomatous inflammation, a small randomized trial was conducted.87 Thirteen symptomatic patients were randomized to receive 12 weeks of nicotine treatment plus usual care or usual care alone, and were compared with six asymptomatic and six disease-free patients. The nicotine-treated symptomatic group assumed the immune phenotype of asymptomatic group after the 12-week study period, suggesting a potential therapeutic role for nicotine for pulmonary sarcoidosis as well as elucidating immunologic processes underlying the disease. Larger studies should investigate clinical endpoints to follow up on these intriguing findings.
It is an exciting time for sarcoidosis epidemiological research with the recent emergence of new large population datasets. Examples from Sweden, Taiwan and the USA demonstrate the power of existing data to study a relatively rare disease. We must purposefully leverage these data in order to conduct in-depth, etiological studies with the goal of advancing the field towards understanding and preventing disease.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: E.V.A. is supported by funding from the Swedish Heart-Lung Foundation (Hjärt-Lungfonden), the Swedish Research Council (Vetenskapsrådet), the Strategic Research Area in Epidemiology (SFO Epi), the Swedish Society of Medicine (Svenska Läkaresällskapet) and Karolinska Institutet’s Research Foundation. Y.C.C. is supported by National Institutes of Health (NIH) grants R01 CA058420 and UM1 CA164974.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Elizabeth V Arkema  https://orcid.org/0000-0002-3677-9736
https://orcid.org/0000-0002-3677-9736
Contributor Information
Elizabeth V. Arkema, Clinical Epidemiology Unit, Department of Medicine Solna, Karolinska Institutet, Stockholm 17176, Sweden.
Yvette C. Cozier, Slone Epidemiology Center at Boston University, Boston, MA, USA
References
- 1. Prasse A, Katic C, Germann M, et al. Phenotyping sarcoidosis from a pulmonary perspective. Am J Respir Crit Care Med 2008; 177: 330–336. [DOI] [PubMed] [Google Scholar]
- 2. du Bois RM, Goh N, et al. Is there a role for microorganisms in the pathogenesis of sarcoidosis? J Intern Med 2003; 253: 4–17. [DOI] [PubMed] [Google Scholar]
- 3. Aschengrau A SG. Essentials of epidemiology in public health. 3rd ed. Sudbury, MA: Jones and Bartlett, 2014. [Google Scholar]
- 4. Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med 2001; 164: 1885–1889. [DOI] [PubMed] [Google Scholar]
- 5. Design of a case control etiologic study of sarcoidosis (ACCESS). ACCESS research group. J Clin Epidemiol 1999; 52: 1173–1186. [DOI] [PubMed] [Google Scholar]
- 6. Arkema EV, Grunewald J, Kullberg S, et al. Sarcoidosis incidence and prevalence: a nationwide register-based assessment in Sweden. Eur Respir J 2016; 48: 1690–1699. [DOI] [PubMed] [Google Scholar]
- 7. Wu CH, Chung PI, Wu CY, et al. Comorbid autoimmune diseases in patients with sarcoidosis: a nationwide case-control study in Taiwan. J Dermatol 2017; 44: 423–430. [DOI] [PubMed] [Google Scholar]
- 8. Baughman RP, Field S, Costabel U, et al. Sarcoidosis in America. Analysis based on health care use. Ann Am Thorac Soc 2016; 13: 1244–1252. [DOI] [PubMed] [Google Scholar]
- 9. Dumas O, Abramovitz L, Wiley AS, et al. Epidemiology of sarcoidosis in a prospective cohort study of U.S. women. Ann Am Thorac Soc 2016; 13: 67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hillerdal G, Nou E, Osterman K, et al. Sarcoidosis: epidemiology and prognosis. A 15-year European study. Am Rev Respir Dis 1984; 130: 29–32. [DOI] [PubMed] [Google Scholar]
- 11. Ungprasert P, Crowson CS, Matteson EL. Influence of gender on epidemiology and clinical manifestations of sarcoidosis: a population-based retrospective cohort study 1976-2013. Lung 2017; 195: 87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cozier YC, Berman JS, Palmer JR, et al. Sarcoidosis in black women in the United States: data from the black women’s health study. Chest 2011; 139: 144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Duchemann B, Annesi-Maesano I, Jacobe de Naurois C, et al. Prevalence and incidence of interstitial lung diseases in a multi-ethnic county of Greater Paris. Eur Respir J 2017; 50. [DOI] [PubMed] [Google Scholar]
- 14. Coquart N, Cadelis G, Tressieres B, et al. Epidemiology of sarcoidosis in Afro-Caribbean people: a 7-year retrospective study in Guadeloupe. Int J Dermatol 2015; 54: 188–192. [DOI] [PubMed] [Google Scholar]
- 15. Hennessy TW, Ballard DJ, DeRemee RA, et al. The influence of diagnostic access bias on the epidemiology of sarcoidosis: a population-based study in Rochester, Minnesota, 1935-1984. J Clin Epidemiol 1988; 41: 565–570. [DOI] [PubMed] [Google Scholar]
- 16. McKinlay JB. Some issues associated with migration, health status and the use of health services. J Chronic Dis 1975; 28: 579–592. [DOI] [PubMed] [Google Scholar]
- 17. Beghe D, Dall’Asta L, Garavelli C, et al. Sarcoidosis in an Italian province. Prevalence and environmental risk factors. PLoS One 2017; 12: e0176859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brito-Zeron P, Sellares J, Bosch X, et al. Epidemiologic patterns of disease expression in sarcoidosis: age, gender and ethnicity-related differences. Clin Exp Rheumatol 2016; 34: 380–388. [PubMed] [Google Scholar]
- 19. Lill H, Kliiman K, Altraja A. Factors signifying gender differences in clinical presentation of sarcoidosis among Estonian population. Clin Respir J 2016; 10: 282–290. [DOI] [PubMed] [Google Scholar]
- 20. Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999; 160: 736–755. [DOI] [PubMed] [Google Scholar]
- 21. Sawahata M, Sugiyama Y, Nakamura Y, et al. Age-related and historical changes in the clinical characteristics of sarcoidosis in Japan. Respir Med 2015; 109: 272–278. [DOI] [PubMed] [Google Scholar]
- 22. Jamilloux Y, Maucort-Boulch D, Kerever S, et al. Sarcoidosis-related mortality in France: a multiple-cause-of-death analysis. Eur Respir J 2016; 48: 1700–1709. [DOI] [PubMed] [Google Scholar]
- 23. Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW, et al. Trends and patterns of differences in chronic respiratory disease mortality among US counties, 1980-2014. JAMA 2017; 318: 1136–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mirsaeidi M, Machado RF, Schraufnagel D, et al. Racial difference in sarcoidosis mortality in the United States. Chest 2015; 147: 438–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Birnbaum AD, French DD, Mirsaeidi M, et al. Sarcoidosis in the national veteran population: association of ocular inflammation and mortality. Ophthalmol 2015; 122: 934–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ungprasert P, Carmona EM, Utz JP, et al. Epidemiology of sarcoidosis 1946-2013: a population-based study. Mayo Clin Proc 2016; 91: 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ungprasert P, Matteson EL, Crowson CS. Increased risk of multimorbidity in patients with sarcoidosis: a population-based cohort study 1976 to 2013. Mayo Clin Proc 2017; 92: 1791–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ungprasert P, Crowson CS, Matteson EL. Sarcoidosis increases risk of hospitalized infection: a population-based study 1976-2013. Ann Am Thorac Soc 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ungprasert P, Crowson CS, Matteson EL. Risk of cardiovascular disease among patients with sarcoidosis: a population-based retrospective cohort study, 1976-2013. Eur Respir J 2017; 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nowinski A, Puscinska E, Goljan A, et al. The influence of comorbidities on mortality in sarcoidosis: a observational prospective cohort study. Clin Respir J 2017; 11: 648–656. [DOI] [PubMed] [Google Scholar]
- 31. Brito-Zeron P, Acar-Denizli N, Siso-Almirall A, et al. The Burden of Comorbidity and Complexity in Sarcoidosis: Impact of Associated Chronic Diseases. Lung 2017; 2: 239–238. [DOI] [PubMed] [Google Scholar]
- 32. Dureault A, Chapelon C, Biard L, et al. Severe infections in sarcoidosis: incidence, predictors and long-term outcome in a cohort of 585 patients. Medicine (Baltimore) 2017; 96: e8846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ungprasert P, Crowson CS, Matteson EL. Risk of malignancy among patients with sarcoidosis: a population-based cohort study. Arthritis Care Res (Hoboken) 2017; 69: 46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sogaard KK, Svaerke C, Thomsen RW, et al. Sarcoidosis and subsequent cancer risk: a Danish nationwide cohort study. Eur Respir J 2015; 45: 269–272. [DOI] [PubMed] [Google Scholar]
- 35. Ungprasert P, Crowson CS, Matteson EL. Association of sarcoidosis with increased risk of vte: a population-based study, 1976 to 2013. Chest 2017; 151: 425–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yaqoob ZJ, Al-Kindi SG, Zein JG. Sarcoidosis and risk of VTE: validation with big data. Chest 2017; 151: 1398–1399. [DOI] [PubMed] [Google Scholar]
- 37. Bours S, de Vries F, van den Bergh JP, et al. Risk of vertebral and non-vertebral fractures in patients with sarcoidosis: a population-based cohort. Osteoporos Int 2016; 27: 1603–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ungprasert P, Crowson CS, Matteson EL. Risk of fragility fracture among patients with sarcoidosis: a population-based study 1976-2013. Osteoporos Int 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ungprasert P, Crowson CS, Achenbach SJ, et al. Hospitalization among patients with sarcoidosis: a population-based cohort study 1987-2015. Lung 2017; 195: 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pohle S, Baty F, Brutsche M. In-hospital disease burden of sarcoidosis in Switzerland from 2002 to 2012. PLoS One 2016; 11: e0151940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brincker H. Sarcoid reactions and sarcoidosis in Hodgkin’s disease and other malignant lymphomata. Br J Cancer 1972; 26: 120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Suen JS, Forse MS, Hyland RH, et al. The malignancy-sarcoidosis syndrome. Chest 1990; 98: 1300–1302. [DOI] [PubMed] [Google Scholar]
- 43. Seersholm N, Vestbo J, Viskum K. Risk of malignant neoplasms in patients with pulmonary sarcoidosis. Thorax 1997; 52: 892–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Romer FK, Hommelgaard P, Schou G. Sarcoidosis and cancer revisited: a long-term follow-up study of 555 Danish sarcoidosis patients. Eur Respir J 1998; 12: 906–912. [DOI] [PubMed] [Google Scholar]
- 45. Ji J, Shu X, Li X, et al. Cancer risk in hospitalized sarcoidosis patients: a follow-up study in Sweden. Ann Oncol 2009; 20: 1121–1126. [DOI] [PubMed] [Google Scholar]
- 46. Askling J, Grunewald J, Eklund A, et al. Increased risk for cancer following sarcoidosis. Am J Respir Crit Care Med 1999; 160: 1668–1672. [DOI] [PubMed] [Google Scholar]
- 47. Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med 2007; 357: 2153–2165. [DOI] [PubMed] [Google Scholar]
- 48. Sorensen HT, Mellemkjaer L, Nielsen GL, et al. Skin cancers and non-Hodgkin lymphoma among users of systemic glucocorticoids: a population-based cohort study. J Natl Cancer Inst 2004; 96: 709–711. [DOI] [PubMed] [Google Scholar]
- 49. Bonifazi M, Bravi F, Gasparini S, et al. Sarcoidosis and cancer risk: systematic review and meta-analysis of observational studies. Chest 2015; 147: 778–791. [DOI] [PubMed] [Google Scholar]
- 50. Cozier YC, Coogan PF, Govender P, et al. Obesity and weight gain in relation to incidence of sarcoidosis in US black women: data from the Black Women’s Health Study. Chest 2015; 147: 1086–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dumas O, Boggs KM, Cozier YC, et al. Prospective study of body mass index and risk of sarcoidosis in US women. Eur Respir J 2017; 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ungprasert P, Crowson CS, Matteson EL. Smoking, obesity and risk of sarcoidosis: a population-based nested case-control study. Respir Med 2016; 120: 87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Harpsoe MC, Basit S, Andersson M, et al. Body mass index and risk of autoimmune diseases: a study within the Danish National Birth Cohort. Int J Epidemiol 2014; 43: 843–855. [DOI] [PubMed] [Google Scholar]
- 54. Wozniak SE, Gee LL, Wachtel MS, et al. Adipose tissue: the new endocrine organ? A review article. Dig Dis Sci 2009; 54: 1847–1856. [DOI] [PubMed] [Google Scholar]
- 55. Cancello R, Clement K. Is obesity an inflammatory illness? Role of low-grade inflammation and macrophage infiltration in human white adipose tissue. BJOG 2006; 113: 1141–1147. [DOI] [PubMed] [Google Scholar]
- 56. Subramanian V, Ferrante AW., Jr. Obesity, inflammation, and macrophages. Nestle Nutr Workshop Ser Pediatr Program 2009; 63: 151–159; discussion 9–62; 259–268. [DOI] [PubMed] [Google Scholar]
- 57. Juge-Aubry CE, Henrichot E, Meier CA. Adipose tissue: a regulator of inflammation. Best Pract Res Clin Endocrinol Metab 2005; 19: 547–566. [DOI] [PubMed] [Google Scholar]
- 58. Schipper HS, Prakken B, Kalkhoven E, et al. Adipose tissue-resident immune cells: key players in immunometabolism. Trends Endocrinol Metab 2012; 23: 407–415. [DOI] [PubMed] [Google Scholar]
- 59. Kanneganti TD, Dixit VD. Immunological complications of obesity. Nat Immunol 2012; 13: 707–712. [DOI] [PubMed] [Google Scholar]
- 60. Strissel KJ, DeFuria J, Shaul ME, et al. T-cell recruitment and Th1 polarization in adipose tissue during diet-induced obesity in C57BL/6 mice. Obesity (Silver Spring) 2010; 18: 1918–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lugogo NL, Hollingsworth JW, Howell DL, et al. Alveolar macrophages from overweight/obese subjects with asthma demonstrate a proinflammatory phenotype. Am J Respir Crit Care Med 2012; 186: 404–411. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62. Umetsu DT. Mechanisms by which obesity impacts upon asthma. Thorax 2017; 72: 174–177. [DOI] [PubMed] [Google Scholar]
- 63. Newman LS, Rose CS, Bresnitz EA, et al. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med 2004; 170: 1324–1330. [DOI] [PubMed] [Google Scholar]
- 64. Douglas JG, Middleton WG, Gaddie J, et al. Sarcoidosis: a disorder commoner in non-smokers? Thorax 1986; 41: 787–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hance AJ, Basset F, Saumon G, et al. Smoking and interstitial lung disease. The effect of cigarette smoking on the incidence of pulmonary histiocytosis X and sarcoidosis. Ann N Y Acad Sci 1986; 465: 643–656. [DOI] [PubMed] [Google Scholar]
- 66. Valeyre D, Soler P, Clerici C, et al. Smoking and pulmonary sarcoidosis: effect of cigarette smoking on prevalence, clinical manifestations, alveolitis, and evolution of the disease. Thorax 1988; 43: 516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Murin S, Bilello KS, Matthay R. Other smoking-affected pulmonary diseases. Clin Chest Med 2000; 21: 121–137, ix. [DOI] [PubMed] [Google Scholar]
- 68. Miller FW, Alfredsson L, Costenbader KH, et al. Epidemiology of environmental exposures and human autoimmune diseases: findings from a National Institute of Environmental Health Sciences Expert Panel Workshop. J Autoimmun 2012; 39: 259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Vihlborg P, Bryngelsson IL, Andersson L, et al. Risk of sarcoidosis and seropositive rheumatoid arthritis from occupational silica exposure in Swedish iron foundries: a retrospective cohort study. BMJ Open 2017; 7: e016839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rafnsson V, Ingimarsson O, Hjalmarsson I, et al. Association between exposure to crystalline silica and risk of sarcoidosis. Occup Environ Med 1998; 55: 657–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kern DG, Neill MA, Wrenn DS, et al. Investigation of a unique time-space cluster of sarcoidosis in firefighters. Am Rev Respir Dis 1993; 148: 974–980. [DOI] [PubMed] [Google Scholar]
- 72. Prezant DJ, Dhala A, Goldstein A, et al. The incidence, prevalence, and severity of sarcoidosis in New York City firefighters. Chest 1999; 116: 1183–1193. [DOI] [PubMed] [Google Scholar]
- 73. Crowley LE, Herbert R, Moline JM, et al. ‘Sarcoid like’ granulomatous pulmonary disease in World Trade Center disaster responders. Am J Ind Med 2011; 54: 175–184. [DOI] [PubMed] [Google Scholar]
- 74. Izbicki G, Chavko R, Banauch GI, et al. World Trade Center ‘sarcoid-like’ granulomatous pulmonary disease in New York City Fire Department rescue workers. Chest 2007; 131: 1414–1423. [DOI] [PubMed] [Google Scholar]
- 75. Jordan HT, Stellman SD, Prezant D, et al. Sarcoidosis diagnosed after September 11, 2001, among adults exposed to the World Trade Center disaster. J Occup Environ Med 2011; 53: 966–974. [DOI] [PubMed] [Google Scholar]
- 76. Webber MP, Yip J, Zeig-Owens R, et al. Post-9/11 sarcoidosis in WTC-exposed firefighters and emergency medical service workers. Respir Med 2017; 132: 232–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Benatar SR. Sarcoidosis in South Africa. A comparative study in Whites, Blacks and Coloureds. S Afr Med J 1977; 52: 602–606. [PubMed] [Google Scholar]
- 78. Jacyk WK. Sarcoidosis in the West African. A report of eight Nigerian patients with cutaneous lesions. Trop Geogr Med 1984; 36: 231–236. [PubMed] [Google Scholar]
- 79. Awotedu AA, George AO, Oluboyo PO, et al. Sarcoidosis in Africans: 12 cases with histological confirmation from Nigeria. Trans R Soc Trop Med Hyg 1987; 81: 1027–1029. [DOI] [PubMed] [Google Scholar]
- 80. Gerke AK, Tang F, Cozier YC, et al. A web-based registry for patients with sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2017; 34: 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Brownell I, Ramirez-Valle F, Sanchez M, et al. Evidence for mycobacteria in sarcoidosis. Am J Respir Cell Mol Biol 2011; 45: 899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Dubaniewicz A. Mycobacterium tuberculosis heat shock proteins and autoimmunity in sarcoidosis. Autoimmun Rev 2010; 9: 419–424. [DOI] [PubMed] [Google Scholar]
- 83. Gupta D, Agarwal R, Aggarwal AN, et al. Sarcoidosis and tuberculosis: the same disease with different manifestations or similar manifestations of different disorders. Curr Opin Pulm Med 2012; 18: 506–516. [DOI] [PubMed] [Google Scholar]
- 84. Oswald-Richter KA, Beachboard DC, Zhan X, et al. Multiple mycobacterial antigens are targets of the adaptive immune response in pulmonary sarcoidosis. Respir Res 2010; 11: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Fang C, Huang H, Xu Z. Immunological evidence for the role of mycobacteria in sarcoidosis: a meta-analysis. PLoS One 2016; 11: e0154716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Esteves T, Aparicio G, Garcia-Patos V. Is there any association between Sarcoidosis and infectious agents? A systematic review and meta-analysis. BMC Pulmonary Medicine 2016; 16: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Julian MW, Shao G, Schlesinger LS, et al. Nicotine treatment improves Toll-like receptor 2 and Toll-like receptor 9 responsiveness in active pulmonary sarcoidosis. Chest 2013; 143: 461–470. [DOI] [PubMed] [Google Scholar]