Abstract
Objective:
People with nondialysis-dependent chronic kidney disease (CKD) and renal transplant recipients (RTRs) have compromised physical function and reduced physical activity (PA) levels. Whilst established in healthy older adults and other chronic diseases, this association remains underexplored in CKD. We aimed to review the existing research investigating poor physical function and PA with clinical outcome in nondialysis CKD.
Data sources:
Electronic databases (PubMed, MEDLINE, EMBASE, Web of Science, Cochrane Central Register of Controlled Trials) were searched until December 2017 for cohort studies reporting objective or subjective measures of PA and physical function and the associations with adverse clinical outcomes and all-cause mortality in patients with nondialysis CKD stages 1–5 and RTRs. The protocol was registered on the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42016039060).
Review methods:
Study quality was assessed using the Newcastle-Ottawa Scale and the Agency for Healthcare and Research Quality (AHRQ) standards.
Results:
A total of 29 studies were included; 12 reporting on physical function and 17 on PA. Only eight studies were conducted with RTRs. The majority were classified as ‘good’ according to the AHRQ standards. Although not appropriate for meta-analysis due to variance in the outcome measures reported, a coherent pattern was seen with higher mortality rates or prevalence of adverse clinical events associated with lower PA and physical function levels, irrespective of the measurement tool used. Sources of bias included incomplete description of participant flow through the study and over reliance on self-report measures.
Conclusions:
In nondialysis CKD, survival rates correlate with greater PA and physical function levels. Further trials are required to investigate causality and the effectiveness of physical function and PA interventions in improving outcomes. Future work should identify standard assessment protocols for PA and physical function.
Keywords: kidney diseases, kidney transplantation, mortality, physical activity, physical function
Introduction
Chronic kidney disease (CKD) is a long-term condition affecting approximately three million people in the UK and over 61,000 people have end-stage renal disease and require dialysis or a renal transplant.1 Research into kidney disease has historically tended to concentrate on patients with severe renal impairment requiring renal replacement therapy, however there is a significant proportion of the UK population living with earlier stage CKD and interventions to promote a healthy lifestyle with this group are starting to emerge.
People living with nondialysis CKD experience a high symptom burden with progressively impaired physical function and low levels of physical activity (PA). These negatively affect quality of life (QoL) and independence.2,3 In patients with nondialysis CKD, even a small increase in regular PA levels can improve self-reported quality of health and life, as well as improving exercise tolerance and cardiovascular reactivity.4 In older adults,5 and in other chronic disease populations such as diabetes,6,7 it is well established that both reduced physical function and PA are associated with an increased risk of cardiovascular disease (CVD) and all-cause mortality.8,9 Whilst evidence is limited in nondialysis CKD populations, it is well established in patients undergoing dialysis that both self-reported10–13 and objective13–15 physical function is a significant and independent predictor of all-cause mortality and future hospitalization. Notably, regularly physically active dialysis patients have a decreased risk of CVD and death,16 however the physiological and social impact of dialysis is such that findings in this group are not directly transferable to a patient population that does not require renal replacement therapy. Although renal transplant recipients (RTRs) generally report improved physical function, PA, and QoL following transplantation, it often remains poor,17,18 and patients who have undergone transplantation remain at high risk of CVD.19
Physical function and PA are two key ‘modifiable’ lifestyle factors that may reduce mortality and clinical adverse events and have a positive impact on quality of life in nondialysis CKD and RTRs. Furthermore, early identification, using simple physical function or PA measures, of patients at risk of clinical adverse events may focus interventions (e.g. exercise or nutrition) designed to improve such outcomes.
Physical function and PA should be viewed as two independent concepts. Physical function is the ability to perform activities of daily living, and is assessed using simple tests to reflect these tasks (e.g. getting out of a chair) or by subjectively rating competency in completing different tasks.13 PA is any bodily movement produced by contraction of skeletal muscle that increases energy expenditure above a basal level.20 PA and physical function correlate significantly and both concepts are important to clinicians and patients, hence this review will explore the relationship of each with clinical outcomes.
We performed a systematic review to identify the association between physical function and PA with all-cause mortality and other adverse clinical outcomes in nondialysis CKD (i.e. including RTRs). No systematic review of the current literature has been performed on this association in this patient group. We hypothesized that patients with nondialysis CKD who are functionally limited or less physically active will demonstrate a higher risk of all-cause mortality and adverse clinical outcomes.
Methods
Protocol and registration
The protocol was registered on the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42016039060). Data are reported in line with the ‘Preferred Reporting Items for Systematic Reviews and Meta-Analyses’ (PRISMA) guidelines.21
Search strategy and selection criteria
We aimed to identify observational studies that explored the link between physical function, PA, and adverse clinical outcomes and all-cause mortality in nondialysis CKD. The primary question of interest was the association between objective and subjective measures of physical function, PA, and the likelihood of death (i.e. all-cause mortality) and adverse clinical outcomes in patients with CKD not currently requiring dialysis therapy. For the purpose of this review, an ‘adverse clinical outcome’ was defined as one (or more) of the following events: end-stage renal disease (i.e. the need for/time to dialysis), unforeseen hospital admission, or nonfatal cardiovascular event (e.g. myocardial infarction, stroke, etc.). The primary outcome of interest was all-cause mortality.
Data sources and search strategy
The following electronic databases were searched from their date of establishment to July 2016 and a further search was performed in December 2017 to gather any new literature. National Centre for Biotechnology Information (NCBI) PubMed [which includes the Medical Literature Analysis and Retrieval System Online (MEDLINE)], Excerpta Medica dataBASE (EMBASE), Web of Science (WOS) (which includes the KCI-Korean Journal Database, MEDLINE, Russian Science Citation Index, and SciELO Citation Index), and the Cochrane Central Register of Controlled Trials (CENTRAL). The search strategy was tailored to each database and used a combination of key words and medical subject headings (MeSH). MeSH search terms were: ‘kidney diseases’, ‘kidney transplantation’, ‘physical activity’, ‘mortality’, ‘death’, ‘cardiovascular event’. Other non-MeSH search terms used were: ‘renal impairment’, ‘physical function’, ‘physical performance’, ‘disability’, ‘all-cause mortality’, ‘cardiovascular diseases’, ‘adverse event’, ‘hospital admission’.
As per the PRISMA statement, an example full electronic search strategy can be found for the NCBI PubMed database in supplementary material 1.
Article eligibility criteria
The eligibility for full text review of each citation was independently evaluated by two authors (HJM, TJW) on the basis of title and abstract. Any article deemed potentially relevant was retrieved for full text review. The reference lists of any relevant articles were also screened to identify studies which may have been missed in the search.
Inclusion criteria
Human adults (aged 18 years or over).
CKD (any stage) or RTRs.
Cohort studies including secondary analysis of randomized control trials and abstracts.
Reporting physical function or PA outcome measures.
Reporting association with adverse clinical outcomes and all-cause mortality in either unadjusted or adjusted terms.
Specific exclusion criteria
Renal failure; any dialysis modality.
Review articles.
Animal trials.
Non-English articles.
Data extraction
Following a preliminary pilot search in NCBI PubMed, a data extraction form was created to capture relevant information from included studies. Each article was reviewed by two independent members of the research team during the data extraction process. The following information was extracted for each study:
Study characteristics: such as the year of publication, study design, and sample size.
Patient characteristics, such as mean age, sex distribution, race, and comorbidities.
Definitions and incidences of CKD, physical function (or its associated domains), PA, clinical adverse events, and all-cause mortality.
Reported association of physical function or PA with adverse clinical outcomes or all-cause mortality in either unadjusted or adjusted terms (e.g. hazard or odds ratio).
Evaluation of quality and risk of bias
Each study was evaluated for quality and risk of bias using the Newcastle-Ottawa Scale (NOS)22 independently by two reviewers. Discrepancies in scoring were settled by mutual agreement. The primary authors (HJM and TJW) had the final verdict decision. The NOS is a quality evaluation method for nonrandomized studies which uses three criteria: selection, comparability, and outcome. Each study is designated a number of stars for each section, based on predetermined queries.22 The NOS has been extensively used to evaluate quality and bias for systematic reviews and meta-analyses and is recommended by the Cochrane Collaboration.23 Scores from the NOS were transformed into Agency for Healthcare Research and Quality (AHRQ) standards (‘good’, ‘fair’, and ‘poor’ quality).22
Results
Study selection
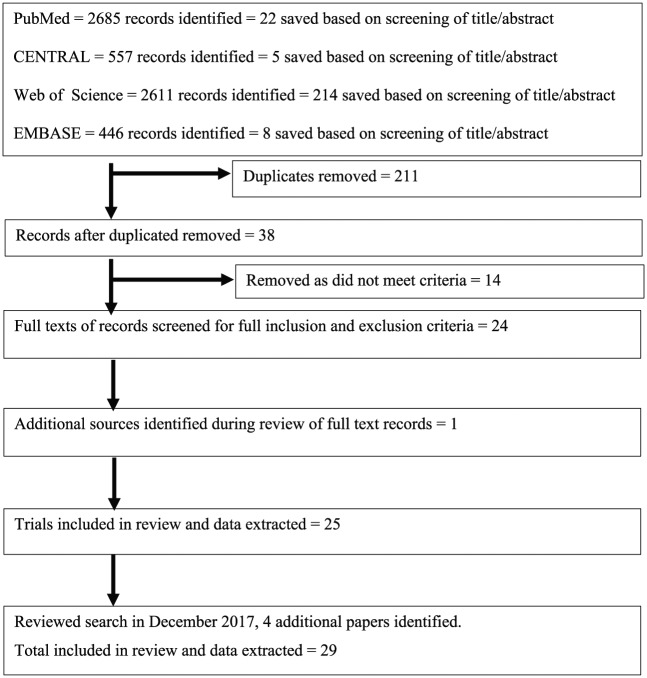
A total of 6299 records were identified by systematic searching and 249 were deemed appropriate based upon the title and abstract alone. Upon removal of 211 duplicates, 38 records were assessed against the full eligibility criteria and 14 records were removed. One additional source was identified during the original review process. In December 2017, a re-search found four additional studies. A total of 29 trials were reviewed (see Figure 1).
Figure 1.
PRISMA flow diagram.
PubMed = National Centre for Biotechnology Information (NCBI) PubMed [which includes the Medical Literature Analysis and Retrieval System Online (MEDLINE)]; CENTRAL = Cochrane Central Register of Controlled Trials; WoS = Web of Science (which includes the KCI-Korean Journal Database, MEDLINE, Russian Science Citation Index, and SciELO Citation Index); EMBASE = Excerpta Medica database.
Study characteristics
Overall, the articles demonstrated a range of follow-up times (median follow up = 7.0 years; range 124–15.9 years25) and sample sizes (median = 719; range 2626–50, 620.27) Studies were conducted in the USA,25,28–41Taiwan,27,42–44 Estonia,26 the Netherlands,45 Korea,46 the UK,47,48 Italy,24 Hungary,49 Brazil,50 Finland,51 and Slovakia,52 ensuring data from a variety of cultures are included which, although increasing generalizability, may mean culturally specific behaviour trends are masked. Studies included single-25,26,38,42–45,47,49,50,52 and multicentre investigations,24,30,33–37,39–41,48,51 and population-wide surveys.27–29,31,32,46 The majority of these studies are observational, except Pechter and colleagues26 who described a 10-year programme of supervised hydrotherapy exercise, and Chen and colleagues30 who reported observational data collected as part of a randomized controlled trial investigating the effects of different diets in kidney disease.
The disease populations studied varied, with 19 investigations conducted in nondialysis CKD,24,26–33,35,37,39,40,42,43,46,47,50 7 with RTRs,36,38,41,45,48,49,52 and Tikkanen-Dolenc and colleagues51 studied both CKD and RTRs. Some studied all five stages of CKD,24,26–28,34,39,43,44,46,47,51 whilst others studied a fixed estimated glomerular filtration rate (eGFR) range.29–34,37,40,42,50 Gulati and colleagues25 studied a female-only population with no pre-existing diagnosis of CKD, however the mean eGFR of the study population was 53.7 ml/min/1.73 m² and 79% were found to have an eGFR less than 60 ml/min/1.73 m². Further, Robinson-Cohen and colleagues34 conducted a general population study but calculated hazard ratio (HR) for stratified eGFR bands and, henceforth, both of these papers have been included in the review. Two papers39,50 investigated physical function as a subset of another concept: Delgado and colleagues39 investigated frailty in CKD, whilst Periera and colleagues50 studied the incidence of sarcopenia. Similarly Chang and colleagues43 measured hand-grip strength (HGS) to investigate the effects of protein-energy wasting.
The associations between physical function and all-cause mortality or adverse clinical outcomes are summarized in Table 1, and studies reporting the association between PA and outcomes can be found in Table 2. Ten papers investigated physical function, whilst 15 studied PA. Two papers used cumulative measures using both PA and physical function,34,38 however these have been included in the table corresponding to the main emphasis of the individual trial. Tsai and colleagues44 investigated physical function as ‘indices’ of the person’s ability to engage in PA in addition to reporting PA behaviour.
Table 1.
Summary of findings; association between physical function and all-cause mortality or adverse clinical outcomes.
| Study | Patient characteristics: N, CKD stage or RTR; mean age (years); % male; mean eGFR [SD] | Mean follow-up duration (years) | Comparison, control, or comparator | Outcome measures/intervention | Main findings | Mortality HR [95% CI] |
|---|---|---|---|---|---|---|
| Chin et al. (2014)46 | 984, CKD 1–5; 76.0 years [9.1]; 44%; 72.3 [17.0] | 5 | eGFR groups | Self-report: Korean version of ADLs, Instrumental ADL | ↓renal function associated with 29.5% ↓ADL/IADL
scores ↓ADL/IADL scores associated with ↑MR |
eGFR ⩾60: HR=1.87‡ [1.10–3.20] eGFR <59: HR=2.53‡ [1.57–4.09] |
| Delgado et al. (2015)39 | 812, CKD; 52 years (median); 60.5%, mGFR=33.1 [11.7] | 17 (median) |
Sample divided into 3 categories: not frail, immediate frail, frail |
Self-report: MDRD LTPAQ; MDRD quality of wellbeing measure |
↓eGFR correlates with ↑levels of self-report frailty and ↑MR | Intermediate frail: HR=1.47 [1.14–1.90]$,‡,§,||
Frail: HR=1.71 [1.26–2.30]$,‡,§,|| Intermediate frail: HR=1.43 [1.11–1.83] $,‡,§,¶,|| Frail: HR=1.48 [1.08–2.00]$,‡,§,¶,|| |
| Griva et al. (2013)48 | 347, RTRs; 46.55 years [13.96]; 54.2%; 38.54 [14.07] | 8.57 [6.55] | N/A | Self-report: HRQoL and SF-36 | ↓Physical HRQoL and ↓PF associated with ↑MR and graft failure | All-cause mortality: HR=4.3 [2.72–6.78]*, p<0.001 HR=1.82 [1.04–2.86]$,||, p=0.04 Graft failure: HR=2.99 [2.08–4.3]*, p<0.001 HR=1.57 [1.04–2.38]$,||, p=0.03 |
| Molnar-Varga et al. (2011)49 | 879, RTRs; 49 years [13]; 58%; 50 [22] | 7.83 (median) | N/A | Self-report: KDQoL, including HRQoL, SF-36 and CES-D scale | PCS and PF independently associated with mortality or graft
loss. However, associations were not significant after
adjustment for depression 10-point ↑PCS yields 18% ↓MR; 10-point ↑PF associated with 11% ↓MR |
SF-36 PCS: HR=0.66 [0.59–0.75]*, p<0.001 HR=0.8 [0.7–0.91]$,‡,¶,||, p=0.001 Adjusted for depression score: HR= 0.82 [0.71–0.95]$,‡,¶,||, p=0.008 PF score: HR=0.84 [0.80–0.87]*, p<0.001 HR=0.88 [0.83–0.93]$,‡,¶,||, p<0.001 Adjusted for depression score: HR=0.89 [0.84–0.94]$,‡,¶,||, p<0.001 |
| Prihodova et al. (2014)52 | 151, RTRs; 47.09 years [13.2]; 56.3%; 51.16 [15.6] | 7.1 [2.2] | N/A | Self-report: SF-36 | ↑survival with ↑eGFR (2% per point), ↑PCS (4% per point) | Survival analysis: PCS: HR=1.04, p<0.05* |
| Doyle et al. (2015)47 | 3012, CKD 1–5; 84 years; 41% | 12 | Comparison across eGFR groups | Self-report/objective: Barthel score | ↑discharge Barthel score (i.e. ↑PF) were associated with ↓all-cause MR | Barthel score ⩾10, eGFR <30: HR=7.0 eGFR 45-59: HR=3.0 Barthel score 19–20, eGFR <30: HR: 1.5 eGFR 45–90: HR=1.25 |
| Chang et al. (2011) | 128, CKD 1–5; 60.7 years [14.8]; 46.8%; 46.6 [28.2] | 2.825 | eGFR groups | Objective: HGS | HGS used as measure of protein-energy wasting HGS is independent predictor of outcome |
Risk of all-cause mortality or dialysis
initiation: HR=0.9, p=0.004 (CKD 1–5) HR=0.91 [0.83–0.99], p=0.031 (CKD 3b–5) |
| Gulati et al. (2012)25 | 5716; 52.5 years [10.8]; 0%; 53.7 [8.3] | 15.9 | N/A | Objective: treadmill test using Bruce protocol to measure cardiorespiratory fitness | Cardiorespiratory fitness significantly modified the
association between eGFR and mortality (p
< 0.001) eGFR< 45 + fitness level < 5 METs: MR=7.6 deaths/1000 person years eGFR ⩾ 60 + fitness level of > 8 METs: MR=0.56 deaths/1000 person years |
|
| Lattanzio et al. (2015)24 | 487, CKD post hospital discharge; 80.1 years [6.0]; 45.8%; 50.4 [14.7] | 1 | none | Objective: SPPB | ↑MR with older age, hypoalbuminemia, cognitive impairment, impaired ADLs, eGFR <30, anaemia and SPPB < 5 | SPPB=5–8: HR = 1.96 [0.63-6.07]$,‡
SPPB=0–4: HR = 5.70 [1.98–12.4]$,‡ SPPB=5–8: HR = 1.45 [0.53–4.27]$,‡,§,|| SPPB=0–4: HR = 2.93 [1.07–8.63]$,‡,§,|| |
| Nastasi et al. (2017)41 | 719, RTRs; 51.6 years [14.2]; 62.3% | 2 (median) | N/A | Objective: SPPB | 1 point reduction in chair stand or walking speed score correlates with 1.21- and 1.5-fold increase in mortality risk | SPPB <10: HR=3.57 [1.83–6.98]*,
p<0.001 HR=2.3 [1.1–4.74]$,‡,§,||, p=0.02 |
| Pereira et al. (2015)50 | 287, CKD 3–5; 59.9 years [10.5]; 62%; 25.0 [15.8] | Up to 3.33 | N/A | Objective: sarcopenia measured using HGS and bioelectrical impedance analysis | Presence of sarcopenia significantly predicts all-cause mortality | HR=2.89 [1.4–5.96]*,
p=0.004 HR=3.58 [1.43–8.31]$,‡,§, p=0.003 |
| Roshanravan et al. (2013)40 | 385, CKD 2–4; 61 years [13]; 84%; 41 [19] | 3 (median) | N/A | Objective: TUAG; HGS; 6MWD; gait speed | PF measures reliant on lower limb strength ↓30–39% compared
to normative values but grip strength relatively
preserved MR=47 deaths per 1000 person years 0.1 m/s ↓gait speed associated with 26% ↑risk of death 1 s longer TUAG associated with ~8% ↑MR |
Gait speed ⩽ 0.8 m/s: HR=2.45 [1.09–5.54$,‡,§,¶,|| (per 0.1 m/s slower HR=1.26) TUAG ⩾12 s: HR=1.81 [0.92–3.56]$,‡,§,¶,|| (HR=1.08 increases per 1 s slower) 6MWD <350 m: HR=2.82 [1.17–6.92]$,‡,§,¶,|| (HR=1.15 per 50 m reduction) |
6MWD, 6 min walk distance; ADL, activities of daily living; CES-D, Centre for Epidemiologic Centres Scale for Depression; CI, confidence interval; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate (ml/min/1.73 m²); HGS, hand grip strength; HR, hazard ratio; HRQoL, health-related quality of life; KDQoL, Kidney Disease Quality of Life; MDRD LTPAQ, Modification of Diet in Renal Disease Leisure Time Physical Activity Questionnaire; MET, metabolic equivalent task; MR, mortality rate; N/A, not applicable; PA, physical activity; PCS, physical composite score; PF, physical function; RTR, renal transplant recipient; SD, standard deviation; SF-36, 36-item Short Form survey; SPPB, Short Physical Performance Battery; TUAG, timed-up-and-go; IADL, instrumental activities of daily living; mGFR, measured glomerular filtration.
Unadjusted model; $adjusted for age; ‡adjusted for sex; §adjusted for body mass index; ¶adjusted for eGFR; ||adjusted for additional covariants (see reference for full analysis).
Table 2.
Summary of findings; association between physical activity level and all-cause mortality or adverse clinical outcomes.
| Study | Patient characteristics: N, CKD stage or RTR; mean age (years); % male; mean eGFR [SD] | Mean follow-up duration (years) | Comparison, control, or comparator | Outcome measures/intervention | Main findings | Mortality HR [95% CI] |
|---|---|---|---|---|---|---|
| Beddhu et al. (2009)28 | 15,368 in full study Non-CKD: Inactive: 48 years; 37%; 95.6 Insufficiently active: 42 years; 47%; 94.9 Recommended activity: 43 years; 54%; 92.9 CKD, n=907 (eGFR<60): Inactive: 73 years; 27% male; 46.9 Insufficiently active: 66 years; 40% male; 50.8 Recommended activity: 68 years; 43% male; 49.8 |
7 for CKD group (8.8 years for non-CKD group) |
Non-CKD population: divided into inactive, insufficiently active, recommended activity | Self-report: interviewer administered HAQ | CKD was associated with a ↑prevalence of low PA (odds ratio 1.30 [1.03–1.64]) | CKD Insufficiently active: HR=0.58 [0.42–0.79]$,‡,§,¶,|| Recommended activity: HR=0.44 [0.33–0.58]$,‡,§,¶,|| Non-CKD group Insufficiently active: HR=0.6 [0.45–0.81] $,‡,§,¶,|| Recommended activity: HR=0.59 [0.45–0.77]$,‡,§,¶,|| |
| Chen et al. (2008)30 | 811, CKD 3–4; 52 years, 61%; 32.5 | Not explicitly stated | Nil | Self-report: interviewer administered MDRD LTPAQ | No change in MR with PA category | Indoor activity: HR=0.94 [0.77–1.14]$,‡,¶,||
Exercise: HR=1.01 [0.84–1.10]$,‡,¶,|| Outdoor activity: HR=0.94 [0.80–1.10]$,‡,¶,|| |
| Chen et al. (2014)42 | 6363, CKD 3–5; 70.1 years; 57% | 10 | Self-report: exercise activity with 3-month recall; confirmed by family/caregiver | ↓MR in groups that walked regularly ↑frequency of walking correlated with ↓MR |
Walking: HR=0.65 [0.51–0.81], p<0.001 RRT risk: HR=0.75 [0.69–0.80], p<0.001 ↑duration of exercise: HR=0.77 [0.70–0.85], p<0.001 RRT risk: HR=0.89 [0.86–0.92], p<0.001 for each 30 min increase ↑frequency of exercise: HR=0.83 [0.78–0.90], p<0.001 RRT risk: HR=0.92 [0.90–0.94], p<0.001 for each category increase |
|
| Navaneethan et al. (2014)31 | 11,586 9433, non-CKD; 43.9 years [0.3]; 50.6%; 96.8 (0.4) 2153, CKD; 60.7 years [0.7]; 43.1% [1.0]; 72.9 [0.9] |
4.5 | Non-CKD | Self-report: interviewer-administered PA questionnaire | PA below recommended levels mortality: HR=1.36
[1.00–1.85]$,‡,§,¶,||
For each log unit ↑METs/week: HR=0.97 [0.95–1.00]$,‡,§,¶,|| PA <450 METs/week CKD: HR=1.34 [0.98–1.84]$,‡,§,¶,|| Non-CKD: HR=1.65 [1.19–2.28]$,‡,§,¶,|| PA <450 METs/week CKD: HR=1.36 [1–1.85]$,‡,§,¶,|| Non-CKD: HR=1.65 [1.21–2.26]$,‡,§,¶,|| |
|
| Ricardo et al. (2013)32 | 2288, CKD 1–4; 59 years; 40%; 78 | 13 | Self-report: interviewer-administered HAQ | Individuals in the highest eGFR strata were less likely to adhere to the recommended level of PA than those in the lowest eGFR strata (42% versus 34%) | Insufficient PA: HR=0.76 [0.6–0.96]$,‡ HR=0.86 [0.67–1.10]$,‡,¶,||, p = 0.22 Recommended PA: HR=0.73 [0.57–0.92] $,‡ HR=0.80 [0.65–0.99]$,‡,¶,||, p=0.04 |
|
| Ricardo et al. (2015)33 | 3006, CKD eGFR 20–70; 58 years [11]; 52%; 43 [14] | 4 (median) | N/A | Self-report: MESA | Less than ideal PA: HR=0.74 [0.57–0.96]$,‡,||
Ideal PA: HR 0.60 [0.49–0.74]$,‡,|| |
|
| Robinson-Cohen et al. (2014)35 | 256, CKD 3–4; 82%; 0 min/week, 61.8 [11.3] years; 37.8 [20.1] 1–60 min/week, 58.8 [12.8] years; 41.0 [18.6] 60–150 min/week, 61.7 [12] years; 37.4 [18.2] >150 min/week, 61.7 [12.5] years; 40.5 [14.0] |
3.7 (median) | Subdivided into groups based on min/week PA | Self-report: four-week Physical Activity History Questionnaire | ↓annual decline in eGFR (2.8%) in highest PA
categories Each ↑60 min PA associated with ~0.5%/year slower decline |
HR for incident ESRD Any PA: HR=0.59 [0.28–1.24]$,‡, p=0.19 Per 60 min/week increment: HR=0.9 [0.74–1.10]$,‡, p=0.32 |
| Rosas et al. (2012)36 | 507, RTR; 47.8 years [12.8]; 61% | 8.4 | Self-report: PA scales for the elderly | Inactive: MR 36.3% Moderate: MR 23.3% Active: MR 16.3% |
METs (per 10 unit change): HR=0.91 [0.87–0.96]*, p<0.001 HR=0.93 [0.88–0.97]$,‡, p=0.002 Moderate tertile: HR=0.81 [0.55–1.2]*, p=0.3 HR=0.91 [0.61–1.36] $,‡,||, p=0.7 Active tertile: HR=0.45 [0.29–0.72]*, p=0.001 HR=0.53 [0.33–0.84] $,‡,||, p=0.01 |
|
| Shlipak et al. (2005)37 | 6495; CKD group (eGFR<60) 1249; 75 years [6]; 47%; 50
[1.73] Non-CKD group 4559; 72 [5] years; 41%; 87 [20] |
8.6 | Non-CKD | Self-report: MLTPAQ | CV MR = 32 deaths per 1000 person years in CKD; 16 deaths per 1000 person years in non-CKD | CKD Low PA: HR=1.58 [1.25–2.01] $,‡,||, p<0.001 Non-CKD Low PA: HR=1.31 [1.10–1.57] $,‡,|| p=0.003 |
| Tikkanen-Dolenc et al. (2017) | 310 CKD, including RTR (n=64) and dialysis-dependent patients (n=36) (2639 in full study) | 11.4 | Self-report: Finnish version of MLTPAQ |
HR for CKD and RTR (excluding dialysis dependent)
LTPA (moderate/high LTPA used as
reference) Low: HR=1.99 [0.95–4.15]* Low: HR=2.12 [0.99–4.57]‡,|| Exercise intensity (moderate/high intensity used as reference) Low: HR=3.11 [1.31–7.38]* Low: HR=2.4 [0.99–5.81]‡,|| Exercise frequency (moderate–high frequency used as reference) Low: HR=2.85 [1.4–5.8]* Low: HR=2.6 [1.15–5.84]‡,|| Exercise duration (high duration used as reference) Low: HR=4.03 [1.8–9.01]* Low: HR=2.87 [1.21–6.84]‡,|| |
||
| Wang et al. (2013)27 | 445,075; 41.1 years [13.8]; 50% 42,757, CKD, no DM; 49.4 years [16.5]; 52.3%; 69.4 7863, CKD + DM; 59.3 years [11.8]; 54.9%; 66.2 |
Up to 12 | Healthy population; CKD; CKD + DM | Self-report: questionnaire (not specified) | MR per 100,000 person years: Healthy population: inactive: 362 [352–372] Low active: 314 [300–328] Fully active: 281.4 [269–295] CKD + DM Inactive: 1317.2 [1191–1456] Low active: 912.2 [744–1118] Fully active: 871 [745–1018] |
DM/CKD Low active: HR= 0.78 [0.65–0.92] $,‡ Fully active: HR=0.63 [0.55–0.73] $,‡ |
| Yango et al. (2006)38 | 402 RTR however data only presented for n=64 >60 years; 64 years [4]; 65% | 3 | Retrospective cohort study | Self-report: PA level assessed based on history obtained by the examining physician | 1-year survival rate: Overall 78% Active 94% Inactive 24% 3-year survival rate: Overall 71% Active 24% Inactive 24% |
None calculated |
| Zelle et al. (2011)45 | 540, RTR patients; 51 years [12]; 54% | 5.3 [4.7–5.7] | N/A | Self-report: interviewer-led Tecumseh Occupational Activity Questionnaire; MLTPAQ | HR=0.58 [0.4–0.70]*,
p<0.001 HR=0.67 [0.54–0.83]$,‡, p<0.001 |
|
| Robinson-Cohen et al. (2009)34 | 4011 PA score 2–3: 896; 72.8 years [5.4]; 30.7%; 75.1 [18.3] PA score 4–6: 2137; 72.0 years [5.1]; 40.3%; 78.9 [17.2] PA score 7–8: 896; 71.2 years [4.4]; 56.7%; 81.1 [16.2] |
7 (median) | Divided into categories based on PA score | Objective and self-report: gait speed used in combination with PA questionnaires to give cumulative PA score. | Lower risk of RDKF was found with increased PA
score$,‡,§,||
Same relationship could be seen when the results were stratified into groups using eGFR |
HR of developing RDKF$,‡,§,||
eGFR<60 PA score 4–6: HR=0.75 [1.45–1.27] PA score 7–8: HR=0.78 [1.4–1.51], p=0.44 eGFR 60–89 PA score 4–6: HR=0.88 [0.71–1.09] PA score 7–8: HR=0.63 [0.47–0.85], p=0.02 eGFR 90–119 PA score 4–6: HR=0.72 [0.56–0.92] PA score 7–8: HR=0.69 [0.51–0.94], p=0.04 |
| Beddhu et al. (2015)29 | 3626 in full study; 383, CKD | 2.86 [0.64] | Non-CKD population | Objective: accelerometry | ↑sedentary duration was associated with ↑mortality | Non-CKD: HR=1.18 [1.09–1.28]$,‡,||
CKD subgroup: HR=1.16 [1.04–1.13] $,‡,|| |
| Pechter et al. (2014)26 | 26, CKD; Intervention: 7; 52 years; 42%; 50.9
[9.2] Control group: 9; 48 years; 50%; 51.6 [7.1] |
10 | Sedentary control group who did not consent to exercise | Intervention: regular aquatic exercise for 10 years (>32 weeks a year, 30 min, 2× a week) | Active group: 0% MR; 0% commenced dialysis Control group: 55% MR; 22% commenced dialysis |
Not reported |
| Tsai et al. (2017)44 | 161, CKD 1–5; 67.2 years [7.8]; 54%; 34.5 [28.8] | 2.425 | CKD 1–3 versus CKD 4–5 comparison |
Objective:
HGS 30 s chair stand 2 min step Subjective: Taiwan version of the WHO QoL-BREF Interviewer administered HAQ |
COMBINED PA and PF
No relationship between PA and outcomes |
Risk of initiation of dialysis High HGS: HR=0.89 [0.84–0.96] High 2 min step: HR=0.304 [0.01–0.95] |
CKD, chronic kidney disease; CI, confidence interval; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HAQ, Household Adult Questionnaire; HGS, hand-grip strength; HR, hazard ratio; MDRD LTPAQ, Modification of Diet in Renal Disease Leisure Time Physical Activity Questionnaire; MESA, Multi-Ethnic Study of Atherosclerosis Typical Week Physical Activity Survey; MET, Metabolic Equivalent of Task; MLTPAQ, Minnesota Leisure Time Physical Activity Questionnaire; MR, mortality rate; N/A, not applicable; PA, physical activity; PF, physical function; RDKF, rapid decline in kidney function; RRT risk, risk of requiring renal replacement therapy; RTR, renal transplant recipient; SD, standard deviation.
Unadjusted model; $adjusted for age; ‡adjusted for sex; §adjusted for body mass index; ¶adjusted for eGFR; ||adjusted for plus additional covariants (see reference for full analysis). ESRD, end-stage renal disease; WHO QoL-BREF, World Health Organization Quality of Life BREF; LTPA, leisure time physical activity.
Outcomes reported
The majority of the papers studied mortality, either as all-cause24–33,36,38–43,45–52 or cardiovascular mortality.37,45 Other outcomes reported included prevalence of frailty,39 sarcopenia,50 protein-energy wastage,43 major adverse cardiovascular event,44 first hospitalization,44 rate of decline of renal function,34,35 or risk of requiring dialysis.26,42-44 One study reported an odds ratio of developing diabetic nephropathy.27
Overall, the results showed that poorer physical function and lower PA were associated with increased mortality rates, however differing methodologies preclude meta-analysis. HRs were reported in some studies (summarized in Table 1) varying from 1.0452 to 5.7,24 dependent on measurement type and population studied.
Only 4 papers36,38,45,51 reviewed the importance of being active with a renal transplant and 441,48,49,52 of the 10 papers investigating physical function studied RTRs. Outcomes studied were all-cause mortality,36,38,41,45,48,49,51,52 cardiovascular mortality,45 graft failure,38,45,48,49 and death with a functioning transplant.36,49 Higher PA levels both prior to transplantation36 and post transplant38,45 were associated with lower mortality rates. Similarly lower physical function levels were associated with increased mortality HRs.48,49,52
Objective physical function
Six papers used objective measures of physical function, including the Short Physical Performance Battery (SPPB),24,41 HGS,43,44,50 using a Bruce protocol treadmill test to determine cardiorespiratory fitness,25 the ‘timed-up-and-go’ (TUAG),40 the 6 min walk test (6MWT),40 30 s chair stands,44 2 min step44 and gait speed.40 The Short Physical Performance Battery (SPPB), TUAG, the 6MWT, and gait speed were independently associated with increased all-cause mortality.24,40,41 Greater scores in the 2 min step were correlated with a reduced risk of commencing dialysis.44 The number of chair stands achieved in 30 s was shown to correlate with reduced risk of a major adverse cardiovascular event and with all-cause hospitalization.44 Since both TUAG and the SPPB include measures of a person’s gait speed and ability to stand from a chair, it may be inferred that a measure of physical function utilizing walking and standing provides a useful measure of physical function in CKD when outcomes are to be studied.
HGS was measured in three studies,40,43,50 with inconsistent results. Pereira and colleagues50 measured HGS as a marker of sarcopenia which was demonstrated to correlate with mortality risk; Roshanravan and colleagues40 found HGS was relatively preserved compared with lower limb strength, as 6MWT, gait speed, and TUAG had greater area under the Receiver Operating Characteristic (ROC) values than HGS. However, Chang and colleagues43 found that HGS was an independent outcome predictor in CKD.
Cardiorespiratory fitness was found to modify the association between eGFR and mortality.25 A maximum cardiorespiratory fitness level of less than 5 METs (metabolic equivalent of task ~17.5 ml/kg/min) combined with an eGFR of under 45 ml/min/1.73 m² was associated with increased mortality rates compared with those with better fitness and higher eGFR.25
Objective physical activity
Only one study29 used an objective PA measure (i.e. accelerometry), whilst another34 combined gait speed with a questionnaire to give a cumulative PA score.
Subjective physical function
Self-report measures of physical function were used by six papers.39,46–49,52 The 36-item Short Form survey ‘SF-36’ was used in three of these,48,49,52 and a significant relationship between the ‘Physical Component Score’ (PCS) and outcomes was consistently demonstrated. The other subjective methods used included ‘Modification of Diet in Renal Disease (MDRD) Quality of Wellbeing measure’,39 ‘Korean version of ADL’s’,46 ‘Instrumental ADL’,46 ‘Health Related Quality of Life (HRQoL)’48 and ‘Kidney Disease Quality of Life measure (KDQoL)’,49 which included ‘HRQoL’, ‘SF-36’ and ‘Center for Epidemiologic Studies Depression Scale’ (CES-D). Similar trends were seen between poorer outcomes and lower physical function.
Subjective physical activity
Thirteen studies which explored PA associations used questionnaires, including ‘Household Adult’ questionnaire28,32 (a translated version was used by Tsai and colleagues44), ‘Leisure Time Physical Activity Questionnaire’,31 ‘Modified Diet in Renal Disease Leisure Time Physical Activity Questionnaire’ (MDRD LTPAQ),30 ‘Four-Week Physical Activity History Questionnaire (FWH)’,35 ‘Multi-Ethnic Study of Atherosclerosis (MESA) Typical Week Physical Activity Survey’,33 ‘Physical Activity Scales for the Elderly’ (PASE),36 ‘Minnesota Leisure Time PA’ questionnaire37,45 (a translated version was used by Tikkanen-Dolenc and colleagues51), and ‘Tecumseh Occupational Activity Questionnaire’.45 One study27 failed to report which method was used and two used clinician judgement to classify PA.38, 42 Questionnaires were frequently used in conjunction with a compendium of activities to give MET score for further analysis.27,28,31–33,36,44,45,51
The most common PA reported was walking, with data showing that increasing walking duration and intensity correlates with favourable health benefits. The dose–response relationship remains unclear. Navaneethan and colleagues31 and Ricardo and colleagues33 demonstrated reduced mortality risk only when guideline PA levels were achieved (i.e. >150 min/week moderately vigorous PA) whilst Beddhu and colleagues29 found replacing sedentary time with light activity resulted in a lower mortality risk but upgrading to moderate or vigorous PA did not reduce the risk further. Robinson-Cohen and colleagues35 found the risk of developing end-stage renal disease decreased with every 60 min/week increase in PA, with the largest reduction when more than 150 min was achieved. Similarly, Tikkanen-Dolenc and colleagues51 stratified HRs according to intensity, duration, and frequency of PA, and demonstrated increased HRs when each of these differed from the guideline amounts, with the greatest increase in risk when target duration of PA was not achieved. In contrast, Tsai and colleagues44 found no change in HRs with PA levels as measured by questionnaires, but found that various functional measures were significant. Whilst these studies demonstrate interesting, although conflicting, conclusions, despite large sample sizes, the p values reported are often not significant35 or not specified.29,31,33
Risk of bias
Each study was evaluated for quality and risk of bias using the Newcastle-Ottawa Scale and Agency for Healthcare Research and Quality (AHRQ) standards. These results are summarized in Table 3. Overall the quality of these papers was mixed, with 20 classed as ‘good’, 7 as ‘fair’, and 2 determined to be ‘poor’ quality. Sources of potential bias identified included not fully describing the participant flow through the study and the use of self-report measures.
Table 3.
Papers reviewed, Newcastle-Ottawa Scale score, and bias criteria.
| Study | Selection /4 | Comparability /2 | Outcome /3 | AHRQ criteria | |
|---|---|---|---|---|---|
| 1 | Beddhu et al. 200928 | *** | ** | ** | Good |
| 2 | Beddhu et al. 201529 | *** | ** | *** | Good |
| 3 | Chang et al. 201143 | *** | ** | *** | Good |
| 4 | Chen et al. 200830 | ** | ** | ** | Fair |
| 5 | Chen et al. 201442 | ** | ** | *** | Fair |
| 6 | Chin et al. 201446 | ** | ** | * | Poor |
| 7 | Delgado et al. 201539 | ** | ** | *** | Fair |
| 8 | Doyle et al. 201547 | *** | ** | ** | Good |
| 9 | Griva et al. 201348 | *** | ** | *** | Good |
| 10 | Gulati et al. 201225 | *** | ** | *** | Good |
| 11 | Lattanzio et al. 201524 | *** | ** | *** | Good |
| 12 | Molnar-Varga et al. 201149 | ** | ** | ** | Fair |
| 13 | Nastasi et al. 2017 | *** | ** | ** | Good |
| 14 | Navaneethan et al. 201431 | **** | ** | *** | Good |
| 15 | Pechter et al. 201426 | **** | - | * | Poor |
| 16 | Pereira et al. 201550 | *** | ** | *** | Good |
| 17 | Prihodova et al. 201452 | *** | ** | ** | Good |
| 18 | Ricardo et al. 201332 | ** | ** | ** | Fair |
| 19 | Ricardo et al. 201533 | ** | ** | *** | Fair |
| 20 | Robinson-Cohen et al. 200934 | *** | ** | *** | Good |
| 21 | Robinson-Cohen et al. 201435 | *** | ** | *** | Good |
| 22 | Rosas et al. 201236 | *** | ** | *** | Good |
| 23 | Rosharavan et al. 201340 | **** | ** | ** | Good |
| 24 | Shlipak et al. 200537 | *** | ** | ** | Good |
| 25 | Tikkanen-Dolenc et al. 2017 | *** | ** | ** | Good |
| 26 | Tsai et al. 201744 | *** | ** | *** | Good |
| 27 | Wang et al. 201327 | *** | ** | *** | Fair |
| 28 | Yango et al. 200638 | *** | ** | *** | Good |
| 29 | Zelle et al. 201145 | *** | ** | ** | Good |
AHRQ, Agency for Healthcare and Research Quality.
Discussion
Summary of review findings
Overall, our review has shown that, in patients with nondialysis CKD, reduced physical function and PA levels are associated with increased mortality and adverse clinical events, including decline in renal function, increased risk of requiring renal replacement therapy, and poor renal graft survival (RTRs only). Similar observations have been observed in dialysis patients16 and in other chronic populations.6,7 This has important clinical implications, potentially providing an opportunity to improve outcomes. The concepts of PA and physical function have significant overlap and although engagement in functional tasks can be considered a category of PA, function can also be considered an antecedent of activity. The two concepts are also frequently intertwined in the literature, which necessitated the consideration of the two ideas in the same review.
Bias was assessed in this review using the NOS. Interestingly, the papers studying disease progression were scored negatively by the NOS as the outcome (i.e. CKD) was present at the start of the trial and this represents a weakness for this scoring system. HRs were reported in many, but not all, papers; however some studies reported mortality risk whilst others reported survival analysis, making the data unsuitable for meta-analysis. Studies which yielded HRs were calculated both as unadjusted models and adjusted for confounding variables, such as age, body mass index (BMI), sex, depression, and kidney function levels; however sensitivity analysis to confirm these findings was poorly reported.
It is important to state the difficulty deducing causality from the data presented, as patients with greater illness burden are often less active and have a reduced functional level. Further longitudinal studies are needed whereby interventions increase PA or physical function to assess resultant changes in outcomes. Further research is needed into the potential dose–response of PA, and whilst it appears that being active on most days, in line with the current PA recommendations, is beneficial, even low levels of PA may confer some benefit in renal patients. It is also important to consider that there is a physical function minima, below which PA is impossible. While, in principle, encouraging patients to be more active may be a straightforward suggestion, the complexity of successful behaviour change interventions should not be underestimated.
The data were not appropriate for meta-analysis due to the variance in the measurement outcomes and the analysis methods used. This demonstrates the need to identify accepted norm assessments of physical function and PA to use in the renal community to allow comparison between interventions. The paucity of research in the transplant population is also demonstrated in regard to both PA and physical function.
Physical function and outcome
Reduced physical function was found to correlate with frailty, sarcopenia, and protein-energy wastage which, in turn, are associated with mortality. Despite the potential confounders introduced by investigating these wider concepts, the value of maintaining functional ability and activity levels remained clear. Only one paper49 assessed depression as a covariant when exploring the relationship between physical function and mortality. Once the HR analysis was adjusted, the significance of the model dropped. Due to the frequent concomitance of depression and functional loss, further investigations are required to determine whether this is a trend as yet uncharted, or a coincident pattern.
Doyle and colleagues47 assessed physical function using the Barthel score, where ability to engage in activities of daily living is assigned an ordinal score, and demonstrating a higher score on hospital discharge was associated with a lower risk of all-cause mortality. Whilst this score is frequently used by clinicians as an objective measure, it is unclear in this paper whether it was used objectively or as a self-report tool. Ricardo and colleagues32 calculated a ‘healthy lifestyle score’, based on BMI, PA levels, dietary intake, and smoking behaviour. Their results demonstrated a positive relationship between a ‘healthy’ lifestyle and mortality rates, but it is difficult to isolate the effect of PA.
Objective tests were more commonly used to measure physical function. A gait speed reduction of 0.1 m/s was associated with a 26% increased mortality risk, whilst a 1 s longer TUAG score correlated with an 8% increased risk of death.40 Thus these objective tests could be useful prognostic tools in CKD, and may provide interventional targets yielding direct patient benefit. HGS measurement generated inconsistent results and hence requires further investigation before recommendations can be made about its use as an outcome measure in nondialysis CKD.
Physical activity and outcome
Interestingly, Pechter and colleagues26 found 100% survival in patients who maintained engagement in a 10-year hydrotherapy programme compared with 55% in the control group (no exercise) who either died or required renal replacement therapy. However, it may be argued that only the patient group with a low comorbidity burden are able to engage continuously in this type of intervention, which may confound these results. It must be considered that financing such supervised exercise for the entire CKD population is untenable under modern health systems. Conversely, Chen and colleagues30 reported no change in mortality risk with higher PA levels, although the authors acknowledge the data’s wide confidence intervals. Also, the sample studied was generally more active than a general CKD cohort, with 50% walking or exercising regularly.
Measurement of PA should be conducted using objective accelerometry if possible, however only one paper utilized this outcome measure. This diversity of PA measures also means that cutoffs determining ‘activity’ or ‘inactivity’ vary widely, and as such, different constructs are being compared. This also limits exploration of dose–response effects and potential benefits.
Outcome measure use
A key finding from this review was the large breadth of measures used to assess both physical function and PA. Both objective and subjective measures were used, and whilst each confer their own strength and limitations, the heterogeneity makes it difficult to compare effects and prevents meta-analysis. In many instances, questionnaire-based assessments were used, particularly in the measurement of PA level. This has substantial limitations in regard to recall bias and desirable responses, and for some of these questionnaires validity in the renal population remains undemonstrated. Some questionnaires were administered by interviewers,30–32,37,45 which may have increased completion rates and corrected one of the common criticisms of questionnaire use. In Yango and colleagues,38 retrospective clinician judgement on patient PA level was used, and such subjectivity means minimal conclusions can be drawn from this trial. Methodological flaws were also demonstrated by Chen and colleagues42 who asked participants and their caregivers to recall a 3-month history of PA. The ‘Minnesota Leisure Time Physical Activity’ questionnaire, used by three studies,37,45,51 has been criticized as it requires a full year’s recall which has been previously demonstrated to be limited by recall bias.53 We propose future researchers should use commonly reported and validated measures to aid synthesis of data between clinical trials. The SF-36 was used by three papers48,49,52 and a one-point increase in the PCS correlated with a decrease in mortality risk in RTRs of between 1.8%49 and 4%,52and hence this subjective outcome measure is recommended for further use.
Despite a consensus among nephrologists that PA is important for patients, assessment of physical function or PA advice is not a part of the routine management of CKD. Efforts to improve both physical function and PA by intervention should be actively encouraged in this group. In regard to physical function, it appears simple objective tests, such as the TUAG and gait speed (but perhaps not HGS), and self-reported measures, in particular the SF-36 (PCS), are useful prognostic tools in CKD. As such, research or clinical practice should use these physical function tests when assessing intervention effects. Complex and ‘laboratory’-based measures, such as those measuring VO2 or using an accelerometer or isokinetic dynamometer, provide high-quality and reliable data, however these assessments are often impractical in a clinical setting and poorly tolerated by patients. More pragmatic measures of physical function and physical activity, such as the TUAG, gait speed, or via self-report, can be quickly and cheaply conducted in a clinic waiting room and hence provide a real-world method of assessing the patient’s functional status which correlates with morbidity and mortality. When assessing either physical functioning or activity, a researcher or healthcare professional should be aware of the relative strengths and limitations of each assessment.
Conclusion
This is the first systematic review elucidating the relationship between physical function and PA with clinical outcomes in the underexplored area of nondialysis CKD. Better physical function and greater PA levels both correlate with improved outcomes, including both reduced all-cause and cardiovascular mortality risk, reduced risk of rapid decline in renal function, reduced prevalence of frailty and sarcopenia, and graft survival in transplant recipients. However, causality as yet remains unproved and further research is needed.
Clinical messages
Reduced physical function and PA levels are associated with increased mortality risk and increased risk of adverse clinical outcomes in both nondialysis CKD and in RTRs. Further work is needed to investigate causality within this relationship. Consistent use of outcome assessments is critical to allow meta-analysis.
Supplemental Material
Supplemental material, Supplementary_material_1_-_Example_PubMed_search_strat for The association of physical function and physical activity with all-cause mortality and adverse clinical outcomes in nondialysis chronic kidney disease: a systematic review by Heather J. MacKinnon, Thomas J. Wilkinson, Amy L. Clarke, Douglas W. Gould, Thomas F. O’Sullivan, Soteris Xenophontos, Emma L. Watson, Sally J. Singh and Alice C. Smith in Therapeutic Advances in Chronic Disease
Acknowledgments
Initial design: ACS, TJW, HJM; conducting searches: TJW, HJM; article retrieval: TJW, HJM; article review: HJM, TJW, ALC, DWG, ELW, TFO, SX, ACS; drafting manuscript: HJM, TJW; editing manuscript: HJM, TJW, ALC, DWG, ELW, TFO, ACS; mentoring: SJS, ACS. The views expressed are those of the authors and not necessarily those of the NHS, the National Institute for Health Research Leicester BRC, CLAHRC EM or the Department of Health and Social Care
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This report is independent research supported by the National Institute for Health Research Leicester Biomedical Research Centre and the NIHR Collaboration for Leadership in Applied Health Research and Care East Midlands (CLAHRC EM). We also gratefully acknowledge additional funding from The Stoneygate Trust.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Heather J MacKinnon  https://orcid.org/0000-0002-3530-0471
https://orcid.org/0000-0002-3530-0471
Supplementary material: Supplementary material for this article is available online.
Contributor Information
Heather J. MacKinnon, Leicester Kidney Lifestyle Team, Department of Infection, Immunity and Inflammation, University of Leicester, Leicester, UK John Walls Renal Unit, Leicester General Hospital, University Hospitals of Leicester NHS Trust, Leicester, UK.
Thomas J. Wilkinson, Leicester Kidney Lifestyle Team, Department of Infection, Immunity and Inflammation, University of Leicester, LE1 7RH Leicester, UK.
Amy L. Clarke, Leicester Kidney Lifestyle Team, Department of Infection, Immunity and Inflammation, University of Leicester, Leicester, UK John Walls Renal Unit, Leicester General Hospital, University Hospitals of Leicester NHS Trust, Leicester, UK.
Douglas W. Gould, Leicester Kidney Lifestyle Team, Department of Infection, Immunity and Inflammation, University of Leicester, Leicester, UK John Walls Renal Unit, Leicester General Hospital, University Hospitals of Leicester NHS Trust, Leicester, UK.
Thomas F. O’Sullivan, Leicester Kidney Lifestyle Team, Department of Infection, Immunity and Inflammation, University of Leicester, Leicester, UK John Walls Renal Unit, Leicester General Hospital, University Hospitals of Leicester NHS Trust, Leicester, UK.
Soteris Xenophontos, Leicester Kidney Lifestyle Team, Department of Infection, Immunity and Inflammation, University of Leicester, Leicester, UK; John Walls Renal Unit, Leicester General Hospital, University Hospitals of Leicester NHS Trust, Leicester, UK.
Emma L. Watson, Leicester Kidney Lifestyle Team, Department of Infection, Immunity and Inflammation, University of Leicester, Leicester, UK John Walls Renal Unit, Leicester General Hospital, University Hospitals of Leicester NHS Trust, Leicester, UK.
Sally J. Singh, School of Sport, Exercise and Health Sciences, National Centre for Sport and Exercise Medicine, Loughborough University, Loughborough, UK Centre for Exercise and Rehabilitation Services, University Hospitals of Leicester NHS Trust, Leicester, UK.
Alice C. Smith, Leicester Kidney Lifestyle Team, Department of Infection, Immunity and Inflammation, University of Leicester, Leicester, UK John Walls Renal Unit, Leicester General Hospital, University Hospitals of Leicester NHS Trust, Leicester, UK.
References
- 1. MacNeill SJ, Ford D. UK Renal Registry 19th Annual Report: Chapter 2 UK Renal Replacement Therapy Prevalence in 2015: National and Centre-specific Analyses. Nephron 2017; 137(Suppl. 1):45–71. [DOI] [PubMed] [Google Scholar]
- 2. Padilla J, Krasnoff J, DaSilva M, et al. Physical Functioning in patients with chronic kidney disease. J Nephrol 2008; 21: 550–559. [PubMed] [Google Scholar]
- 3. Heiwe S, Tollback A, Clyne N. Twelve weeks of exercise training increases muscle function and walking capacity in elderly predialysis patients and healthy subjects. Nephron 2001; 88: 48–56. [DOI] [PubMed] [Google Scholar]
- 4. Kosmadakis GC, John S, Clapp EL, et al. Benefits of regular walking exercise in advance pre-dialysis chronic kidney disease. Nephrol Dial Transplant 2012; 27: 997–1004. [DOI] [PubMed] [Google Scholar]
- 5. Morie M, Reid KF, Miciek R, et al. Habitual physical activity levels are associated with performance in measures of physical function and mobility in older men. J Am Geriat Soc 2010; 58: 1727–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Glenn KR, Slaughter JC, Fowke JH, et al. Physical activity, sedentary behavior and all-cause mortality among blacks and whites with diabetes. Ann Epidemiol 2015; 25: 649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martinez-Gomez D, Guallar-Castillon P, Mota J, et al. Physical activity, sitting time and mortality in older adults with diabetes. Int J Sports Med 2015; 36: 1206–1211. [DOI] [PubMed] [Google Scholar]
- 8. Wu C-Y, Hu H-Y, Chou Y-C, et al. The association of physical activity with all-cause, cardiovascular, and cancer mortalities among older adults. Prev Med 2015; 72: 23–29. [DOI] [PubMed] [Google Scholar]
- 9. Kokkinos P. Physical activity, health benefits, and mortality risk. ISRN Cardiol 2012; 2012: 718789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Knight EL, Ofsthun N, Teng M, et al. The association between mental health, physical function, and hemodialysis mortality. Kidney Int 2003; 63: 1843–1851. [DOI] [PubMed] [Google Scholar]
- 11. DeOreo PB. Hemodialysis patient-assessed functional health status predicts continued survival, hospitalization, and dialysis-attendance compliance. Am J Kidney Dis 1997; 30: 204–212. [DOI] [PubMed] [Google Scholar]
- 12. de Oliveira MP, Kusumota L, Haas VJ, et al. Health-related quality of life as a predictor of mortality in patients on peritoneal dialysis. Rev Lat Am Enfermagem 2016; 24: e2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Painter P, Roshanravan B. The association of physical activity and physical function with clinical outcomes in adults with chronic kidney disease. Curr Opin Nephrol Hypertens 2013; 22: 615–623. [DOI] [PubMed] [Google Scholar]
- 14. Kutner NG, Zhang R, Huang Y, et al. Gait speed and mortality, hospitalization, and functional status change among hemodialysis patients: a US renal data system special study. Am J Kidney Dis 2015; 66: 297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johansen KL, Dalrymple LS, Glidden D, et al. Association of performance-based and self-reported function-based definitions of frailty with mortality among patients receiving hemodialysis. Clin J Am Soc Nephrol 2016; 11: 626–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stack AG, Molony DA, Rives T, et al. Association of physical activity with mortality in the US dialysis population. Am J Kidney Dis 2005; 45: 690–701. [DOI] [PubMed] [Google Scholar]
- 17. Greenwood SA, Lindup H, Taylor K, et al. Evaluation of a pragmatic exercise rehabilitation programme in chronic kidney disease. Nephrol Dial Transplant 2012; 27(Suppl. 3): iii126–iii34. [DOI] [PubMed] [Google Scholar]
- 18. Dontje ML, de Greef MHG, Krijnen WP, et al. Longitudinal measurement of physical activity following kidney transplantation. Clin Trans 2014; 28: 394–402. [DOI] [PubMed] [Google Scholar]
- 19. Aakhus S, Dahl K, Widerøe TE. Cardiovascular disease in stable renal transplant patients in Norway: morbidity and mortality during a 5-yr follow-up. Clin Trans 2004; 18: 596–604. [DOI] [PubMed] [Google Scholar]
- 20. Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep 1985; 100: 126–131. [PMC free article] [PubMed] [Google Scholar]
- 21. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wells GS, O’Connell B, Peterson D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses 2011, http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 18 December 2017).
- 23. Higgins JG. (ed.) Cochrane handbook for systematic reviews of interventions. The Cochrane Collaboration, Wiley, 2011; 391–431. [Google Scholar]
- 24. Lattanzio F, Corsonello A, Montesanto A, et al. Disentangling the impact of chronic kidney disease, anemia, and mobility limitation on mortality in older patients discharged from hospital. J Gerontol A Biol Sci Med Sci 2015; 70: 1120–1127. [DOI] [PubMed] [Google Scholar]
- 25. Gulati M, Black HR, Arnsdorf MF, et al. Kidney dysfunction, cardiorespiratory fitness, and the risk of death in women. J Womens Health 2012; 21: 917–924. [DOI] [PubMed] [Google Scholar]
- 26. Pechter U, Raag M, Ots-Rosenberg M. Regular aquatic exercise for chronic kidney disease patients: a 10-year follow-up study. Int J Rehabil Res 2014; 37: 251–255. [DOI] [PubMed] [Google Scholar]
- 27. Wang IK, Tsai MK, Liang CC, et al. The role of physical activity in chronic kidney disease in the presence of diabetes mellitus: a prospective cohort study. Am J Nephrol 2013; 38: 509–516. [DOI] [PubMed] [Google Scholar]
- 28. Beddhu S, Baird BC, Zitterkoph J, et al. Physical activity and mortality in chronic kidney disease (NHANES III). Clin J Am Soc Nephrol 2009; 4: 1901–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Beddhu S, Wei G, Marcus RL, et al. Light-intensity physical activities and mortality in the United States general population and CKD subpopulation. Clin J Am Soc Nephrol 2015; 10: 1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen JL, Lerner D, Ruthazer R, et al. Association of physical activity with mortality in chronic kidney disease. J Nephrol 2008; 21: 243–252. [PubMed] [Google Scholar]
- 31. Navaneethan SD, Kirwan JP, Arrigain S, et al. Adiposity measures, lean body mass, physical activity and mortality: NHANES 1999–2004. BMC Nephrol 2014; 15: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ricardo AC, Madero M, Yang W, et al. Adherence to a healthy lifestyle and all-cause mortality in CKD. Clin J Am Soc Nephrol 2013; 8: 602–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ricardo AC, Anderson CA, Yang W, et al. Healthy lifestyle and risk of kidney disease progression, atherosclerotic events, and death in CKD: findings from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis 2015; 65: 412–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Robinson-Cohen C, Katz R, Mozaffarian D, et al. Physical activity and rapid decline in kidney function among older adults. Arch Intern Med 2009; 169: 2116–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Robinson-Cohen C, Littman AJ, Duncan GE, et al. Physical activity and change in estimated GFR among persons with CKD. J Am Soc Nephrol 2014; 25: 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rosas SE, Reese PP, Huan Y, et al. Pretransplant physical activity predicts all-cause mortality in kidney transplant recipients. Am J Nephrol 2012; 35: 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shlipak MG, Fried LF, Cushman M, et al. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA 2005; 293: 1737–1745. [DOI] [PubMed] [Google Scholar]
- 38. Yango AF, Gohh RY, Monaco AP, et al. Excess risk of renal allograft loss and early mortality among elderly recipients is associated with poor exercise capacity. Clin Nephrol 2006; 65: 401–407. [DOI] [PubMed] [Google Scholar]
- 39. Delgado C, Grimes BA, Glidden DV, et al. Association of Frailty based on self-reported physical function with directly measured kidney function and mortality. BMC Nephrol 2015; 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Roshanravan B, Robinson-Cohen C, Patel KV, et al. Association between physical performance and all-cause mortality in CKD. J Am Soc Nephrol 2013; 24: 822–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nastasi AJ, McAdams-DeMarco MA, Schrack J, et al. Pre-kidney transplant lower extremity impairment and post-kidney transplant mortality. Am J Transplant 2018; 18: 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen IR, Wang S-M, Liang C-C, et al. Association of walking with survival and RRT among patients with CKD stages 3–5. Clin J Am Soc Nephrol 2014; 9: 1183–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chang YT, Wu HL, Guo HR, et al. Handgrip strength is an independent predictor of renal outcomes in patients with chronic kidney diseases. Nephrol Dial Transplant 2011; 26: 3588–3595. [DOI] [PubMed] [Google Scholar]
- 44. Tsai YC, Chen HM, Hsiao SM, et al. Association of physical activity with cardiovascular and renal outcomes and quality of life in chronic kidney disease. PLoS One. 2017; 12: e0183642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zelle DM, Corpeleijn E, Stolk RP, et al. Low physical activity and risk of cardiovascular and all-cause mortality in renal transplant recipients. Clin J Am Soc Nephrol 2011; 6: 898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chin HJ, Ahn SY, Ryu J, et al. Renal function and decline in functional capacity in older adults. Age Ageing 2014; 43: 833–838. [DOI] [PubMed] [Google Scholar]
- 47. Doyle EM, Sloan JM, Goodbrand JA, et al. Association between kidney function, rehabilitation outcome, and survival in older patients discharged from inpatient rehabilitation. Am J Kidney Dis 2015; 66: 768–774. [DOI] [PubMed] [Google Scholar]
- 48. Griva K, Davenport A, Newman SP. Health-related quality of life and long-term survival and graft failure in kidney transplantation: a 12-year follow-up study. Transplantation 2013; 95: 740–749. [DOI] [PubMed] [Google Scholar]
- 49. Molnar-Varga M, Molnar MZ, Szeifert L, et al. Health-related quality of life and clinical outcomes in kidney transplant recipients. Am J Kidney Dis 2011; 58: 444–452. [DOI] [PubMed] [Google Scholar]
- 50. Pereira RA, Cordeiro AC, Avesani CM, et al. Sarcopenia in chronic kidney disease on conservative therapy: prevalence and association with mortality. Nephrol Dial Transplant 2015; 30: 1718–1725. [DOI] [PubMed] [Google Scholar]
- 51. Tikkanen-Dolenc H, Waden J, Forsblom C, et al. Physical activity reduces risk of premature mortality in patients with type 1 diabetes with and without kidney disease. Diabetes Care 2017; 40: 1727–1732. [DOI] [PubMed] [Google Scholar]
- 52. Prihodova L, Nagyova I, Rosenberger J, et al. Health-related quality of life 3 months after kidney transplantation as a predictor of survival over 10 years: a longitudinal study. Transplantation 2014; 97: 1139–1145. [DOI] [PubMed] [Google Scholar]
- 53. Richardson MT, Leon AS, Jacobs DR, et al. Comprehensive evaluation of the Minnesota leisure time physical activity questionnaire. J Clin Epidemiol 1994; 47: 271–281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_material_1_-_Example_PubMed_search_strat for The association of physical function and physical activity with all-cause mortality and adverse clinical outcomes in nondialysis chronic kidney disease: a systematic review by Heather J. MacKinnon, Thomas J. Wilkinson, Amy L. Clarke, Douglas W. Gould, Thomas F. O’Sullivan, Soteris Xenophontos, Emma L. Watson, Sally J. Singh and Alice C. Smith in Therapeutic Advances in Chronic Disease