Abstract
OBJECTIVE:
The objective of this study was to compare the efficacy and safety of radiofrequency ablation (RFA), cryoablation, and microwave ablation (MWA) for patients with lung malignancies.
METHODS:
We performed a network meta-analysis to identify both direct and indirect evidence from relevant trials by searching PubMed, Embase, and the Cochrane Library to December 31, 2017, for the treatment of malignant lung tumors with the use of RFA, MWA, or cryoablation. We extracted the relevant information from the published studies with a predefined data sheet and assessed the risk of bias with the Cochrane risk of bias tool. The primary outcomes were efficacy (local progression rate and overall survival rate) and safety (major complications rate). We did a random-effects network meta-analysis within a Bayesian framework as well as assessed the quality of evidence contributing to each network estimate using GRADE framework.
RESULTS:
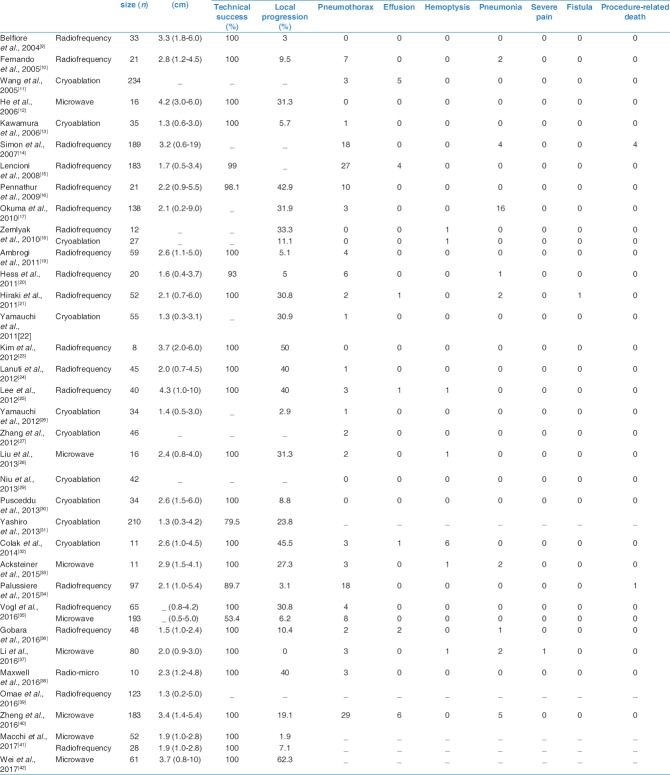
We collected 34 studies eligible which included 1840 participants and 2520 lung malignancies (1318 primary lung cancer and 1202 pulmonary metastatic tumors). The quality of evidence was rated as very low in most comparisons. From the point of local progression rate, RFA and MWA were significantly more effective than cryoablation with odds ratio (OR) of 0.04 (95% confidence interval [CI]: 0.004, 0.38; P = 0.005) and 0.02 (95% CI: 0.002, 0.24; P = 0.001), respectively. No significant difference was found between MWA and RFA with an OR of 0.63 (95% CI: 0.04, 10.39; P = 0.745). Regarding the major complications, RFA, MWA, and cryoablation showed the comparable safety (P > 0.05).
CONCLUSION:
RFA and MWA offer an advantage over cryoablation for patients with malignant lung tumors.
Keywords: Cryoablation, lung tumors, microwave ablation, radiofrequency ablation
Primary lung cancers are the most common malignancies and the leading cause of cancer-related death worldwide. In addition, the lung is also the second-most common site of metastasis.[1] Surgical resection is universally accepted as the first-line therapy in an early-stage non-small cell lung cancer (NSCLC) and selected metastatic lung tumors. However, surgery is not suitable for most patients due to strict surgical criteria. Although chemotherapy, radiation therapy, and a combination of these modalities are alternative treatments for such patients, complete tumor remission is rarely achieved.[2]
During recent decades, thermal ablation has increasingly been performed on solid tumors of the liver, kidney, mammary, adrenal glands, and also for lung tumors.[3] Image-guided thermal ablation offers clinicians and patients a repeatable, effective, low-cost, and safe treatment for effective palliation and in some cases, cure of both primary and metastatic thoracic malignancies. The principal modalities in clinical practice are radiofrequency ablation (RFA), cryoablation, and microwave ablation (MWA). RFA, currently the most widely used ablative technique, uses a high-frequency current to heat and coagulate tissues.[4] Cryoablation uses extreme cold to cause tissue destruction through a complex combination of cellular damage during a freezing and thawing cycles.[5] MWA is a relatively new form of ablation treatment for lung tumors by increasing polar water molecules kinetic energy and converting this energy into heat which increases tissue temperatures to cytotoxic levels.[6]
It is clear that patients who have lung malignancies with limited treatment options are benefiting from image-guided ablation therapy.[7] However, the exact subset of patients who will benefit the most from such procedures and with ablative technology remains unknown. The purpose of this current study is to systematically evaluate and compare the efficacy (local tumor control or progression and survival rates) and safety (major complications rates) of radiofrequency, cryoablation, and MWA for patients with lung malignancies.
Methods
For this network meta-analysis, we searched PubMed, Embase, and the Cochrane Library through December 31, 2017, using the following medical terms are: (“radiofrequency” OR “cryoablation” OR “microwave” OR “thermal ablation”) AND “lung”. Reference lists of obtained articles were searched as well.
Inclusion and exclusion criteria
We included studies as follows: patients with primary lung cancer or pulmonary metastases from other primary tumors; thermal ablation (radiofrequency, microwave, or cryoablation) was used to treat these patients; and studies reported outcomes of patients after thermal therapy which included local control or progression rates, survival rates, and/or major complications (medical intervention required for pneumothorax, effusion, hemoptysis, pneumonia, severe pain and bronchopleural fistula, and procedure-related death). Studies that used other treatments combined with thermal ablation were excluded from the study.
Data extraction and quality assessment
Two investigators (a radiologist and a pneumologist) independently abstracted data using a predefined sheet and the final decision was made by consensus of the all authors. We extracted the following data: author, year of publication, design of study (prospective or retrospective and single-arm or case–control.), population region, age, gender, sample size, tumor size, follow-up duration, and clinical outcomes including tumor local control rates, survival rates, and major complications. The risk of bias was assessed with the Cochrane risk of bias tool.[8] We also assessed the quality of evidence contributing to each network estimate using the GRADE framework (Grade Working Group, USA).
Statistical analysis
Direct pairwise meta-analysis was not performed for unavailable head-to-head comparisons. Bayesian hierarchical modeling of the present network meta-analysis complied with the National Institute for Health and Excellence Decision Support Units guidelines. Count statistics of the local progression rate and major complications rate was analyzed using a Bayesian random effects model to calculate relative effects expressed as Odds Ratios (OR) with a 95% of confidence interval (CI) for pairwise comparisons of different ablation modalities. We assessed statistical heterogeneity with the I2 statistic and P value as well as publication bias with an Egger's test. We did not fit the survival curves because that the detailed 5-year survival data were unavailable.
To determine whether the results were affected by study characteristics, we performed subgroup network meta-analyses by the following variables: study design (prospective/retrospective), population region (Europe/America, East Asia), sample size, tumor size, gender ratio, age, and follow-up duration. The Stata version 14.0 (StataCorp LP, Texas, USA) was used in this network meta-analysis, and the level of statistical significance was set at α =0.05.
Results
There were 34 studies[9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42] identified in this network meta-analysis [Figure 1]. These included 17 studies from Europe/America and 17 studies from East Asia. These studies included a total of 1840 patients (mean age, 67.9; male, 64.5%) with 1318 primary lung tumors and 1202 pulmonary metastatic tumors. The median follow-up was 29 months [Table 1]. Of the 34 studies, only three studies were direct pairwise comparisons design [Figure 2]. The quality of evidence was rated as very low.
Figure 1.
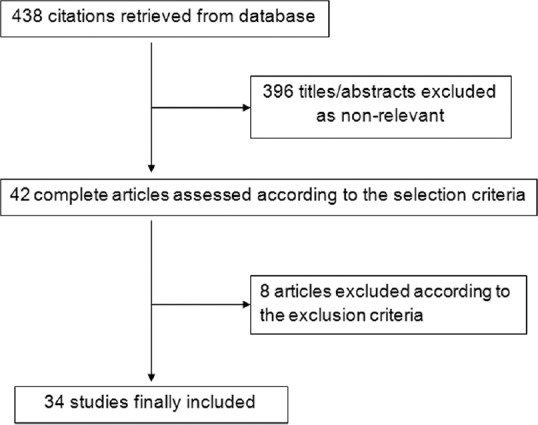
Flow diagram of the reviewing process
Table 1.
Characteristics for thermal ablation studies included in the present meta-analysis
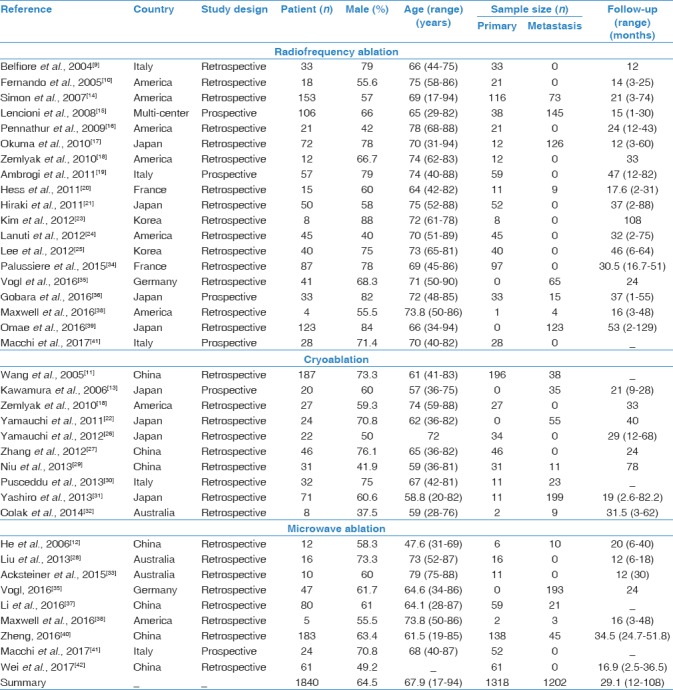
Figure 2.
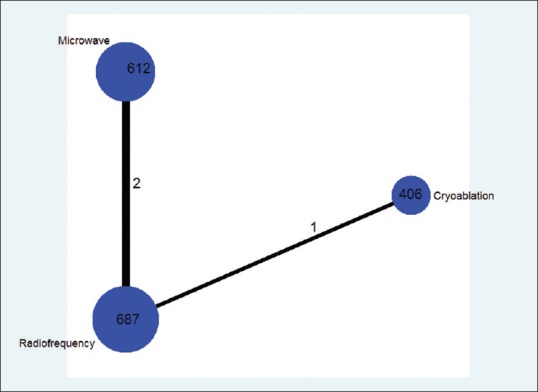
Comparison network of the included studies The width of the lines is proportional to the number of direct comparisons from original studies, and the size of every circle is proportional to the number of randomly assigned participants (sample size)
Local progression
The weighted average local progression rate of thermal ablation was 19.2% (19.8% to RFA, 23.7% to cryoablation, and 10.9% to MWA, respectively) [Table 2]. RFA and MWA were significantly more effective than cryoablation with an OR of 0.04 (95% CI: 0.004, 0.38; P = 0.005) and 0.02 (95% CI: 0.002, 0.24; P = 0.001), respectively. In addition, a comparable efficacy was found between MWA and RFA with an OR of 0.63 (95% CI: 0.04, 10.39; P = 0.745). The I2 value was 75.1% (P < 0.001). Meta-regression identified the study design (P = 0.02) and population region (P = 0.015) were significantly associated with local progression. Publication bias presented using the Egger's test (P = 0.007).
Table 2.
Efficacy and safety of thermal ablation studies included in the present meta-analysis
Major complications
The weighted average major complications rate of thermal ablation was 11.5% (11.6% to RFA, 4.6% to cryoablation, and 22.5% to MWA, respectively) [Table 2]. However, the network meta-analysis showed the comparable safety between cryoablation and RFA (P = 0.974), microwave and RFA (P = 0.979), respectively. The I2 value was 76.4% (P < 0.001). Meta-regression identified none of the potential factors for major complications. The Egger's test (P = 0.089) indicated no publication bias.
Overall survival rate
The 1, 2, 3, 4, and 5 year-weighted average overall survival rate for RFA was 84.3%, 66.8%, 62.4%, 55.1%, and 43.5%, respectively. The 1, 2, and 3 years weighted average overall survival rate for cryoablation was 86.5%, 73.5%, and 71.2%, respectively. The 1, 2, 3, 4, and 5 years weighted average overall survival rate for MWA was 82.5%, 54.6%, 35.7%, 29.6%, and 16.6%, respectively [Figure 3].
Figure 3.
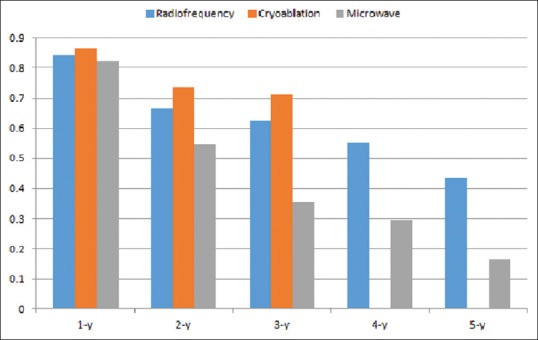
The weighted average overall survival rate
Discussion
In the current network meta-analysis, we conclude that RFA and MWA were more effective to decrease the progression rate of lung malignance than cryoablation with OR of 0.04 (95% CI: 0.004, 0.38; P = 0.005) and 0.02 (95% CI: 0.002, 0.24; P = 0.001), respectively. Regarding the major complications, RFA, MWA, and cryoablation showed comparable safety (P > 0.05).
In the past 10 years, different technologies have been developed for image-guided percutaneous thermal ablation of lung malignancies, mainly including RFA, MWA, and cryoablation. These image-guided ablation techniques are considered safe, cost-effective, and minimally invasive for patients that are not eligible for the surgery.[43] RFA is an electric current-based technique that heats tissue due to fractioning electrons at a frequency of 400 KHz. The air-filled lung spaces insulate the heated volume resulting to low thermal inertia and high electrical impedance compared to other tissues. A multi-tined expandable electrode has been shown to reduce the local recurrence rate.[44] MWA is a field-based technology. The electromagnetic field creates frictional heat by rotating water molecules. Therefore, the temperature rises higher and more rapidly with wider active heating zone than RFA. In addition, MWA relies less on conduction into tissues and less influenced using the heat-sink effect, yielding a more uniform ablation zone. Cryoablation damages tumor cells through a complex combination of different mechanisms during tissue freezing and thawing.
Our results showed that RFA and MWA were more effective than cryoablation for local control rate, while MWA and RFA showed comparable efficacy. The weighted average local recurrence rate was 23.7% for cryoablation, 19.8% for RFA, and 10.9% for MWA. However, the obvious heterogeneity and publication bias in this network meta-analysis weakened the significance. We also found that study design (prospective or retrospective) and population region (Europe/America or East Asia) influenced the local control rate of thermal ablation.
Bi et al. compared the local control rate of stage I NSCLC between RFA and stereotactic body radiation therapy (SBRT), and reported the uncorrected pooled 1, 2 3, and 5 years local control rate for RFA were all significantly lower than those for SBRT.[45] In addition, Bi et al. also reported the pooled 1, 2, 3, and 5 year-overall survival rate, and neither treatment had significant difference. Due to the tremendous heterogeneity, we failed to perform a statistic analysis for the pooled 1, 2, 3, 4, and 5 years overall survival rate.
Although thermal ablation of lung tumors is generally safe, it may cause various complications.[46] Most of the complications can be treated conservatively or with minimal therapy. However, the rare but serious complications should be known, including massive hemorrhage, intractable pneumothorax, pneumonitis, pulmonary artery pseudo aneurysm, injury of nearby important tissues, systemic air embolism, lung abscess and empyema, and skin burn.
This study has some limitations. First, we fail to distinguish the subgroups based on different stages (e.g., stage I, stage II, stage III, and stage IV) of primary lung cancer and metastatic lung tumors from different primary malignancies. This may significantly obscure the overall survival rate among different ablations. Second, in the GRADE framework, most of the comparisons are assessed as low or very low quality. Third, the inclusion of retrospective and single-arm studies may introduce the patient selection bias. Finally, details on patient demographics and tumor characteristics (number/size) are not accounted for (especially worthy of note since tumor size is known to be one of the most important factors that limit the efficacy of ablation).
Conclusion
RFA and MWA may offer an advantage over cryoablation for patients with malignant lung tumors.
Financial support and sponsorship
This research was financially supported by China Postdoctoral Science Foundation (Grant No.: 2017M621677).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Reck M, Heigener DF, Mok T, Soria JC, Rabe KF. Management of non-small-cell lung cancer: Recent developments. Lancet. 2013;382:709–19. doi: 10.1016/S0140-6736(13)61502-0. [DOI] [PubMed] [Google Scholar]
- 3.Dupuy DE. Image-guided thermal ablation of lung malignancies. Radiology. 2011;260:633–55. doi: 10.1148/radiol.11091126. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed M, Liu Z, Afzal KS, Weeks D, Lobo SM, Kruskal JB, et al. Radiofrequency ablation: Effect of surrounding tissue composition on coagulation necrosis in a canine tumor model. Radiology. 2004;230:761–7. doi: 10.1148/radiol.2303021801. [DOI] [PubMed] [Google Scholar]
- 5.Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology. 1998;37:171–86. doi: 10.1006/cryo.1998.2115. [DOI] [PubMed] [Google Scholar]
- 6.Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: Principles and applications. Radiographics. 2005;25(Suppl 1):S69–83. doi: 10.1148/rg.25si055501. [DOI] [PubMed] [Google Scholar]
- 7.Jahangeer S, Forde P, Soden D, Hinchion J. Review of current thermal ablation treatment for lung cancer and the potential of electrochemotherapy as a means for treatment of lung tumours. Cancer Treat Rev. 2013;39:862–71. doi: 10.1016/j.ctrv.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 8.The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions. Ver. 5.1.0. Available from: http://handbook.-5-l .cochrane.org/
- 9.Belfiore G, Moggio G, Tedeschi E, Greco M, Cioffi R, Cincotti F, et al. CT-guided radiofrequency ablation: A potential complementary therapy for patients with unresectable primary lung cancer – A preliminary report of 33 patients. AJR Am J Roentgenol. 2004;183:1003–11. doi: 10.2214/ajr.183.4.1831003. [DOI] [PubMed] [Google Scholar]
- 10.Fernando HC, De Hoyos A, Landreneau RJ, Gilbert S, Gooding WE, Buenaventura PO, et al. Radiofrequency ablation for the treatment of non-small cell lung cancer in marginal surgical candidates. J Thorac Cardiovasc Surg. 2005;129:639–44. doi: 10.1016/j.jtcvs.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Littrup PJ, Duan Y, Zhang Y, Feng H, Nie Z, et al. Thoracic masses treated with percutaneous cryotherapy: Initial experience with more than 200 procedures. Radiology. 2005;235:289–98. doi: 10.1148/radiol.2351030747. [DOI] [PubMed] [Google Scholar]
- 12.He W, Hu XD, Wu DF, Guo L, Zhang LZ, Xiang DY, et al. Ultrasonography-guided percutaneous microwave ablation of peripheral lung cancer. Clin Imaging. 2006;30:234–41. doi: 10.1016/j.clinimag.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Kawamura M, Izumi Y, Tsukada N, Asakura K, Sugiura H, Yashiro H, et al. Percutaneous cryoablation of small pulmonary malignant tumors under computed tomographic guidance with local anesthesia for nonsurgical candidates. J Thorac Cardiovasc Surg. 2006;131:1007–13. doi: 10.1016/j.jtcvs.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 14.Simon CJ, Dupuy DE, DiPetrillo TA, Safran HP, Grieco CA, Ng T, et al. Pulmonary radiofrequency ablation: Long-term safety and efficacy in 153 patients. Radiology. 2007;243:268–75. doi: 10.1148/radiol.2431060088. [DOI] [PubMed] [Google Scholar]
- 15.Lencioni R, Crocetti L, Cioni R, Suh R, Glenn D, Regge D, et al. Response to radiofrequency ablation of pulmonary tumours: A prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study) Lancet Oncol. 2008;9:621–8. doi: 10.1016/S1470-2045(08)70155-4. [DOI] [PubMed] [Google Scholar]
- 16.Pennathur A, Luketich JD, Heron DE, Abbas G, Burton S, Chen M, et al. Stereotactic radiosurgery for the treatment of stage I non-small cell lung cancer in high-risk patients. J Thorac Cardiovasc Surg. 2009;137:597–604. doi: 10.1016/j.jtcvs.2008.06.046. [DOI] [PubMed] [Google Scholar]
- 17.Okuma T, Matsuoka T, Yamamoto A, Oyama Y, Hamamoto S, Toyoshima M, et al. Determinants of local progression after computed tomography-guided percutaneous radiofrequency ablation for unresectable lung tumors: 9-year experience in a single institution. Cardiovasc Intervent Radiol. 2010;33:787–93. doi: 10.1007/s00270-009-9770-9. [DOI] [PubMed] [Google Scholar]
- 18.Zemlyak A, Moore WH, Bilfinger TV. Comparison of survival after sublobar resections and ablative therapies for stage I non-small cell lung cancer. J Am Coll Surg. 2010;211:68–72. doi: 10.1016/j.jamcollsurg.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 19.Ambrogi MC, Fanucchi O, Cioni R, Dini P, De Liperi A, Cappelli C, et al. Long-term results of radiofrequency ablation treatment of stage I non-small cell lung cancer: A prospective intention-to-treat study. J Thorac Oncol. 2011;6:2044–51. doi: 10.1097/JTO.0b013e31822d538d. [DOI] [PubMed] [Google Scholar]
- 20.Hess A, Palussière J, Goyers JF, Guth A, Aupérin A, de Baère T, et al. Pulmonary radiofrequency ablation in patients with a single lung: Feasibility, efficacy, and tolerance. Radiology. 2011;258:635–42. doi: 10.1148/radiol.10100771. [DOI] [PubMed] [Google Scholar]
- 21.Hiraki T, Gobara H, Mimura H, Matsui Y, Toyooka S, Kanazawa S, et al. Percutaneous radiofrequency ablation of clinical stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2011;142:24–30. doi: 10.1016/j.jtcvs.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 22.Yamauchi Y, Izumi Y, Kawamura M, Nakatsuka S, Yashiro H, Tsukada N, et al. Percutaneous cryoablation of pulmonary metastases from colorectal cancer. PLoS One. 2011;6:e27086. doi: 10.1371/journal.pone.0027086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SR, Han HJ, Park SJ, Min KH, Lee MH, Chung CR, et al. Comparison between surgery and radiofrequency ablation for stage I non-small cell lung cancer. Eur J Radiol. 2012;81:395–9. doi: 10.1016/j.ejrad.2010.12.091. [DOI] [PubMed] [Google Scholar]
- 24.Lanuti M, Sharma A, Willers H, Digumarthy SR, Mathisen DJ, Shepard JA, et al. Radiofrequency ablation for stage I non-small cell lung cancer: Management of locoregional recurrence. Ann Thorac Surg. 2012;93:921–7. doi: 10.1016/j.athoracsur.2011.11.043. [DOI] [PubMed] [Google Scholar]
- 25.Lee H, Jin GY, Han YM, Chung GH, Lee YC, Kwon KS, et al. Comparison of survival rate in primary non-small-cell lung cancer among elderly patients treated with radiofrequency ablation, surgery, or chemotherapy. Cardiovasc Intervent Radiol. 2012;35:343–50. doi: 10.1007/s00270-011-0194-y. [DOI] [PubMed] [Google Scholar]
- 26.Yamauchi Y, Izumi Y, Hashimoto K, Yashiro H, Inoue M, Nakatsuka S, et al. Percutaneous cryoablation for the treatment of medically inoperable stage I non-small cell lung cancer. PLoS One. 2012;7:e33223. doi: 10.1371/journal.pone.0033223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X, Tian J, Zhao L, Wu B, Kacher DS, Ma X, et al. CT-guided conformal cryoablation for peripheral NSCLC: Initial experience. Eur J Radiol. 2012;81:3354–62. doi: 10.1016/j.ejrad.2012.04.035. [DOI] [PubMed] [Google Scholar]
- 28.Liu H, Steinke K. High-powered percutaneous microwave ablation of stage I medically inoperable non-small cell lung cancer: A preliminary study. J Med Imaging Radiat Oncol. 2013;57:466–74. doi: 10.1111/1754-9485.12068. [DOI] [PubMed] [Google Scholar]
- 29.Niu L, Chen J, Yao F, Zhou L, Zhang C, Wen W, et al. Percutaneous cryoablation for stage IV lung cancer: A retrospective analysis. Cryobiology. 2013;67:151–5. doi: 10.1016/j.cryobiol.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Pusceddu C, Sotgia B, Fele RM, Melis L. CT-guided thin needles percutaneous cryoablation (PCA) in patients with primary and secondary lung tumors: A preliminary experience. Eur J Radiol. 2013;82:e246–53. doi: 10.1016/j.ejrad.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 31.Yashiro H, Nakatsuka S, Inoue M, Kawamura M, Tsukada N, Asakura K, et al. Factors affecting local progression after percutaneous cryoablation of lung tumors. J Vasc Interv Radiol. 2013;24:813–21. doi: 10.1016/j.jvir.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 32.Colak E, Tatlı S, Shyn PB, Tuncalı K, Silverman SG. CT-guided percutaneous cryoablation of central lung tumors. Diagn Interv Radiol. 2014;20:316–22. doi: 10.5152/dir.2014.13440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Acksteiner C, Steinke K. Percutaneous microwave ablation for early-stage non-small cell lung cancer (NSCLC) in the elderly: A promising outlook. J Med Imaging Radiat Oncol. 2015;59:82–90. doi: 10.1111/1754-9485.12251. [DOI] [PubMed] [Google Scholar]
- 34.Palussiere J, Lagarde P, Aupérin A, Deschamps F, Chomy F, de Baere T, et al. Percutaneous lung thermal ablation of non-surgical clinical N0 non-small cell lung cancer: Results of eight years' experience in 87 patients from two centers. Cardiovasc Intervent Radiol. 2015;38:160–6. doi: 10.1007/s00270-014-0999-6. [DOI] [PubMed] [Google Scholar]
- 35.Vogl TJ, Eckert R, Naguib NN, Beeres M, Gruber-Rouh T, Nour-Eldin NA, et al. Thermal ablation of colorectal lung metastases: Retrospective comparison among laser-induced thermotherapy, radiofrequency ablation, and microwave ablation. AJR Am J Roentgenol. 2016;207:1340–9. doi: 10.2214/AJR.15.14401. [DOI] [PubMed] [Google Scholar]
- 36.Gobara H, Arai Y, Kobayashi T, Yamakado K, Inaba Y, Kodama Y, et al. Percutaneous radiofrequency ablation for patients with malignant lung tumors: A phase II prospective multicenter study (JIVROSG-0702) Jpn J Radiol. 2016;34:556–63. doi: 10.1007/s11604-016-0557-z. [DOI] [PubMed] [Google Scholar]
- 37.Li B, Wang Z, Zhou K, Gao Q, Li X. Safety and feasibility within 24 h of discharge in patents with inoperable malignant lung nodules after percutaneous microwave ablation. J Cancer Res Ther. 2016;12:C171–5. doi: 10.4103/0973-1482.200608. [DOI] [PubMed] [Google Scholar]
- 38.Maxwell AW, Healey TT, Dupuy DE. Percutaneous thermal ablation for small-cell lung cancer: Initial experience with ten tumors in nine patients. J Vasc Interv Radiol. 2016;27:1815–21. doi: 10.1016/j.jvir.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 39.Omae K, Hiraki T, Gobara H, Iguchi T, Fujiwara H, Matsui Y, et al. Long-term survival after radiofrequency ablation of lung oligometastases from five types of primary lesions: A retrospective evaluation. J Vasc Interv Radiol. 2016;27:1362–70. doi: 10.1016/j.jvir.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 40.Zheng A, Ye X, Yang X, Huang G, Gai Y. Local efficacy and survival after microwave ablation of lung tumors: A retrospective study in 183 patients. J Vasc Interv Radiol. 2016;27:1806–14. doi: 10.1016/j.jvir.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 41.Macchi M, Belfiore MP, Floridi C, Serra N, Belfiore G, Carmignani L, et al. Radiofrequency versus microwave ablation for treatment of the lung tumours: LUMIRA (lung microwave radiofrequency) randomized trial. Med Oncol. 2017;34:96. doi: 10.1007/s12032-017-0946-x. [DOI] [PubMed] [Google Scholar]
- 42.Wei Z, Ye X, Yang X, Huang G, Li W, Wang J, et al. Advanced non small cell lung cancer: Response to microwave ablation and EGFR status. Eur Radiol. 2017;27:1685–94. doi: 10.1007/s00330-016-4474-4. [DOI] [PubMed] [Google Scholar]
- 43.Palussière J, Catena V, Buy X. Percutaneous thermal ablation of lung tumors-radiofrequency, microwave and cryotherapy: Where are we going? Diagn Interv Imaging. 2017;98:619–25. doi: 10.1016/j.diii.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 44.Ihara H, Gobara H, Hiraki T, Mitsuhashi T, Iguchi T, Fujiwara H, et al. Radiofrequency ablation of lung tumors using a multitined expandable electrode: Impact of the electrode array diameter on local tumor progression. J Vasc Interv Radiol. 2016;27:87–95. doi: 10.1016/j.jvir.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 45.Bi N, Shedden K, Zheng X, Kong FS. Comparison of the effectiveness of radiofrequency ablation with stereotactic body radiation therapy in inoperable stage I non-small cell lung cancer: A systemic review and pooled analysis. Int J Radiat Oncol Biol Phys. 2016;95:1378–90. doi: 10.1016/j.ijrobp.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hiraki T, Gobara H, Fujiwara H, Ishii H, Tomita K, Uka M, et al. Lung cancer ablation: Complications. Semin Intervent Radiol. 2013;30:169–75. doi: 10.1055/s-0033-1342958. [DOI] [PMC free article] [PubMed] [Google Scholar]