Abstract
Pleomorphic adenoma is the most frequently encountered benign mixed tumor of the minor salivary gland and usually presents in the parotid; however, in the minor salivary gland, it is more common in the palate. Tumors of the minor salivary glands are uncommon, with the most common intraoral site reported being the hard and soft palate owing to the preponderance of minor salivary glands in this region followed by the lips. Pleomorphic adenoma arising from minor salivary glands of the lips tends to occur at an earlier age than it does at other sites. Pleomorphic adenoma of the lip is a rare neoplasm, and thus its diagnosis requires a high index of suspicion and a long-term follow-up. Here, the authors present a case of pleomorphic adenoma of the upper lip.
Keywords: Lip, minor salivary gland, neoplasm, parotid, pleomorphic adenoma
INTRODUCTION
Minor salivary glands exists in large numbers in the oral cavity, especially in the cheek, lip, palate, gingival, floor of the mouth and the retromolar trigone. Overall, they account for 8–10% of the daily total saliva volume. Minor salivary gland tumors are uncommon and represent important intraoral lesions. Pleomorphic adenomas (PAs) are the most common of all salivary neoplasms, accounting for 40–70% of all minor salivary neoplasia.[1] PAs are defined as benign epithelial tumors with varying capsulation.[1] The name “pleomorphic adenoma” was suggested by Willis because of its unique histopathologic characteristics.[2] Although this lesion most often arises in the superficial lobe of the parotid gland, it may be also seen in the submandibular and minor salivary glands.[2] Tumors of the minor salivary glands are uncommon, and the most common intraoral site reported is the hard and soft palate owing to the preponderance of minor salivary glands in this region, followed by the lips.[3] Other sites of occurrence include the floor of the mouth, cheek and retromolar region areas, where minor salivary glands exists in large numbers. Furthermore, malignant lesions are more common in minor salivary glands than benign lesions. However, about 5% of benign lesions undergo malignant transformation. Although the onset of PAs vary, they most commonly occur during the third and fourth decades. There is a female preponderance in PAs, with a female-to-male ratio of 1.9:1.[3]
Histologically, PAs show a diverse range of microscopic appearances, consisting essentially of a capsule, epithelial, myoepithelial and mesenchymal or stromal elements.[4] Although the exact etiology of PA remains unclear, the cells of origin are thought to be reserve cells from the intercalated ducts, while cytogenetics and molecular studies have implicated chromosomal aberration of 8q12 and 12q15.[1,4] Further, using the human androgen receptor gene, it has been shown that the PA stromal and epithelial cells originate from the same precursor.[2] Here, the authors present a case of a patient with PA in the upper lip, thereby reporting a common neoplasm in a rare site.
CASE REPORT
A 33-year-old male presented to the Department of Dental and Maxillofacial Surgery at our hospital with a 3-year history of painless, progressive right upper lip swelling [Figure 1]. There was no history of previous trauma, and past medical and dental history was unremarkable. Clinical examination revealed a rubbery, well-circumscribed, freely mobile and lobulated mass in the right half of the right upper lip, measuring 4 cm at its widest diameter. The overlying labial mucosal was intact and appeared pink and smooth [Figure 2]. The general condition of the patient was normal, with negative regional lymphadenopathy and no abnormalities were detected on further examination. A provisional diagnosis of lipoma was established.
Figure 1.
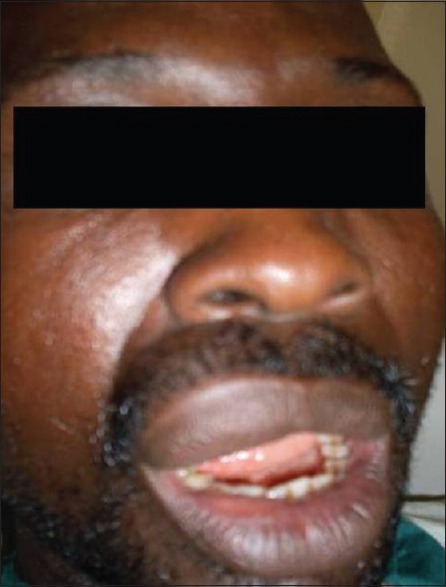
Clinical photograph of the patient
Figure 2.
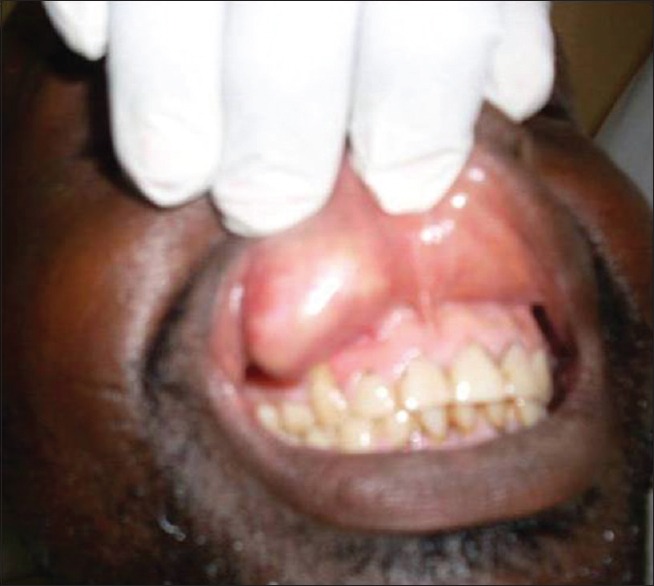
Intraoral clinical photograph
Surgery was performed through regional block of the right infraorbital nerve and augmented with infiltration of the surrounding area of the lesion with 2% lignocaine with 1:100,000 adrenaline hydrochloride. A horizontal labial transmucosal incision was made over the lesion with the upper lip cervically retracted. Complete excision of the lesion was achieved intraorally without compromising the surrounding tissues and overlying skin. Following hemostasis, 3.0 Vicryl sutures were used to close the labial mucosa. The excised lesion was oval, multinodular, yellowish and encapsulated, measuring 3.5 cm in diameter. Early postoperative recovery was uneventful. The patient was subsequently discharged on the same day and consecutively followed up monthly over a period of 6 months. No evidence of recurrence was recorded.
Histopathologic examination of the specimen showed an encapsulated tumor composed of an island and sheets of epithelial and myoepithelial cells arranged in duct, acini, irregular tubules and strands, dispersed within a background of loose myxoid stroma exhibiting chondroid differentiation. There was no evidence of mitosis or necrosis [Figures 3 and 4]. Based on the histological findings, a diagnosis of PA was made.
Figure 3.
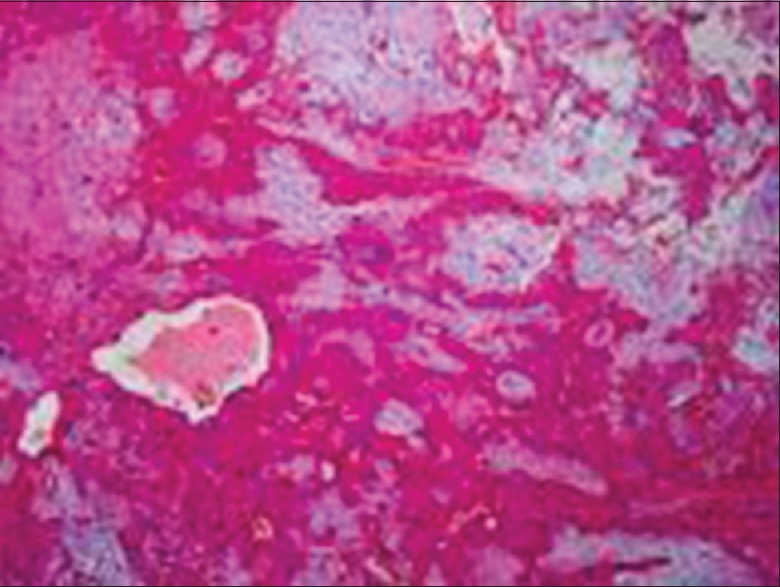
Photomicrograph of specimen at ×4 objective
Figure 4.
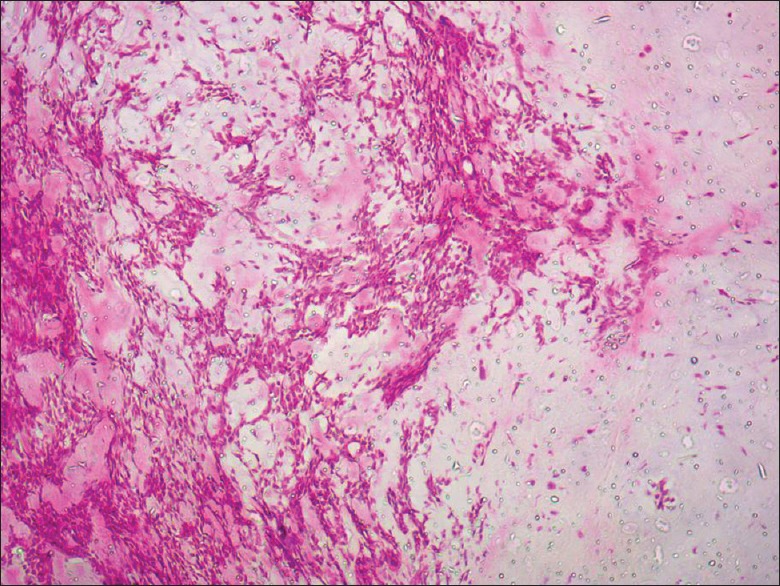
Photomicrograph of the specimen at ×100 objective
DISCUSSION
PA is the most frequently encountered benign mixed tumor of the salivary gland and usually presents in the parotid.[3] In general, tumors originating from the minor salivary gland are uncommon, but PAs account for nearly 40% of the benign tumors here.[5,6] Similar to other tumors in the minor salivary glands, PA most commonly occurs in the palate.[3,6] In our case, PA occurred in the upper lip. Earlier reports found that there is female predilection,[7] but our case was a male. The peak incidence of PA of the lips has also been reported to occur in the third to fourth decades of life, with an average age of 33.2 years,[7] which was also the age of our patient (33 years).
The onset of PA in the minor salivary glands of the lips is at an earlier age than other sites.[8] Previous studies have demonstrated that the prevalence of PA on upper lips is remarkably higher that that on lower lips, with a ratio of 6:1. Further, there is a propensity for benign tumors to occur on the upper lip, whereas malignant lesions predominantly occur on the lower lip.[8] This contrast is possibly because of differences in embryonic development between the upper and lower lips.[8] Lip lesions are found inside the mouth, where they present as a well-circumscribed, nodular, mobile, sessile, rubbery mass. The overlying mucosa is usually pink and not ulcerated, unless severely traumatized. The lesion may appear as a bump externally.[2] Differential diagnosis of PA in the upper lip is canalicular adenoma and it preferentially occurs in the upper lip.[9] Canalicular adenoma lacks chondroid or myxoid matrix, distinguishing it from PA. Histologically, canalicular adenomas differ from PA by showing tumor cell beading and intraluminal squamous balls or morules.[9]
Histopathologically, PA of the lip is not different from the other sites, wherein it presents as a morphologically complex tumor with the epithelial and myoepithelial cells arranged in diverse patterns and embedded in the mucopolysaccharide stroma.[10] Usually, the epithelium forms sheets or ductal structures comprising an inner layer of large, cuboidal cells surrounded by an abluminal layer of myoepithelial cells. The ducts often contain eosinophilic secretory material. The myoepithelial cells could appear as spindle shaped, hyaline, polygonal or clear. The mesenchymal cells are hyalinized, myxoid/mucoid or cartilaginous; mesenchymal cells are sometimes the predominant element of the tumor. Myoepithelial cells are thought to be responsible for production of the myxoid, chondroid and hyalinized stroma.[4]
For differential diagnosis, the immunohistochemical profile of PA is not of significant value. Nevertheless, often, the PA luminal cells stain positively for cytokeratins 3, 6, 10, 11, 13 and 16. In addition, the myoepithelial cells irregularly stain positively for cytokeratins 13, 14 and 16 and pan-cytokeratin as well as, with varying degrees, stain positively for alpha-smooth muscle actin, vimentin, glial acidic fibrillary protein, S-100 protein and calponin.[10]
The surgical treatment for PA is a complete wide surgical excision with adequate safety margins. An inadequate resection or rupture of the capsule or tumor spillage during excision can lead to local recurrence.[2] Carcinoma ex PA arises from untreated PA.[6] However, they have rarely been reported in the lips, perhaps as it is in a conspicuous area where esthetics is compromised, the patient might hasten to present for treatment.
In summary, PAs of the lips are rare, and thus practitioners must have a high index of suspicion for correctly diagnosing it. The best treatment modality is complete wide surgical excision. However, practitioners should note that PA can recur even several years after the surgical excision. In addition, the transformation to the malignant state is possible, and thus long-term follow-up is essential.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient has given his consent for his images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Eveson JW, Kusafuka K, Stenman G, Nagao T. Head and Neck Tumours. Lyon, France: 2005. Pleomorphic Adenoma. Paper Presented at: World Health Organization Classification of Tumours: Pathology & Genetics. [Google Scholar]
- 2.Sood A, Chung S, Datiashvili RO. An incidental finding of pleomorphic adenoma of the minor salivary glands in the skin area of the lower lip. Eplasty. 2014;14:e39. [PMC free article] [PubMed] [Google Scholar]
- 3.Gbotolorun OM, Arotiba GT, Effiom OA, Omitola OG. Minor salivary gland tumours in a Nigerian hospital: A retrospective review of 146 cases. Odontostomatol Trop. 2008;31:17–23. [PubMed] [Google Scholar]
- 4.Debnath SC, Adhyapok AK. Pleomorphic adenoma (benign mixed tumour) of the minor salivary glands of the upper lip. J Maxillofac Oral Surg. 2010;9:205–8. doi: 10.1007/s12663-010-0052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchner A, Merrell PW, Carpenter WM. Relative frequency of intra-oral minor salivary gland tumors: A study of 380 cases from Northern California and comparison to reports from other parts of the world. J Oral Pathol Med. 2007;36:207–14. doi: 10.1111/j.1600-0714.2007.00522.x. [DOI] [PubMed] [Google Scholar]
- 6.Jaber MA. Intraoral minor salivary gland tumors: A review of 75 cases in a Libyan population. Int J Oral Maxillofac Surg. 2006;35:150–4. doi: 10.1016/j.ijom.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Mortazavi H, Alirezaei S, Azari-Marhabi S, Baharvand M, Eshghpour M. Upper lip pleomorphic adenoma: Comparison of reported cases between 1990 and 2012. J Dent Mater Tech. 2013;2:125–9. [Google Scholar]
- 8.Shrestha A, Reddy NS, Ganguly SN. Pleomorphic adenoma of the upper lip: A case report. Nepal Med Coll J. 2010;6:51–3. [Google Scholar]
- 9.Thompson LD, Bauer JL, Chiosea S, McHugh JB, Seethala RR, Miettinen M, et al. Canalicular adenoma: A clinicopathologic and immunohistochemical analysis of 67 cases with a review of the literature. Head Neck Pathol. 2015;9:181–95. doi: 10.1007/s12105-014-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eveson JW, Nagao T. Diseases of the salivary glands. In: Barnes L, editor. Surgical Pathology of the Head and Neck. New York: Informa; 2009. pp. 475–648. [Google Scholar]


