Abstract
This study was initiated as a component of a larger undertaking designed to study bone healing in microgravity aboard the International Space Station (ISS). Spaceflight experimentation introduces multiple challenges not seen in ground studies, especially with regard to physical space, limited resources, and inability to easily reproduce results. Together, these can lead to diminished statistical power and increased risk of failure. It is because of the limited space, and need for improved statistical power by increasing sample size over historical numbers, NASA studies involving mice require housing mice at densities higher than recommended in the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011). All previous NASA missions in which mice were co-housed, involved female mice; however, in our spaceflight studies examining bone healing, male mice are required for optimal experimentation. Additionally, the logistics associated with spaceflight hardware and our study design necessitated variation of density and cohort make up during the experiment. This required the development of a new method to successfully co-house male mice while varying mouse density and hierarchical structure.
For this experiment, male mice in an experimental housing schematic of variable density (Spaceflight Correlate) analogous to previously established NASA spaceflight studies was compared to a standard ground based housing schematic (Normal Density Controls) throughout the experimental timeline. We hypothesized that mice in the Spaceflight Correlate group would show no significant difference in activity, aggression, or stress when compared to Normal Density Controls. Activity and aggression were assessed using a novel activity scoring system (based on prior literature, validated in-house) and stress was assessed via body weights, organ weights, and veterinary assessment. No significant differences were detected between the Spaceflight Correlate group and the Normal Density Controls in activity, aggression, body weight, or organ weight, which was confirmed by veterinary assessments. Completion of this study allowed for clearance by NASA of our bone healing experiments aboard the ISS, and our experiment was successfully launched February 19, 2017 on SpaceX CRS-10.
Keywords: mouse housing density, spaceflight, behavior, stress, aggression
1. INTRODUCTION
Spaceflight investigations are inherently limited in scope due to many constraints not present in traditional laboratory settings. These constraints include: limited time for manipulations, limited reagents/tools available for utilization, limited ability to adjust study design once in spaceflight, inability to readily repeat the study, and limited space/caging hardware for rodent studies (Andreev-Andrievskiy et al., 2014; Kovo, 2017). The latter is a primary focus of the preliminary ground-based study presented here, which was conducted in preparation for spaceflight investigations.
In order to increase our statistical power to detect differences in bone healing in spaceflight versus on Earth, we wanted to utilize the maximum number of mice that NASA’s hardware could accommodate. There are two separate spaceflight hardware units which cage mice (Kovo, 2017). The Transporter is the unit that transports the mice on the Space-X rocket from Kennedy Space Center to the International Space Station (ISS). Currently, a maximum of two Transporters can be flown at one time. Each Transporter has two sides which can house 10 mice/side for a total of 20 mice/Transporter. Therefore, a total of 40 mice can be transferred to the ISS. Soon after the mice arrive to the ISS, the astronauts transfer them into the hardware maintained on the ISS, the Habitat. There are four Habitat units on the ISS, each Habitat has two side which can house 5 mice/side for a total of 10 mice/Habitat. Thus, a total of 40 mice can also be housed on the ISS and used in spaceflight investigations (Kovo, 2017).
Prior to our studies, NASA had only co-housed female mice in spaceflight as previous studies with male mice had shown significant loss of life in spaceflight (Andreev-Andrievskiy et al., 2014). Specifically, a Russian Bion-M 1 biosatellite co-housed groups of 3 male mice, which had undergone training/acclimation to the specialized food delivery system. Only 16 of 45 mice survived the spaceflight. In their report, the researchers suggested that aggression was low under these conditions; however, the same report showed that of the surviving 16 mice, 25 had limb injuries and 38% had tail injuries. Although it was not clear how those injuries occurred, it may be that the loss of life and/or injury could be attributed in part to aggression of male mice, perhaps compounded by animal stress. Regardless of the cause, these results increased NASA’s concern regarding allowing investigators to use male mice in spaceflight studies. For our bone healing studies, all of our previous ground-based testing had been conducted in male C57BL/6 mice. We chose male mice over female mice as their femur is larger, which improves the surgical outcomes compared to using smaller female mice (unpublished observation, MAK). However, obtaining approvals from NASA to utilize male C57BL/6 mice in spaceflight required a series of investigations to mitigate possible risks.
One of the main studies required by NASA was to confirm we could obtain a stable cohort of 10 male C57BL/6 mice that were healthy following segmental bone defect surgery. Another important aspect of the study was to confirm that after 10 male mice had been co-housed in the Transporter for approximately one week (actual time for spaceflight studies varies based on launch schedule and docking schedule of the Space-X Dragon with the ISS), they could be successfully divided into two groups of 5 mice. Success here was considered a lack of aggressive activity above that typically observed with 5 male mice group housed as is standard in research laboratories. A final goal of these studies was to assess the housing density recommendations outlined in the Guide for the Care and Use of Laboratory Animals, in the context of the spaceflight hardware. The “Guide” recommends a cage footprint of 51.6 cm2 per 15-25 g mouse and 77.4 cm2 per >25 g mice (National Research Council, 2011). Designing and validating a protocol to accomplish these tasks was the focus of this work. Therefore, we hypothesized that mice in the Spaceflight Correlate group (which mimics spaceflight experimental design timeline, changes in mouse density, and hierarchy) would show no significant difference in activity, aggression, or stress compared to Normal Density Controls. Of note, we successfully proved this hypothesis and completed these goals as detailed below.These accomplishments were critical in obtaining NASA approval of our spaceflight studies, which launched on SpaceX CRS-10 (February 19, 2017).
2. METHODS
2.1. Ethics Statement:
All rodent experiments were conducted according to our protocol approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee (IACUC study number 10861) and followed the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011). Of note, for spaceflight investigations, the housing density of mice in the Transporter units is under the “Guide’s” recommendation (Table 1) while in our ground-based “Transporter Correlate” studies described here (see below for more details) we minimally exceed the Guide’s recommendation.
Table 1.
Dimensions for N10 cages, N40 cages, NASA Transporter units, and NASA Habitat units.
| N10 |
N40 |
Transporter |
Habitat |
Guide Recommendation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cage | aCage/5 Mice | bCage/10 Mice | Cage | cCage/15 Mice | Cage | Cage/10 Mice | Cage | Cage/5 Mice | ||
| Footprint (cm2) | 556.5 | 111.3 | 55.7 | 1287.1 | 85.8 | 385.2 | 38.5 | 385.7 | 77.0 | ≥51.6 cm2/mouse (<25 g) |
| Habitable Surface Area (cm2) | 556.5 | 111.3 | 55.7 | 1287.1 | 85.8 | 4612.9 | 461.3 | 5690.3 | 1135.5 | |
a Normal Density Control group throughout the study and Spaceflight Correlate group during the Habitat Correlate Phase.
b Spaceflight Correlate group during the Transporter Correlate Phase.
c Spaceflight Correlate group during the Acclimation Phase.
2.2. Animal and Overview of Timeline and Housing Strategy:
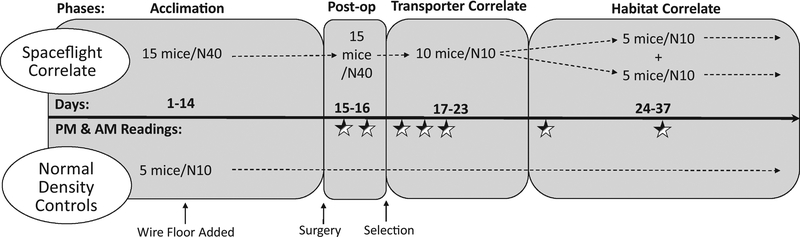
Two hundred, 8-week-old, male C57BL/6 mice were recruited for this study (Jackson Laboratories). This study contained two groups. The control group was termed the “Normal Density Control” group and the experimental group was termed the “Spaceflight Correlate” group. The Spaceflight Correlate group timeline and caging strategy were developed in conjunction with the NASA Ames Research Center Rodent Research IV team to model the likely timeline and caging constraints of the Rodent Research IV spaceflight mission (SpaceX CRS-10). Ten groups of 5 or 15 allo-reared animals were purchased so that from the time of weaning, mice were maintained in same-sex groups. As detailed in Figure 1, groups containing 5 mice were housed in standard shoebox cages or N10 cages (Ancare, N10 polycarbonate rodent cage, 48.4 cm × 74.2 cm × 32.3 cm) upon receipt at Indiana University School of Medicine and throughout the course of this investigation and are considered our “Normal Density Control” group.
Figure 1.
Experimental Timeline. Mice arrived from Jackson Laboratories and were assigned to the Spaceflight Correlate group (n=150 mice, 10 cages of 15 mice) or to the Normal Density Control Group (n=50 mice, 10 cages of 5 mice) based on their grouping during transport (15 mice/group or 5 mice/group, respectively). After 7 days of acclimation, wire grid floors were placed into the bottom of each cage (small arrow) and mice were allowed to acclimate to the wire flooring for an additional 7 days. On day 14 mice underwent perioperative management and surgical treatment (small arrow). Experimental phases are labeled at the top of each rounded box and the width of the phase corresponds to the phase duration. Experiment phases include: Post-op (2 days post-surgery), Transporter Correlate (1 week following Post-op phase), and the Habitat Correlate (2 weeks following the Transporter Correlate phase). Experimental days are denoted above the timeline (dark, horizontal arrow). The days upon which video recordings were collected are labeled below the timeline, marked with a half-shaded star( ).
).
The “Spaceflight Correlate” group, consisted of groups containing 15 allo-reared mice. As shown in Figure 1, the Spaceflight Correlate mice were initially housed in N40 cages (Ancare, N40 polycarbonate rodent cage, 67.7 cm × 122.6 cm × 39.5 cm) during the Acclimation Phase, at the time of surgery, and during the early post-operative period (first 2 days post-surgery). Then 10 of the 15 mice were “selected” to continue in the study and were placed together in an N10 cage for 1 week (mice not selected to continue were humanely euthanized). This one-week period of time is referred to as the Transporter Correlate Phase. The Transporter Correlate Phase uses commercially available N10 rodent cages to approximate the size of the footprint of the NASA Transporter unit (each side of the unit). The NASA Transporter unit is specially designed spaceflight hardware to safely transport mice from Kennedy Space Center to the ISS on the SpaceX Dragon (Kovo, 2017). For spaceflight studies, 10 mice are loaded into each side of the Transporter 1 day before scheduled launch and remain in the Transporter unit for approximately 1 week. As detailed in Table 1, during the Transporter Correlate Phase of the study, the mice are at double the density of that of the Normal Density Control group.
Once the SpaceX Dragon docks to the ISS and the Dragon capsule is unloaded, the astronauts transfer the mice from the Transporter unit to the Rodent Habitat unit. To model this, after being in the Transporter Correlate Phase for 1 week, the 10 mice were randomly divided into 2 groups of 5 mice and each group of 5 mice was housed in an N10 cage for the remainder of the study. This final portion of the study is termed the Habitat Correlate Phase and during this phase the Spaceflight Correlate and Normal Density Control groups are housed at identical densities (Figure 1 and Table 1). The Habitat Correlate Phase also uses commercially available N10 rodent cages to approximate the size of the footprint of the NASA Rodent Habitat unit (each side of the unit). The NASA Rodent Habitat unit is NASA’s spaceflight hardware designed to house the mice while they are onboard the ISS (Kovo, 2017). For clarity, mice in the Spaceflight Correlate arm of the study experience two phases: the Transporter Correlate and Habitat Correlate phases. For a complete description of the spaceflight hardware, please refer to Kovo et al (Kovo, 2017). In brief, flight certified NASA Rodent Habitats and the NASA Transport Unit are powered units built and validated to spaceflight specifications. These Units have active air flow (which facilitates collection of urine, feces, food particulate, etc on the filter beneath the wire grid flooring), lighting, video monitoring (Habitat only), and filtered inlet and exhaust air. The Habitat and Transporter Units are outfitted with NASA Nutrient-upgraded Rodent Food Bar and contain a specialized water delivery system with specialized Lixits (for spaceflight mice must be acclimated to both the Lixits and food). Additional descriptions of the spaceflight hardware and photos showing the cages/NuRFB were recently published (Childress et al., 2018).
The Indiana University School of Medicine animal facility maintained a 12hr:12hr light:dark cycle transitioning at 07:00 and 19:00. The mouse colonies at this institution are tested quarterly for the pathogens listed in our Supplementary Health Report using indirect sentinel exposure. At the time of the study, the mice did not have any of the listed pathogens. The macroenvironment was maintained at 22ºC, and 30 to 70% relative humidity. All mice had ad libitum access to water and Teklad Rodent Diet (Envigo 8604 Indianapolis, IN). Mice were moved into clean cages 3X/week at 09:15. Upon delivery, all cages received shredded paper enrichment (1 Enviropak/5 mice, W.F. Fisher & Son, Inc). As shown in Figure 1, mice were allowed to acclimate for 1 week before introducing a raised wire floor containing 3 openings per inch (Ancare, N10SSRWF and N40SSRWF, respectively for N10 and N40 cages). The raised wire floor was placed on the bottom of all cages to simulate the structure of cages used for spaceflight, which have similar wire walls and floors. Subsequently, mice underwent perioperative management and surgical treatment analogous to our spaceflight experimental design. Resting boards (7.6 cm × 15.2 cm polycarbonate, Bio-Serve, K3392 Rest Stops) were placed in the cages during surgical recovery to provide a surface upon which animals can walk and rest to ease the transition to ambulating after surgery. Resting boards and Enviropak material were removed 2 days post-surgery at which time 10 mice from each of the Spaceflight Correlate cages were selected (and all mice from Normal Density Controls, 5 mice/cage), and all selected mice were transferred into fresh N10 cages with raised wire floors for one week. After one week, the selected ten experimental mice in the Spaceflight Correlate groups were then randomly divided into groups of 5 and moved into clean N10 cages (the Normal Density Controls were also moved into clean N10 cages at this time), and the experiment was completed 2 weeks later (23 days post-surgery). It should be noted that due to the number of animal surgeries and the limited camera systems, surgeries were completed on 5 separate dates (groups were randomized across these surgical dates).
2.3. Surgical Protocol:
One day prior to surgery, mice received Buprenorphine SR (3.0-3.5 mg/kg, SQ, 72 hours of analgesic coverage/injection, Patterson Veterinary). It should be noted that NSAIDs were not used because they are contraindicated for bone healing. On the day of surgery, mice were anesthetized with Ketamine-Xylazine (Patterson Veterinary, 125-20 mg/kg, IP), ophthalmic ointment applied to each eye, and the right hindlimb was shaved and prepared with ethanol and betadine. A 1cm incision was made laterally over the right hindlimb thigh. Blunt dissection was carried down to expose the femur and the muscle bluntly stripped in the diaphyseal region. Next, the knee was flexed and the patellar tendon split with a 27-gauge needle, which was then manually advanced between the femoral condyles into the femoral intramedullary canal. A sterile Dremel rotary cutting tool was then used to remove a 2mm intercalary segment from the femoral diaphysis, and the needle was advanced over a poly(propylene fumarate)/tricalcium phosphate synthetic scaffold (2 mm × 1 mm ID/2 mm ED, 0.7 mm side port to allow for fluid flow) and through the greater trochanter. This synthetic scaffold is identical to that previously described (Chu, Warden, Turner, & Stewart, 2007), reduced in size from that used in rats. The tip of the needle was bent and pulled against the greater trochanter. Next, a collagen sponge imbued with saline (negative control), 5 μg of BMP-2 (positive control, Medtronic), or 5 μg of thrombopoietin (experimental group, Peprotech) was wrapped around the synthetic graft and sutured into place with 3-0 vicryl suture (Ethicon, J215H). The muscle fascia was then closed using 3-0 vicryl suture and the skin was closed using 7mm wound clips (Braintree Scientific, RF7CS). Note: Although comparison of these three treatments was not the aim of this study, this protocol increased fidelity to the planned spaceflight study. Additionally, treatment groups were randomized across our groups for analysis, and although we did not specifically compare treatment with behavior, no overt differences were noted (data not shown).
The non-operated control mice were treated as follows: the mice were prepared as above until just before the incision (no incision was made and surgery was not completed). However, wound clips were placed on the intact skin matching the location in the surgery mice.
Following placement of wound clips, mice were returned to new N10 cages (Normal Density Controls) or N40 cages (Spaceflight Correlate) which contained resting boards and nesting material from their previous cage. Cages were placed half-on/half-off warming pads until all mice recovered from anesthetic. Respiratory rate, body warmth (warm vs. cold as determined by touch), and signs of discomfort were monitored until all mice were independently ambulating within the cage. The mice were then returned to the animal facility and research personnel and/or veterinary staff monitored mice twice daily for the first 48 hours post-surgery, and daily thereafter. Two days post-surgery mice received a second dose of slow release buprenorphine, providing a total of 5 days of analgesia coverage for the mice from the time of surgery. Although not required in these studies, if animals had been identified as in pain or distress by research personnel or veterinary staff (at least daily monitoring), animals would have been provided with additional analgesia.
2.4. Assessment Procedure:
All mice were examined through daily health checks by veterinary staff and research personnel. Specifically, mice were examined for visual evidence of fighting and were evaluate using standard Mouse Health Check Forms (assessing activity, extremities, coat/fur, skin, respiration, eyes and nose), and weighed twice a week for the duration of the study.
Digitally recorded video was obtained for all cages from 07:00-09:00 (immediately after lights turn on, light cycle or AM readings) and from 19:00-21:00 (immediately after lights turn off, dark cycle or PM readings) with wide-angle cameras positioned to observe each cage (Lorex HD security cameras with infrared night-vision capability). Videos were collected immediately after light cycle changes as this is a known stressor and our study is interested in capturing any potential triggers of aggression (Dudley et al., 2003; Hascoet, Bourin, & Nic Dhonnchadha, 2001). As shown in Figure 1, both PM and AM videos were recorded on post-surgery days 1 and 2 during the Post-Op Phase (days 15-16 from mouse arrival). Videos were also recorded the first 3 days after 10 selected mice were placed in N10 cages during the Transporter Correlate Phase (PM and AM videos, days 3-5 post-surgery or days 17-19 after mouse arrival). Finally, videos were obtained the first day after mice were split into groups of 5 mice during the Habitat Correlate Phase and a week later (PM & AM videos, days 10&17 post-surgery or days 24&31 days after mouse arrival).
The observed behaviors/interactions between mice in cages were scored by 3 independent reviewers from a pool of 7 reviewers. The scoring was completed as follows: points were assigned to each instance of behavior observed during the 2-hour video recording and the points were totaled to provide the PM or AM “activity score” for each cage. It is important to note that while we are denoting this as the activity score, it is a combination of active behaviors that may not be considered aggressive, as well as aggressive behaviors. Therefore, the percentage of aggressive behaviors (scores of 7 or 10 for each incident) was also calculated. Table 2 provides details of the behaviors and their assigned scores. Non-aggressive behaviors such as eating, sleeping, or grooming were given a score of “0”. Following or persistent chasing resulted in a score of “3”. Any of the threat postures described by Miczek and O’Donnell (ethograms can be found in their reference (Miczek & O′Donnell, 1978)), as well as urogenital contact or rear-biting were assigned a score of “7”. If a cluster of aggressive behaviors with tumbling to denote fighting was observed a score of “10” was assigned. Readers were trained by veterinary staff to recognize these behaviors and postures and then the scoring system was validated for acceptable inter-observer reliability. All readers were blinded to which cage they were scoring and to what scores other reviewers assigned the cage (current or other time points).
Table 2.
Mouse behavior scoring system.
| Category behavior | Example behavior | Behavior point |
|---|---|---|
| Maintenance behavior | Eating, sleeping, grooming | 0 |
| Increased activity | Following Cagemate | 3 |
| Aggressive interactions/poses | Threat postures, Horizontal threat jumps, Urogenital-biting, Rear-biting, | 7 |
| Aggressive combination of interactions | Sequence of aggressive behaviors with concomitant tumbling | 10 |
2.5. Endpoint Procedure:
Mice were euthanized 23 days post-surgery (37 days after mouse arrival) via CO2 inhalation followed by cervical dislocation. Mice were weighed and adrenal glands, spleen, and thymus were dissected and weighed for 88 mice from the Spaceflight Correlate group and 26 mice from the Normal Density Control cages. Due to operational errors, not all organ weights were collected. The sample numbers in Table 2 are thus lower than the number of animals that began the trial.
2.6. Statistical Analysis:
Unless otherwise indicated, all data are presented as the mean ± standard error. Student’s t-tests and one-way ANOVAs were performed using Microsoft Excel 2016 Data Analysis Toolkit for bilateral and multiple comparisons, respectively. For analysis of animal body weights over time, a repeated measures ANOVA with a Bonferroni post-hoc test (α=0.05) was performed using SPSS (IBM SPSS 24; SPSS Inc., Armonk, NY).
Prior to conducting this study, a power analysis of our primary endpoint (Spaceflight Correlate vs. Normal Density Controls) indicated that a sample size of 8 was required for this study (assuming power of 0.8 and alpha=0.05). As the subject of the study was the behavior of the cage of mice, 8 cages per group was required. Since this was speculative and operational errors could occur we began the study with 10 cages/group.
3. RESULTS
3.1. Validation of Activity and Aggression Scoring System:
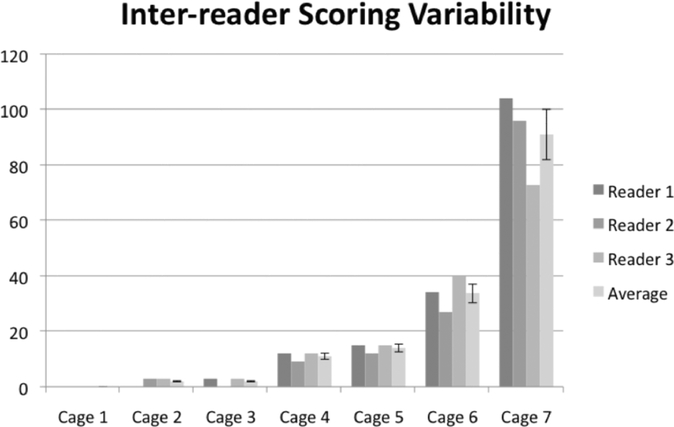
The scores of three independent, blinded readers for seven validation cages are displayed in Figure 2 and summarized in Table 3. The large gap between the different activity cages, and narrow range of scores within each cage, indicate that readers could consistently discriminate among the different behavior types (p<0.05). Importantly, no statistically significant differences were detected between readers for any of the video recordings analyzed (p>0.05).
Figure 2.
Inter-reader scoring variability on low, moderate, and high activity cages. Three independent readers were tasked with scoring 7 cages that had been pre-selected for exemplifying different stages of behavior as detailed in Table 2. No significant differences were observed for scores on the same cage between reviewers (ANOVA, p>0.05). Reviewers were able to reliably delineate between low, moderate, and high activity cages (Student’s t-test, p<0.05).
Table 3.
Mouse behavioral scoring averages and ranges.
| Activity level | Average score | SEM | Range |
|---|---|---|---|
| Low | 1.3 | 0.7 | 0–3 |
| Moderate | 12.5 | 1.5 | 9–15 |
| High (Aggressive) | 62.3 | 28.7 | 27–104 |
The range of the high, aggressive cages reflects the difference between Cage 6 (average 4.8 aggressive behaviors during a 2 hour video recording), and Cage 7 (average 13 aggressive behaviors during a 2 hour video recording). The high standard error for the combined score is likely owing to the very different activity in the cages, as seen in Figure 2, with some contribution from the difficulty of documenting every aggressive display in a highly aggressive group (qualitative observation by readers). Note: This method was further validated by the correlation of physical evidence of fighting (e.g. bite marks, scratching, and scarring) with the high, aggressive score seen in video recording of Cage 7. Based on observations in the video and physical examination of the animals, the aggressor was identified and removed from the cage. Upon removal of the aggressor, wounds healed and aggressive activities were no longer observed.
3.2. Activity and Aggression:
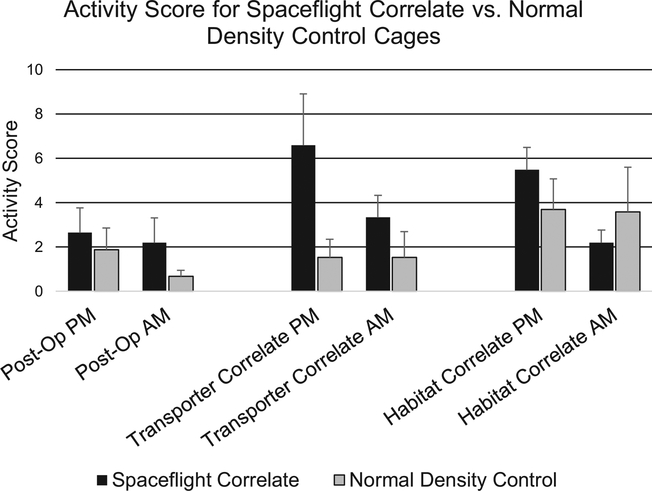
As indicated in our methods and demonstrated in Figure 1, cages were scored during both light (AM) and dark (PM) cycles. Of note, activity was generally higher in dark cycles versus light cycles, an expected finding given that mice are nocturnal animals (Figure 3).
Figure 3.
Average activity scores for Spaceflight Correlate vs. Normal Density Control cages. No significant differences were observed between Spaceflight Correlate and Normal Density Control cages during any phase of the experimental design when reviewing like cycle videos (e.g. AM or PM, Student’s t-test, p>0.05). Data are presented as the mean and error bars represent the standard error of the mean.
Activity data were analyzed during each experimental phase: Post-Operative, Transporter Correlate, and Habitat Correlate. For all analyses, mice in the experimental group (Spaceflight Correlate) were compared to mice in the control group (Normal Density Controls) at each of the phases studied. As seen in Figure 3, activity trended higher in the Spaceflight Correlate cages compared to Normal Density Controls, especially in the PM Transporter Correlate phase, but no significant differences were detected (p≥0.2). As a reminder these scores reflect the activity of each cage, a composite of both the aggressive and non-aggressive behaviors such as following of cage mates as detailed above.
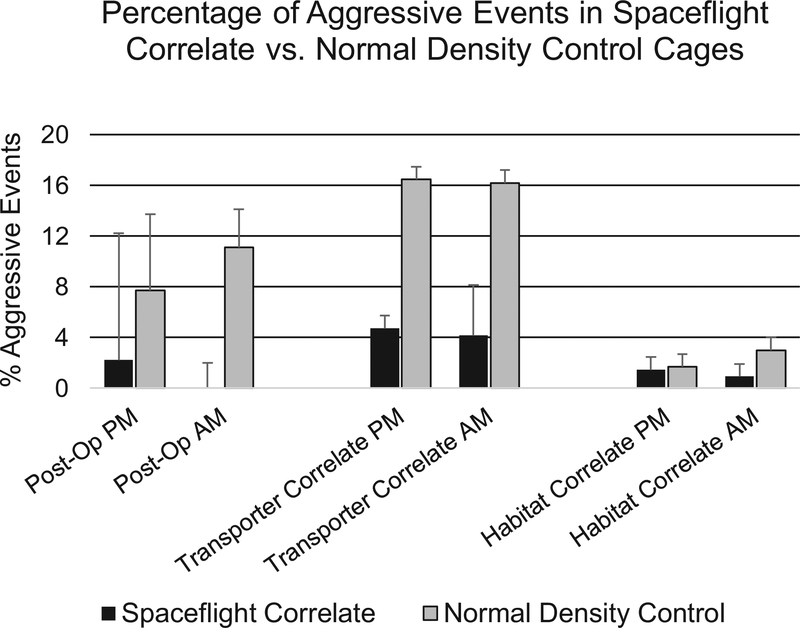
Next, to further evaluate aggressive behavior, aggressive events were tabulated for each cage at each experimental phase: Post-Operative, Transporter Correlate, and Habitat Correlate. The percentage aggressive events was then determined from the total events calculated for each cage. It should be noted that 0’s are not tallied in this calculation, and therefore the percentage of aggression, if anything is overly represented (e.g. falsely elevated). As illustrated in Figure 4, aggression trended lower in the Spaceflight Correlate cages compared to Normal Density Controls, especially during the Post-Operative and Transporter Correlate phases, but no significant differences were detected (p≥0.05).
Figure 4.
Average percentage of aggressive events observed in Spaceflight Correlate vs. Normal Density Control cages. No significant differences were observed between Spaceflight Correlate and Normal Density Control cages during any phase of the experimental design when reviewing like cycle videos (e.g. AM or PM, Student’s t-test, p>0.05). Data are presented as the mean and error bars represent the standard error of the mean.
Overall, these data reflect the veterinary staff’s assessment that the aggression seen in the Spaceflight Correlate cages was not significantly different from standard laboratory housing configurations (Normal Density Controls).
3.3. Organ and Body Weights:
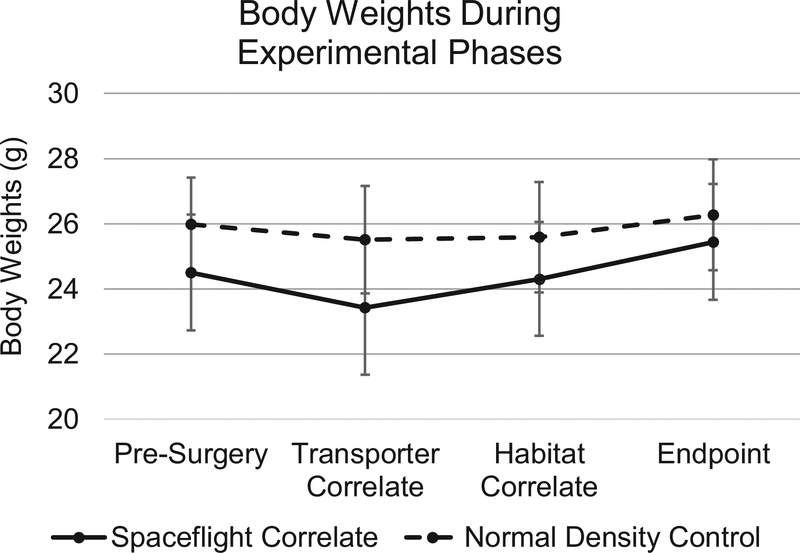
It is known that changes in housing density and hierarchical relationships can contribute to elevated stress level, which can manifest as changes in body and organ size (Whittaker, Howarth, & Hickman, 2012). To assess these possible impacts, we compared Spaceflight Correlate animals with Normal Density Controls. Mouse body weights were recorded immediately before surgery for all animal, at the start of the Transporter Correlate Phase, at the start of the Habitat Correlate Phase, and at the end of the study. Additionally, at the end of the study thymus, spleen, and adrenal gland weights were obtained. As shown in Figure 5, the animal weights did not significantly vary between groups throughout the study duration. Individual organ weights were corrected for animal body weight, and again were not significantly different between groups (Table 4).
Figure 5.
Mouse body weights over time. Body weights were measured pre-surgery, when mice were selected for the Transporter Correlate phase, when mice were moved into the Habitat Correlate phase, and at the end of the experiment. The mean body weight and standard error of the mean at each time period for both the Spaceflight Correlate and Normal Density Control group are shown. No significant differences were detected (repeated measures ANOVA with Bonferroni post-hoc test, p>0.05).
Table 4.
Mouse body and proportional organ weights.
| Group | N | Body Weight (g) |
Adrenal Weight/Body Weight |
Thymus Weight/ Body Weight |
Spleen Weight/ Body Weight |
|---|---|---|---|---|---|
| Spaceflight Correlate |
88 | 25.2 ± 0.2 | 0.30 ± 0.02 | 1.8 ± 0.1 | 3.8 ± 0.1 |
| Normal Density Controls |
26 | 26.5 ± 0.3 | 0.36 ± 0.05 | 1.5 ± 0.1 | 3.6 ± 0.1 |
No significant difference were detected.
4. DISCUSSION AND CONCLUSIONS
The behavior analysis, body weight, and organ weight changes in the Spaceflight Correlate and Normal Density Control groups were similar, which suggests that higher density housing is a viable option for group housing of laboratory mice. This report adds to the body of work demonstrating that mice can be maintained at higher housing densities than typically utilized in the laboratory setting (following the Guide’s recommendation). The current recommendation in the Guide is for mice less than 25 g to be allocated 51.6 cm2 (National Research Council, 2011). As shown in Table 1, while being housed in the transporter they are technically at a higher than recommended density (38.5 cm2/mouse) when using the footprint to calculate surface area. However, the habitable surface area is actually 461.3 cm2/mouse when including the walls and ceiling, which are also accessible to the mice. Indeed, in our recent spaceflight simulation study using the same hardware, NASA personnel observed mice climbing the walls to sit on top of the food (Childress et al., 2018). This indicates that the effective surface area is larger than the published footprint of the cage (Table 1). That said, there are also studies which indicate that higher densities do not lead to aggressive behavior in male mice (Morgan et al., 2014; Nicholson et al., 2009). Yet, debate in the field exists (Foltz & DeLong, 2010; Whittaker et al., 2012), and it is in part because of this debate, as well as NASA’s goal to de-risk our spaceflight experimental design, that this study was completed to confirm that acceptably low levels of aggression would be observed when altering both the density of mice and their hierarchy as detailed in Figure 1 (Spaceflight Correlate group).
As shown in Figures 3 and 4, we observed similar activity and aggression levels between Spaceflight Correlate and Normal Density Control groups suggesting that varying the animal density (first higher, then lower) and cohort composition did not induce aggressive behavior. Of notice, in Figure 3, although not significantly different, there was an apparent increase in the activity of mice in the Spaceflight Correlate group as compared to the Normal Density Controls during the Transporter Correlate Phase. This may be explained in part by the fact that overall cage scores are provided so with 10 mice compared to 5 mice in the same sized cage (N10), there are more mice being observed and likely more activity. Indeed if activity or aggression scores had been normalized by the number of mice/cage, the Spaceflight Correlate cage scores would be virtually identical to those observed in the Normal Density Control cages or lower. That said, although per mouse normalization may be a valid way of presenting the data, the reality of our study is that a single aggressor can trigger failure of the entire cage.
Changes in mouse housing density and hierarchy can result in stress. Chronic stress in mice can lead to weight loss (Whittaker et al., 2012) or slower than average weight gain in growing mice. In our study, as shown in Figure 5, the Spaceflight Correlate mice had lower apparent body weights during all phases of our experimental study design than did the Normal Density Control mice. However, none of the differences were significantly different.
Past research has also shown that chronically elevated levels of stress may be correlated with weight changes in adrenal glands, spleen, and thymus due to elevated production of and circulating glucocorticoids (Domínguez-Gerpe & Rey-Méndez, 1997; Kubera et al., 1998; Retana-Márquez et al., 2003). As detailed in Table 4, no significant differences in normalized organ weights were observed between mice contained within our Spaceflight Correlate and Normal Density Control groups (a non-significant 16.7% decrease in Spaceflight Correlate adrenal weight compared to normal density controls was calculated). It is important to note that while adrenal weights are a sensitive indicator of severe stress, conditions of mild stress are not necessarily reflected in the adrenal weight (Everds et al., 2013; Tuli, Smith, & Morton, 1995). In fact, adrenal weight differences similar to those reported here have been shown to correlate with large differences in corticosterone blood concentrations (Pérez Mera, Guerra Pestonit, & Rey Méndez, 1993). Taken together, these data show no significant differences in severe stress between the experimental and control groups, and may indicate decreased mild stress in the experimental group as compared to the control group. Corticosterone measurements would have better elucidated the differences in mild stress between the Spaceflight Correlate mice and the Normal Density Controls, though this realization was discovered after experimental completion. As such, in future studies we recommend measuring corticosterone concentrations to assist in evaluating differences in stress between experimental and control groups.
Although a main focus of this study was whether housing density impacts aggressive behavior, it is recognized that housing density potentially influences many other physiologic processes. As an example, group housed rats had minimal bone loss (Wronski et al., 1998), whereas significant bone loss was observed in singly housed rats (Jee, Wronski, Morey, & Kimmel, 1983). Aside from bone specific physiologic changes, studies have also reported that high density housing may have a favorable impact on immunological function and can even reduce mortality (Fullwood, Hicks, Brown, Norman, & McGlone, 1998; Gonder & Laber, 2007; McGlone, Anderson, & Norman, 2001).
An initial increase in activity was observed between the Post-operative and Transporter Correlate phases for the Spaceflight Correlate cages. Specifically, as shown in Figure 3, there was a 249% increase in activity among the Spaceflight Correlate cages when mice were moved from the Post-operative phase to the Transporter Correlate phase on day 17. However, this increase was not significant (p>0.1). This apparent increase in activity may be indicative of exploring a new environment and re-establishing hierarchical order (Whittaker et al., 2012).
In conclusion, these data suggest that male C57BL/6 mice can be successfully cohoused without significant differences in activity or aggression even when caged at higher than typical densities and even when 1/3 to 1/2 of the mice are removed (hierarchical order is changed twice over a 1 week period of time). Not only did these findings result in NASA approval of our spaceflight study design, but we used this “step-down method” (15 mice at the recommended density to 10 mice at high density to two groups of 5 mice at the recommended density) during our recent spaceflight investigations which successfully launched February 19, 2017 on SpaceX CRS-10.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Ms. Jane Han and Mr. Evan Himes (Indiana University School of Medicine) for their technical assistance with this project. Dr. Sungshin Choi (Wyle Laboratories, NASA Ames Research Center) provided valuable advice and assistance. Drs. Ruth Globus (NASA Ames Research Center) and Cynthia May Gong (Wyle Laboratories, NASA Ames Research Center) provided expert review and editorial feedback. This work was supported in part by the Medical Student Affairs Summer Research Program in Academic Medicine, Indiana University School of Medicine, funded in part by NIH NIAMS T32 AR065971 (JDR), a postdoctoral NIH T32 Training Grant in Hematopoiesis, T32 DK007519 (PC), Center for Research and Learning RISE Scholarship, Indiana University Purdue University Indianapolis (FK), and the Department of Orthopaedic Surgery, Indiana University School of Medicine (MAK). This work was supported in part by a grant from the Orthopaedic Trauma Association (MAK, TOM) and the Ralph W. and Grace M. Showalter Research Trust Fund (MAK). In addition, research reported in this publication was supported in part by the following grants: NIH NIAMS R01 AR060863 (MAK) and GA-2015-217 from the Center for the Advancement of Sciences in Space (CASIS, MAK). The views, opinions, and/or findings contained in this report are those of the author(s) and should not be construed as official NIH, NASA, or CASIS position, policy, or decision, unless so designated by other official documentation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andreev-Andrievskiy A, Popova A, Boyle R, Alberts J, Shenkman B, Vinogradova O, … Sychev V (2014). Mice in Bion-M 1 space mission: training and selection. PLoS One, 9(8), e104830. doi: 10.1371/journal.pone.0104830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress P, Brinker A, Gong C-MS, Harris J, Olivos DJ, Rytlewski JD, … Kacena MA (2018). Forces associated with launch into space do not impact bone fracture healing. Life Sciences in Space Research, 16, 52–62. doi: 10.1016/j.lssr.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu TM, Warden SJ, Turner CH, & Stewart RL (2007). Segmental bone regeneration using a load-bearing biodegradable carrier of bone morphogenetic protein-2. Biomaterials, 28(3), 459–467. doi: 10.1016/j.biomaterials.2006.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council NR (2011). Guide for the Care and Use of Laboratory Animals: Eighth Edition. Washington, DC: The National Academies Press. [Google Scholar]
- Domínguez-Gerpe L, & Rey-Méndez M (1997). Time-course of the murine lymphoid tissue involution during and following stressor exposure. Life Sciences, 61(10), 1019–1027. doi: 10.1016/S0024-3205(97)00606-1 [DOI] [PubMed] [Google Scholar]
- Dudley CA, Erbel-Sieler C, Estill SJ, Reick M, Franken P, Pitts S, & McKnight SL (2003). Altered patterns of sleep and behavioral adaptability in NPAS2-deficient mice. Science, 301(5631), 379–383. doi: 10.1126/science.1082795 [DOI] [PubMed] [Google Scholar]
- Everds NE, Snyder PW, Bailey KL, Bolon B, Creasy DM, Foley GL, … Sellers T (2013). Interpreting stress responses during routine toxicity studies: a review of the biology, impact, and assessment. Toxicol Pathol, 41(4), 560–614. doi: 10.1177/0192623312466452 [DOI] [PubMed] [Google Scholar]
- Foltz C, & DeLong D (2010). The response of C57BL/6J and BALB/cJ mice to increased housing density. J Am Assoc Lab Anim Sci, 49(2), 136; author reply 136–137. [PMC free article] [PubMed] [Google Scholar]
- Fullwood S, Hicks TA, Brown JC, Norman RL, & McGlone JJ (1998). Floor Space Needs for Laboratory Mice: C56BL/6 Males in Solid-bottom Cages with Bedding. Ilar j, 39(1), 29–36. [DOI] [PubMed] [Google Scholar]
- Gonder JC, & Laber K (2007). A renewed look at laboratory rodent housing and management. Ilar j, 48(1), 29–36. [DOI] [PubMed] [Google Scholar]
- Hascoet M, Bourin M, & Nic Dhonnchadha BA (2001). The mouse light-dark paradigm: a review. Prog Neuropsychopharmacol Biol Psychiatry, 25(1), 141–166. [DOI] [PubMed] [Google Scholar]
- Jee WS, Wronski TJ, Morey ER, & Kimmel DB (1983). Effects of spaceflight on trabecular bone in rats. Am J Physiol, 244(3), R310–314. doi: 10.1152/ajpregu.1983.244.3.R310 [DOI] [PubMed] [Google Scholar]
- Kovo Y (2017, August 3, 2017). Rodent Research Hardware System | NASA. Retrieved October 1, 2017, 2017, from https://www.nasa.gov/ames/research/space-biosciences/rodent-research-hardware
- Kubera M, Basta-Kaim A, Holan V, Simbirtsev A, Roman A, Pigareva N, … Sham J (1998). Effect of mild chronic stress, as a model of depression, on the immunoreactivity of C57BL⧹6 mice. International Journal of Immunopharmacology, 20(12), 781–789. doi: 10.1016/S0192-0561(98)00050-2 [DOI] [PubMed] [Google Scholar]
- McGlone JJ, Anderson DL, & Norman RL (2001). Floor space needs for laboratory mice: BALB/cJ males or females in solid-bottom cages with bedding. Contemp Top Lab Anim Sci, 40(3), 21–25. [PubMed] [Google Scholar]
- Miczek KA, & O′Donnell JM (1978). Intruder-evoked aggression in isolated and nonisolated mice: effects of psychomotor stimulants and L-dopa. Psychopharmacology (Berl), 57(1), 47–55. [DOI] [PubMed] [Google Scholar]
- Morgan JL, Svenson KL, Lake JP, Zhang W, Stearns TM, Marion MA, … Donahue LR (2014). Effects of housing density in five inbred strains of mice. PLoS One, 9(3), e90012. doi: 10.1371/journal.pone.0090012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A, Malcolm RD, Russ PL, Cough K, Touma C, Palme R, & Wiles MV (2009). The response of C57BL/6J and BALB/cJ mice to increased housing density. J Am Assoc Lab Anim Sci, 48(6), 740–753. [PMC free article] [PubMed] [Google Scholar]
- Pérez Mera M, Guerra Pestonit B, & Rey Méndez M (1993). Thymic response of C57BL/6 mice to three different stressors. [Google Scholar]
- Retana-Márquez S, Bonilla-Jaime H, Vázquez-Palacios G, Domínguez-Salazar E, Martínez-García R, & Velázquez-Moctezuma J (2003). Body weight gain and diurnal differences of corticosterone changes in response to acute and chronic stress in rats. Psychoneuroendocrinology, 28(2), 207–227. doi: 10.1016/S0306-4530(02)00017-3 [DOI] [PubMed] [Google Scholar]
- Tuli JS, Smith JA, & Morton DB (1995). Corticosterone, adrenal and spleen weight in mice after tail bleeding, and its effect on nearby animals. Lab Anim, 29(1), 90–95. doi: 10.1258/002367795780740339 [DOI] [PubMed] [Google Scholar]
- Whittaker AL, Howarth GS, & Hickman DL (2012). Effects of space allocation and housing density on measures of wellbeing in laboratory mice: a review. Lab Anim, 46(1), 3–13. doi: 10.1258/la.2011.011049 [DOI] [PubMed] [Google Scholar]
- Wronski TJ, Li M, Shen Y, Miller SC, Bowman BM, Kostenuik P, & Halloran BP (1998). Lack of effect of spaceflight on bone mass and bone formation in group-housed rats. J Appl Physiol (1985), 85(1), 279–285. doi: 10.1152/jappl.1998.85.1.279 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.